08 February 2022: Database Analysis
Evaluation of Structural Neural Connectivity Between the Primary Auditory Cortex and Cognition-Related Brain Areas Using Diffusion Tensor Tractography in 43 Normal Adults
Sung Ho JangDOI: 10.12659/MSM.936131
Med Sci Monit 2022; 28:e936131
Abstract
BACKGROUND: Little is known about the structural neural connectivity between the primary auditory cortex and cognition-related brain areas in the human brain. This study aimed to evaluate the structural neural connectivity between the primary auditory cortex and cognition-related brain areas in normal subjects, using diffusion tensor tractography (DTT).
MATERIAL AND METHODS: Forty-three healthy subjects with no prior history of audiological, neurological, physical, or psychiatric illnesses were recruited for this study. Diffusion tensor imaging data analysis was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library. In each subject, a region of interest was set on the primary auditory cortex, including the subcortical white matter. We assessed the neural connectivity between the primary auditory cortex and cognition-related brain areas (the dorsolateral prefrontal cortex [DLPFC]; ventrolateral prefrontal cortex [VLPFC]; orbitofrontal cortex [OFC]; hippocampus; parahippocampal cortex; amygdala, anterior and posterior cingulate gyrus; and fornix).
RESULTS: According to the results of DTT, the primary auditory cortex showed neural connectivity (over 50%) with the following areas: the threshold of 1 streamline – the VLPFC (94.2%), OFC (84.9%), fornix (80.2%), hippocampus (76.7%), parahippocampal cortex(74.4%) and DLPFC (58.1%); the threshold of 5 streamlines – the VLPFC (88.4%), OFC (81.4%), fornix (66.3%), hippocampus (55.8%), and parahippocampal cortex (53.5%); and the threshold of 15 streamlines – the VLPFC (82.6%), OFC (74.4%), and fornix (53.5%).
CONCLUSIONS: In normal human subjects, DTT showed that the primary auditory cortex had a high degree of neural connectivity with the prefrontal cortex, fornix, hippocampus, and parahippocampal cortex, which are brain areas associated with cognition and memory.
Keywords: Auditory Cortex, diffusion tensor imaging, Neural Pathways, Cognition, Adult, Brain, Female, Humans, Male, Neuroimaging, Reference Values, young adult
Background
The auditory cortex, which is part of the auditory system, processes auditory information, performing the basic and higher functions of hearing [1,2]. The auditory cortex, which is in the temporal lobe, is classified as the primary, secondary, and tertiary auditory cortices [3]. The primary auditory cortex (Brodmann areas 41 and 42), which plays a crucial role in sound perception, is the most highly organized processing area of sound in the human brain [4]. Previous studies have reported that the primary auditory cortex is also related to cognitive functions, such as decision-making, auditory nonverbal attention, prediction, learning, and working memory [5–11].
The prevalence of hearing loss is estimated at 1.57 billion people globally, and the incidence is higher in older adults; more than 60% of people with hearing loss are over 50 years old [12,13]. Recent studies have reported that hearing loss is associated with cognitive impairment and the exacerbation of dementia [13–17]. Changes in the neural network between the primary auditory cortex and cognition-related brain areas have been suggested as an important pathophysiological hypothesis for cognitive impairment following hearing loss [18–20]. Thus, clarification of the neural connections of the primary auditory cortex with cognition-related brain areas would be important in neuro-rehabilitation.
Previous studies using resting-state functional magnetic resonance imaging (fMRI) have reported that the auditory cortex has functional connectivity with various brain areas, including the cognition-related brain areas (dorsolateral prefrontal cortex [DLPFC], orbitofrontal cortex [OFC], cingulate cortex, hippocampus, and amygdala) [11,21,22]. On the other hand, resting-state fMRI has a limitation in identifying the structural neural connections because it results from functional connectivity between brain areas based on an evaluation of coordinated neural activity using the synchronous firing of transient neural signals [23,24]. Recently developed diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), is a technique used to reveal the structural neural connectivity in three-dimensional visualization by the detection of the translational displacement of water molecules [25,26]. In particular, the estimation of local uncertainty could be enabled by using the probabilistic tracking method in the fiber orientation of each voxel [25,26]. In other words, it considers the distribution of the underlying fiber structure. Accordingly, probabilistic DTI tractography has been used widely to investigate structural connectivity between neural structures, including the amygdala, red nucleus, lateral geniculate body, and vestibular nucleus, in the human brain [27–31]. Some DTT-based studies have demonstrated structural connectivity between the auditory cortex and several specific brain areas (the amygdala, superior temporal sulcus, supramarginal gyrus, intraparietal sulcus, occipital cortex and secondary somatosensory cortex) [32–34]. On the other hand, there has been no study on the structural neural connectivity of the primary auditory cortex with the cognition-related brain areas in the human brain.
This study aimed to evaluate the structural neural connectivity between the primary auditory cortex and cognition-related brain areas in normal subjects, using DTT.
Material and Methods
ETHICS APPROVAL:
The retrospective study was performed in accordance with the requirements of the Declaration of Helsinki research guidelines and the institutional review board of a university hospital approved the study protocol. All subjects understood the purpose of the study and provided written, informed consent prior to participation.
SUBJECTS:
Forty-three subjects (22 males, 21 females; mean age 33.37±8.37 years, range 20–48 years) with no history of audiological, neurological, physical, or psychiatric illnesses were recruited for this study.
DIFFUSION TENSOR IMAGING:
DTI scanning was performed using a 1.5T Philips Gyroscan Intera scanner (Hoffman-LaRoche, Best, Netherlands) with a six-channel head coil. A single-shot, spin-echo planar imaging method was used in 67 contiguous slices acquiring parallel to the anterior commissure-posterior commissure line for each of the 32 non-collinear diffusion-sensitizing gradients (acquisition matrix=96×96; reconstructed to matrix=192×192; field of view=240×240 mm2; repetition time=10,398 ms; echo time=72 ms; parallel imaging reduction factor [sensitivity encoding factor]=2; echo planar imaging factor=59; b=1000 s/mm2; number of excitations=1; and slice thickness=2.5 mm).
PROBABILISTIC FIBER TRACKING:
The Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL: www.fmrib.ox.ac.uk/fsl) was used for DTI data analysis [35–37]. Affine multi-scale two-dimensional registration was applied to correct for head motion effects and image distortions. A probabilistic tractography method based on a multifiber model provided in FMRIB diffusion software with BedpostX method was applied using the software routines option, consisting of 5000 streamline samples, 0.5 mm step lengths, and curvature thresholds=0.2, for fiber tracking.
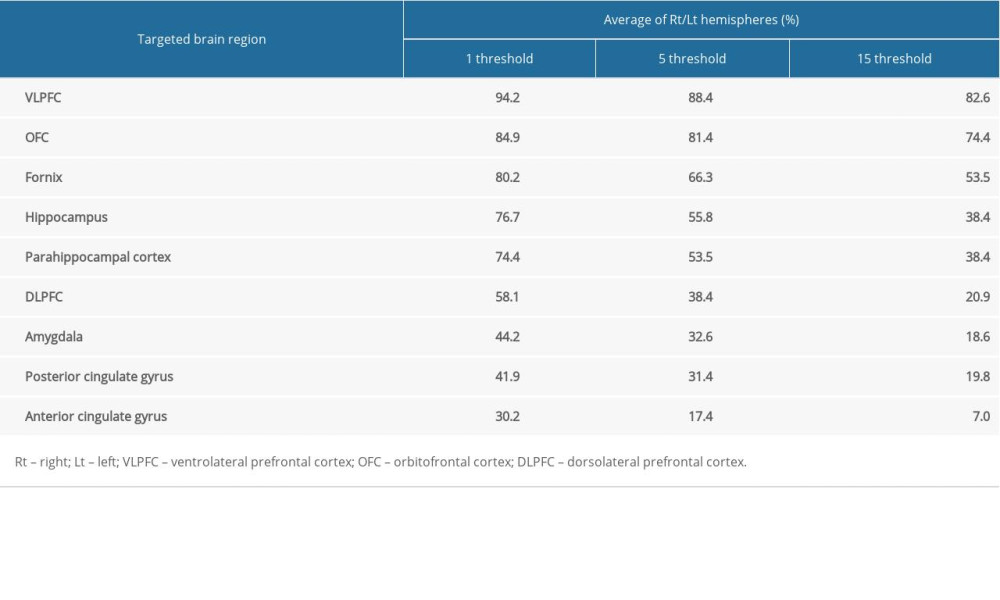
In each subject, a region of interest was set on the primary auditory cortex (Brodmann area 41 and 42), including the subcortical white matter for elucidating the connectivity between the primary auditory cortex and cognition-related brain areas (Figure 1). The results were visualized at the threshold level of 1, 5, and 15 streamlines through each voxel. The neural connectivity was defined as the incidence of connection between the primary auditory cortex and the following brain regions closely related to cognition: the DLPFC; ventrolateral prefrontal cortex (VLPFC); OFC; hippocampus; parahippocampal cortex; amygdala; anterior cingulate gyrus and posterior cingulate gyrus; and fornix (Figure 1).
Results
Table 1 summarizes the neural connectivity of the primary auditory cortex. At the threshold level of one streamline, the primary auditory cortex showed more than 50% connectivity to the VLPFC (94.2%), OFC (84.9%), fornix (80.2%), hippocampus (76.7), parahippocampal cortex (74.4%), and DLPFC (58.1%) (Figure 2). In contrast, the VLPFC (88.4%), OFC (81.4%), fornix (66.3%), hippocampus (55.8%), and parahippocampal cortex (53.5%) revealed more than 50% connectivity with the primary auditory cortex at the threshold level of 5 streamlines. At the threshold level of 15 streamlines, the primary auditory cortex presented more than 50% connectivity to the VLPFC (82.6%), OFC (74.4%), and fornix (53.5%).
Discussion
This study examined the neural connectivity between the primary auditory cortex and cognition-related brain areas in normal subjects using DTT. According to these results, the primary auditory cortex showed high connectivity (over 50%) with the following areas: the threshold of 1 streamline – the VLPFC, OFC, fornix, hippocampus, parahippocampal cortex, and DLPFC; the threshold of 5 streamlines – the VLPFC, OFC, fornix, hippocampus, and parahippocampal cortex; the threshold of 15 streamlines – the VLPFC, OFC, and fornix. As a result, the primary auditory cortex was closely connected to the prefrontal lobe (VLPFC, OFC, and DLPFC) and the declarative memory-related neural structures (fornix, hippocampus, and parahippocampal cortex).
Recent studies using human and animal models have reported that the auditory cortex is involved in various cognitive processes (decision-making, auditory nonverbal attention, prediction, learning, and working memory) [5,9–11]. In the present study, the prefrontal cortex (VLPFC, OFC, and DLPFC) and declarative memory-related neural structures (fornix, hippocampus, and parahippocampal cortex) had high connectivity with the primary auditory cortex. The prefrontal cortex is mainly involved in high cognition: the VLPFC decision-making, goal-directed behavior, and auditory non-spatial processes; the OFC-cognitive process of decision-making and involvement in the reward system; and the DLPFC-working memory and auditory spatial localization [38–40]. On the other hand, the fornix, hippocampus, and parahippocampal cortex are involved in the declarative memory: the fornix – connection of the hippocampus with several subcortical areas; the hippocampus – the consolidation of information from short-term memory to long-term memory; the parahippocampal cortex – the encoding and retrieval of declarative memory [41–46]. Therefore, these results suggest that the primary auditory cortex had a high degree of neural connectivity with several brain areas related to cognition, particularly high cognition and declarative memory function [38–46].
A few studies have examined the neural connectivity of the auditory regions with other brain regions using resting-state fMRI [11,21,22]. In 2018, Yuan et al reported the connectivity between Heschl’s gyrus and various brain regions, including the superior temporal gyrus, DLPFC, parietal cortex, occipital lobe, cingulate cortex, hippocampus, and amygdala [22]. In 2020, Wang et al reported the connectivity between the auditory network (the superior temporal gyrus and Heschl’s gyrus) and reward network (nucleus accumbens, caudate, putamen, and OFC) in 47 patients with mild cognitive impairment, 11 patients with Alzheimer disease, and 47 normal subjects [21]. In 2021, Jianxun et al reported the connectivity of the auditory regions around Geschl’s gyrus with low variability with the sensory-motor cortex and the auditory regions in the superior temporal gyrus with high variability with the inferior frontal gyrus and temporal pole in 30 normal subjects [11]. Regarding DTT, a few studies have reported the neural connectivity of the auditory cortex with specific brain areas [32–34]. In 2010, Crippa et al reported the neural connection between the auditory cortex and amygdala in 10 tinnitus patients and 15 normal subjects [32]. In 2011, Beer et al observed projections from Heschl’s region to the superior temporal sulcus, supramarginal gyrus, intraparietal sulcus, and occipital cortex in 10 normal subjects [33]. In 2013, Tony et al reported a neural connection of the primary auditory cortex with the primary and secondary somatosensory cortex in one patient with acquired auditory-tactile synesthesia and 17 normal subjects [34]. The above DTT-based studies investigated the structural connectivity between the auditory cortex and specific brain regions. Direct comparison our results with previous studies is impossible because there has been no study investigating the neural connectivity between the primary auditor cortex and cognition-related brain regions, like our study. However, previous studies have reported the neural connectivity to some brain areas (the DLPFC, hippocampus, and OFC), which coincided with our results [11,21,22]. As a result, to the best of our knowledge, this is the first study to demonstrate the neural connectivity of the primary auditory cortex with the cognition-related brain areas using DTT.
This study had some limitations. First, the afferent and efferent fibers between the primary auditory cortex and cognition-related brain regions could not be divided because DTT cannot detect the direction. Second, the strength of the connectivity between the primary auditory cortex and each brain region could not be quantified. Third, the use of probabilistic DTT tractography can result in false-positive and false-negative findings because of the fiber complexity or partial volume effect [47,48]. Fourth, the angle size could affect the structural neural connectivity and lead to false-negative findings. Therefore, further studies to overcome these limitations are warranted.
Conclusions
In conclusion, this study examined the structural neural connectivity between the primary auditory cortex and the cognition-related brain areas in the 43 normal adult human subjects. DTT showed that the primary auditory cortex had a high degree of neural connectivity with the prefrontal cortex, fornix, hippocampus, and parahippocampal cortex, which are brain areas associated with cognition and memory. These results are expected to be helpful in neuro-rehabilitation. For example, the primary auditory cortex stimulation using transcranial direct current stimulation or repetitive transcranial magnetic stimulation can activate the prefrontal cortex and declarative memory-related neural structures. Further clinical studies on this topic are needed.
Figures
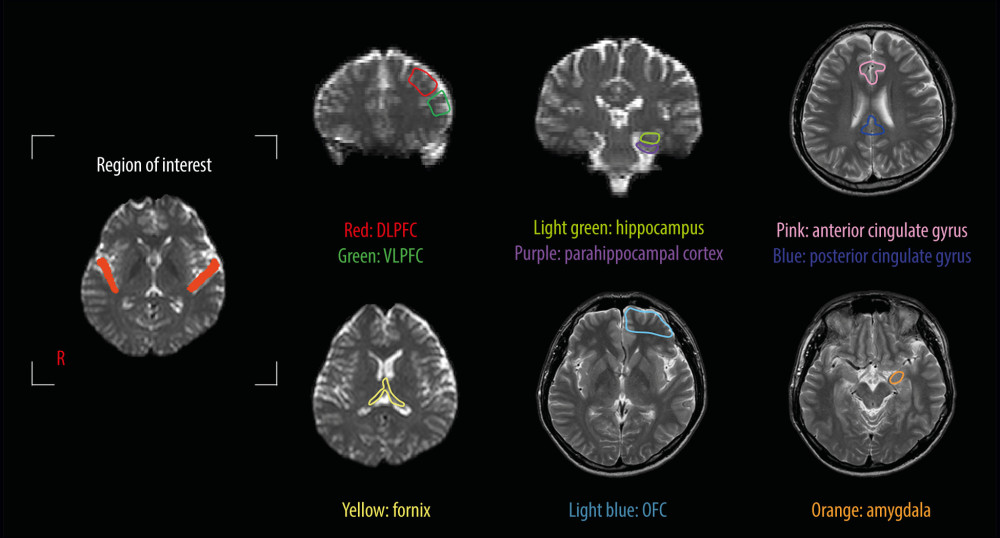 Figure 1. Region of interest and the cognition-related brain areas. Region of interest: the primary auditory cortex. The cognition-related brain areas: the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior and posterior cingulate gyri, orbitofrontal cortex (OFC), amygdala, hippocampus, and parahippocampal cortex.
Figure 1. Region of interest and the cognition-related brain areas. Region of interest: the primary auditory cortex. The cognition-related brain areas: the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior and posterior cingulate gyri, orbitofrontal cortex (OFC), amygdala, hippocampus, and parahippocampal cortex. 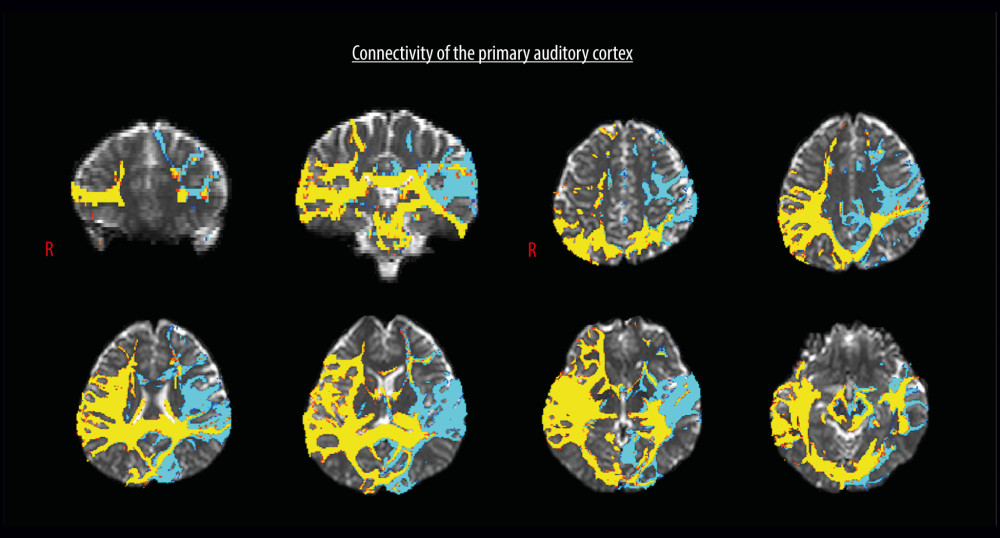 Figure 2. Diffusion tensor tractography for the structural neural connectivity between the primary auditory cortex and the cognition-related brain areas in a representative normal subject (a 33-year-old man).
Figure 2. Diffusion tensor tractography for the structural neural connectivity between the primary auditory cortex and the cognition-related brain areas in a representative normal subject (a 33-year-old man). References
1. Pickles JO: An Introduction to the physiology of hearing, 2012, Bingley, UK, Emerald
2. Blanco-Elorrieta E, Pylkkanen L, Bilingual language switching in the laboratory versus in the wild: the spatiotemporal dynamics of adaptive language control: J Neurosci, 2017; 37; 9022-36
3. Gulban OF, Goebel R, Moerel M, Improving a probabilistic cytoarchitectonic atlas of auditory cortex using a novel method for inter-individual alignment: Elife, 2020; 9; e56963
4. Purves D, Williams SM: Neuroscience, 2001, Sunderland (Mass), Sinauer Associates
5. Budinger E, Scheich H, Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex: Hear Res, 2009; 258; 16-27
6. Munoz-Lopez MM, Mohedano-Moriano A, Insausti R, Anatomical pathways for auditory memory in primates: Front Neuroanat, 2010; 4; 129
7. Weinberger NM: The oxford handbook of auditory science: The auditory brain, 2010, New York (NY), Oxford University Press
8. Weinberger NM, Plasticity in the primary auditory cortex, not what you think it is: implications for basic and clinical auditory neuroscience: Otolaryngol (Sunnyvale), 2012; 002
9. King AJ, Teki S, Willmore BDB, Recent advances in understanding the auditory cortex: F1000Res, 2018; 7; F1000
10. Barton B, Brewer AAStavros Hatzopoulos ACaPHS, Attention and working memory in human auditory cortex: The human auditory system – basic features and updates on audiological diagnosis and therapy, 2019, IntechOpen
11. Ren J, Xu T, Wang D, Individual variability in functional organization of the human and monkey auditory cortex: Cereb Cortex, 2021; 31; 2450-65
12. Fulton SE, Lister JJ, Bush AL, Mechanisms of the hearing-cognition relationship: Semin Hear, 2015; 36; 140-49
13. GBD 2019 Hearing Loss Collaborators, Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the global burden of disease study 2019: Lancet, 2021; 397; 996-1009
14. Hardy CJ, Marshall CR, Golden HL, Hearing and dementia: J Neurol, 2016; 263; 2339-54
15. Uchida Y, Sugiura S, Nishita Y, Age-related hearing loss and cognitive decline – the potential mechanisms linking the two: Auris Nasus Larynx, 2019; 46; 1-9
16. Livingston G, Huntley J, Sommerlad A, Dementia prevention, intervention, and care: 2020 report of the lancet commission: Lancet, 2020; 396; 413-46
17. Johnson JCS, Marshall CR, Weil RS, Hearing and dementia: From ears to brain: Brain, 2021; 144; 391-401
18. Pichora-Fuller MK, Mick P, Reed M, Hearing, cognition, and healthy aging: Social and public health implications of the links between age-related declines in hearing and cognition: Semin Hear, 2015; 36; 122-39
19. Golub JS, Brain changes associated with age-related hearing loss: Curr Opin Otolaryngol Head Neck Surg, 2017; 25; 347-52
20. Slade K, Plack CJ, Nuttall HE, The effects of age-related hearing loss on the brain and cognitive function: Trends Neurosci, 2020; 43; 810-21
21. Yuan G, Liu G, Wei D, Functional connectivity corresponding to the tonotopic differentiation of the human auditory cortex: Hum Brain Mapp, 2018; 39; 2224-34
22. Wang D, Belden A, Hanser SB, Geddes MR, Loui P, Resting-state connectivity of auditory and reward systems in Alzheimer’s disease and mild cognitive impairment: Front Hum Neurosci, 2020; 14; 280
23. Biswal B, Yetkin FZ, Haughton VM, Hyde JS, Functional connectivity in the motor cortex of resting human brain using echo-planar mri: Magn Reson Med, 1995; 34; 537-41
24. Jang SH, Kwon YH, Lee MY, Difference of neural connectivity for motor function in chronic hemiparetic stroke patients with intracerebral hemorrhage: Neurosci Lett, 2012; 531; 80-85
25. Parker GJM, Alexander DC, Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue: Philos Trans R Soc Lond B Biol Sci, 2005; 360; 893-902
26. Behrens TE, Berg HJ, Jbabdi S, Probabilistic diffusion tractography with multiple fibre orientations: what can we gain?: Neuroimage, 2007; 34; 144-55
27. Muthusamy KA, Aravamuthan BR, Kringelbach ML, Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting: J Neurosurg, 2007; 107; 814-20
28. Nucifora PG, Verma R, Lee SK, Melhem ER, Diffusion-tensor mr imaging and tractography: Exploring brain microstructure and connectivity: Radiology, 2007; 245; 367-84
29. Jang SH, Kwon HG, Neural connectivity of the amygdala in the human brain: A diffusion tensor imaging study: Neural Netw World, 2014; 24; 591-99
30. Kwon HG, Jang SH, Neural connectivity of the lateral geniculate body in the human brain: Diffusion tensor imaging study: Neurosci Lett, 2014; 578; 66-70
31. Jang SH, Lee MY, Yeo SS, Kwon HG, Structural neural connectivity of the vestibular nuclei in the human brain: A diffusion tensor imaging study: Neural Regen Res, 2018; 13; 727-30
32. Crippa A, Lanting CP, van Dijk P, Roerdink JB, A diffusion tensor imaging study on the auditory system and tinnitus: Open Neuroimag J, 2010; 4; 16-25
33. Beer AL, Plank T, Greenlee MW, Diffusion tensor imaging shows white matter tracts between human auditory and visual cortex: Exp Brain Res, 2011; 213; 299-308
34. Ro T, Ellmore TM, Beauchamp MS, A neural link between feeling and hearing: Cereb Cortex, 2013; 23; 1724-30
35. Woolrich MW, Jbabdi S, Patenaude B, Bayesian analysis of neuroimaging data in FSL: Neuroimage, 2009; 45; S173-86
36. Smith SM, Jenkinson M, Woolrich MW, Advances in functional and structural MR image analysis and implementation as FSL: Neuroimage, 2004; 23(Suppl 1); S208-19
37. Jenkinson M, Beckmann CF, Behrens TE, FSL: Neuroimage, 2012; 62; 782-90
38. Kaas JH, Hackett TA, Subdivisions of auditory cortex and processing streams in primates: Proc Natl Acad Sci USA, 2000; 97; 11793-99
39. Plakke B, Romanski LM, Auditory connections and functions of prefrontal cortex: Front Neurosci, 2014; 8; 199
40. Rudebeck PH, Rich EL, Orbitofrontal cortex: Curr Biol, 2018; 28(18); R1083-88
41. Cassel JC, Duconseille E, Jeltsch H, Will B, The fimbria-fornix/cingular bundle pathways: A review of neurochemical and behavioural approaches using lesions and transplantation techniques: Prog Neurobiol, 1997; 51; 663-716
42. Eichenbaum H: A brain system for declarative memory The cognitive neuroscience of memory: an introduction, 2002; 213-36, New York, Oxford University Press
43. Karnik M, Medial temporal lobe structure and function: PhD diss Dissertation, 2009, Washington University All theses and dissertations
44. Cutsuridis V, Yoshida M, Editorial: Memory processes in medial temporal lobe: Experimental, theoretical and computational approaches: Front Syst Neurosci, 2017; 11; 19
45. Choi YJ, Lee EJ, Lee JE, The fornix: functional anatomy, normal neuroimaging, and various pathological conditions: Investig Magn Reson Imaging, 2021; 25; 59-75
46. Loughrey DG, Feeney J, Kee F, Social factors may mediate the relationship between subjective age-related hearing loss and episodic memory: Aging Ment Health, 2021; 25; 824-31
47. Yamada K, Sakai K, Akazawa K, MR tractography: A review of its clinical applications: Magn Reson Med Sci, 2009; 8; 165-74
48. Fillard P, Descoteaux M, Goh A, Quantitative evaluation of 10 tractography algorithms on a realistic diffusion mr phantom: Neuroimage, 2011; 56; 220-34
Figures
 Figure 1. Region of interest and the cognition-related brain areas. Region of interest: the primary auditory cortex. The cognition-related brain areas: the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior and posterior cingulate gyri, orbitofrontal cortex (OFC), amygdala, hippocampus, and parahippocampal cortex.
Figure 1. Region of interest and the cognition-related brain areas. Region of interest: the primary auditory cortex. The cognition-related brain areas: the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior and posterior cingulate gyri, orbitofrontal cortex (OFC), amygdala, hippocampus, and parahippocampal cortex. Figure 2. Diffusion tensor tractography for the structural neural connectivity between the primary auditory cortex and the cognition-related brain areas in a representative normal subject (a 33-year-old man).
Figure 2. Diffusion tensor tractography for the structural neural connectivity between the primary auditory cortex and the cognition-related brain areas in a representative normal subject (a 33-year-old man). In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952