06 March 2023: Review Articles
Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review
Anthony Georges Matta12ADEF*, Didier Carrié2ADEFGDOI: 10.12659/MSM.939020
Med Sci Monit 2023; 29:e939020
Abstract
ABSTRACT: Takotsubo cardiomyopathy, also known as stress-induced cardiomyopathy, is a reversible syndrome commonly found among patients presenting for acute coronary syndromes, especially women. With the COVID-19 pandemic, the incidence of takotsubo cardiomyopathy was dramatically increased. However, this clinical cardiac entity remains underdiagnosed, largely due to the interplay with acute coronary syndrome. The pathophysiology of takotsubo cardiomyopathy is miscellaneous, including coronary vasospasm, microcirculatory dysfunction, catecholamine surge, and sympathetic overdrive. Diagnosing takotsubo cardiomyopathy requires a high index of clinical suspicion and multimodality tests. To date, there are no guidelines for the management of takotsubo cardiomyopathy. Thus, available data are derived from case series, retrospective analyses, and experts’ opinions. Heart failure medicines were investigated in takotsubo cardiomyopathy patients. Evidence supports the benefits of angiotensin-converting enzyme inhibitors and angiotensin receptors blockers use on mortality and recurrence rates, while results from use of beta-blockers are controversial. In complicated cases, inotropes are preferred over vasopressors, except in the presence of left ventricular outflow tract obstruction, in which medical therapy is limited to fluids administration and beta-blockers. Use of oral vitamin K antagonist can benefit patients at high thrombo-embolic risk for up to 3 months. Mechanical supports are reserved for refractory hemodynamically unstable cases. This review aims to provide an update on the epidemiology, diagnosis, and outcomes of takotsubo cardiomyopathy, and an extended discussion on the management of complicated and non-complicated cases.
Keywords: Clinical Trial, Case Management, takotsubo cardiomyopathy, Humans, Female, COVID-19, Microcirculation, Pandemics, acute coronary syndrome, COVID-19 Testing
Background
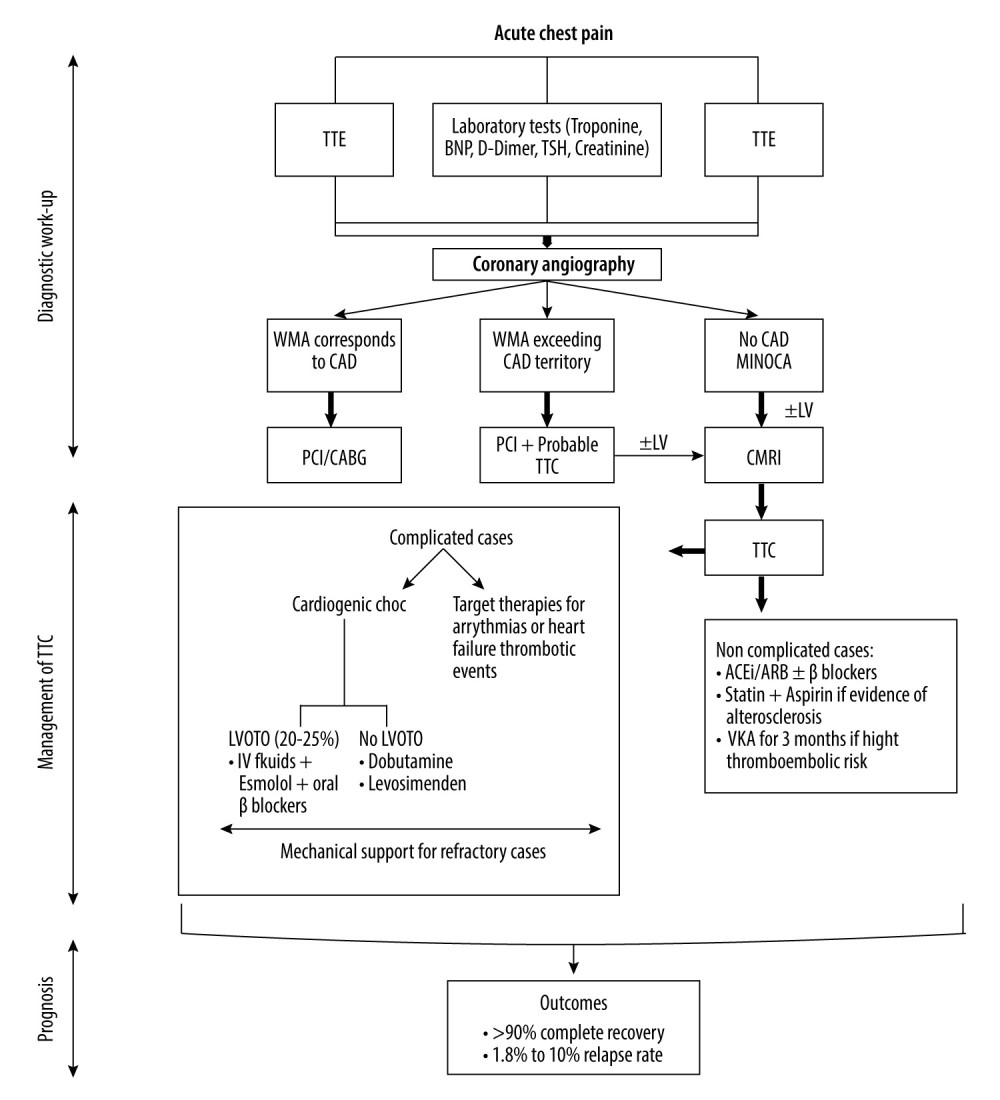
Numerous terms like “broken heart syndrome”, “stress-induced cardiomyopathy”, and “apical ballooning syndrome” have been used to describe takotsubo cardiomyopathy [1]. Takotsubo cardiomyopathy is a reversible acute cardiac condition related to transient regional left ventricular wall motion abnormalities extending beyond a single epicardial coronary artery territory [2]. It usually mimics acute coronary syndrome, and early coronary angiography is generally performed [3]. Due to this overlap between the initial clinical presentation of takotsubo cardiomyopathy and acute coronary syndrome, the Inter Tak Diagnostic Score has been recently developed to discriminate these 2 different entities in the acute stage [4]. Then, according to this score and, unlike the modified Mayo Clinic diagnostic criteria, the presence of significant coronary artery disease does not exclude takotsubo cardiomyopathy diagnosis. Four major patterns or variants of takotsubo cardiomyopathy are described in the literature: the apical ballooning form (typical form), accounting for 80% of takotsubo cardiomyopathy cases with the classical Japanese octopus trap feature [5]; mid-ventricular form with Hawk’s beak appearance [6,7]; and basal and focal forms [1,8]. Formerly considered a benign self-limiting state, takotsubo cardiomyopathy is now known to be related to short- and long-term adverse cardiovascular outcomes [9–11]. Male sex, advanced age, reduced left ventricular ejection fraction below 35% at initial presentation, prolonged QT interval on electrocardiogram, identification of a physical trigger, atrial fibrillation, and development of acute complications are predictors of poor prognosis [2]. Management of takotsubo cardiomyopathy is based on anecdotal evidence from experts’ opinions and case series, largely due to the absence of randomized clinical trials [12,13]. With the era of COVID-19 and the increased number of takotsubo cardiomyopathy cases, this review provides an update and short discussion about the epidemiology, pathophysiology, and diagnostic tests, and an extended discussion about the management of complicated and non-complicated cases based on the previously published expert consensus [12], recent reviews [13], and available studies. We also suggest a stepwise approach to the management of takotsubo cardiomyopathy. Physicians must deal with the underlying trigger when present, clinical cardiac manifestations, acute complications, and long-term recurrence risk. In general, heart failure medications are conventionally used in the setting of stress-induced cardiomyopathy. Herein, we review the management of and therapeutic approaches for takotsubo cardiomyopathy during the acute phase to call attention to the potential associated complications and to promote physician awareness, recognition, and care of this rare process. Therefore, this review aims to provide an update on the epidemiology, pathophysiology, diagnosis, management, and outcomes of takotsubo cardiomyopathy, also known as stress-induced cardiomyopathy.
Epidemiology
Takotsubo cardiomyopathy accounts 1% to 3% of acute coronary syndrome [1] and 0.5% to 0.9% of ST-segment elevation myocardial infarction [12]. It occurs predominantly in women, particularly in the post-menopausal period [13]. Women over 50 years old account for 80% to 90% of patients who develop takotsubo cardiomyopathy [2,14,15]. Takotsubo cardiomyopathy is usually underdiagnosed, especially in patients who have co-existing coronary artery disease, largely due to the interplay between acute coronary syndrome and takotsubo cardiomyopathy [16]. Precipitating physical or emotional or mixed stressful events have been identified in two-thirds of takotsubo cardiomyopathy cases [17]. The incidence of takotsubo cardiomyopathy has dramatically increased during the COVID-19 pandemic in association with psychological stressors such as social isolation, financial issues, and anxiety [18,19]. Moreover, takotsubo cardiomyopathy has also been reported as a rare complication after administration of novel messenger-RNA COVID-19 vaccines [20,21].
Pathophysiology
Although described 30 years ago, the exact pathophysiological mechanism of takotsubo cardiomyopathy remains unclear. Its pathophysiology varies, including coronary vasospasm, microcirculatory dysfunction, catecholamine surge, and sympathetic overdrive [22–24]. During the acute phase, the massive direct release of catecholamine by the sympathetic nerve endings into myocardium results in ventricular dysfunction and myocardial contraction band necrosis, a hallmark histological finding of takotsubo cardiomyopathy [25–27]. Some authors have suggested that this local catecholamine excess dysregulates myocardial calcium-handling and has more cardiotoxic effects than the circulating one [28,29]. This local catecholamine overexpression may also explain why the catecholamine bloodstream level is not always elevated. The difference in the distribution of β1 and β2 adrenoreceptors densities between the apical and basal cardiac segments could explain the observed left ventricular contraction abnormalities in the apical ballooning variant of takotsubo cardiomyopathy [28,30]. Apart from the most established adrenergic hypothesis, it is still debated whether coronary microvascular dysfunction is a consequence of or the primary cause of acute episodes of takotsubo cardiomyopathy. Repeated provocation tests showed reproducible coronary vasospasm in 20% of takotsubo cardiomyopathy patients [31].
Diagnostic Investigations in Takotsubo Cardiomyopathy
Electrocardiogram is the primary diagnostic test performed after the first medical contact with takotsubo cardiomyopathy patients, who commonly present with acute-onset chest pain and/or dyspnea mimicking acute coronary syndrome. Electrocardiogram reveals persistent or dynamic ischemic changes like ST-segment elevation, ST depression, prolonged QT interval, and T wave inversion [32,33]. ECG changes are usually not localized to a particular territory and progress over 3 stages [34–36]. The stage 1 is marked by ST-segment deviation in the early hours of symptoms onset, stage 2 involves T wave inversion and QT interval prolongation occurring within the first 72 hours, and stage 3 is characterized by gradual regression of abnormalities over weeks or months. Systematic transthoracic echocardiography has several advantages: it delineates ventricular wall motion abnormalities, evaluates left ventricular ejection fraction, and assesses acute complications (thrombus formation, mitral regurgitation, ventricular rupture, and left ventricular outflow tract obstruction) [37–39]. Almost all takotsubo cardiomyopathy patients undergo coronary angiography, that reveals normal or nearly normal coronary arteries or obstructive coronary artery disease incongruent with myocardial kinetic abnormalities [13]. Co-existing coronary artery disease is reported in 15% of takotsubo cardiomyopathy cases [40,41]. The diagnostic investigation tests routinely end with cardiac magnetic resonance imaging with gadolinium contrast administration, which helps to exclude other differential diagnoses or pathologic states (eg, acute myocardial infarction and myocarditis), and identifies the potential complications (eg, thrombi formation, right ventricle involvement, and pericardial and pleural effusion) [2,13]. Myocardial edema is the principal cardiac magnetic resonance feature of takotsubo cardiomyopathy. Levels of cardiac biomarkers (BNP/NT-pro BNP and troponin T) are generally increased in a disproportion way. A large increase in BNP/NT-pro BNP level is associated with a slight rise in troponin T concentration, and this discrepancy can help differentiate takotsubo cardiomyopathy from acute myocardial infarction in the acute presentation. Indeed, high NT-pro BNP/troponin T ratio is suggestive of takotsubo cardiomyopathy, with a sensitivity of 91% and specificity of 95% [42].
Till now, there are no guidelines for the management of TTC, and heart failure medications have been investigated in different retrospective analyses. The cardiovascular outcome of antithrombotic therapy remains debatable, especially in the absence of obstructive CAD. First, the use of ACEi (angiotensin-converting enzyme inhibitors) or ARB (angiotensin receptor blockers) has proved positive effects on 1-year survival in TTC patients [43] and reducing TTC recurrence risk [44,45]. Second, no beneficial effects for beta-blockers against mortality and recurrence rate have been observed [41–46]. However, recent studies described long-term (up to 3-years) improvement in left ventricular systolic function [47] and reduction of cardiovascular mortality [48] in conjunction with beta-blockers use. Lastly, standard heart failure treatment combining beta-blockers and ACEi or ARBs with or without aspirin and statin has revealed controversial results. While numerous studies have reported favorable result with this combination [49–51], no reduction in TTC recurrence at 3-year follow-up and an absence of significant improvement in left ventricular function and duration of hospital stay have been revealed by others [52–54]. Interestingly, the reported rate of recurrence of TTC is low [48]. Focusing on antithrombotic therapy, the early use of low-molecular-weight heparin in the acute phase followed at hospital discharge by up to 3 months of oral anticoagulant treatment has benefits in stroke prevention, especially in patients at high thromboembolic risk [55,56]. Although aspirin or dual antiplatelet (aspirin + clopidogrel) intake may reduce the major cardiovascular events including stroke [48], a recent study conducted on 1553 TTC patients found no effects of aspirin use on prognosis and complications development [57]. Thus, statins and aspirin are conventionally recommended in the setting of co-existing CAD [2]. A fast reduction in inflammatory biomarkers was associated with 600 mg daily use of alpha-lipoic acid [58]. In general, the optimal duration of TTC treatment ranges from 3 months to 1 year, depending on cardiac function recovery [59]. There are 2 ongoing prospective randomized clinical trials. The first, NACRAM trial, is investigating the benefits of early intravenous administration of n-acetylcysteine followed by oral ramipril for 3 months on myocardial edema reduction and cardiac function improvement [60]. The second, an interventional trial, is evaluating outcomes of early TTC patient rehabilitation [61]. The advantages of hormone therapy (estrogens) in the long-term management of TTC are still being studied. Angiotensin-neprilysin receptor inhibitors, SGLT2 inhibitors, and mineralocorticoid receptor antagonists, which are recommended in the context of heart failure, have not tested in TTC patients to date. Physicians must also deal with precipitating triggers when present, such as psychiatrist referral and antidepressant prescription. To summarize, the available evidence supports the use of ACEi or ARB in hemodynamically stable uncomplicated cases of TTC. The outcomes from the use of all other pharmacological agents remain unclear.
Management of Complicated TTC
ACUTE HEART FAILURE:
The management of acute HF after TTC is driven by the standard international guidelines for HF, comprising oxygen and respiratory support, ACEi, ARB, ARNI, beta-blockers, mineralocorticoid receptors antagonist, and diuretics. Thus, the only difference is to avoid pharmacological therapies for preload and afterload reduction in the setting of left ventricular outflow tract obstruction (LVOTO). While beta-blockers did not show an effect on survival in HF-TTC patients [46], they showed a reduction of cardiac rupture incidence [62]. The average duration of medical treatment is up to 4 weeks.
CARDIOGENIC SHOCK:
Within the first 72 hours of hospital admission for TTC, cardiogenic shock occurs in 11% of hospitalized patients, and 20% to 25% present with left ventricular outflow tract obstruction (LVOTO) [63]. Positive inotropic agents (eg, dopamine/dobutamine) are preferred over sympathomimetic cholinergic vasopressors (eg, ephedrine/norepinephrine) to restore cardiac function and blood pressure. Exacerbated in-hospital and long-term mortality rates have been observed with catecholamine administration for circulatory support in hemodynamically unstable TTC patients [64]. Mechanical support is considered for refractory and/or severe cases with and without LVOTO. However, it is mandatory to check for LVOTO before initiating therapy, as this condition contraindicates inotropic drugs and intra-aortic balloon pump. In case of LVOTO, the first medical treatment is limited to fluid resuscitation and beta-blockers. Santoro et al revealed the efficacy of early intravenous administration of esmolol followed by daily bisoprolol in decreasing LVOTO gradient and alleviating obstruction [65]. Intravenous injection of levosimendan, a calcium-sensitizing agent and ATP-potassium channel-opening mediator, significantly improves left ventricular function in the setting of TTC-cardiogenic shock [66].
THROMBOEMBOLIC EVENTS:
Left ventricular thrombus formation and subsequent systemic embolization occur in 1$ to 2% of TTC cases, especially in those with severely altered left ventricular systolic function. When left ventricular thrombus is detected either by transthoracic echocardiography or cardiac MRI, oral anticoagulation therapy based on vitamin K antagonist is recommended for at least 3 months to prevent thrombus migration [55]. The efficacy of novel non-vitamin K oral anticoagulant has not been studied in TTC.
ARRHYTHMIAS:
Ventricular tachycardia or ventricular fibrillation represents two-thirds of TTC-associated arrhythmia [67,68]. Closed QT interval and telemetry monitoring during the acute phase were suggested. Intravenous magnesium is the first therapeutic approach for torsade de pointes, followed by isoproterenol for non-responders. Amiodarone is the preferred anti-arrhythmic drug compared to other drugs that may also prolonged QT interval. Finally, the question concerning permanent pacemaker or defibrillator implantation versus temporary device use in case of severe arrhythmias (high-grade atrio-ventricular block, ventricular arrhythmias) is still debatable [59].
Takotsubo Cardiomyopathy Patient Outcomes
The overall prognosis of TTC is favorable with complete recovery in more than 90% of patients within 1–2 months. However, the reported prevalence of relapse ranges from 1.8% to 10% [43,45,69,70]. Women with advanced age (>50 years), severely altered left cardiac function, vulnerability to emotional stress, and fluctuations in cardiac baroreceptors were identified as independent predictors of recurrence [71]. In addition, male sex, high-grade Killip on hospital admission, and diabetes mellitus were recognized as independent predictors of mortality [72]. A recently published study showed lower mortality and recurrence rates at 5.2-year follow-up in TTC patients treated with beta-blockers only compared to those receiving ACEi or ARB alone [70]. Finally, modifying lifestyle by reducing caffeine consumption and smoking cessation help to prevent TTC recurrence [73].
Conclusions
To summarize, diagnosing TTC requires a high index of clinical suspicion and multimodality tests. The management of TTC is personalized care analyzed on a case-by-case basis. A primary diagnostic work-up is essential to screen for the presence of potential complications, especially for LVOTO, which may drive the therapeutic approach. ACEi/ARB and beta-blockers are commonly used in non-complicated patients, while mechanical supports, vasopressors, and inotropes are reserved for severe cases. Statins and aspirin are advised if atherosclerotic signs co-exist. Long-term management includes screening for triggers or precipitant factors, monitoring for recurrence, and cardiac rehabilitation. Further prospective randomized clinical trials are warranted to establish standard guidelines.
References
1. Matta A, Delmas C, Campelo-parada F, Takotsubo cardiomyopathy: Rev Cardiovasc Med, 2022; 23; 38
2. Ghadri J, Wittstein IS, Prasad A, International expert consensus document on takotsubo syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology: Eur Heart J, 2018; 39; 2032-46
3. Luscher TF, Templin C, Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition: Eur Heart J, 2016; 37; 2816-20
4. Ghadri JR, Cammann VL, Jurisic S, A novel clinical score (Inter TAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry: Eur J Heart Fail, 2017; 19; 1036-42
5. Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy: JAMA, 2011; 306; 277-86
6. Roncalli J, Carrie D, Fauvel JM, Losordo D, A hawk’s beak to identify the new transient midventricular Tako-Tsubo syndrome: Int J Cardiol, 2008; 127; 179-80
7. Matta A, Roncalli J, Elbaz M, Mid-ventricular takotsubo cardiomyopathy with hawk’s beak appearance: A case report: Am J Case Rep, 2020; 21; e919563
8. Ennezat PV, Pesenti-Rossi D, Aubert JM, Transient left ventricular basal dysfunction without coronary stenosis in acute cerebral disorders: A novel heart syndrome (inverted takotsubo): Echocardiography, 2005; 22; 599-602
9. Redfors B, Jha S, Thorleifsson S, Short- and long-term clinical outcomes for patients with takotsubo syndrome and patients with myocardial infarction: A report from the swedish coronary angiography and angioplasty registry: J Am Heart Assoc, 2021; 10; e017290
10. Uribarri A, Nunez-Gil IJ, Conty DA, Short- and long-term prognosis of patients with takotsubo syndrome based on different triggers: Importance of the physical nature: J Am Heart Assoc, 2019; 8; e013701
11. Ghadri JR, Kato K, Cammann VL, Long-term prognosis of patients with takotsubo syndrome: J Am Coll Cardiol, 2018; 72; 874-82
12. Sattar Y, Woei Siew KS, Connerney M, Management of takotsubo syndrome: A comprehensive review: Cureus, 2020; 12; e6556
13. Assad J, Femia G, Pender P, Takotsubo syndrome: A review of presentation, diagnosis and management: Clin Med Insights Cardiol, 2022; 16; 11795468211065782
14. Cammann VL, Szawan KA, Stahli BE, Age-related variations in takotsubo syndrome: J Am Coll Cardiol, 2020; 75; 1869-77
15. Pelliccia F, Kaski JC, Crea F, Camici PG, Pathophysiology of takotsubo syndrome: Circulation, 2017; 135; 2426-41
16. Napp LC, Cammann VL, Jaguszewski M, Coexistence and outcome of coronary artery disease in takotsubo syndrome: Eur Heart J, 2020; 41; 3255-68
17. Gianni M, Dentali F, Grandi AM, Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review: Eur Heart J, 2007; 27; 1523-29
18. Jabri A, Kalra A, Kumar A, Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic: JAMA Netw open, 2020; 3; e2014780
19. Eftekharzadeh P, Patel A, Sokolova E, Takotsubo cardiomyopathy: A COVID-19 complication: Cureus, 2022; 14; e22803
20. Ahmed SK, Mohamed MG, Essa RA, Global reports of takotsubo (stress) cardiomyopathy following COVID-19 vaccination: A systematic review and meta-analysis: Int J Cardiol Heart Vasc, 2022; 43; 101108
21. Fazlollahi A, Zahmatyar M, Noori M, Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series: Rev Med Virol, 2022; 33; e2318
22. Hasan SM, Patel JD, Faluk M, Takotsubo cardiomyopathy and its variants: A case series and literature review: J Community Hosp Intern Med PersPerspect, 2020; 10; 210-15
23. Boyd B, Solh T, Takotsubo cardiomyopathy: JAAPA, 2020; 33; 24-29
24. Amin HZ, Amin LZ, Pradipta A, Takotsubo cardiomyopathy: A brief review: J Med Life, 2020; 13; 3-7
25. Kume T, Kawamoto T, Okura H, Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction: Circ J, 2008; 72; 106-8
26. Akashi YJ, Nakazawa K, Sakakibara M, 123i-mibg myocardial scintigraphy in patients with “takotsubo” cardiomyopathy: J Nucl Med, 2004; 45; 1121-27
27. Mizutani K, Shioya A, Hirose Y, Serious takotsubo cardiomyopathy: An autopsy case presenting severe irreversible myocardial damage after frequent episodes of recurrence: Diagn Pathol, 2020; 15; 90
28. Mori H, Ishikawa S, Kojima S, Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli: Cardiovasc Res, 1993; 27; 192-98
29. Nef HM, Mollmann H, Troidl C, Abnormalities in intracellular Ca2+ regulation contribute to the pathomechanism of tako-tsubo cardiomyopathy: Eur Heart J, 2009; 30; 2155-64
30. Vriz O, Minisini R, Citro R, Analysis of beta1 and beta2-adrenergic receptors polymorphism in patients with apical ballooning cardiomyopathy: Acta Cardiol, 2011; 66; 787-90
31. Rivero F, Cuesta J, Garcia-Guimaraes M, Time-related microcirculatory dysfunction in patients with takotsubo cardiomyopathy: JAMA Cardiol, 2017; 2; 699-700
32. Ghadri JR, Wittstein IS, Prasad A, International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and management: Eur Heart J, 2018; 39; 2047-62
33. Yoshikawa T, Takotsubo cardiomyopathy, a new concept of cardiomyopathy: Clinical features and pathophysiology: Int J Cardiol, 2015; 182; 297-303
34. Namgung J, Electrocardiographic findings in takotsubo cardiomyopathy: ECG evolution and its difference from the ECG of acute coronary syndrome: Clin Med Insights Cardiol, 2014; 8; 29-34
35. Frangieh AH, Obeid S, Ghadri JR, Ecg criteria to differentiate between takotsubo (stress) cardiomyopathy and myocardial infarction: J Am Heart Assoc, 2016; 5; e003418
36. Scally C, Choo W, Rudd A, The early dynamic of ecg in takotsubo syndrome presenting with st-elevation: A comparison with age and gender-matched ST-elevation myocardial infarction: Int J Cardiol, 2020; 320; 7-11
37. Citro R, Lyon AR, Meimoun P, Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: Clinical and prognostic implications: J Am Soc Echocardiogr, 2015; 28; 57-74
38. De Gregorio C, Grimaldi P, Lentini C, Left ventricular thrombus formation and cardioembolic complications in patients with takotsubo-like syndrome: A systematic review: Int J Cardiol, 2008; 131; 18-24
39. Akashi YJ, Tejima T, Sakurada H, Left ventricular rupture associated with takotsubo cardiomyopathy: Mayo Clin Proc, 2004; 79; 821-24
40. Uribarri A, Nunez-Gil IJ, Conty DA, Short- and long-term prognosis of patients with takotsubo syndrome based on different triggers: Importance of the physical nature: J Am Heart Assoc, 2019; 8; e013701
41. Redfors B, Jha S, Thorleifsson S, Short- and long-term clinical outcomes for patients with takotsubo syndrome and patients with myocardial infarction: A report from the swedish coronary angiography and angioplasty registry: J Am Heart Assoc, 2021; 10; e017290
42. Fröhlich GM, Schoch B, Schmid F, Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy: Int J Cardiol, 2012; 154; 328-32
43. Templin C, Ghadri JR, Diekmann J, Clinical features and outcomes of takotsubo (stress) cardiomyopathy: N Engl J Med, 2015; 373; 929-38
44. Regnante RA, Zuzek RW, Weinsier SB, Clinical characteristics and four-year outcomes of patients in the Rhode Island takotsubo cardiomyopathy registry: Am J Cardiol, 2009; 103; 1015-19
45. Singh K, Carson K, Usmani Z, Systematic review and metanalysis of incidence and correlates of recurrence of takotsubo cardiomyopathy: Int J Cardiol, 2014; 174; 696-701
46. Isogai T, Matsui H, Tanaka H, Early β-blocker use and in-hospital mortality in patients with Takotsubo cardiomyopathy: Heart, 2016; 34; 161-66
47. Ghalyoun BA, Guragai R, Garris R, Abstract11626: Faster recovery in takotsubo with prior beta-blocker use: Circulation, 2019; 140
48. Medias JE, Cardioselective ultra-short-acting β-blockers for patients with takotsubo syndrome?: Letters to the editor: Geriatr Gerontol Int, 2018; 18; 816-17
49. Yayehd K, N’da NW, Belle L, Management of Takotsubo cardiomyopathy in non-academic hospitals in France: The observational French syndromes of takotsubo (OFSETT) study: Arch Cardiovasc Dis, 2016; 109; 4-12
50. Abanador-Kamper N, Kamper L, Wolfertz J, Evaluation of therapy management and outcome in takotsubo syndrome: BMC Cardiovasc Disord, 2017; 17; 225
51. Dias A, Franco E, Koshkelashvili N, Antiplatelet therapy in takotsubo cardiomyopathy: Does it improve cardiovascular outcomes during index event?: Heart Vessels, 2016; 31; 1285-90
52. Santoro F, Ieva R, Musaico F, Lack of efficacy of drug therapy in preventing takotsubo cardiomyopathy recurrence: A meta-analysis: Clin Cardiol, 2011; 34; 672-76
53. Fazio G, Pizzuto C, Barbaro G, Chronic pharmacological treatment in takotsubo cardiomyopathy: Int J Cardiol, 2008; 127; 121-23
54. Kummer M, ElBattrawy I, Ansari U, The use of beta blockers in takotsubo syndrome as compared to acute coronary syndrome: Front Pharmacol, 2020; 11; 681
55. Santoro F, Stiermaier T, Tarantino N, Left ventricular thrombi in takotsubo syndrome: Incidence, predictors, and management: Results from the GEIST (German Italian Stress Cardiomyopathy) registry: J Am Heart Assoc, 2017; 6; 006990
56. De Gregorio C, Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: A systematic review of 26 clinical studies: Int J Cardiol, 2010; 141; 11-17
57. D’Ascenzo F, Gili S, Bertaina M, Impact of aspirin on takotsubo syndrome: A propensity score-based analysis of the InterTAK Registry: Eur J Heart Fail, 2020; 22; 330-37
58. Marfella R, Barbieri M, Sardu C, Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy: J Cardiol, 2016; 67; 153-61
59. Bairashevskaia AV, Belogubova SY, Kondratiuk MR, Update of takotsubo cardiomyopathy: Present experience and outlook for the future: Int J Cardiol Heart Vasc, 2022; 39; 100990
60. Ong GJ, Nuguyen TH, Stansborough J, The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and design of a randomised controlled trial of sequential NAcetylcysteine and ramipril for the management of takotsubo Syndrome: Contemporary Clinical Trials, 2020; 90; 105894
61. Dawson D, Physical exercise and mental wellbeing rehabilitation for acute stress-induced takotsubo cardiomyopathy: The PLEASE Study (PLEASE), 2021
62. Kumar S, Kaushik S, Nautiyal A, Cardiac rupture in takotsubo cardiomyopathy: A systematic review: Clin Cardiol, 2011; 34; 672-76
63. P’keefe EL, Torres-Acosta N, O’keefe JH, Takotsubo syndrome: Cardiotoxic stress in the COVID era, Mayo Clinic proceedings: innovations: Qual Outcomes, 2020; 4; 775-85
64. Ansari U, Elbattrawy I, Fastner C, Clinical outcomes associated with catecholamine use in patients diagnosed with takotsubo cardiomyopathy: BMC Cardiovasc Disord, 2018; 18; 54
65. Santoro F, Ieva R, Ferraretti A, Hemodynamic effects, safety, and feasibility of intravenous esmolol infusion during Takotsubo cardiomyopathy with left ventricular outflow tract obstruction: Results from a multicenter registry: Cardiovasc Ther, 2016; 34; 161-66
66. Santoro F, Ieva R, Ferraretti A, Safety and feasibility of levosiemndan administration in Takotsubo cardiomyopathy: A case series: Cardiovasc Ther, 2013; 31; 133-37
67. Moller C, Eitel C, Thiele H, Eitel I, Stiermaier T, Ventricular arrhythmias in patients with Takotsubo syndrome: J Arrhythmia, 2018; 34; 369-75
68. Brown KH, Trohman RG, Madias C, Arrhythmias in takotsubo cardiomyopathy: Card Electrophysiol Clin, 2015; 7; 331-40
69. Kato K, Kitahara H, Kobayashi Y, Recurrence of takotsubo cardiomyopathy: International Cardiovascular Forum Journal, 2016; 5; 50-52
70. Lau C, Chiu S, Nayak R, Survival and risk of recurrence of takotsubo syndrome: Heart, 2021; 107; 1160-66
71. Sager HB, Schunkert H, Kurowski V, Recurrent mid-ventricular Tako-Tsubo cardiomyopathy: Three episodes of a uniform cardiac response to varying stressors: Int J Cardiol, 2011; 152; 22-24
72. Stiermaier T, Moeller C, Oehler K, Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences: Eur J Heart Fail, 2016; 18; 650-56
73. Harris KM, Rosman L, Burg MM, Salmoirago-Blotcher , Modifiable lifestyle factors in women with Takotsubo syndrome: A case-control study: Heart, 2020; 49; 524-29
In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952