21 May 2022: Clinical Research
Curative Analysis of Patients with Hepatocellular Carcinoma Using Transcatheter Arterial Chemoembolization Combined with Radiofrequency Ablation
Yifan Li1AE, Diwen Zhu1BCD, Weixin Ren1AE*, Junpeng Gu1BCD, Weizheng Ji1BCD, Haixiao Zhang1BCD, Yingjun Bao1BCD, Gengfei Cao1BCD, Asihaer Hasimu1BCDDOI: 10.12659/MSM.936246
Med Sci Monit 2022; 28:e936246
Abstract
BACKGROUND: Transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) can improve the survival of patients with hepatocellular carcinoma (HCC). The purpose was to explore the characteristics of high-risk and low-risk groups of HCC patients receiving combination therapy using a decision tree model.
MATERIAL AND METHODS: This retrospective cohort study investigated HCC patients treated with a combination of TACE and RFA at our hospital from 2012 to 2018. Decision tree analysis was used to study the 1-year prognosis of patients, and patients were divided into high-risk and low-risk groups.
RESULTS: We included a total of 142 patients with HCC, 21.83% female and 78.17% male, with the median age of 60 years old. The median follow-up was 13.5 months; 39.44% of patients had progressive disease or death (high-risk group) and 60.56% of patients did not have progressive disease or survival (low-risk group). The area under the curve (AUC) of the decision tree model was 0.846. There were significant differences in sex (P=0.003), age (P=0.038), tumor number (P=0.043), number of RFAs in the first treatment cycle (P<0.001), alanine transaminase (ALT) (P<0.001), and aspartate transaminase (AST) (P=0.041) between the high-risk and the low-risk groups. Risk of progressive disease or death in the high-risk group was 12.232 times higher than in the low-risk group.
CONCLUSIONS: To improve individual survival, clinicians should pay attention to the identification of high-risk HCC patients receiving combination therapy, especially those with less frequent use of RFA during the first treatment and higher ALT and AST levels.
Keywords: High-Risk Groups, hepatocellular carcinoma, radiofrequency ablation, transarterial chemoembolization, Carcinoma, Hepatocellular, Catheter Ablation, Chemoembolization, Therapeutic, Female, Humans, Liver Neoplasms, Male, Middle Aged
Background
Hepatocellular carcinoma (HCC) accounts for approximately 75–85% of primary liver cancers; it was the sixth most common cancer and the third leading cause of cancer-related death in 2020 [1]. In Western populations, 90% of patients progress from the chronic inflammatory fibrotic stage to cirrhosis, through low-grade and high-grade dysplastic nodules, and eventually to tumors [2]. In China, HCC has high morbidity and mortality due to the high incidence of hepatitis B [3,4]. Patients with HCC generally have no typical clinical symptoms in the early stage. Many patients are in the middle and late stages when they are diagnosed [5]. Moreover, HCC patients often also have various degrees of liver cirrhosis, liver insufficiency, and other manifestations of chronic liver damage, or lose the opportunity for radical surgical resection due to the location, number, size, and other related characteristics of the tumor, so the prognosis is very poor and the mortality rate is higher [6,7].
For the treatment of advanced HCC, transcatheter arterial chemoembolization (TACE) is currently widely used as an effective local palliative treatment [8]. Several studies have proved the curative effect of TACE on HCC and it is widely used in clinical practice, which could significantly improve the survival rate of patients with advanced HCC [9,10]. Radiofrequency ablation (RFA) technology is widely used in the treatment of HCC patients at various stages of disease due to its advantages of being minimally invasive, safe, convenient, and repeatable [11]. RFA provides similar odds of survival as liver resection or liver transplantation [12,13], but these latter 2 treatments are associated with local recurrence or a high recurrence rate [14,15]. At present, studies suggest the combination of TACE and RFA treatment for HCC patients to improve their survival and quality of life. However, many researchers have reported that in patients with HCC, TACE therapy combined with RFA therapy is associated with significantly better long-term survival compared with TACE or RFA therapy alone [16]. Few studies have explored the characteristics of the better-prognosis and poor-prognosis populations of patients treated with the combination of TACE and RFA [17–19]. It is important to identify high-risk groups receiving combination therapy and to intervene accordingly.
This study aimed to use machine learning methods to stratify HCC patients into high-risk and low-risk groups, and to explore the efficacy of TACE combined with RFA therapy in various populations.
Material and Methods
STUDY DESIGN AND POPULATION:
This retrospective cohort study enrolled HCC patients receiving TACE combined with RFA between 2012 and 2018 in the First Affiliated Hospital of Xinjiang Medical University. TACE combined with RFA treatment in our study was defined as performing TACE treatment for the lesions selected for treatment, followed by re-examination after 1 month, and then RFA adjuvant therapy was used. A total of 142 patients were included in this study.
Follow-up was performed approximately 1 month, 3 months, and 6 months after HCC treatment. If patients feel unwell during the follow-up period, they were encouraged to quickly seek medical attention. All patients were required to be followed up in hospital and undergo physical examination, including routine blood tests, coagulation function, liver and kidney function, tumor markers, and abdominal contrast-enhanced CT/MRI.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
OUTCOME VARIABLE:
The endpoint of this study was progressive disease or overall mortality of the patients within 1 year, who were defined as high-risk group. Progressive disease was defined as a 20% increase in the sum of the diameters of the viable target lesions or the appearance of new lesions.
DATA COLLECTION:
Data on the demographic and clinical characteristics of the study population were collected. Demographic characteristics included age and sex. Clinical characteristics included tumor location, tumor number, tumor size, portal vein tumor thrombus (PVTT), hepatitis, number of TACEs in the first treatment cycle, number of RFAs in the first treatment cycle, alpha-fetoprotein (AFP) level (ng/ml), total bilirubin (Tbil) level (μmol/L), albumin level (g/L), alanine transaminase (ALT) level (U/L), aspartate transaminase (AST) level (U/L), lactic dehydrogenase (LDH) level (U/L), alkaline phosphatase (ALP) level (U/L), Child-Pugh score, Barcelona Clinic Liver Cancer (BCLC) stage, and China liver cancer staging (CNLC). One RFA treatment, regardless of the number of HCC lesions during the treatment period, was counted as 1 RFA.
STATISTICAL ANALYSIS:
Quantitative data were tested for normality using the Kolmogorov-Smirnov test. Normally distributed data were described as mean±standard deviation (mean±SD), and the
We used SAS v. 9.4 (SAS Institute, Cary, North Carolina) and Python software v. 3.7.4 (Python Software Foundation, DE, USA) for analyses.
Results
CHARACTERISTICS OF THE STUDY POPULATION:
The characteristics of the studied patients are shown in Table 1. We found that 60.56% (86/142) of patients who received the combination of TACE and RFA were free of progressive disease or survived within 1 year, and 39.44% (56/142) of patients had progressive disease or death within 1 year. The median age was 60 years and 21.83% (31/142) patients were female and 78.17% (111/142) were male. Most of the patients (79.58% [113/142]) had hepatitis. The median AFP was14.35 ng/ml, the median ALT was 25.36 U/L, and the median AST was 30.60 U/L. Most patients (48.59% [69/142]) were BCLC stage A, and 38.03% (54/142) were CNLC stage Ia.
DEVELOPMENT AND PERFORMANCE OF THE DECISION TREE MODEL TO DISTINGUISH HIGH-RISK AND LOW-RISK PATIENTS:
After LASSO regression analysis to screen out variables, we developed the decision tree model (Figure 1). A total of 142 samples were divided into 2 groups according to the number of RFA in the first treatment cycle ≤1.5. One group (samples=28) had reached the decision endpoint, and the other 114 samples were further divided into 95 samples and 19 samples according to Tbil ≤26.795. The dataset of 95 samples was divided into 2 groups according to ALT ≤14.55, 1 of which reached the decision endpoint (samples=14). The dataset of 19 samples continued to be classified according to ALT ≤39.12, and both datasets reached the decision endpoint (samples=12 and 7). As shown in Figure 1, the remaining samples were classified according to ALP level ≤152.45, AST ≤23.85, AST ≤28.95, AFP ≤1.95, and sex. Value=[] is the frequency of outcomes, the number on the left was progression-free and survival (low-risk group) and the number on the right was progressive disease or death (high-risk group) within 1 year. The cut-off of the decision tree model was 0.571, the area under the curve (AUC) and the positive predictive value (PPV) with 95% confidence interval (CI) was 0.846 (95% CI: 0.780–0.911) and 0.750 (95% CI: 0.637–0.863), respectively (Table 2).
COMPARISON OF THE HIGH-RISK AND LOW-RISK GROUPS:
The comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy is shown in Table 1. The ALT and AST levels were significantly higher in the high-risk group compared to the low-risk group (Table 1). There were significant differences in sex, age, tumor number, and number of RFA in the first treatment cycle between the high-risk and low-risk groups. Differences between the data before and after imputation were not statistically significant, indicating that our results were reliable (Supplementary Table 1). Figure 2 shows the cumulative incidence of progressive disease or death in the low-risk and high-risk groups. The risk of progressive disease or death in the high-risk group was 12.232 times (HR=12.232, [95% CI: 6.005–24.916]) that of the low-risk group after adjusting for the number of TACEs during the first treatment, LDH, tumor location, tumor size, PVTT, hepatitis, CP, BCLC, and CNLC (Table 3).
Discussion
HCC is the most common pathological type of liver cancer. TACE and RFA are commonly used local treatment options. The combined use of these 2 can make up for their respective deficiencies and further exert their respective curative effects. Identifying the characteristics of high-risk groups from the patients undergoing combined TACE and RFA treatment and intervening in a timely manner is important to improve the quality of life and prognosis of HCC patients. Our decision tree model for dividing patients into low-risk and high-risk groups performs well. Compared with the low-risk group, the high-risk group was younger, had a higher proportion of males, had more tumors, received less RFA in the first treatment cycle, and had higher ALT and AST levels.
A retrospective study of HCC patients by Yuan et al [20] showed that the number of tumors was an independent factor affecting the overall survival of patients, which was consistent with the relevant literature [21]. Our results also showed that the high-risk group had more tumors than the low-risk group because the number of tumors can predict the degree of intrahepatic spread of HCC. The greater the liver tumor burden, the greater the size and extent of liver tumor infiltration [22].
The patients in the high-risk group were those with progressive disease or death, and we found these patients were younger than those in the low-risk group. The study by Gu et al showed that younger patients (<60 years old) were associated with recurrence of HCC, which was similar to our results [23]. Clinically, the pathological types are usually low in differentiation for young HCC patients, and poorly differentiated HCC is generally more malignant [24,25], so the prognosis after treatment is poor.
The results showed that the high-risk group received fewer RFA treatments in the first treatment cycle than the low-risk group. High-risk patients were younger, with lower tumor differentiation and higher malignancy. Clinically, these patients also had larger tumors and more obviously abnormal blood vessels. Therefore, TACE treatment is the main treatment, and sometimes multiple TACE treatments are required. If the embolization effect is obvious after repeated TACE treatment and the blood supply is reduced, RFA treatment can be performed on the area with blood supply [26–28]. RFA can regulate local tumor tissue to above 60°C, resulting in complete tumor degeneration and necrosis [29]. High temperature is the fundamental mechanism by which RFA to achieves tumor treatment [26]. If the embolization effect is not particularly satisfactory after each TACE, the TACE treatment is repeated. Therefore, high-risk patients received fewer RFA treatments.
In patients with chronic hepatitis and cirrhosis, the AST/ALT ratio was associated with progressive hepatic impairment [30]. As reported in previous studies, elevated AST levels were also found to be one of the prognostic factors for poor prognosis [31]. A retrospective paired case-control study conducted in a tertiary cancer center from January 2006 to December 2010 showed that ALT was an important prognostic factor in HCC patients treated with combined TACE and RFA [32]. Our study also found that ALT and AST levels were higher in the high-risk group than in the low-risk group. This may be because the combined therapy does not significantly damage liver function, and clinicians can monitor ALT and AST levels and perform appropriate interventions.
The main advantage lies in the risk stratification of HCC patients receiving TACE and RFA combined treatment by using the decision tree model and exploring the characteristics of patients under different risk stratification to provide a reference for clinical intervention. The present study has some limitations. The sample size of the enrolled cases in this study was small, and this was a retrospective single-center study. We did not collect data on clinical variables after the administration of combined TACE and RFA in HCC patients, so we could not demonstrate the treatment response. Large-scale, high-quality, prospective controlled trials are needed for further research in clinical practice.
Conclusions
To improve the prognosis and reduce the risk of progressive disease or death in HCC patients receiving combined TACE and RFA therapy, clinicians may need to consider the frequency of RFA administration during the first treatment and focus on the patients with higher ALT and AST levels.
Figures
![Decision tree model to distinguish high-risk and low-risk patients. Patients were classified according to the indicated cut-off values of the variables. Value=[] is the frequency of outcomes, the number on the left is progression-free and survival (low-risk group), and the number on the right is progressive disease or death (high-risk group) within 1 year. The grid without judgment conditions has reached the endpoint of the decision path. Samples is the number of samples in the node. Num_RFA – number of RFA in the first treatment cycle; Tbil – total bilirubin; ALT – alanine transaminase; ALP – alkaline phosphatase; AST – aspartate transaminase; AFP – α-fetoprotein. (Python v. 3.7.4 sklearn package plot tree, Python Software Foundation, DE, USA).](https://jours.isi-science.com/imageXml.php?i=medscimonit-28-e936246-g001.jpg&idArt=936246&w=1000) Figure 1. Decision tree model to distinguish high-risk and low-risk patients. Patients were classified according to the indicated cut-off values of the variables. Value=[] is the frequency of outcomes, the number on the left is progression-free and survival (low-risk group), and the number on the right is progressive disease or death (high-risk group) within 1 year. The grid without judgment conditions has reached the endpoint of the decision path. Samples is the number of samples in the node. Num_RFA – number of RFA in the first treatment cycle; Tbil – total bilirubin; ALT – alanine transaminase; ALP – alkaline phosphatase; AST – aspartate transaminase; AFP – α-fetoprotein. (Python v. 3.7.4 sklearn package plot tree, Python Software Foundation, DE, USA).
Figure 1. Decision tree model to distinguish high-risk and low-risk patients. Patients were classified according to the indicated cut-off values of the variables. Value=[] is the frequency of outcomes, the number on the left is progression-free and survival (low-risk group), and the number on the right is progressive disease or death (high-risk group) within 1 year. The grid without judgment conditions has reached the endpoint of the decision path. Samples is the number of samples in the node. Num_RFA – number of RFA in the first treatment cycle; Tbil – total bilirubin; ALT – alanine transaminase; ALP – alkaline phosphatase; AST – aspartate transaminase; AFP – α-fetoprotein. (Python v. 3.7.4 sklearn package plot tree, Python Software Foundation, DE, USA). 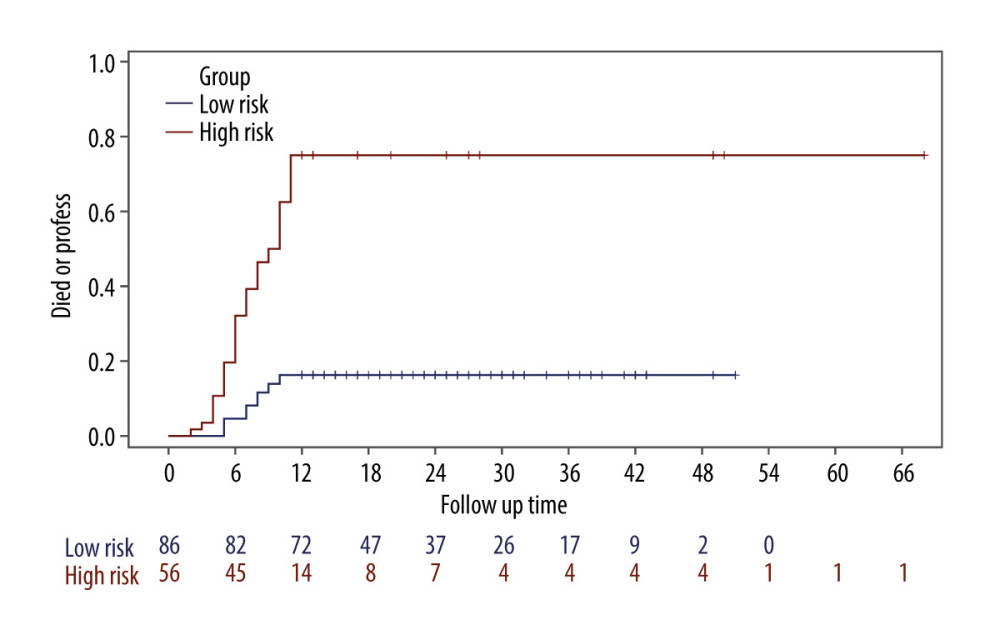 Figure 2. Kaplan-Meier curves for progressive disease or death according to patients in low-risk and high-risk groups (SAS v. 9.4, SAS Institute, Cary, North Carolina).
Figure 2. Kaplan-Meier curves for progressive disease or death according to patients in low-risk and high-risk groups (SAS v. 9.4, SAS Institute, Cary, North Carolina). Tables
Table 1. Comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy.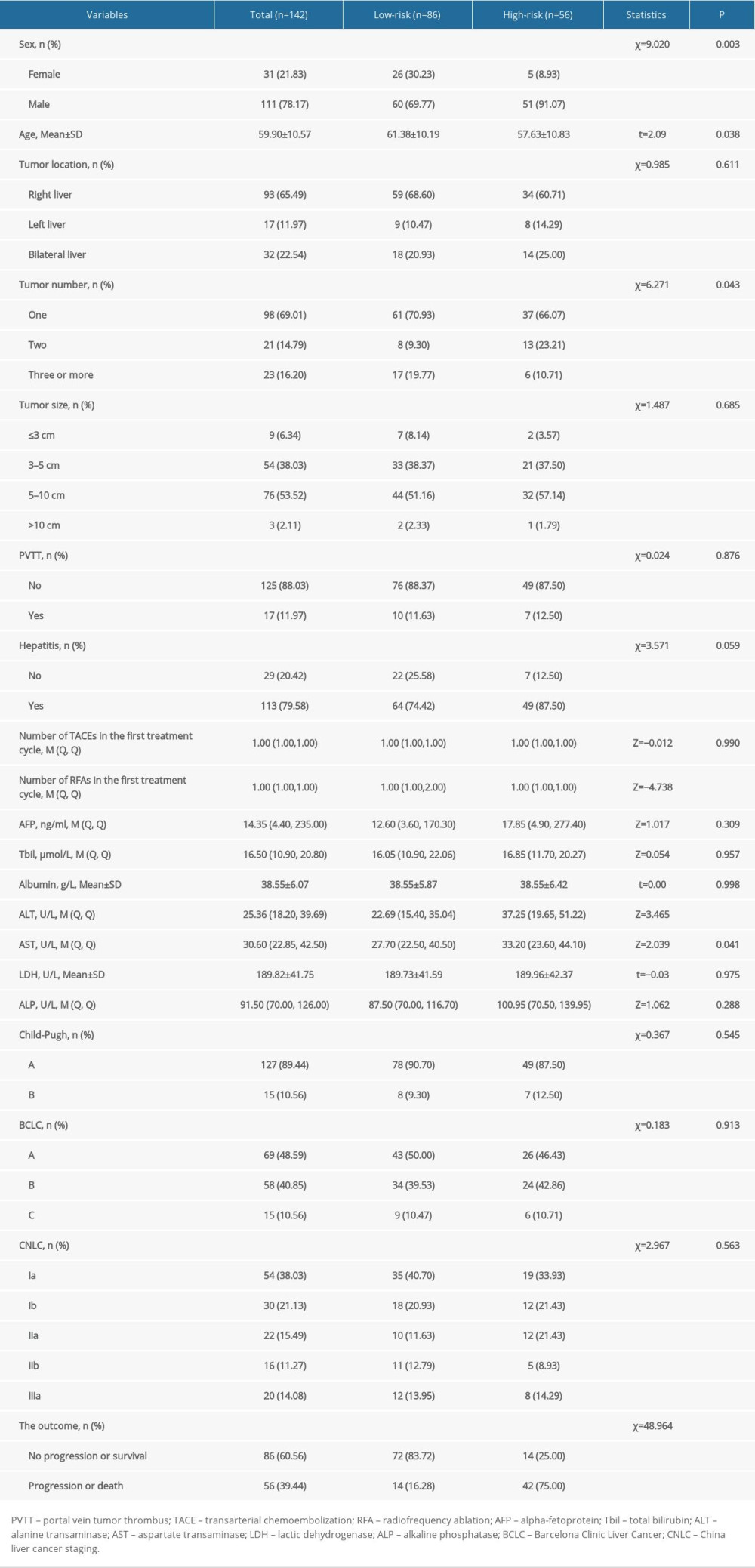 Table 2. Predictive performance of decision tree model.
Table 2. Predictive performance of decision tree model.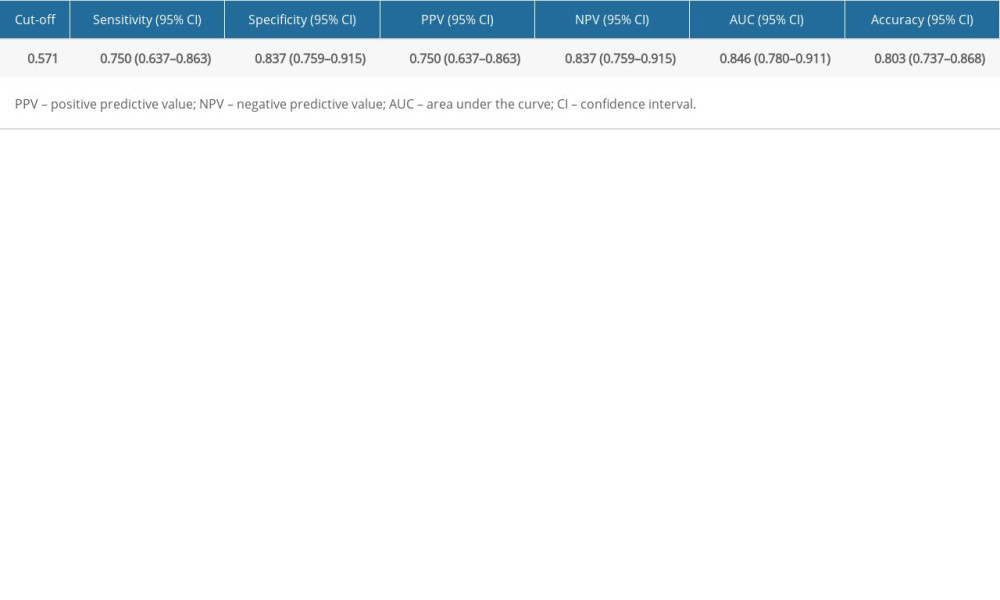 Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group.
Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group.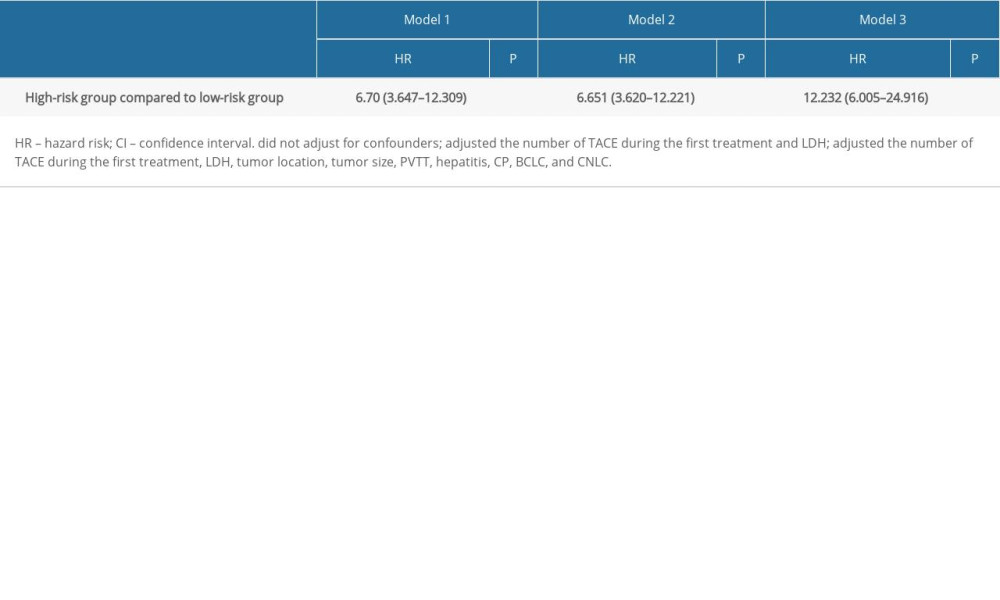 Supplementary Table 1. Comparison between groups before and after data imputation.
Supplementary Table 1. Comparison between groups before and after data imputation.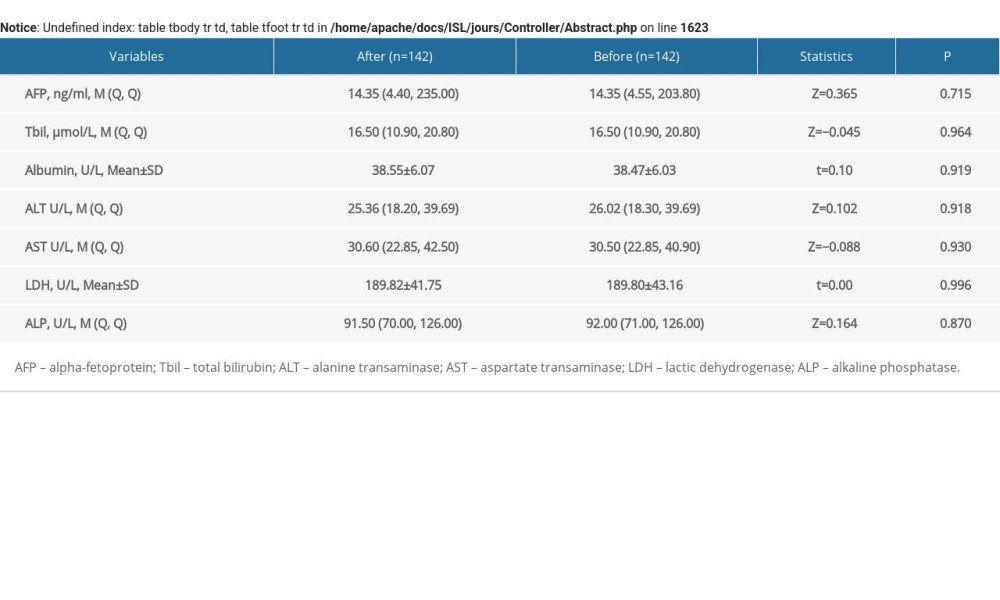
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71; 209-49
2. öcal O, Rössler D, Ricke J, Seidensticker M, Advances in diagnostic and interventional radiology in hepatocellular carcinoma: Dig Dis, 2021 [Online ahead of print]
3. Caines A, Selim R, Salgia R, The changing global epidemiology of hepatocellular carcinoma: Clin Liver Dis, 2020; 24; 535-47
4. Liu JF, Chen TY, Zhao YR, Vertical transmission of hepatitis B virus: Propositions and future directions: Chin Med J (Engl), 2021; 134(23); 2825-31
5. Zhang X, Guan L, Tian H, Risk factors and prevention of viral hepatitis-related hepatocellular carcinoma: Frontiers in Oncology, 2021; 11; 686962
6. Matsumoto M, Mouli S, Saxena P, Comparing real world, personalized, multidisciplinary tumor board recommendations with BCLC algorithm: 321-patient analysis: Cardiovasc Intervent Radiol, 2021; 44; 1070-80
7. Zhang C, Liu S, Yang M, Hepatocellular carcinoma and obesity, type 2 diabetes mellitus, cardiovascular disease: Causing factors, molecular links, and treatment options: Front Endocrinol (Lausanne), 2021; 12; 808526
8. Ghanaati H, Mohammadifard M, Mohammadifard M, A review of applying transarterial chemoembolization (TACE) method for management of hepatocellular carcinoma: J Family Med Prim Care, 2021; 10; 3553-60
9. Miyayama S, Treatment strategy of transarterial chemoembolization for hepatocellular carcinoma: Applied Sciences, 2020; 10; 7337
10. Camma C, Schepis F, Orlando A, Transarterial chemoembolization for unresectable hepatocellular carcinoma: Meta-analysis of randomized controlled trials: Radiology, 2002; 224; 47-54
11. Nault J, Sutter O, Nahon P, Ganne-Carrié N, Séror O, Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations: J Hepatol, 2018; 68; 783-97
12. Liu P, Hsu C, Hsia C, Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a propensity score model: Ann Surg, 2016; 263; 538-45
13. Kim Y, Lim H, Rhim H, Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: Analysis of prognostic factors: J Hepatol, 2013; 58; 89-97
14. Chen J, Peng K, Hu D, Tumor location influences oncologic outcomes of hepatocellular carcinoma patients undergoing radiofrequency ablation: Cancers, 2018; 10; 378
15. Kang T, Lim H, Lee M, Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: Risk factors and clinical significance: Radiology, 2015; 276; 274-85
16. Jiang C, Cheng G, Liao M, Huang J, Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: A time-to-event meta-analysis: World J Surg Oncol, 2021; 19; 81
17. Yuan P, Wang F, Zhu G, Chen B, The clinical efficiency of TACE combined with simultaneous computed tomography guided radiofrequency ablation for advanced hepatocellular carcinoma: Invest New Drugs, 2021; 39; 1383-88
18. Keshavarz P, Raman SS, Comparison of combined transarterial chemoembolization and ablations in patients with hepatocellular carcinoma: A systematic review and meta-analysis: Abdom Radiol (NY), 2022; 47; 1009-23
19. Liu W, Xu H, Ying X, Radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) for patients with medium-to-large hepatocellular carcinoma: A retrospective analysis of long-term outcome: Med Sci Monit, 2020; 26; e923263
20. Yuan H, Cao P, Li H-L, Transarterial chemoembolization with radiofrequency ablation versus hepatectomy in hepatocellular carcinoma beyond the Milan criteria: A retrospective study: Cancer Manag Res, 2018; 10; 5545-52
21. Hu H, Kim J, Lee L, Chemoembolization for hepatocellular carcinoma: Multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort: J Vasc Intervent Radiol, 2011; 22; 917-23
22. Yang Y, Chen Y, Ye F, Late recurrence of hepatocellular carcinoma after radiofrequency ablation: A multicenter study of risk factors, patterns, and survival: Eur Radiol, 2021; 31; 3053-64
23. Gu L, Shen Z, Ji L, High-intensity focused ultrasound alone or combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with unsuitable indications for hepatectomy and radiofrequency ablation: A phase II clinical trial: Surg Endosc, 2022; 36; 1857-67
24. Yamazaki Y, Kakizaki S, Sohara N, Hepatocellular carcinoma in young adults: The clinical characteristics, prognosis, and findings of a patient survival analysis: Dig Dis Sci, 2007; 52; 1103-7
25. Pu J-L, Chen Z, Yao L-Q, Long-term oncological prognosis after curative-intent liver resection for hepatocellular carcinoma in the young versus the elderly: Multicentre propensity score-matching study: BJS Open, 2022; 6; zrab145
26. Izzo F, Granata V, Grassi R, Radiofrequency ablation and microwave ablation in liver tumors: An update: Oncologist, 2019; 24; e990-e1005
27. Habibollahi P, Sheth RA, Cressman ENK, Histological correlation for radiofrequency and microwave ablation in the local control of hepatocellular carcinoma (HCC) before liver transplantation: A comprehensive review: Cancers, 2020; 13; 104
28. Kasper H, Bangard C, Gossmann A, Pathomorphological changes after radiofrequency ablation in the liver: Pathol Int, 2010; 60; 149-55
29. Knavel EM, Brace CL, Tumor ablation: Common modalities and general practices: Tech Vasc Interv Radiol, 2013; 16; 192-200
30. Rigopoulou E, Gatselis N, Arvaniti P, Alcoholic liver disease and autoimmune hepatitis: Sometimes a closer look under the surface is needed: Eur J Intern Med, 2021; 85; 86-91
31. Gianni E, Risso D, Botta F, Validity and clinical utility of the aspartate aminiransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease: Arch Intern Med, 2003; 163; 218-24
32. Bholee A, Peng K, Zhou Z, Radiofrequency ablation combined with transarterial chemoembolization versus hepatectomy for patients with hepatocellular carcinoma within Milan criteria: A retrospective case-control study: Clin Transl Oncol, 2017; 19; 844-52
Figures
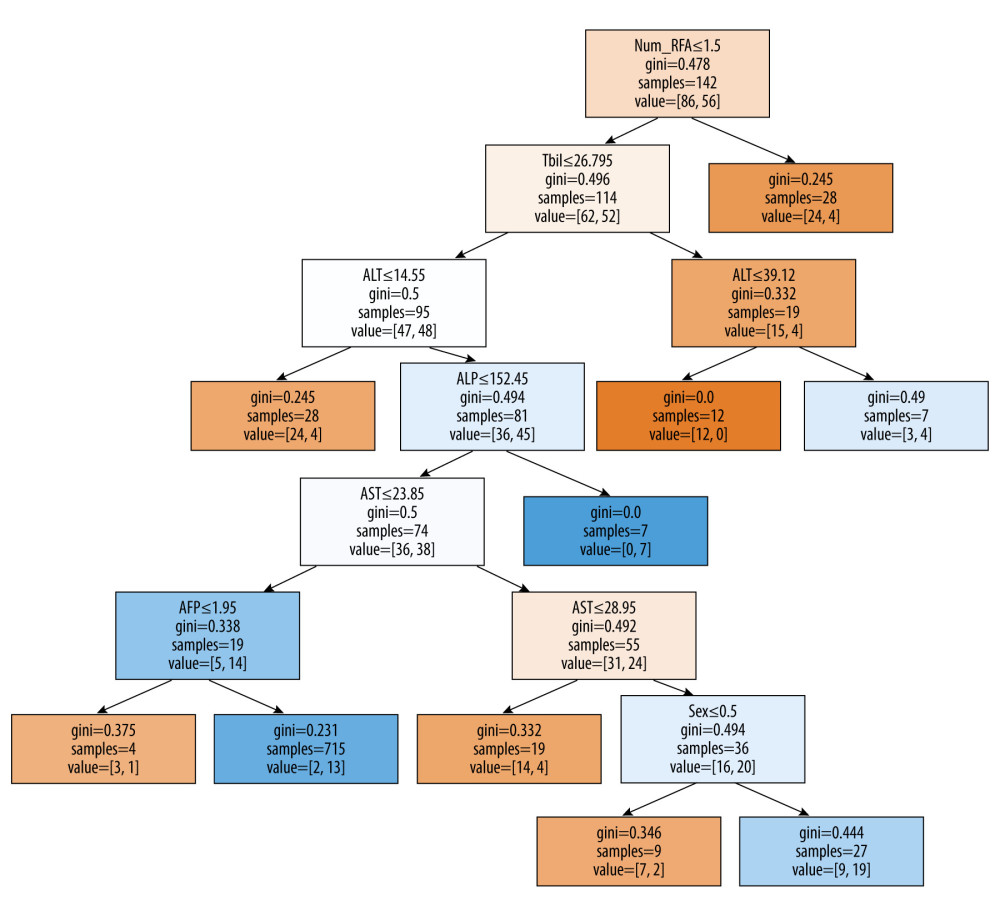 Figure 1. Decision tree model to distinguish high-risk and low-risk patients. Patients were classified according to the indicated cut-off values of the variables. Value=[] is the frequency of outcomes, the number on the left is progression-free and survival (low-risk group), and the number on the right is progressive disease or death (high-risk group) within 1 year. The grid without judgment conditions has reached the endpoint of the decision path. Samples is the number of samples in the node. Num_RFA – number of RFA in the first treatment cycle; Tbil – total bilirubin; ALT – alanine transaminase; ALP – alkaline phosphatase; AST – aspartate transaminase; AFP – α-fetoprotein. (Python v. 3.7.4 sklearn package plot tree, Python Software Foundation, DE, USA).
Figure 1. Decision tree model to distinguish high-risk and low-risk patients. Patients were classified according to the indicated cut-off values of the variables. Value=[] is the frequency of outcomes, the number on the left is progression-free and survival (low-risk group), and the number on the right is progressive disease or death (high-risk group) within 1 year. The grid without judgment conditions has reached the endpoint of the decision path. Samples is the number of samples in the node. Num_RFA – number of RFA in the first treatment cycle; Tbil – total bilirubin; ALT – alanine transaminase; ALP – alkaline phosphatase; AST – aspartate transaminase; AFP – α-fetoprotein. (Python v. 3.7.4 sklearn package plot tree, Python Software Foundation, DE, USA). Figure 2. Kaplan-Meier curves for progressive disease or death according to patients in low-risk and high-risk groups (SAS v. 9.4, SAS Institute, Cary, North Carolina).
Figure 2. Kaplan-Meier curves for progressive disease or death according to patients in low-risk and high-risk groups (SAS v. 9.4, SAS Institute, Cary, North Carolina). Tables
 Table 1. Comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy.
Table 1. Comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy. Table 2. Predictive performance of decision tree model.
Table 2. Predictive performance of decision tree model. Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group.
Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group. Table 1. Comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy.
Table 1. Comparison of low-risk and high-risk groups receiving combined TACE and RFA therapy. Table 2. Predictive performance of decision tree model.
Table 2. Predictive performance of decision tree model. Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group.
Table 3. Association of the high-risk group with progression or death in 1 year compared with the low-risk group. Supplementary Table 1. Comparison between groups before and after data imputation.
Supplementary Table 1. Comparison between groups before and after data imputation. In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








