21 November 2021: Clinical Research
Total Fluoroscopy Time Reduction During Ultrasound- and Fluoroscopy-Guided Percutaneous Transhepatic Biliary Drainage Procedure: Importance of Adjusting the Puncture Angle
Aleksandar N. Filipović12ABDEF*, Dragan Mašulović12ACDEF, Miloš Zakošek1BCDF, Tamara Filipović23ACDEF, Danijel Galun24ABDEFDOI: 10.12659/MSM.933889
Med Sci Monit 2021; 27:e933889
Abstract
BACKGROUND: The purpose of this observational cohort study was to assess patient and operator-dependent factors which could have an impact on total fluoroscopy time during ultrasound and fluoroscopy-guided percutaneous transhepatic biliary drainage (PTBD).
MATERIAL AND METHODS: Between October 2016 and November 2020, 127 patients with malignant biliary obstruction underwent ultrasound- and fluoroscopy-guided PTBD with the right-sided intercostal approach. The initial bile duct puncture was ultrasound-guided in all patients, and the puncture angle was measured by ultrasound. Any subsequent steps of the procedure were performed under continuous fluoroscopy (15 fps). The patients were divided in 2 groups based on the puncture angle: ≤30° (group I) and >30° (group II). In a retrospective analysis, both groups were compared for inter- and intragroup variability, technical success, total fluoroscopy time, and complications.
RESULTS: In group II, the recorded total fluoroscopy time (232.20±140.94 s) was significantly longer than that in group I (83.44±52.61 s) (P<0.001). In both groups, total fluoroscopy time was significantly longer in cases with a lesser degree of bile duct dilatation, intrahepatic bile duct tortuosity, presence of liver metastases, and multiple intrahepatic bile duct strictures.
CONCLUSIONS: The initial bile duct puncture angle was identified as an operator-dependent factor with the possible impact on total fluoroscopy time. The puncture angle of less than 30° was positively correlated with overall procedure efficacy and total fluoroscopy time reduction.
Keywords: Administration, Cutaneous, Biliary Tract Neoplasms, Fluoroscopy, Aged, 80 and over, Bile Ducts, Cholestasis, Cohort Studies, Drainage, Female, Humans, Male, Radiography, Interventional, Time Factors
Background
Malignant biliary hilar obstruction is primarily caused by hilar cholangiocarcinoma, gall bladder cancer, and hepatocellular carcinoma, and less frequently caused by metastatic lymphadenopathy and advanced gastric and duodenal malignancies [1]. Most patients with hilar malignancy present late in the advanced stage of the disease when curative-intent surgery is no longer feasible [2]. In selected cases, percutaneous biliary drainage (PTBD) is performed as a preoperative procedure or prior to neoadjuvant chemotherapy; however, in most cases, it is performed as a palliative intervention [3]. The main goals of PTBD are to decompress the obstructed biliary system, improve liver function, and, if possible, provide physiological bile flow [1]. Although endoscopic techniques for biliary drainage are widely used, PTBD remains the criterion standard procedure in patients with endoscopically inaccessible bile ducts, particularly in patients with hilar biliary malignancies [4]. According to the available literature, a standard PTBD technique has not been established. In most cases, the employed technique depends on the experience of an institution and its available infrastructure. The initial bile duct puncture is frequently achieved by fluoroscopy or ultrasound guidance [2,4–12]. After initial puncture and entrance to the biliary tree, most interventional radiologists prefer to have fluoroscopy guidance for the remaining steps of PTBD. Repeated fluoroscopy leads to concerns about the control of radiation exposure and to a search for appropriate ways of decreasing radiation doses without compromising the effectiveness and safety of the procedure.
Total fluoroscopy time is one of the few controllable variables that impacts radiation exposure and is frequently used as a parameter for estimating radiation doses for interventional radiology personnel and patients.
To the best of our knowledge, there have been no published data on assessing the variability in total fluoroscopy time during ultrasound- and fluoroscopy-guided PTBD or on the impact of the bile duct puncture angle on reducing fluoroscopy time. The hypothesis of this study was to confirm the positive correlation of the angle between the puncture needle and targeted bile duct with total fluoroscopy time during ultrasound- and fluoroscopy-guided PTBD.
Material and Methods
STUDY DESIGN:
The study was designed as a single-center-based clinical observational cohort study. The study protocol was approved by the Ethics Committee of the Clinical Center of Serbia (protocol no. 717/3) and was reviewed and approved by the
PARTICIPANTS:
The inclusion criteria for this study were pathological confirmation or imaging data confirming malignant disease in the hilar region of the liver causing biliary obstruction with performance status to −1.
The exclusion criteria were terminally ill patients, performance status >2, hepatic decompensation (including ascites), severe underlying cardiac or renal diseases, and coagulation disorders (prothrombin activity <40% or platelet count <50×109/L).
Selection bias was minimized by the inclusion of all consecutive cases that met the selection criteria within a specified time frame. Between October 2016 and November 2020, 127 patients with malignant biliary obstruction underwent PTBD. The patients were divided into 2 groups based on the puncture angle: ≤30° (group I) and >30° (group II).The efficacy of the method was retrospectively analyzed in relation to the puncture angle. Demographic and clinical parameters in relation to the puncture angle are presented in Table 1.
STUDY SAMPLE SIZE AND POWER:
Post hoc power analysis was performed to ensure that the sample size of the study was sufficient to show a clinically significant difference between groups with puncture angles <30° and >30°. The calculation was done using G Power 3.1.9.2 (University Kiel, Germany). The result showed that a final sample size of 127 would achieve 100% power.
CLINICAL AND LABORATORY DATA RECORDING:
Clinical and laboratory data were collected from all patients prior to PTBD. Clinical data included patient age, sex, history of previous intervention, and cause of the obstruction. Imaging data included level of obstruction, dilatation of the intrahepatic bile ducts defined by ultrasound, diameter of the punctured bile duct, and puncture angle. In addition, imaging data were analyzed based on ultrasound, multi-detector computed tomography (CT), or magnetic resonance imaging.
FOLLOW-UP:
After the first follow-up visit 7 days after the intervention, the patients who had normal ultrasound and cholangiography findings with adequate catheter function were followed thereafter on a monthly basis until catheter removal or death. In cases of catheter malposition or malfunction leading to insufficient bile drainage or bile leak, the catheter reposition was performed and the patients were followed for any signs of further leak.
Complications were classified in accordance with the guidelines of the Society of Cardiovascular and Interventional Radiology [7].
PTBD PROCEDURE:
PTBD was performed in patients under sedation and with continual monitoring of vital parameters, including heart rate and oxygen saturation. With patients in the supine position, the initial puncture of the periphery biliary radicle was performed under ultrasound guidance. The Chiba needle (22 G/20 cm with an Echo Tip for enhanced visibility under ultrasound) was placed along the longitudinal axis of the convex, sterile in-plane ultrasound probe (2–6 MHz, Toshiba Xario 200). After puncture of the targeted bile duct, the angle between the needle tip and the targeted bile duct (puncture angle) was measured by an ultrasound angle measurement tool (Figure 1), and the value was recorded. The puncture tract was determined by providing sufficient catheter depth insertion to achieve optimal bile drainage volume and the absence of interposed blood vessels. Any subsequent steps of the procedure were performed with fluoroscopy guidance (Innova 540, General Electric, USA). Fluoroscopy was performed in continuous mode with 15 frames per s (fps) and controlled by the interventional radiologist, by foot pedal. The peripheral bile duct cholangiography was performed by injection of the diluted iodine-based contrast (Iomeron 400 mg/mL, Bracco Imaging, Italy) under fluoroscopy guidance. The opacification of the bile duct confirmed the correct needle tip position. After the cholangiogram was obtained, a 0.018” nitinol guidewire was inserted toward the liver hilum. The needle was then removed, and the sheath (4/6 Fr) was inserted over the wire. The guidewire was changed to a 0.035” guidewire. After the path dilatation over the wire, the catheter was placed. The multipurpose drainage catheter (8.5 Fr with a 10-mm locking loop) was used for external biliary drainage (Figure 2).
Total fluoroscopy time (the total time of exposure to X-rays during intervention) was automatically recorded as a part of the radiation dose report (GE Innova 540 Advantage Workstation software V4.6).
The technical success of the procedure was defined as a successful catheter placement into the biliary system if either internal external or external drainage was accomplished. The criteria for successful clinical management of cholestasis were the following: decrease in serum bilirubin for ≥25% within 7 days and reduction of clinical signs and symptoms (jaundice, pruritus, dark urine).
STATISTICAL ANALYSIS:
All statistical analyses were performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA). The data are presented as arithmetic mean and standard deviation and minimum and maximum values, in the form of absolute and relative numbers. The comparison of numerical variables was performed by the
Results
The study included 127 patients: 71 men (55.9%) and 56 women (44.1%). The causes of biliary obstruction were bile duct carcinoma, gallbladder carcinoma, pancreatic cancer, recurrent disease at the choledochojejunostomy, and metastasis to the liver hilum. The mean values for the examined population were as follows: age 65.26±11.04 years (39 years, 87 years); peripheral bile duct diameter 5.00±1.52 mm; and central bile duct diameter 10.43±2.88 mm. Most of the study population had a PTBD performed with a single puncture (96.9%). A total of 123 interventions were performed with a single ultrasound-guided puncture, and 4 interventions were performed after repeated ultrasound-guided puncture (second puncture). The mean puncture angle was 29.16±13.52°. In 60.6% of patients, the initial puncture angle was ≤30°. Total fluoroscopy time in the whole population was 142.01±121.32 s. There was no statistically significant difference between the study groups in patient age (
Total fluoroscopy time was statistically significantly longer in patients with intrahepatic bile duct tortuosity (
Multivariate regression analysis showed that the time of fluoroscopy was significantly affected by age (beta=0.118,
There was only 1 patient with mild intrahepatic bile duct dilatation and multiple strictures in whom the drainage catheter could not be placed, but there were no patients without bile drainage after catheter placement. Two other patients had insufficient bile drainage after catheter placement in the appropriate position, which was most likely due to liver failure. For those 2 patients, no additional interventions were performed. Thus, sufficient bile drainage was accomplished in 98% of patients (124/127). In this study, the overall rate of procedure-related complications ruled out by clinical findings and follow-up imaging was less than 10%. A few patients had transient hemobilia, and 1 patient had pancreatitis. No major complications, such as bleeding requiring transfusion, bilioarterial fistula, or portal vein thrombosis, were recorded. In the presented series, no procedure-related mortality was recorded. All deaths were related to patients’ poor condition before the procedure and underlying disease.
Discussion
In a recent meta-analysis, Duan et al proposed that selection for endoscopic biliary drainage or PTBD in patients with malignant biliary obstruction should depend on the following: location of the obstruction, purpose of drainage (preoperative procedure or palliative treatment), and level of experience of the treatment center [13]. Interventional radiologist experience decreases the possible impact of the learning curve on the outcome. Therefore, all of the procedures included in the present study were performed in a tertiary care specialist center by a single interventional radiologist with more than 10 years of experience in performing ultrasound- and fluoroscopy-guided PTBD.
The appropriate bile duct dilatation for performing the PTBD procedure is a common bile duct diameter greater than 7 mm and a diameter greater than 3 mm of the intrahepatic segmental branches (measured by ultrasound) [12]. Nondilated ducts were defined as peripheral bile ducts that measured less than 2 mm in diameter or were smaller than the adjacent portal vein [14]. Therefore, patients with mild bile duct dilatation (18/127) were those who had a peripheral bile duct measuring between 2 to 3 mm and a central bile duct measuring 5 to 8 mm. Achieving sterile cannulation of a mildly or nondilated peripheral intrahepatic bile duct during PTBD is a challenging procedure, with a reported success rate of 65% to 95% [6,12,15–35]. In comparison with the reported success rates, in the present study, we achieved a high technical success rate of 94% (17/18), with an acceptable low complication rate.
In our study, patients who had mild bile duct dilatation and a bile duct puncture angle >30° had a statistically significantly longer fluoroscopy time (284.25±163.95 s) than did patients with mild bile duct dilatation and a puncture angle ≤30° (104.90±66.97 s) (
Real-time ultrasound guidance with modern technology is proven to increase accuracy during initial steps of ultrasound- and fluoroscopy-guided PTBD. A reduction in the number of needle passes through the liver to enter bile ducts has been reported when ultrasound guidance was used for the initial bile duct puncture [15–20]. This technique provides an appropriate angle for the biliary tree entrance. Obtaining the precise and planed access to a bile duct is the first and most important step for a successful and safe percutaneous biliary drainage. Whenever possible, our aim was to enter the bile duct with an angle of less than 30° [21] to reduce the risk of vascular lesion (biliovascular fistula) and maintain the adequate needle tip position during the respiratory movement of the liver. This technique provided an almost straight route for the guidewire, thus enhancing the efficacy of the remaining part of the PTBD procedure. Any subsequent step of the procedure was fluoroscopy-guided and dependent from the previous ultrasound-guided step, exaggerating the importance of the initial puncture angle.
Because the greatest part of this procedure is performed under fluoroscopy and is frequently associated with repeated exposure of interventional radiology personnel and patients to radiation, there is a rising concern over radiation exposure. Radiation exposure is linearly correlated to exposure time [36]. Through our literature review, we found a wide range of success rates and an average effective patient radiation dose that could be attributed to the use of different interventional strategies [12,37]. Kühn et al [12] reported various measures that were not included in the standardized protocol used to facilitate PTBD in patients with nondilated bile ducts, such as CT-guided puncture of the bile ducts, and used a fluoroscopy time as an indirect measure of the complexity of the intervention. Knowing that radiation exposure time is a controllable variable, which depends on the complexity of the intervention, our aim was to find a practical technique to simplify the biliary system approach and reduce radiation doses without compromising the safety and efficacy of the procedure. By identifying operator-dependent and controllable factors with a possible impact on fluoroscopy time, we were able to improve the PTBD technique. There are not many studies that have investigated the importance of the initial bile duct puncture angle during PTBD [15,21], and none of them revealed the practical importance of the puncture angle on overall procedure efficacy and radiation exposure.
All procedures in this study were performed by following the already established principle of as low as reasonably achievable (ALARA) and by adjusting image quality by reducing radiation doses to a low level [38].
The current study has several limitations. First, it was a retrospective study, and selection bias would be difficult to determine. Second, we included only cases with malignant hilar biliary obstruction and intercostal approach PTBD procedures without considering cases with other causes of obstruction, different level of biliary obstruction, and a left-sided approach. This might diminish the power of our statistical conclusions. Further investigation is needed to evaluate our findings, regardless of the puncture site and the cause of obstruction. Third, this was a single-center study and all procedures included in this study were performed by single operator with more than 10 years of experience in performing ultrasound- and fluoroscopy-guided procedures. The final limitation was the lack of analysis of inter-observer agreement.
Conclusions
Ultrasound- and fluoroscopy-guided PTBD was shown to be a safe and effective procedure for palliation of biliary obstruction in patients with malignant hilar biliary obstruction. Ultrasound-guided initial puncture and entrance to the biliary tree with an angle ≤30° was an operator-dependent factor with an impact on total fluoroscopy time reduction during ultrasound- and fluoroscopy-guided PTBD. According to the presented results, which need further external validation in larger samples, it can be concluded that adjusting the initial puncture angle could be an additional valuable practical technique in reducing radiation dose without compromising the safety and efficacy of the PTBD procedure, with emphasis on its advantage in reducing complications.
Figures
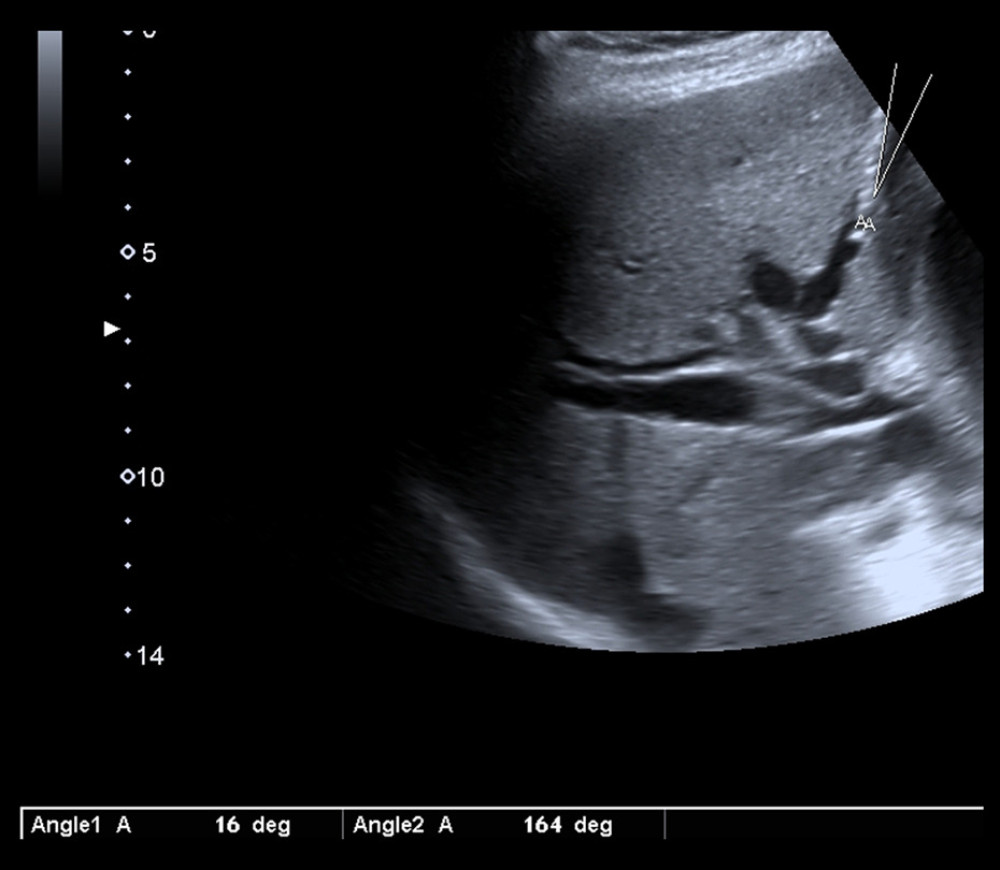 Figure 1. Chiba needle tip and puncture angle measurement. Software used for creation of the figure: Ultrasound Software V2.0, Toshiba Xario 200.
Figure 1. Chiba needle tip and puncture angle measurement. Software used for creation of the figure: Ultrasound Software V2.0, Toshiba Xario 200. 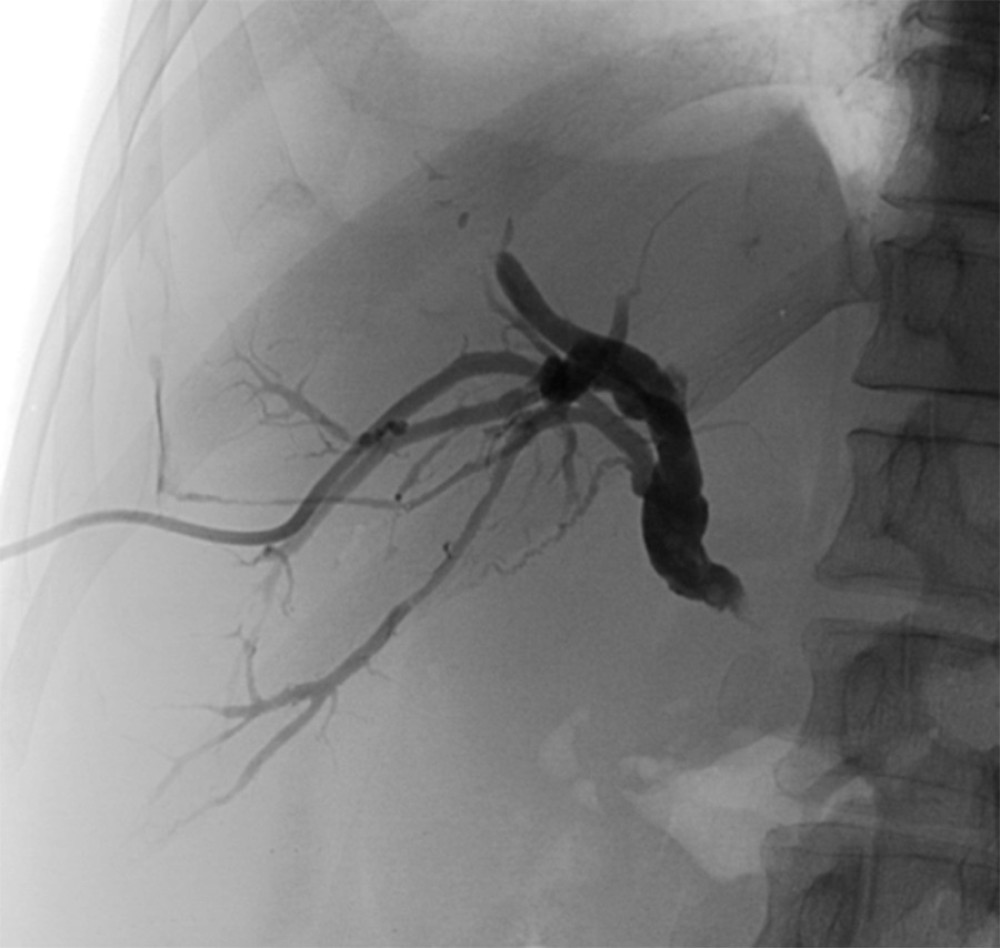 Figure 2. Control radiography performed after drainage catheter placement. Software used for creation of the figure: Advantage Workstation Software V4.6, General Electric.
Figure 2. Control radiography performed after drainage catheter placement. Software used for creation of the figure: Advantage Workstation Software V4.6, General Electric. References
1. Madhusudhan KS, Gamanagatti S, Srivastava DN, Gupta AK, Radiological interventions in malignant biliary obstruction: World J Radiol, 2016; 8(5); 518
2. Nennstiel S, Treiber M, Faber A, Comparison of ultrasound and fluoroscopically guided percutaneous transhepatic biliary drainage: Dig Dis, 2019; 37(1); 77-86
3. George C, Byass OR, Cast JEI, Interventional radiology in the management of malignant biliary obstruction: World J Gastrointest Oncol, 2010; 2(3); 146
4. Dumonceau J-M, Tringali A, Blero D, Biliary stenting: Indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline: Endoscopy, 2012; 44(03); 277-98
5. Saad WE, Transhepatic techniques for accessing the biliary tract: Tech Vasc Interv Radiol, 2008; 11(1); 21-42
6. Saad WE, Wallace MJ, Wojak JC, Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy: J Vasc Interv Radiol, 2010; 21(6); 789-95
7. Burke DR, Lewis CA, Cardella JF, Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage: J Vasc Interv Radiol, 2003; 14(9 Pt 2); S243
8. Laufer U, Kirchner J, Kickuth R, A comparative study of CT fluoroscopy combined with fluoroscopy versus fluoroscopy alone for percutaneous transhepatic biliary drainage: Cardiovasc Intervent Radiol, 2001; 24(4); 240-44
9. Froelich JJ, Wagner H-J, Ishaque N, Comparison of C-arm CT fluoroscopy and conventional fluoroscopy for percutaneous biliary drainage procedures: J Vasc Interv Radiol, 2000; 11(4); 477-82
10. Kim JH, Clinical feasibility and usefulness of CT fluoroscopy-guided percutaneous transhepatic biliary drainage in emergency patients with acute obstructive cholangitis: Korean J Radiol, 2009; 10(2); 144-49
11. Bapaye A, Dubale N, Aher A, Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP: United Eur Gastroenterol J, 2013; 1(4); 285-93
12. Kühn JP, Busemann A, Lerch MM, Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts: Am J Roentgenol, 2010; 195(4); 851-57
13. Duan F, Cui L, Bai Y, Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: A systematic review and meta-analysis: Cancer Imaging, 2017; 17(1); 27
14. Funaki B, Zaleski G, Straus C, Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts: Am J Roentgenol, 1999; 173(6); 1541-44
15. Wagner A, Mayr C, Kiesslich T, Reduced complication rates of percutaneous transhepatic biliary drainage with ultrasound guidance: J Clin Ultrasound, 2017; 45(7); 400-7
16. Dietrich C, Braden B, Ignee A, Perkutane transhepatische Cholangiodrainage: Interventionelle Sonographie Thieme Verlag, 2011; 283-304 [in German]
17. Lee W, Kim GC, Kim JY, Ultrasound and fluoroscopy guided percutaneous transhepatic biliary drainage in patients with nondilated bile ducts: Abdom Imaging, 2008; 33(5); 555-59
18. Koito K, Namieno T, Nagakawa T, Morita K, Percutaneous transhepatic biliary drainage using color Doppler ultrasonography: J Ultrasound Med, 1996; 15(3); 203-6
19. Wimmer B, Hauenstein K, Kauffmann G, Friedburg H, Sonography for percutaneous biliary drainage: RöFo, 1981; 135(4); 466-70
20. Sukigara M, Taguchi Y, Watanabe T, Percutaneous transhepatic biliary drainage guided by color Doppler echography: Abdom Imaging, 1994; 19(2); 147-49
21. Shimizu H, Kato A, Takayashiki T, Peripheral portal vein-oriented non-dilated bile duct puncture for percutaneous transhepatic biliary drainage: World J Gastroenterol, 2015; 21(44); 12628
22. De Jong EA, Moelker A, Leertouwer T, Percutaneous transhepatic biliary drainage in patients with postsurgical bile leakage and nondilated intrahepatic bile ducts: Dig Surg, 2013; 30(4–6); 444-50
23. Righi D, Franchello A, Ricchiuti A, Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split liver transplantation without dilatation of the biliary tree: Liver Transpl, 2008; 14(5); 611-15
24. Aytekin C, Boyvat F, Harman A, Percutaneous management of anastomotic bile leaks following liver transplantation: Diagn Interv Radiol, 2007; 13(2); 101
25. Pedicini V, Poretti D, Mauri G, Management of post-surgical biliary leakage with percutaneous transhepatic biliary drainage (PTBD) and occlusion balloon (OB) in patients without dilatation of the biliary tree: Preliminary results: Eur Radiol, 2010; 20(5); 1061-68
26. Teplick SK, Flick P, Brandon JC, Transhepatic cholangiography in patients with suspected biliary disease and nondilated intrahepatic bile ducts: Gastrointest Radiol, 1991; 16(1); 193-97
27. Cozzi G, Severini A, Civelli E, Percutaneous transhepatic biliary drainage in the management of postsurgical biliary leaks in patients with nondilated intrahepatic bile ducts: Cardiovasc Intervent Radiol, 2006; 29(3); 380-88
28. Kim YH, Cha SJ, US-guided percutaneous transhepatic biliary drainage: Comparative study of right-sided and left-sided approach: J Korean Radiol Soc, 2002; 46(2); 115-18
29. Makuuchi M, Bandai Y, Ultrasonically guided cholangiography and bile drainage: Interventional Ultrasound, 1985, Berlin, Heidelberg, Springer
30. Kozlov A, Polikarpov A, Oleshchuk N, Tarazov P, Comparative assessment of percutaneous transhepatic cholangiodrainage under roentgenoscopy and ultrasound guidance: Vestn Rentgenol Radiol, 2002(4); 30-33
31. Morita S, Kitanosono T, Lee D, Comparison of technical success and complications of percutaneous transhepatic cholangiography and biliary drainage between patients with and without transplanted liver: Am J Roentgenol, 2012; 199(5); 1149-52
32. Honoré C, Vibert E, Hoti E, Management of excluded segmental bile duct leakage following liver resection: HPB (Oxford), 2009; 11(4); 364-69
33. Stampfl U, Hackert T, Radeleff B, Percutaneous management of postoperative bile leaks after upper gastrointestinal surgery: Cardiovasc Intervent Radiol, 2011; 34(4); 808-15
34. Miyazaki M, Shibuya K, Tokue H, Tsushima Y, Percutaneous transhepatic biliary drainage assisted by real-time virtual sonography: A retrospective study: BMC Gastroenterol, 2013; 13(1); 127
35. Schmitz D, Grosse A, Hallscheidt P, Color Doppler ultrasound-guided PTBD with and without metal stent implantation by endoscopic control: Prospective success and early adverse event rates: Z Gastroenterol, 2015; 53(11); 1255-60
36. Berrington de Gonzalez A, Darby S, Risk of cancer from diagnostic X rays: Estimates for the UK and 14 other countries: Lancet, 2004; 363; 345-51
37. Stratakis J, Damilakis J, Hatzidakis A, Radiation dose and risk from fluoroscopically guided percutaneous transhepatic biliary procedures: J Vasc Interv Radiol, 2006; 17(1); 77-84
38. Doyen B, Maurel B, Hertault A, Radiation safety performance is more than simply measuring doses! Development of a radiation safety rating scale: Cardiovasc Intervent Radiol, 2020; 43(9); 1331-41
Figures
 Figure 1. Chiba needle tip and puncture angle measurement. Software used for creation of the figure: Ultrasound Software V2.0, Toshiba Xario 200.
Figure 1. Chiba needle tip and puncture angle measurement. Software used for creation of the figure: Ultrasound Software V2.0, Toshiba Xario 200. Figure 2. Control radiography performed after drainage catheter placement. Software used for creation of the figure: Advantage Workstation Software V4.6, General Electric.
Figure 2. Control radiography performed after drainage catheter placement. Software used for creation of the figure: Advantage Workstation Software V4.6, General Electric. Tables
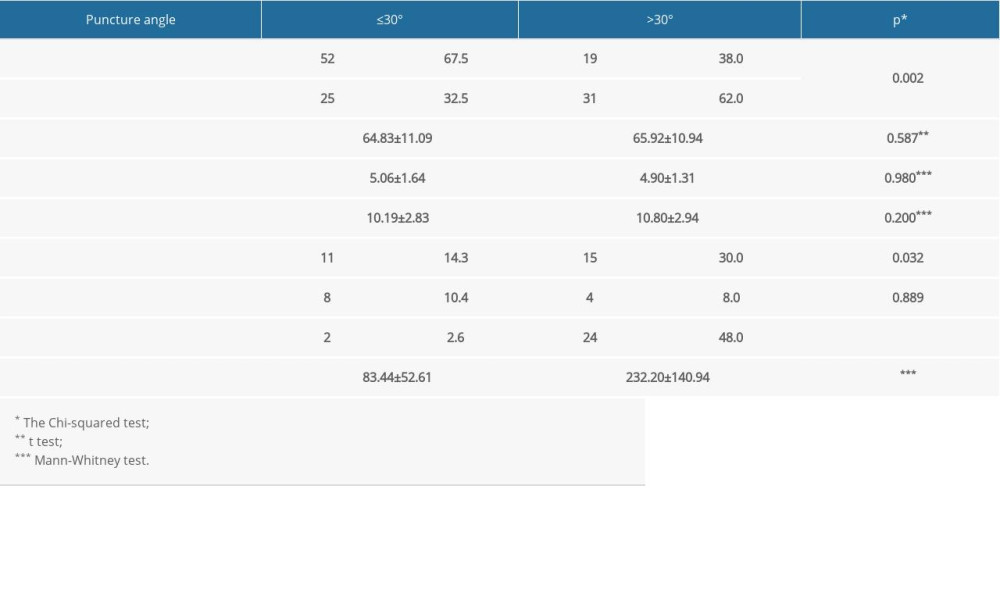 Table 1. Demographic and clinical parameters in relation to puncture angle.
Table 1. Demographic and clinical parameters in relation to puncture angle.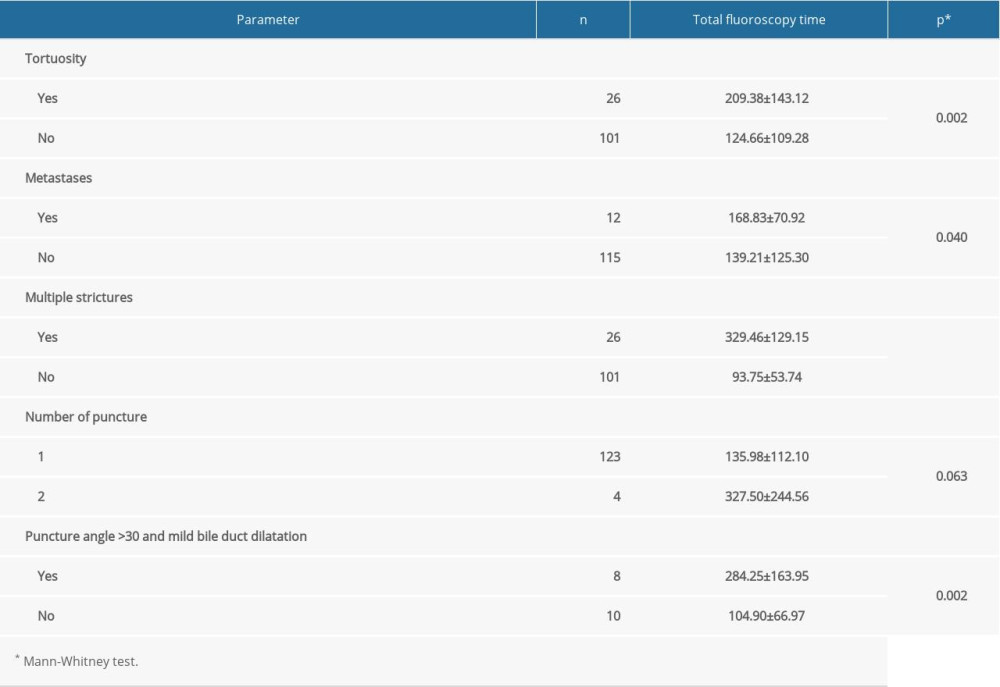 Table 2. Total fluoroscopy time in relation to the examined parameters.
Table 2. Total fluoroscopy time in relation to the examined parameters.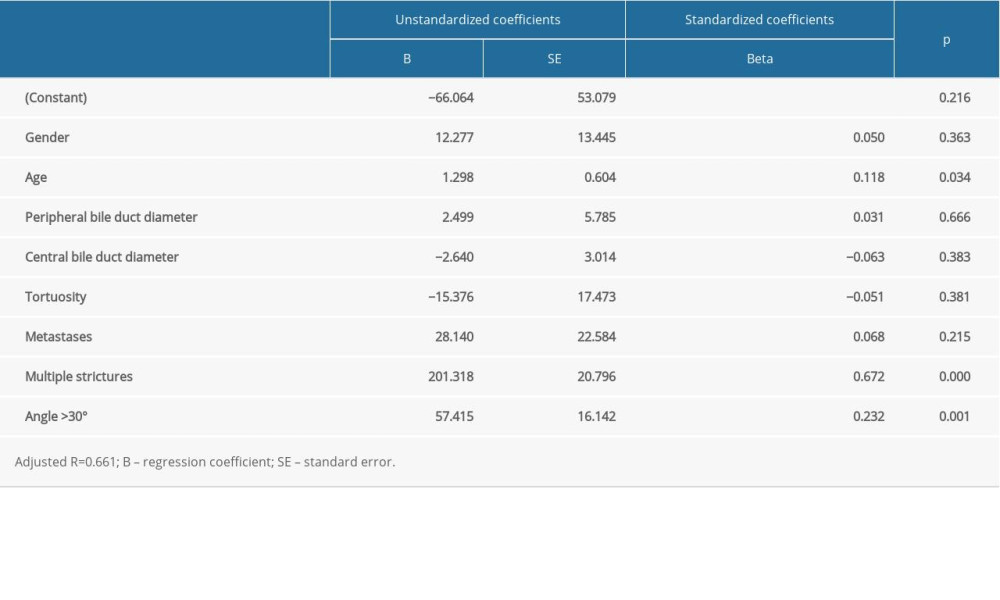 Table 3. The association of total fluoroscopy time and demographic and clinical characteristics.
Table 3. The association of total fluoroscopy time and demographic and clinical characteristics. Table 1. Demographic and clinical parameters in relation to puncture angle.
Table 1. Demographic and clinical parameters in relation to puncture angle. Table 2. Total fluoroscopy time in relation to the examined parameters.
Table 2. Total fluoroscopy time in relation to the examined parameters. Table 3. The association of total fluoroscopy time and demographic and clinical characteristics.
Table 3. The association of total fluoroscopy time and demographic and clinical characteristics. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








