02 March 2023: Clinical Research
Demographic and Clinical Factors Associated with Depression in 140 Hospitalized Diabetic Patients with Distal Symmetric Polyneuropathy Evaluated Using Beck Depression Inventory and 6-Item Neuropathy Total Symptom Score Questionnaire
Piotr Dziemidok1ABCDEF, Daria Gorczyca-Siudak1CDEF*, Marta Makara-Studzińska2ADFDOI: 10.12659/MSM.939043
Med Sci Monit 2023; 29:e939043
Abstract
BACKGROUND: Diabetic distal symmetric polyneuropathy (DSPN) is one of the most common and costliest long-term complications. The pain and function limitations may lead to depression. This study aimed to assess the influence of demographic and clinical factors on the prevalence of depression among diabetic patients with distal symmetric polyneuropathy (DSPN).
MATERIAL AND METHODS: A total of 140 patients with diabetic DSPN completed the 21-item Beck Depression Inventory (BDI) measuring characteristic attitudes and symptoms of depression. The intensity of neuropathic complaints was assessed using the Neuropathy Total Symptom Score- 6 items (NTSS-6). Testing for peripheral neuropathy was performed. All patients completed questionnaires, which included anthropometric measures, social parameters, and medical aspects. Statistical analyses were done using STATISTICA 8 PL software.
RESULTS: Statistically significant relationships were found between the depression symptoms in diabetic patients and the intensity of subjective neuropathy symptoms evaluated by NTSS-6, body mass index (BMI), and education level. On average, each 1-point increase in NTSS-6 predicted a 16% increase in the risk of depression. Each 1 kg/m² increase in the BMI was associated with a 10% increase of depression risk.
CONCLUSIONS: The study showed the positive quantitative relationship between diabetic DSPN and depression symptoms. The BMI, severity of neuropathy symptoms, and lower level of education had a statistically significant association with the level of depression and may be useful in evaluating the risk of depression among DSPN patients.
Keywords: Body Weight, Depression, Diabetic Neuropathies, Education, Humans, Polyneuropathies, Pain, Demography, Diabetes Mellitus
Background
Diabetes mellitus (DM) is a complex condition with a serious impact on the lives and well-being of individuals, their families, and societies worldwide. It requires long-term management to achieve optimal glycemic control and successful prevention of chronic complications [1,2]. DM prevalence is still increasing – nearly half a billion people are living with DM worldwide and the number is projected to increase by 25% in 2030 and 51% in 2045, reaching 700 million. The prevalence is higher in urban (10.8%) than rural (7.2%) areas, and in high-income (10.4%) than low-income countries (4.0%) [3]. Individuals with DM have an increased risk of developing mental health disorders, psychological disturbances, and functional problems associated with living with their condition [4]. There is a 2-fold increase in depressive symptoms observed in people with DM [5] in both type 1 and type 2 diabetics [6]. The prevalence of DM and episodes of depressive symptoms differ widely across countries [7]. There is a strong dominance of studies evaluating the relationship between depression and diabetes in English-speaking populations, which suggests a need for additional studies from non-English-speaking countries.
Diabetic distal symmetric polyneuropathy (DSPN) is one of the costliest long-term complications, with a history of many inadequately treated or untreated patients [8]. Additionally, DSPN is a well-known trigger for developing depression, although the relationship between the intensity of diabetic neuropathy symptoms and depression is not well established [7,9,10]. Pain and unsteadiness are well-known factors of depression among diabetic patients [11]. Observing and establishing the relationship between other sociomedical factors and the risk of depression in patients with diabetic neuropathy is important to prevent development of depression in this group of patients.
The Beck Depression Inventory (BDI) is a 21-item, self-reporting, effective, and simple screening test evaluating the severity of depression in normal and psychiatric populations. The BDI questionnaire can be used to screen for depressive symptoms in people with diabetes in clinical practice [12,13]. The Neuropathy Total Symptom Score - 6-items (NTSS-6) measures the frequency and severity of 6 diabetic peripheral neuropathic symptoms. NTTS-6 correlates well with biothesiometry findings [14]. It provides a valid assessment of diabetic neuropathy sensory symptoms and is useful for symptom evaluation in clinical trials and in practice [15]. In our clinic we evaluate diabetic neuropathy using all these methods as a model of constant neuropathy evaluation for the last 7 years and we explained the method in our last paper [16]. Therefore, the present study aimed to evaluate the demographic and clinical factors associated with depression in 140 diabetic patients in Lublin, Poland with DSP using the BDI and the NTSS-6 questionnaire.
Material and Methods
Ethics approval was given by the Ethics Committee Institute of Rural Health by resolution no. 3/2017 on 22 Feb 2017.
Since all the methods used for the evaluation of our patients were routine everyday tests that we use and perform in our ward, patients gave signed general consent for hospitalization and treatment at the ward according to the hospital rules.
The study included 140 patients with DM (32 patients with DM type 1 and 108 patients with DM type 2) hospitalized at the Diabetes Clinic of the Institute of Rural Health in Lublin during 2010–2011. The study group consisted of 80 males and 60 females with recognized DSPN. Since there is no evidence that diabetic neuropathy and depression differ in symptomatology, etiopathology, or diagnostic methodology between the 2 types of DM, all patients were analyzed together.
The patients had diabetes mellitus recognized long before hospitalization due to the treatment in the out-patient clinic of our center and DSPN (recognized) before or during hospitalization. In the methods described below, DSPN was confirmed during the first day of hospitalization and BDI was applied during first 24 h of hospitalization.
The aim of the study was to evaluate the relationship between several sociomedical factors and/or the severity of neuropathy and risk of depression among patients with DSPN.
Several clinical, social, and anthropometric factors were checked and the severity of DSPN symptoms was evaluated to establish their association with depressive symptoms to identify potential targets for interventions to decrease depression related to diabetic DSPN.
Patients meeting the following criteria were enrolled to the study: (1) at least 18 years old; (2) diagnosed with diabetes mellitus type 1 or 2; (3) symptomatic diabetic polyneuropathy affecting the lower extremities with at least 1 positive sensory symptom such as pain, burning, paresthesia, or prickling; (4) without changes in neuropathy treatment during the last 30 days (stable doses of pain medications or other medications prescribed for diabetic neuropathy); (5) assessed by the investigator as able to maintain compliance and cooperation during hospitalization. Exclusion criteria were as follows: (1) lack of informed written consent to participate in the study; (2) ankle-brachial index (ABI) <0.5 suggesting ischemia; (3) any concomitant severe disease limiting compliance to study procedures or life expectancy. The study participants were asked about the presence of neuropathy symptoms such as burning, a painful feeling of cold or hot, pins and needles, electric shocks, pricking, numbness, and itching.
All patients completed a BDI questionnaire consisting of 21 items to quantify the severity of depression symptoms. Each item is a list of 4 statements arranged in increasing severity about a particular symptom of depression, including 13 items for cognitive and 8 items for somatic symptoms. BDI scores were calculated according to the questionnaire key: 1–11 points indicates symptoms are considered normal, 12–19 indicates mild depression, 20–25 points indicates moderate depression, and 26–63 points indicates severe depression. The patients also filled out a questionnaire including anthropometric and social parameters. Glycated hemoglobin (HbA1c) levels were assessed and other data on medical aspects of DM were collected.
The neurological examination described below was the same as in our previous published work [16]. The severity of neuropathic concerns was assessed using the NTSS-6 to evaluate the frequency and intensity of individual neuropathy sensory symptoms: numbness and/or insensitivity; prickling and/or tingling sensation; burning sensation; aching pain and/or tightness; sharp, shooting, lancinating pain; and allodynia and/or hyperalgesia.
Relationships between depression symptoms and the degree of DM control and the intensity of neuropathic symptoms and signs were analyzed. Variables assessing neuropathic and vascular complications and the level of DM control were evaluated. The severity of DSPN was also measured by performing monofilament tactile sensitivity test, sensation of vibration with a 128-Hz tuning fork, and sensation of temperature and tendon reflexes in the lower extremities.
The tactile sensation was assessed by the 5.07 (10 g) Semmes–Weinstein monofilament test, temperature sensation was assessed by the 10-point Tip-Therm test (warm–cold), and changes in vibration sensation threshold on the participants’ feet were assessed using a manual biothesiometer. Knee and ankle reflexes were checked with a neurological hammer. The instrumental assessment of neuropathy and reflexes was conducted by the same person experienced in this field for years. A 10 sensitive-point test protocol was used for tactile and temperature sensation, with assessment at the big toe, third and fifth toe and 3 (first, third, and fifth) metatarsal head points on both feet, followed by 2 points on metatarsus and 1 on the heel, with 3 examinations per point, including 1 random false stimulation. Foot vibration perception threshold was assessed in 2 points per foot: a toe’s top and the lateral ankle. The effect of treatment was assessed separately for the tactile, temperature, vibration sensation, and reflexes. For each participant, the authors summed up the total scores in tactile sensation, temperature sensation, vibration perception, and reflexes separately. For tactile, temperature sensation, and reflexes, there was 1 point given when a feeling or reflex was present in a certain location and zero points when it was absent. In tactile and temperature sensation, the maximum score was 20 (10 points on each foot). For reflexes the maximum score was 4 (2 points on each leg). The vibration perception was assessed using a manual biothesiometer and there was an average score calculated from 4 examined locations [16].
To examine vascular complications, the ankle-brachial index (ABI) was applied as a simple method to screen for peripheral arterial disease (PAD).
The obtained information was entered into an electronic database and processed using STATISTICA 8 PL software. To assess how anthropometric and social characteristics and methods of DM treatment affect the severity of depression symptoms, multiple logistic regression analysis was used.
The multiple linear regression analysis was applied by standard and stepwise backward elimination method. Respective odds ratios (ORs) of the occurrence of depression were calculated with statistical significance for the whole model of regression and for particular explanatory variables. Using the stepwise backward elimination method, the variables which did not significantly contribute to the adjustment of the regression model (measured by the R2) were eliminated. The presence of collinearity was estimated by tolerance factor. We did not consider the effect modifiers. We did not observe any outliers in scatterplot analysis. The best regression model obtained of a poor adjustment (R2 corrected=0.19248761) explained only less than 20% of variability according to BDI. With the results of linear regression, an attempt was undertaken to estimate the risk of depression (more than 11 points in BDI) in accordance with education and BMI (body mass index) in the constructed model of logistic regression.
Results
Data from 140 adults (aged 20–85 years old, mean age 57±14 years old) with DM were analyzed, including 80 males and 60 females. Most of the patients (72%) were living in an urban area and had at least secondary education (58.5%). The vast majority of participants did not work any longer (70%) and did not receive retirement pension (61%) or sickness allowance (63%). Non-smokers accounted for 86% of patients. Participants’ social characteristics are described in Table 1.
Most study participants had type 2 DM (108 patients vs 32 patients with DM type 1), DM lasted for 14±9 years, and BMI was 30±6 kg/m2. Most participants were treated with insulin injections (81%). Only 29% of patients included in the study administered oral diabetes medications. Ninety-one (65%) participants admitted that they were not following a diabetic diet.
Tables 2 and 3 demonstrate anthropometric and medical characteristics of the patients.
The mean BDI-II score was 15±8 points. According to the results from the BDI questionnaire, no depression was reported by 49 patients (35%), mild depression by 55 patients (39%), moderate by 23 patients (17%), and severe depression by 13 patients (9%).
In the study group, statistically significant regression coefficients were obtained for both BMI and education level in correlation with the severity of depression. Increased level of depression among patients with DSPN was related to obesity. University education was associated with a lower severity of depression among patients with DSPN. Gender, age, place of residence, occupation, retirement pension, type of diabetes and its duration, use of oral drugs and insulin, and following a DM diet were excluded from the model by stepwise regression method (Table 4).
An education level higher by one degree (eg, university vs secondary school) was associated with 50% lower depression risk, whereas a 1 kg/m2 increase in the BMI increased the depression risk in patients with diabetic DPN by nearly 10%. The statistical method applied confirmed that the severity of neuropathic symptoms significantly increases the risk of depression (Table 5).
The stepwise backward elimination analysis confirmed that NTSS-6 as a single variable provides the best statistical adjustment (more than 20% of the explained variability of depression inventory). By performing logistic regression, it was estimated that a 1-point increase in the NTSS-6 was related to a 16% increase of the risk of depression.
Discussion
Distal symmetric polyneuropathy (DSPN) is the most common chronic complication of DM. It is present in up to 10% of patients with the diagnosis of DM2 and affects 50% of patients with long-term diabetes [8–11]. It is now increasingly accepted that it starts to develop even in individuals with prediabetes [17]. Up to 50% of DSPN cases are asymptomatic, which can lead to foot injuries, foot ulcers, and amputations. Therefore, research has been aimed at a deeper understanding of not only the pathogenesis but also risk factors, since this knowledge may help prevent DSPN. The pathogenesis of DSPN is multifactorial and connected with metabolic and microvessel disorders related to risk factors. Major modifiable risk factors of DSPN include hyperglycemia, hypertension, dyslipidemia, obesity, and smoking, followed by insulin resistance, hypoinsulinemia, diabetes duration, age, and height. Strict glycemic control has been shown to be effective in DM1, but to a lesser extent in DM2. The major comorbidities of DSPN are depression, autonomic neuropathy, peripheral arterial disease, cardiovascular disease, nephropathy, retinopathy, and medial arterial calcification [18]. Knowing the relevant risk factors and comorbidities can improve the therapeutic strategy in clinical practice as a part of the overall medical care for patients with DSPN. Clinical practice and consecutive studies indicate a relationship between complications in diabetic patients, especially DSPN, and depression [19]. There are studies which directly connect the development of diabetic neuropathy with depression [11,20].
The study results suggest that, apart from the DSPN symptoms severity, BMI and lower level of education are risk factors for depression in patients with DSPN.
Trento et al suggested that patients with type 2 diabetes are at relatively low risk of psycho-cognitive decline, but being female and on long-term insulin treatment may be risk factors for psychological distress [21]. However, the presence and severity of diabetic neuropathy is associated with depressive symptoms [22]. The painful form of diabetic neuropathy occurs in about one-quarter of patients with DM2 [23]. There is a strong dominance of pain as a major contributor to depression among diabetic patients, and it is related to the multifactorial effect of pain on the human body [20]. Benbow et al found that diabetic neuropathy (especially painful) has the strongest negative influence on diabetic patients’ quality of life [24].
The risk of depression among elderly Japanese diabetic patients (older than 65 years) was assessed by Ishizawa et al in the DIACET study. The greater the depression severity, the higher the crude odds ratios were for peripheral polyneuropathy numbness and pain [25].
Vileikyte and Gonzales in their reports from 2009 and 2014 also confirm that diabetic neuropathy is a risk factor for depression and suggest an instability of walking function rather than the occurrence of pain as a factor aggravating depression [11,26].
According to a population-based mail survey of 4385 patients with type 1 and type 2 diabetes by Katon et al, independent factors associated with a significantly higher likelihood of meeting criteria for depression are, similar to our findings, an elevated BMI and lower level of education [27]. In our study, an increase in the BMI of 1 kg/m2 increased the depression risk in patients with diabetic DSPN by nearly 10%. Katon et al found that the method of DM treatment (insulin therapy) was also an important factor related to risk of depression. In our study, insulin therapy was a relatively frequent method of treatment (81% of participants used insulin) and did not increase the risk of depression. Similar to our results, Berg et al did not find any relationship between the method of DM treatment and the intensification of depression in younger or older patients [28].
The severity of depression was lower if physical activity and education level were higher [28], as in our study.
In practice, this may constitute an important therapeutic premise indicating the need for strict metabolic care of patients, aimed at the treatment of overweight and obesity. In the Utah Neuropathy Study, the level of obesity measured by BMI was positively correlated with the occurrence of neuropathy and was associated with small-nerve fiber injury [29]. In 2015, Papanas and Ziegler listed obesity, among other variables, as a major risk factor for DSPN, followed by diabetes duration, age, hyperglycemia, and lipid disorders [18]. Gehan et al, in a study including 48 obese patients, showed that a weight reduction program in the form of diet control and treadmill exercises can be used as a nonpharmacological agent to improve peripheral nerve function in obese patients with diabetic peripheral neuropathy [30].
The relationship between DSPN and depression is multifactorial [1,2] and unequivocal [26–30]. Is connected with the complicated impact of social, medical, and economic factors.
Providing overweight and obese patients with metabolic care with a weight reduction strategy is a promising method of treatment support. Further studies are needed to evaluate risk factors for depression in this group of patients to minimize their intensity as part of DSPN therapy.
The influence of level of education and BMI index reflects patients coping with everyday life and these may play an important role of coping with everyday discomfort connected with DSPN. Most symptoms in the NTSS-6 are not fully objective since the intensity of pain and discomfort is subjective.
In contrast to Kevc et al, we did not find that age younger and female gender were contributors, but our study group was not very big and consisted only of hospitalized patients [31].
Limitations of the present study are that it was small and descriptive. Patients were also admitted to the hospital for treatment of DSPN symptoms; therefore, their intensity of symptoms was probably higher than those in an out-patient clinic. Obviously, patient treated were not randomized.
Conclusions
There was a positive relationship between the severity of DSPN evaluated by NTSS-6 and symptoms of depression. A 1-point increase in the NTSS-6 was associated with a 16% increase in depression risk. Some demographic variables such as education, as well as the level of obesity, also influence the severity of depression symptoms. An education level higher by one degree (eg, university vs secondary school) was associated with a 50% depression risk reduction.
Every 1 kg/m2 of BMI increased the risk of depression in patients with diabetic DSPN by nearly 10%. Such relationships were not found between the methods of treatment and severity of depression symptoms; therefore, one can assume that daily insulin injection does not influence the severity of depression symptoms.
Tables
Table 1. Characteristics of selected social variables in diabetic patients.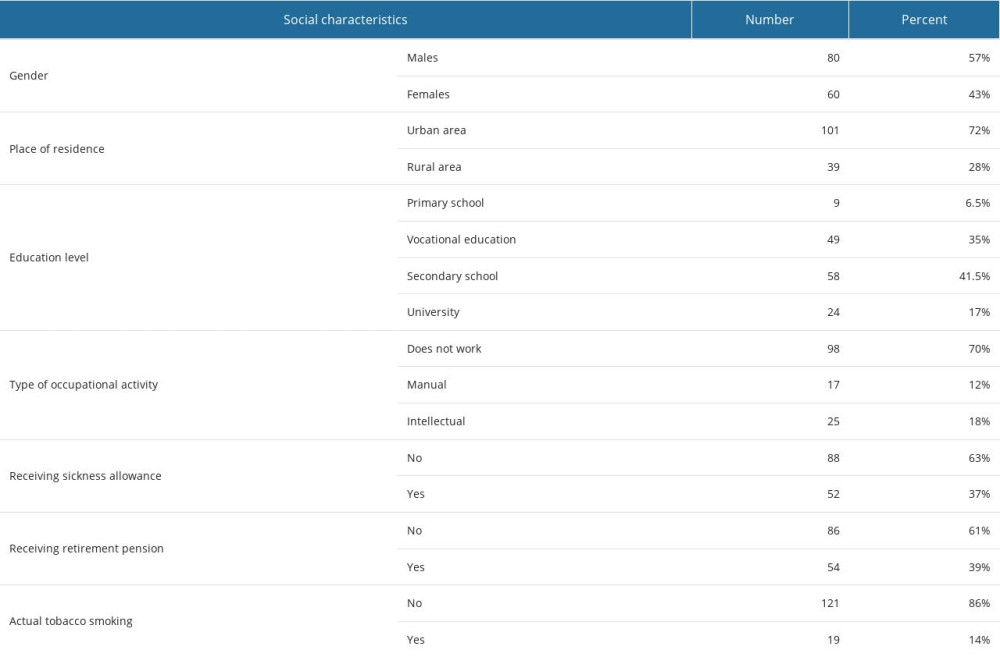 Table 2. Characteristics of selected quantitative variables.
Table 2. Characteristics of selected quantitative variables.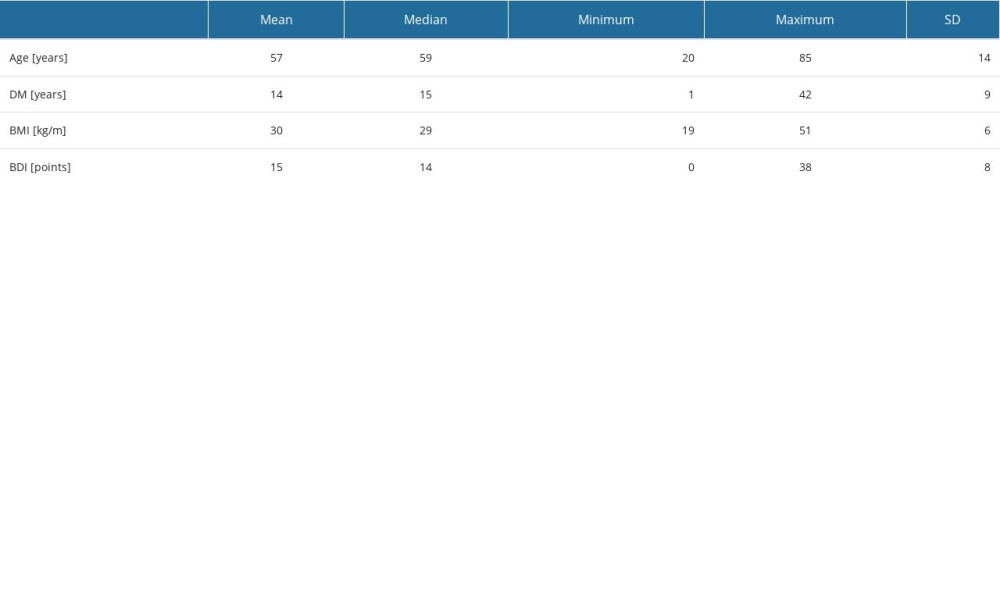 Table 3. Characteristics concerning way of treatment and severity of depression symptoms.
Table 3. Characteristics concerning way of treatment and severity of depression symptoms.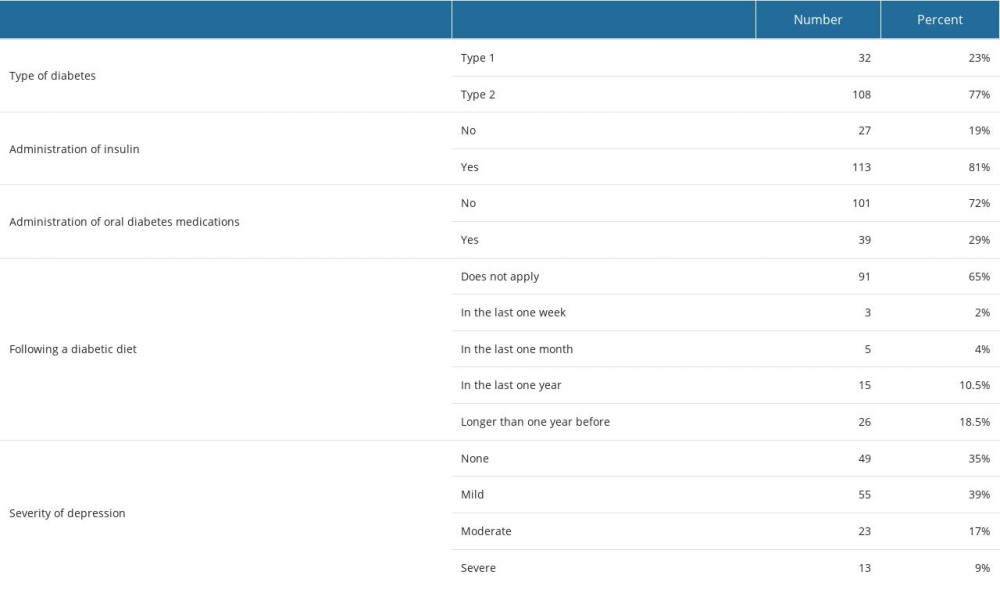 Table 4. Regression analysis of BDI results and patients’ characteristics.
Table 4. Regression analysis of BDI results and patients’ characteristics.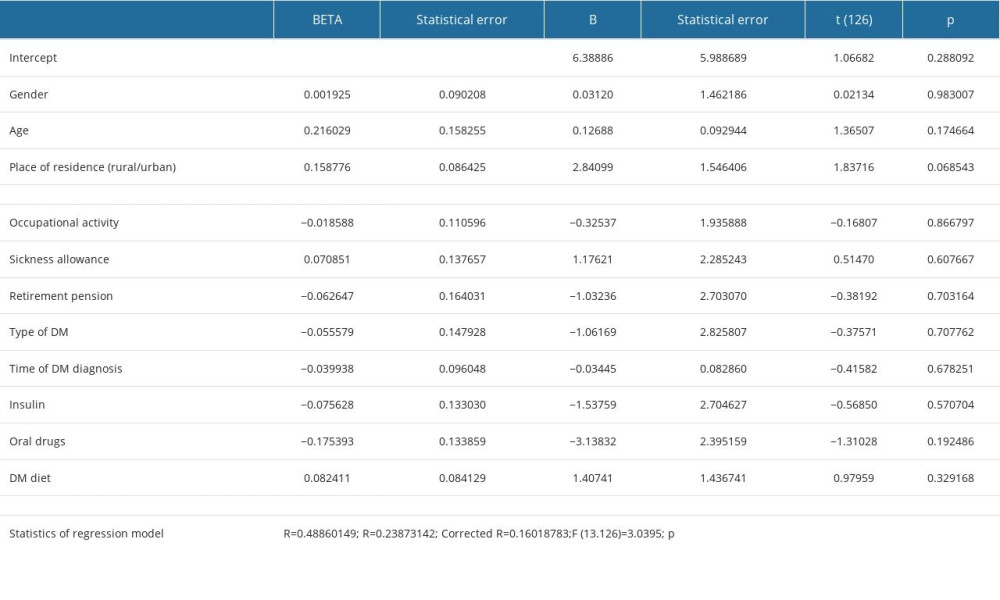 Table 5. Regression analysis of BDI results and DM control and complications.
Table 5. Regression analysis of BDI results and DM control and complications.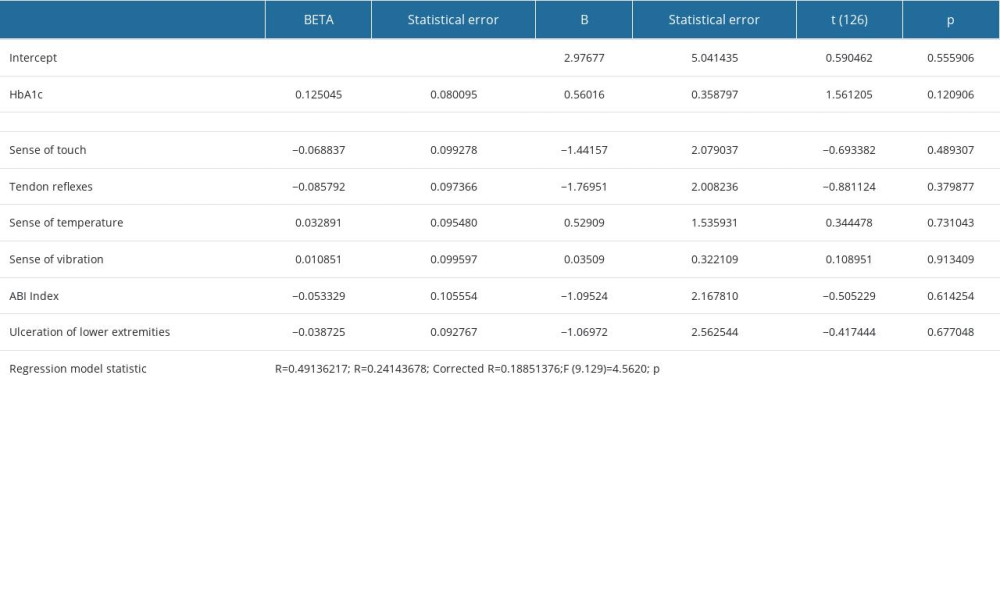
References
1. Alghafri RM, Gatt A, Formosa C, Depression symptoms in patients with diabetic peripheral neuropathy: Rev Diabet Stud, 2020; 16(1); 35-40
2. Dziemidok P, Dąbrowski M, Makara-Studzińska M, Relationship between diabetic neuropathy and occurrence of depression among diabetic patients: Psychiatr Pol, 2016; 50(2); 407-15
3. Saeedi P, Petersohn I, Salpea PIDF Diabetes Atlas Committee, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition: Diabetes Res Clin Pract, 2019; 157; 107843
4. Meurs M, Roest AM, Wolffenbuttel BH, Association of depressive and anxiety disorders with diagnosed versus undiagnosed diabetes: An epidemiological study of 90,686 participants: Psychosom Med, 2016; 78(2); 233-41
5. Mommersteeg PM, Herr R, Pouwer F, The association between diabetes and an episode of depressive symptoms in the 2002 World Health Survey: An analysis of 231,797 individuals from 47 countries: Diabet Med, 2013; 30(6); e208-14
6. Roy T, Lloyd CE, Epidemiology of depression and diabetes: A systematic review: J Affect Disord, 2012; 142(Suppl); S8-S21
7. Lloyd CE, Roy T, Nouwen A, Epidemiology of depression in diabetes: International and cross-cultural issues: J Affect Disord, 2012; 142(Suppl); S22-S29
8. Abbott CA, Malik RA, van Ross ER, Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K: Diabetes Care, 2011; 34(10); 2220-24
9. Harris MD, Psychosocial aspects of diabetes with an emphasis on depression: Curr Diab Rep, 2003; 3(1); 49-55
10. Vedhara K, Miles JN, Wetherell MA, Coping style and depression influence the healing of diabetic foot ulcers: Observational and mechanistic evidence: Diabetologia, 2010; 53(8); 1590-98
11. Vileikyte L, Peyrot M, Gonzalez JS, Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: A longitudinal study: Diabetologia, 2009; 52(7); 1265-73
12. Beck AT, Ward CH, Mendelson M, An inventory for measuring depression: Arch Gen Psychiatry, 1961; 4; 561-71
13. Rauwerda NL, Tovote KA, Peeters ACTM, WHO-5 and BDI-II are acceptable screening instruments for depression in people with diabetes: Diabet Med, 2018; 35(12); 1678-85
14. Lawal-Bello AT, Owolabi FA, Yusuff OT, Diabetic neuropathy: An evaluation using the ntss-6 questionnaire and biothesiometry in type 2 DM patients: Endocrine Abstracts, 2015; 38; 238
15. Bastyr EJ, Price KL, Vera BrilMBBQ Study Group, Development and validity testing of the neuropathy total symptom score-6: Questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy: Clin Ther, 2005; 27(8); 1278-94
16. Gorczyca-Siudak D, Dziemidok P, The 8-week efficacy of Frequency Rhythmic Electrical Modulated System (FREMS) as an add-on therapy in the treatment of symptomatic diabetic peripheral polyneuropathy: Int J Environ Res Public Health, 2022; 20(1); 111
17. Papanas N, Vinik AI, Ziegler D, Neuropathy in prediabetes: Does the clock start ticking early?: Nat Rev Endocrinol, 2011; 7(11); 682-90
18. Papanas N, Ziegler D, Risk factors and comorbidities in diabetic neuropathy: An update 2015: Rev Diabet Stud, 2015; 12(1–2); 48-62
19. de Groot M, Anderson R, Freedland , Association of depression and diabetes complications: A meta-analysis: Psychosom Med, 2001; 63(4); 619-30
20. Jain R, Jain S, Raison CL, Painful diabetic neuropathy is more than pain alone: Examining the role of anxiety and depression as mediators and complicators: Curr Diab Rep, 2011; 11(4); 275-84
21. Trento M, Charrier L, Salassa M, Depression, anxiety and cognitive function in patients with type 2 diabetes: An 8-year prospective observational study: Acta Diabetol, 2015; 2(6); 1157-66
22. Vileikyte L, Leventhal H, Gonzalez JS, Diabetic peripheral neuropathy and depressive symptoms: The association revisited: Diabetes Care, 2005; 28(10); 2378-83
23. Davies M, Brophy S, Williams R, The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes: Diabetes Care, 2006; 29(7); 1518-22
24. Benbow SJ, Wallymahmed ME, MacFarlane IA, Diabetic peripheral neuropathy and quality of life: QJM, 1988; 91(11); 733-37
25. Ishizawa K, Babazono T, Horiba Y, The relationship between depressive symptoms and diabetic complications in elderly patients with diabetes: Analysis using the Diabetes Study from the Center of Tokyo Women’s Medical University (DIACET): J Diabetes Complications, 2016; 30(4); 597-602
26. Vileikyte L, Gonzalez JS, Recognition and management of psychosocial issues in diabetic neuropathy: Handb Clin Neurol, 2014; 126; 195-209
27. Katon W, von Korff M, Ciechanowski , Behavioral and clinical factors associated with depression among individuals with diabetes: Diabetes Care, 2004; 27(4); 914-20
28. Berge LI, Riise T, Tell GS, Depression in persons with diabetes by age and antidiabetic treatment: A cross-sectional analysis with data from the Hordaland Health Study: PLoS One, 2015; 10(5); e0127161
29. Smith AG, Singleton JR, Obesity and hyperlipidemia are risk factors for early diabetic neuropathy: J Diabetes Complications, 2013; 27(5); 436-42
30. Gehan M, Ahmed GM, Mostafa MM, Efficacy of weight reduction program on obese patients with diabetic peripheral neuropathy: Diabetes Technol Ther, 2013; 15(4); A123
31. Kec D, Rajdova A, Raputova J, Risk factors for depression and anxiety in painful and painless diabetic polyneuropathy: A multicentre observational cross-sectional study: Eur J Pain, 2022; 26(2); 370-89
Tables
 Table 1. Characteristics of selected social variables in diabetic patients.
Table 1. Characteristics of selected social variables in diabetic patients. Table 2. Characteristics of selected quantitative variables.
Table 2. Characteristics of selected quantitative variables. Table 3. Characteristics concerning way of treatment and severity of depression symptoms.
Table 3. Characteristics concerning way of treatment and severity of depression symptoms. Table 4. Regression analysis of BDI results and patients’ characteristics.
Table 4. Regression analysis of BDI results and patients’ characteristics. Table 5. Regression analysis of BDI results and DM control and complications.
Table 5. Regression analysis of BDI results and DM control and complications. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








