04 November 2022: Database Analysis
Analysis of Infections Caused by Influenza Viruses and Influenza-Like Viruses in the Population of People over 65 Years of Age in the 2019–2020 Epidemic Season in Poland
Katarzyna Kondratiuk1BCDEF, Ewelina Hallmann2BCDEF*, Katarzyna Łuniewska2BCDEF, Karol SzymańskiDOI: 10.12659/MSM.937953
Med Sci Monit 2022; 28:e937953
Abstract
BACKGROUND: Influenza can be the most dangerous for people in risk groups, for example for seniors, in whom it can lead to serious and life-threatening complications. The aim of this research was to analyze the activity of influenza viruses and influenza-like viruses in patients over 65 years of age in the 2019-2020 epidemic season in Poland.
MATERIAL AND METHODS: A total of 1269 samples collected from patients over 65 years of age with suspected influenza or other respiratory viruses in the 2019-2020 epidemic season (from October 1, 2019, to September 30, 2020) were analyzed. The test material was nose and throat swabs collected during the 2019-2020 epidemic season. Quantitative polymerase chain reaction was used to determine the influenza virus type and subtype for positive samples.
RESULTS: Among the confirmed infections with influenza viruses, cases due to influenza A were dominant, and the dominant subtype was influenza A subtype A/H1N1/pdm09. Infections with influenza-like viruses were also confirmed in the patients participating in the study, with the presence of genetic material of respiratory syncytial viruses confirmed most often.
CONCLUSIONS: Seasonal vaccinations can significantly reduce the number of cases and thus the risk of post-influenza complications and deaths among seniors. This is very important, especially now, due to the current epidemiological situation related to the ongoing SARS-CoV-2 respiratory virus pandemic.
Keywords: Influenza A virus, Influenza B virus, Respiratory Syncytial Viruses, Geriatrics, Epidemiology, Humans, Infant, Influenza, Human, Influenza A Virus, H1N1 Subtype, Seasons, Poland, SARS-CoV-2, COVID-19, Viruses
Background
Influenza is an acute illness caused by infection of the respiratory system with the influenza virus. The disease is caused by viruses from the
Influenza starts rapidly and from the start of illness is associated with the most frequent acute accompanying symptoms, such as a sudden high fever (over 37.8°C), malaise, chills, weakness, headache, joint and muscle pain, dry cough, throat inflammation, and sometimes bronchitis. In general, full recovery can take up to several weeks and patients can experience dry cough, weakness, and increased fatigue. Influenza can be most dangerous for people from the identified risk groups, regardless of their age, in whom the disease is a serious threat to health and even life. These include seniors because, in people over 65 years of age, all symptoms of the disease are usually more intense and can last longer than in young people. Seniors, owing to lowered immunity and frequently accompanying chronic diseases, among other reasons, are much more likely to have influenza complications.
The most effective method of preventing influenza is immunization, which is especially recommended for elderly people. The World Health Organization’s (WHO) Advisory Committee on Immunization Practices, many international and national scientific societies, and the Ministry of Health emphasize that to effectively prevent influenza and its complications, care must be taken to prevent and build immunity in seniors through seasonal influenza vaccinations [4,5]. It is worth remembering that even if vaccinations do not prevent the disease, they can contribute to a much milder course of the disease and help prevent health-threatening complications. The most common complications after influenza in the age group of over 65 years includes pneumonia and bronchitis, secondary bacterial pneumonia caused mainly by
Many scientific studies have shown that influenza vaccination is a safe and effective method of preventing complications from influenza in high-risk groups. It is the main way to prevent influenza and therefore prevent an influenza epidemic. After vaccination, immunity develops after about 7 days, increases with time, and lasts from 6 to 12 months. Although vaccine efficacy can vary, studies show that influenza vaccination reduces the risk of influenza by 40% to 60% in the overall population during periods when most circulating influenza viruses are present [7]. The argument for seasonal influenza vaccinations among the population of people over 65 years of age is the possibility of improving the effectiveness of heart attack prevention in patients with chronic coronary syndromes, improving the prognosis for patients with heart failure, and reducing cardiovascular mortality [8,9]. Influenza vaccinations may prevent, for example, myocardial infarction [10] and stroke [11]. Influenza vaccination has also been shown to prevent recurrent heart ischemia [12] and primary cardiac arrest [10], showing important benefits in patients with cardiovascular disease. The results of Polish studies involving patients with acute cardiovascular events have been evaluated and included in the European cardiologic recommendations for influenza vaccinations [13]. The Department of Influenza Research, National Influenza Center, conducted over 207 scientific studies on the advisability of vaccinations, with particular emphasis on people of various age groups, including children and the elderly [14,15].
According to WHO recommendations, influenza vaccinations among seniors 65 years and older in the WHO European Region should be implemented in individual countries at the level of 75% of vaccination status in this age group [16]. According to the statistics kept by the WHO, the level of vaccination among Polish seniors is alarmingly low and places Poland at the bottom of the list of European countries in this respect. The vaccination rate in the group of people over 65 years of age was only 7.13% in 2020, despite there being free influenza vaccinations offered to seniors in recent years by local governments [17]. Therefore, the WHO urges all member countries to be especially vigilant against influenza, including seasonal influenza A subtypes or influenza viruses that may cause a pandemic, and to maintain routine influenza vaccination programs to protect vulnerable individuals. This is extremely important, especially now, in view of the current epidemiological situation related to the ongoing SARS-CoV-2 respiratory virus pandemic.
The aim of the presented research was to analyze the activity of influenza viruses and influenza-like viruses in patients over 65 years of age in the 2019–2020 epidemic season in Poland.
Material and Methods
ISOLATION OF RNA:
Isolation of RNA was conducted using nasal and throat swabs suspended in 1 mL of saline. For this isolation, the Maxwell 16 Viral Total Nucleic Acid Purification kit (Promega Corporation, Madison, WI, USA) was used in accordance with the manufacturer instructions. From 200 μL of a clinical sample, 50 μL of RNA suspended in RNase-free water was obtained.
REAL-TIME POLYMERASE CHAIN REACTION:
Real-time reverse transcription polymerase chain reaction was used to determine the influenza virus type and subtype. The reaction was performed with the SuperScript Platinum III kit (Invitrogen) using the Rotor-Gene Q thermal cycler (Qiagen). The probes and primers kits influenza A, influenza A/H3N2/, influenza A/H1N1/pdm09, and influenza B were provided by the International Reagent Resource of the Centers for Disease Control and Prevention. Briefly, 20 μL of reaction mixture comprised 5 μL of RNA, 0.4 μM of each primer, 0.4 μM of each probe, 10 μL of 2× reaction buffer mix, 0.4 μL of SuperScript III/Platinum Taq Mix, and 3.4 μL of water. To receive complementary DNA for isolated RNA, reverse transcription reaction was conducted in 50°C for 30 min. Next, the initial denaturation was performed at 95°C for 2 min. Amplification was performed under the following conditions: 45 cycles of denaturation (95°C for 15 s), annealing (55°C for 30 s), and elongation (72°C for 20 s). The positive controls for the reactions were isolated from viruses derived for the vaccine from the 2019–2020 epidemic season: A/Brisbane/02/2018 (H1N1) pdm09, A/Kansas/14/2017/ (H3N2), B/Colorado/06/2017 (B/Victoria/2/87 lineage), and B/Phuket/3073/2013 (B/Yamagata/16/88 lineage). The negative control was the RNase-free water from the SuperScript Platinum III kit (Invitrogen). The Voivodship Sanitary Epidemiological Stations carried out similar analyzes. The details of the methodology are shown in Table 1.
Results
A total of 1269 samples collected from patients over 65 years of age with suspected influenza or other respiratory viruses in the 2019–2020 epidemic season (from October 1, 2019, to September 30, 2020) were analyzed. As part of the sentinel program, 70 samples were tested (which constituted 5.52% of all the samples tested in this age group), while most samples (1199; 94.48% of all the samples taken for the study) came from the non-sentinel program. Positive samples, defined as samples in which the presence of the genetic material of influenza or influenza-like viruses was confirmed, were 28.68% (364) of all the tested samples. The percentage of positive samples in relation to all the tested samples in the age group over 65 years in the 2019–2020 epidemic season in Poland is shown in Figure 1.
After analyzing samples from patients over 65 years of age, 348 cases of influenza were confirmed, of which 345 cases were caused by the influenza A virus. The presence of the genetic material of the influenza B virus was confirmed in only 3 samples. Among cases caused by influenza A, the cases of influenza virus subtype A/H1N1/pdm09 dominated, with 45 confirmations, which constituted 13.04% of all confirmations of influenza infection type A. For influenza A virus of subtype A/H3N2/, 23 infections (6.67% cases due to influenza A) were confirmed. The remaining cases of influenza A virus were not subtyped, of which there were 277, which accounted for 80.29% of influenza A cases. The percentage share of influenza viruses and influenza A virus subtypes in the age group over 65 years in the 2019–2020 epidemic season in Poland is shown in Figure 2.
Among the tested people over the age of 65, infections caused by influenza-like viruses were also confirmed, although infections with influenza viruses constituted the vast majority. Among the analyzed trials, 16 cases caused by influenza-like viruses were confirmed. The greatest number of them were infections with the respiratory syncytial virus, which were found in 13 people, 81.25% of all the infections with influenza-like viruses. There were 2 cases of Coronavirus 229E/NL63 and 1 of adenovirus. The percentage of influenza-like viruses in the over 65 age group in the 2019–2020 epidemic season in Poland is shown in Figure 3. In the tested samples collected from people over 65 years of age in the 2019–2020 epidemic season, coinfections were also confirmed (a situation in which there is a simultaneous infection with 2 or more respiratory viruses, namely influenza and/or influenza-like viruses). The coinfections confirmed in this age group are presented in Table 2.
Discussion
LIMITATIONS OF THE STUDY:
This work is based solely on the analyses of samples that were reported to the Sentinel Influenza Surveillance System. Not all the tested samples are reported to the system; therefore, the number of patients studied in Poland in these seasons could be much higher.
Conclusions
Influenza poses a serious threat to people over the age of 65, because they often have chronic diseases. Patients over 65 years of age constitute the majority of all patients treated for flu, both in Europe and worldwide. This age group also has the highest number of deaths due to severe flu or its complications. Vaccination is the most effective method of preventing and controlling infectious diseases in the age group over 65, so resources should be invested in prevention and education related to vaccines. Today, in the era of the COVID-19 pandemic, the recommendation of influenza vaccination has particular importance.
Figures
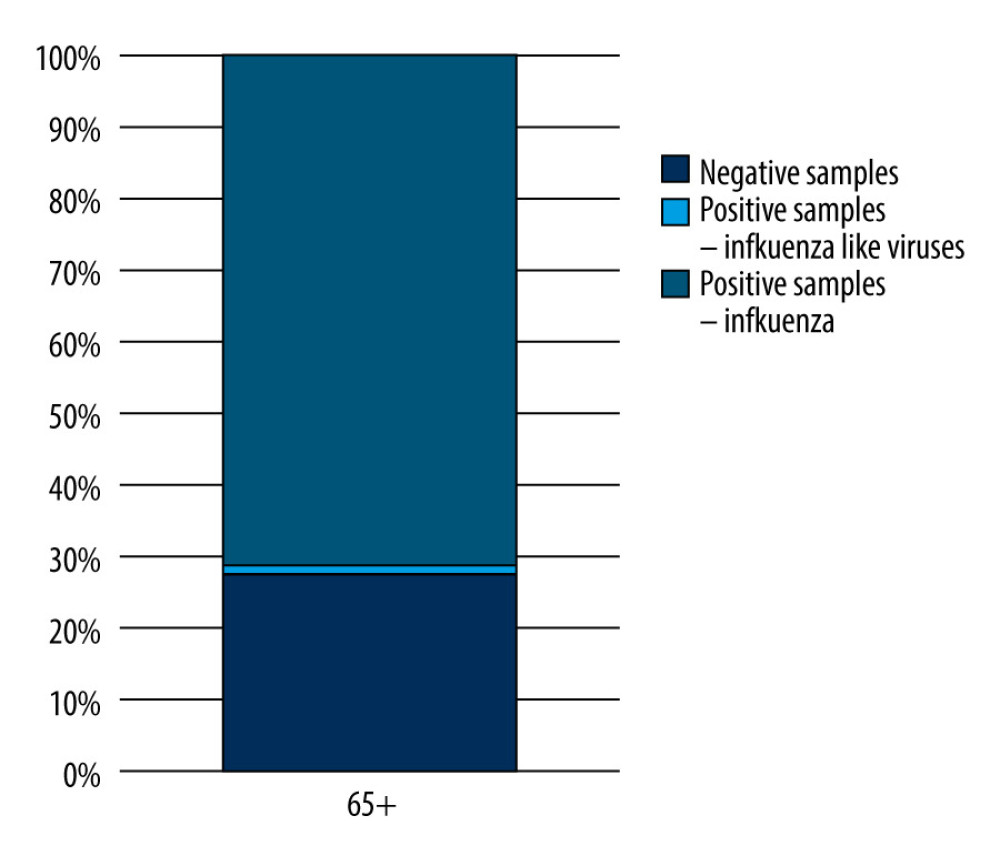 Figure 1. The percentage of positive samples in relation to all tested samples in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 1. The percentage of positive samples in relation to all tested samples in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. 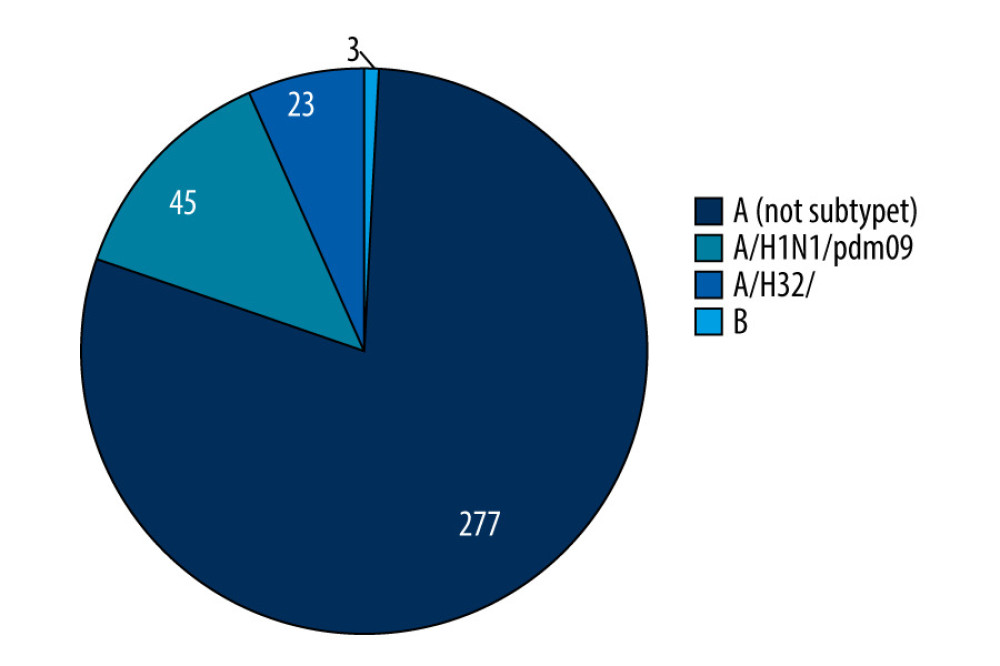 Figure 2. The percentage share of influenza viruses and their subtypes in children and in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 2. The percentage share of influenza viruses and their subtypes in children and in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. 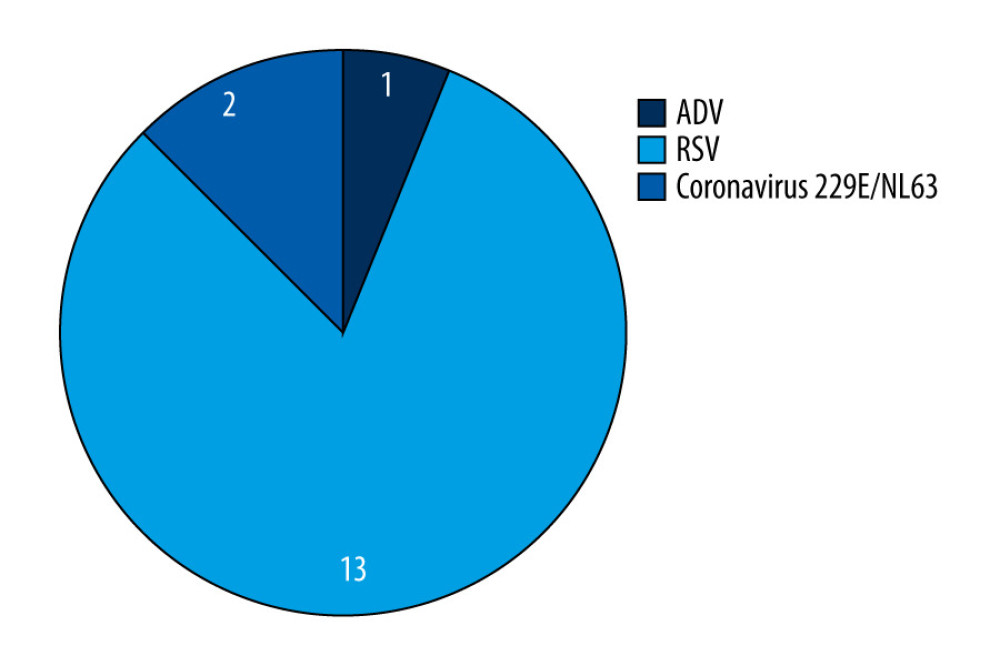 Figure 3. The percentage share of influenza-like viruses in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 3. The percentage share of influenza-like viruses in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. 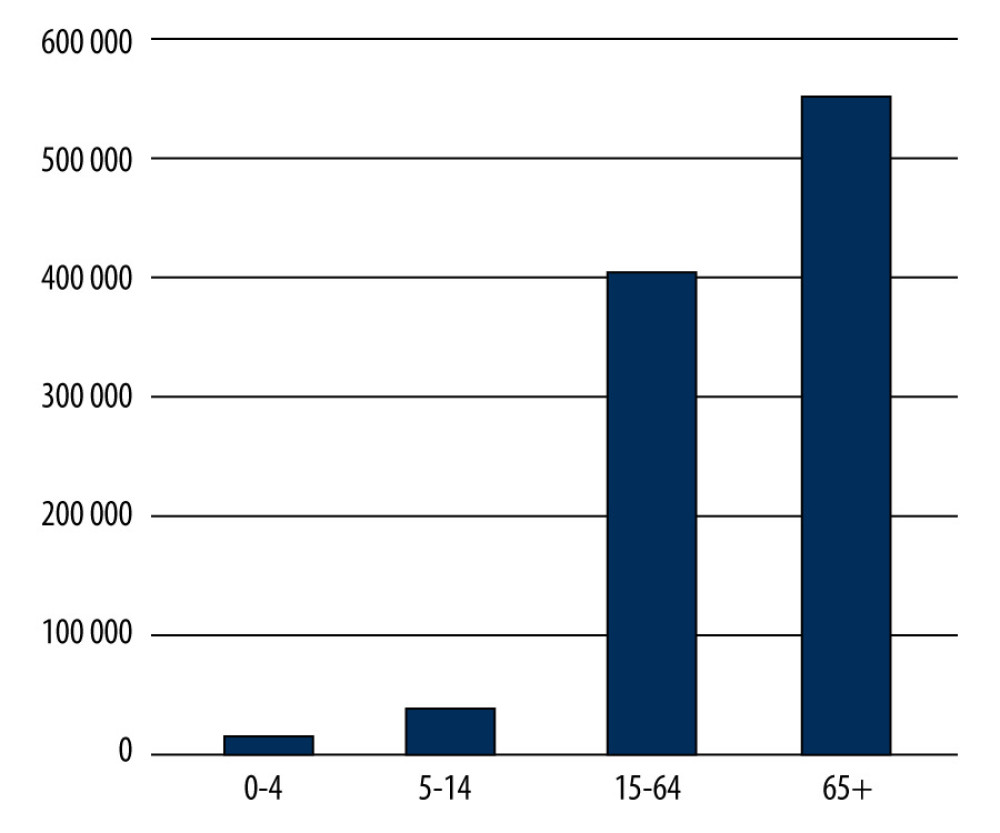 Figure 4. Number of influenza-vaccinated persons by age groups in 2019 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 4. Number of influenza-vaccinated persons by age groups in 2019 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. 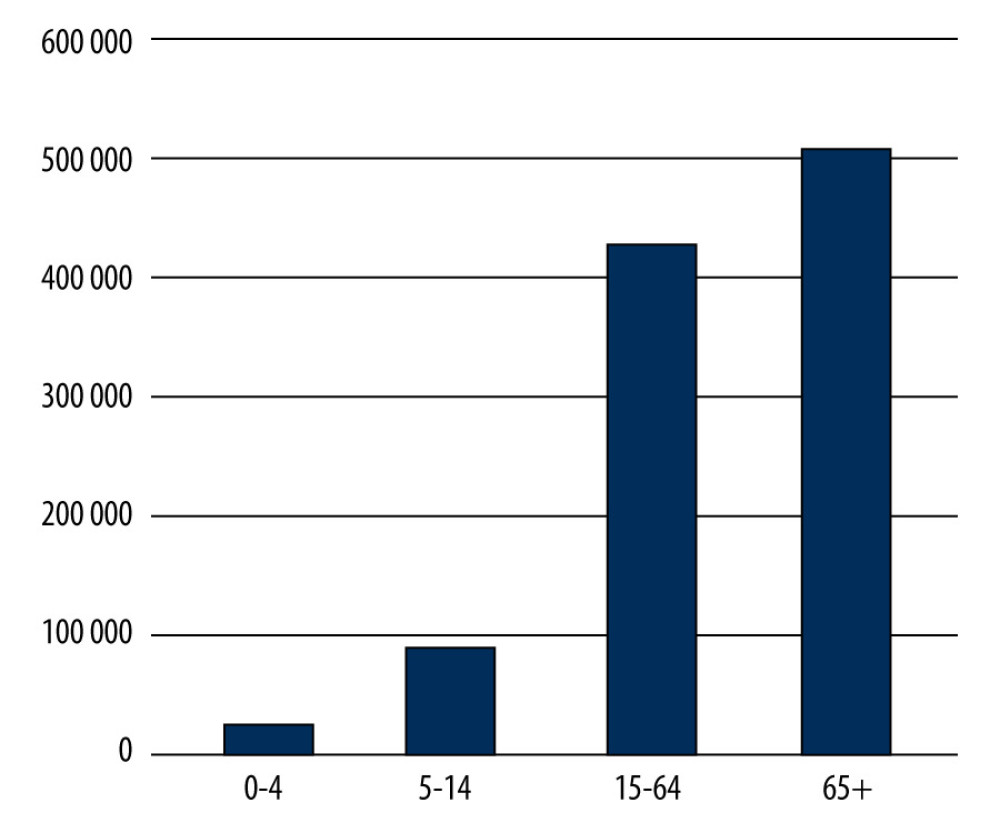 Figure 5. Number of influenza-vaccinated persons by age groups in 2020 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 5. Number of influenza-vaccinated persons by age groups in 2020 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. References
1. Li Q, Sun X, Li Z, Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus: Proc Natl Acad Sci USA, 2012; 109(46); 18897-902
2. Tong S, Shu X, Shi M, New world bats harbor diverse influenza A viruses: PLoS Pathog, 2013; 9(10); 1-12
3. Brydak LBStructure and classification. Influenza, influenza pandemic, myth of a real threat: Rytm Warszawa, 2008; 35-48 [in Polish]
4. Brydak LBClinical characteristics of influenza and post-influenza complications. Influenza, influenza pandemic, myth of a real threat: Rytm Warszawa, 2008; 101-123 [in Polish]
5. Grohskopf LA, Alyanak E, Broder KR, Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on immunization practices – United States, 2019–20 influenza season: MMWR Recomm Rep, 2019; 68(3); 1-21
6. Tuen-Ching Ch, Fan-Ngai Hung I, Ka-Hay Luk J, Effectiveness of influenza vaccination in institutionalized older adults: A systematic review: J Am Med Dir Assoc, 2014; 15(3); 226e1-e6
7. Center for Disease Control and Prevention: Vaccine effectiveness: How well do flu vaccines work? Available from: https:www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
8. Piepoli MF, Hoes AW, Agewall SESC Scientific Document Group, 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR): Eur Heart J, 2016; 37(29); 2315-81
9. Knuuti J, Wijns W, Saraste AESC Scientific Document Group, 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: Eur Heart J, 2020; 41(3); 407-77 Erratum in: Eur Heart J. 2020;41(44)4242
10. Siscovick DS, Raghunathan TE, Kin DY, Influenza vaccination and the risk of primary cardiac arrest: Am J Epidemiol, 2000; 152; 674-77
11. Lavallee PH, Perchaud V, Gautier-Bertrand M, Association between influenza vaccination and reduced risk of brain infraction: Stroke, 2002; 33; 513-18
12. Naghavi M, Barlas Z, Sioadaty S, Association of influenza vaccination and reduced risk of recurrent myocardial infarction: Circulation, 2000; 102; 3039-45
13. Ciszewski A, Bilinska ZT, Brydak LB, Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study: Eur Heart J, 2008; 29(11); 1350-58
14. Brydak LBPrevention and economic effects of influenza. Influenza, influenza pandemic, myth of a real threat: Rytm Warszawa, 2008; 283-418 [in Polish]
15. : NIZP PZH-PIB Home Page [serial online] [cited 02, 02, 2022] Available from: [in Polish]https://www.pzh.gov.pl/zaklady/
16. World Health Organization [serial online] [cited 02, 02, 2022] Available from: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/seasonal-vaccination-policies-and-coverage-in-the-european-region
17. Szczepienia ochronne w Polsce w 2020 roku, Narodowy Instytut Zdrowia Publicznego PZH – Państwowy Instytut Badawczy: Warszawa, 2021; 81-82 [in Polish]
18. Bednarska K, Hallmann-Szelińska E, Kondratiuk K, Novelties in influenza surveillence in Poland: Problemy Higieny i Epidemiologii, 2016; 97(2); 101-5
19. Brydak LB, Jak możemy ustrzec się grypy w XX1 wieku?: Family Medicine & Primary Care Review, 2013; 15(2); 216-20 [in Polish]
20. Dymek-Skoczyńska A, Stanisławska J, Drozd E, Szczepienia przeciw grypie u osób w wieku podeszłym – czynniki determinujące decyzję pacjentów: Nowiny Lekarskie, 2012; 81(1); 21-25 [in Polish]
21. Łuniewska K, Szymański K, Kondratiuk K, Subtypes of influenza virus infection and outcomes in individuals older than 65 years of age in Poland in the 2016/2017 to 2019/2020 epidemic seasons: Med Sci Monit, 2021; 27; e929243
22. Andrew MK, MacDonald S, Godin J, Persistent functional decline following hospitalization with influenza or acute respiratory illness: J Am Geriatr Soc, 2021; 69(3); 696-703
23. Iuliano AD, Roguski KM, Chang HH, Estimates of global seasonal influenza-associated respiratory mortality: A modelling study: Lancet, 2018; 391(10127); 1285-300
24. Szczepienia ochronne w Polsce w 2020 roku, Narodowy Instytut Zdrowia Publicznego PZH – Państwowy Instytut Badawczy: Warszawa, 2021; 81-82 [in Polish]
25. Szczepienia ochronne w Polsce w 2019 roku, Narodowy Instytut Zdrowia Publicznego PZH – Państwowy Instytut Badawczy: Warszawa, 2020; 81-82 [in Polish]
26. Vu T, Farish S, Jenkins M, A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community: Vaccine, 2002; 20(13–14); 1831-36
Figures
 Figure 1. The percentage of positive samples in relation to all tested samples in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 1. The percentage of positive samples in relation to all tested samples in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. Figure 2. The percentage share of influenza viruses and their subtypes in children and in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 2. The percentage share of influenza viruses and their subtypes in children and in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. Figure 3. The percentage share of influenza-like viruses in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 3. The percentage share of influenza-like viruses in the 65 year and older age group in the 2019–2020 epidemic season in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. Figure 4. Number of influenza-vaccinated persons by age groups in 2019 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 4. Number of influenza-vaccinated persons by age groups in 2019 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. Figure 5. Number of influenza-vaccinated persons by age groups in 2020 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022.
Figure 5. Number of influenza-vaccinated persons by age groups in 2020 in Poland. Prepared by L.B. Brydak, based on National Institute of Public Health NIH – National Research Institute, 2022. Tables
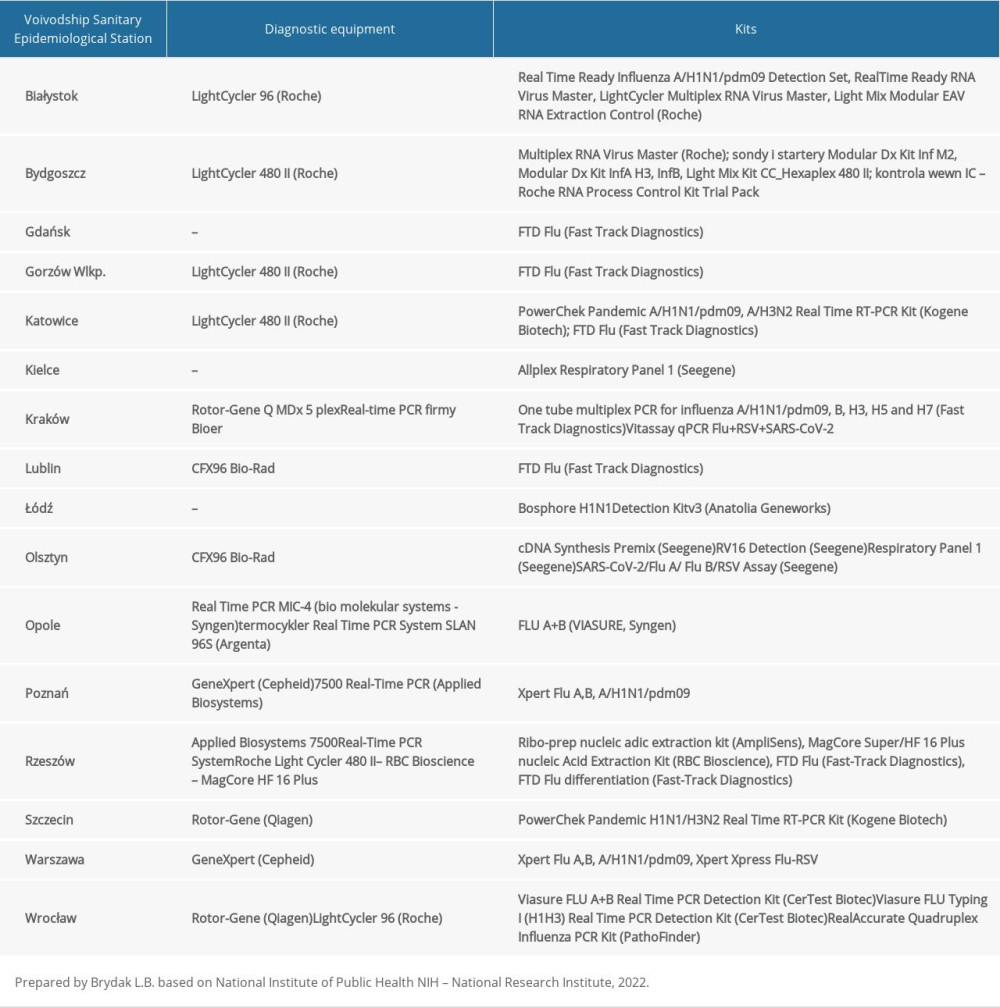 Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the epidemic season 2019–2020 in Poland.
Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the epidemic season 2019–2020 in Poland.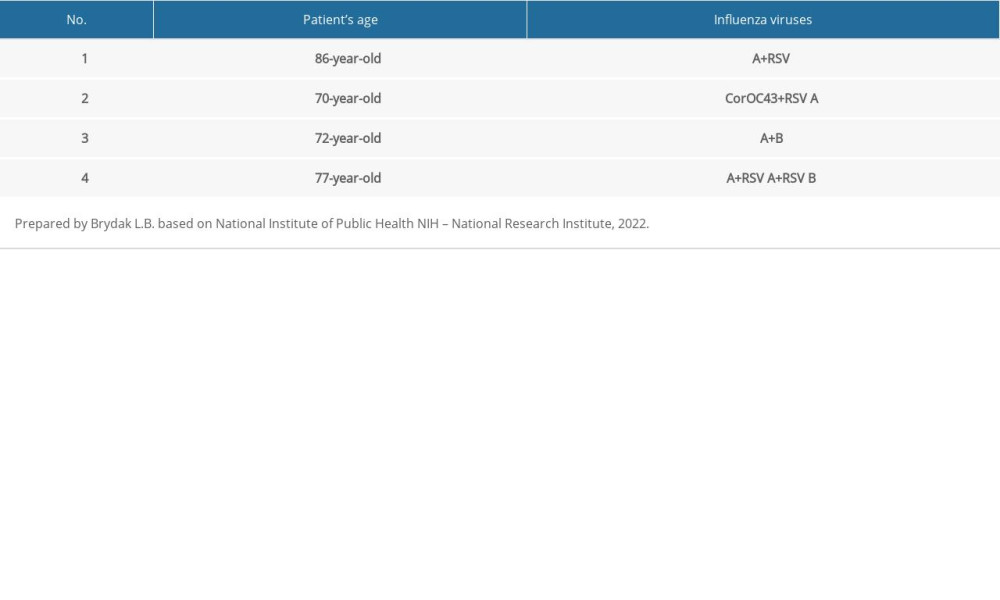 Table 2. Coinfections of respiratory viruses in the 2019–2020 epidemic season among the population of people over 65 years of age in Poland.
Table 2. Coinfections of respiratory viruses in the 2019–2020 epidemic season among the population of people over 65 years of age in Poland. Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the epidemic season 2019–2020 in Poland.
Table 1. The diagnostic methods used in 16 Voivodship Sanitary Epidemiological Stations in the epidemic season 2019–2020 in Poland. Table 2. Coinfections of respiratory viruses in the 2019–2020 epidemic season among the population of people over 65 years of age in Poland.
Table 2. Coinfections of respiratory viruses in the 2019–2020 epidemic season among the population of people over 65 years of age in Poland. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








