07 April 2023: Clinical Research
Comparison of Radifocus versus Silverway Guidewires for Percutaneous Radial Angiography Following Failed Use of a J-Tip Guidewire
Deborah M.F. van den BuijsDOI: 10.12659/MSM.939429
Med Sci Monit 2023; 29:e939429
Abstract
BACKGROUND: During transradial coronary angiography, when conventional J-tip wires fail to deliver catheters to the aortic root due to anatomical obstacles, additional hydrophilic wires, such as Radifocus (Terumo) or Silverway (Asahi), are used. We recently showed that the Silverway guidewire was effective at delivering the catheter to the aortic root. In this study, we aimed to compare the efficacy and safety of Radifocus and Silverway guidewires in 100 patients after failed use of the J-tip guidewire.
MATERIAL AND METHODS: After patients had a failure of a conventional J-tip wire to reach the aortic root, 100 patients were 1:1 randomized to either the Silverway or Radifocus wire. All patients with failure of the J-tip wire were eligible. The primary endpoint was the time between wire entry in the catheter and successful delivery of the catheter to the aortic root. Secondary endpoints included change of access site, number of complications, and questionnaires on subjective wire assessments by the performing interventional cardiologist.
RESULTS: The primary endpoint was significantly shorter in patients randomized to the Silverway arm (median 30 s [21-39] vs 48 s [36-66]; P<0.001)). The percentage of patients with change of access site was not different between the groups (2 vs 2, not significant). Only 1 minor complication (2%) occurred, in the Radifocus group. Questionnaires revealed that torque control, crossing, and support were all significantly better with the Silverway wire (P<0.001).
CONCLUSIONS: Silverway showed superior torque control, resulting in faster catheter delivery to the aortic root when compared with the Radifocus guidewire.
Keywords: cardiac catheterization, Cardiac Catheters, Coronary Angiography, Radial Artery, Humans, Equipment Design, Catheterization, catheters, Treatment Outcome
Background
Radial access is the preferred approach for coronary angiography, unless there are overriding procedural considerations [1–4]. During transradial coronary angiography, it is not always feasible to deliver catheters to the aortic root with the support a conventional J-tip wire, owing to spasm, tortuosity, or loopings at the level of the brachial or subclavian artery or brachiocephalic trunk [5,6]. These anomalies are prone to bleeding and vascular complications and increased procedure time [7,8]. Other hydrophilic wires, such as Radifocus (Terumo, Tokyo, Japan) and Silverway (Asahi Intecc Co., Tokyo, Japan), are used to overcome these anatomical challenges [9,10].
The Radifocus guidewire is a polymer-coated wire for more lubricity and has a nitinol core structure for better torque transmission than a conventional J-tip; however, it is less supportive and can easily enter side branches due to the high lubricity and therefore might cause dissections of perforations [7].
The Silverway guidewire has a hybrid coating, Asahi Cable Tube ONE (ACT ONE) technology on the proximal shaft and double coil structure at the tip that connects the core and coil to ensure one-to-one torque transmission. The combination of these technologies incorporates good deliverability, torque transmission, and safety [5]. We recently showed that the Silverway guidewire is successful at delivering the guidewire to the aortic valve when the J-tip guidewire fails due to anatomical obstacles [9].
To date, there are no data comparing the 2 wires. In this RADifocus VErsus Silverway (RADVES) study, we aimed to compare the efficacy and safety of both the Radifocus and Silverway guidewires for percutaneous transradial coronary angiography in 100 patients following the failed use of a J-tip guidewire.
Material and Methods
ETHICS STATEMENT:
The trial was approved by the Institutional Review Board at Ziekenhuis Oost-Limburg Genk (Z-2021119) and registered at
PARTICIPANTS:
Patients were eligible if the conventional J-tip wire failed to reach to aortic root during an elective non-emergent coronary procedure. ST-elevation myocardial infarction, non-ST elevation myocardial infarction, and hemodynamic instability were exclusion criteria.
After angiographic rule out of first-pass complications by the J-tip wire occurred and informed consent was given, patients were 1:1 randomized by an online module using permuted blocks to either the Silverway or Radifocus guidewire, stratified by whether the J-tip wire failed proximal or distal to the subclavian artery.
MATERIALS:
A 6 French radial sheath was used for radial access in all patients (Radifocus Introducer II Transradial Kit, Terumo). All patients received upfront transradial spasmolytic agents containing 1 mg of nitroglycerin, 1 mg of verapamil, and 5000 units of heparin. Subsequently, a 5 French or 6 French diagnostic catheter was used (Cordis); the choice of catheter was at the operators’ discretion.
The Silverway guidewire was compared with the Radifocus guidewire. No additional spasmolytic agents were administered after the initial dose. Final hemostasis was achieved using a transradial band (Terumo). Timing of entry of the guidewire into the catheter to reaching the aortic root was performed by a timer that was built in to our system (Philips).
DATA COLLECTION:
Baseline, procedural, and clinical outcome data were prospectively collected using Castor EDC software. The primary endpoint was the time between wire entry in the catheter and successful delivery of the catheter to the aortic root within a predefined time limit of 300 seconds. In case the assigned study wire failed to pass, cross-over to the other study wire was mandatory. Secondary endpoints included change of access site to the contralateral radial or femoral arteries and the number of complications (dissection, perforation, or hematoma), as assessed by post-procedural angiography and questionnaires on subjective wire assessments (1, not satisfactory, to 3, satisfactory) by the performing interventional cardiologist.
STATISTICAL ANALYSIS:
All continuous data are reported as mean±standard deviation if normally distributed or median [interquartile range] if not normally distributed. Continuous endpoints were compared with a
Results
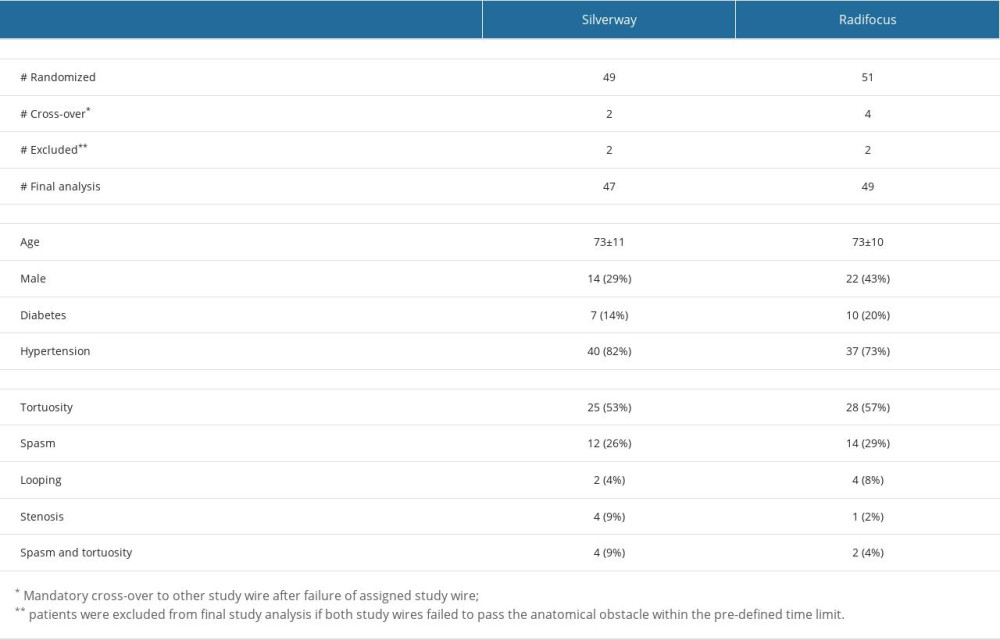
BASELINE CHARACTERISTICS:
Baseline pre-randomization characteristics were well balanced between the 2 groups (Table 1). Patients had a mean age of 73±11 years, the mean body mass index was 27±4.6 kg/m2, most patients were female (64%), and most had a medical history of hypertension (87%).
The indication for the cardiac catheterization was stable angina in 56% of cases, heart failure in 12% of cases, a pre-operative indication in 3%, unstable angina in 9%, and other indications, such as rhythm disorders or silent ischemia, in 20% (Table 1).
PROCEDURAL CHARACTERISTICS:
The reasons for the failure of the initial J-tip wire to cross were tortuosity (n=53), spasm (n=26), loopings (n=10), stenosis (n=5), and a combination of spasm and tortuosity in the remaining 6 patients.
In 6% of the patients, the guidewire was combined with a 4 French catheter to reach the aortic valve, in 24% of patients the guidewire was combined with a 5 French catheter, and in 70% of patients the guidewire was combined with a 6 French catheter.
A total of 4 patients (2 patients randomized to Radifocus and 2 randomized to Silverway), all of whom had an arterial looping, were excluded from the final endpoint analysis because both study wires failed to pass within the pre-defined time limit of 300 s. In 2 additional patients, after initial failure of the assigned Radifocus wire, cross-over to Silverway was successful after 19 and 38 seconds (Figure 1). For these patients, the primary endpoint for the study wire was imputed according to the worst-case scenario (300 s).
Cross-over to a different access site was similar for the Silverway and Radifocus wires (Table 2).
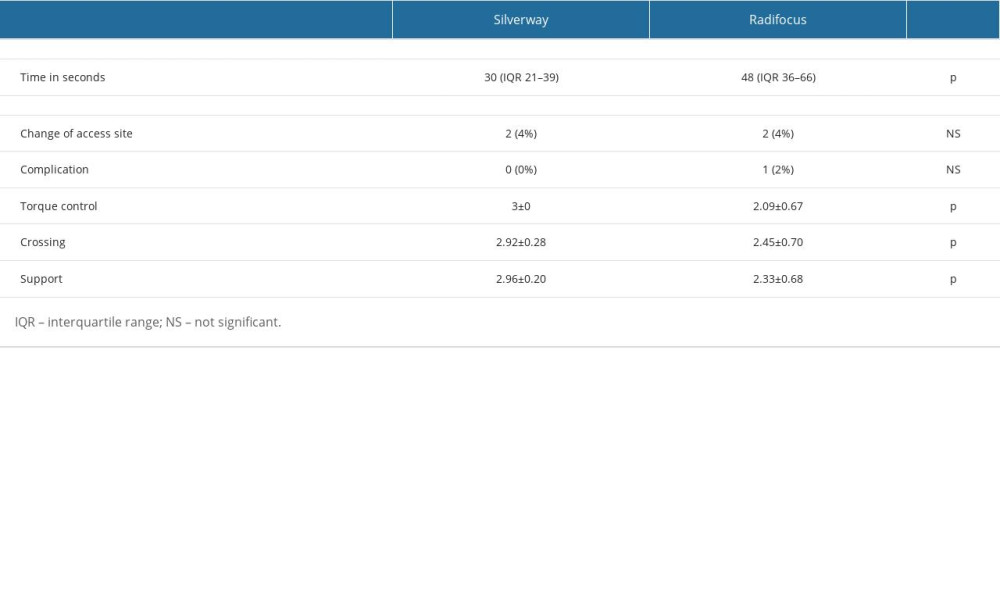
PRIMARY AND SECONDARY ENDPOINTS:
The primary endpoint was significantly shorter in patients randomized to the Silverway arm (median 30 s [21–39] vs 48 s [36–66]; P<0.001; Figure 2). The percentage of patients with a change of access site did not differ between the 2 groups (2 vs 2, not significant).
The only complication was a small dissection without clinical implication in a single patient randomized to the Radifocus group (Table 2). Questionnaires revealed that torque control, crossing, and support were all significantly better with the Silverway wire (Table 2). Retrospective analysis showed no radial artery complications (eg, thrombosis) in our patient population.
Discussion
LIMITATIONS:
The major limitation of this study is the single-center open-label trial design. In addition, we limited our study to 2 different hydrophilic guidewires that are currently on the market (Silverway and Radifocus). The market, however, is not limited to these 2 hydrophilic wires, and other available hydrophilic wires might have similar results. A future trial comparing Silverway and a conventional J-tip as a workhorse wire would be of interest.
Conclusions
This study showed that when compared with the Radifocus guidewire, the Silverway guidewire had significantly better torque control, resulting in more rapid catheter delivery to the aortic root.
Figures
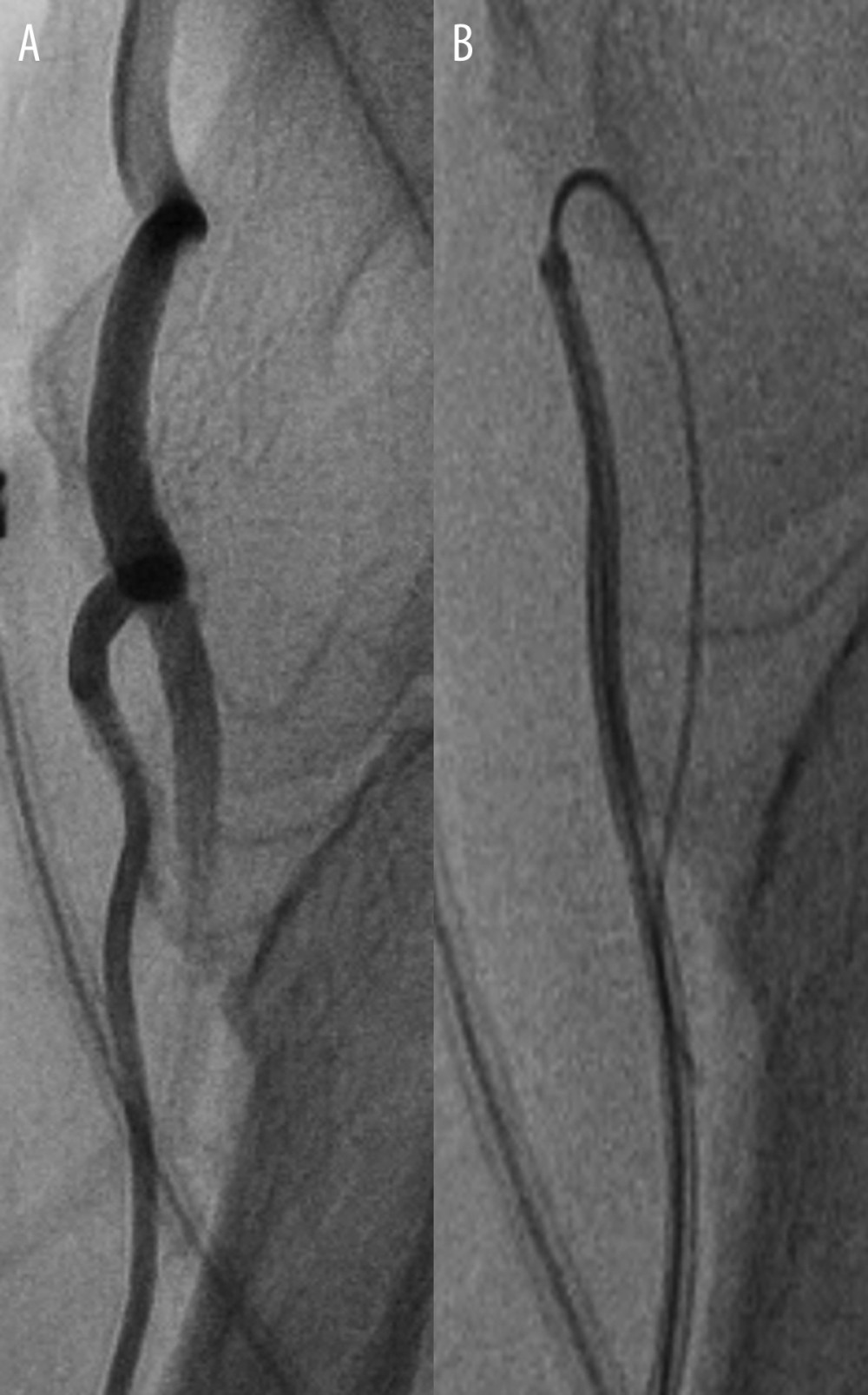 Figure 1. A case of tortuosity in the radial artery which could not be passed with the standard J-tip guidewire (A). The Radifocus wire had low steerability and torque transmission; therefore, it always chose the distal path (B). The Silverway wire was able to cross the tortuosity and reached the aortic valve in 19 seconds (no image available).
Figure 1. A case of tortuosity in the radial artery which could not be passed with the standard J-tip guidewire (A). The Radifocus wire had low steerability and torque transmission; therefore, it always chose the distal path (B). The Silverway wire was able to cross the tortuosity and reached the aortic valve in 19 seconds (no image available). 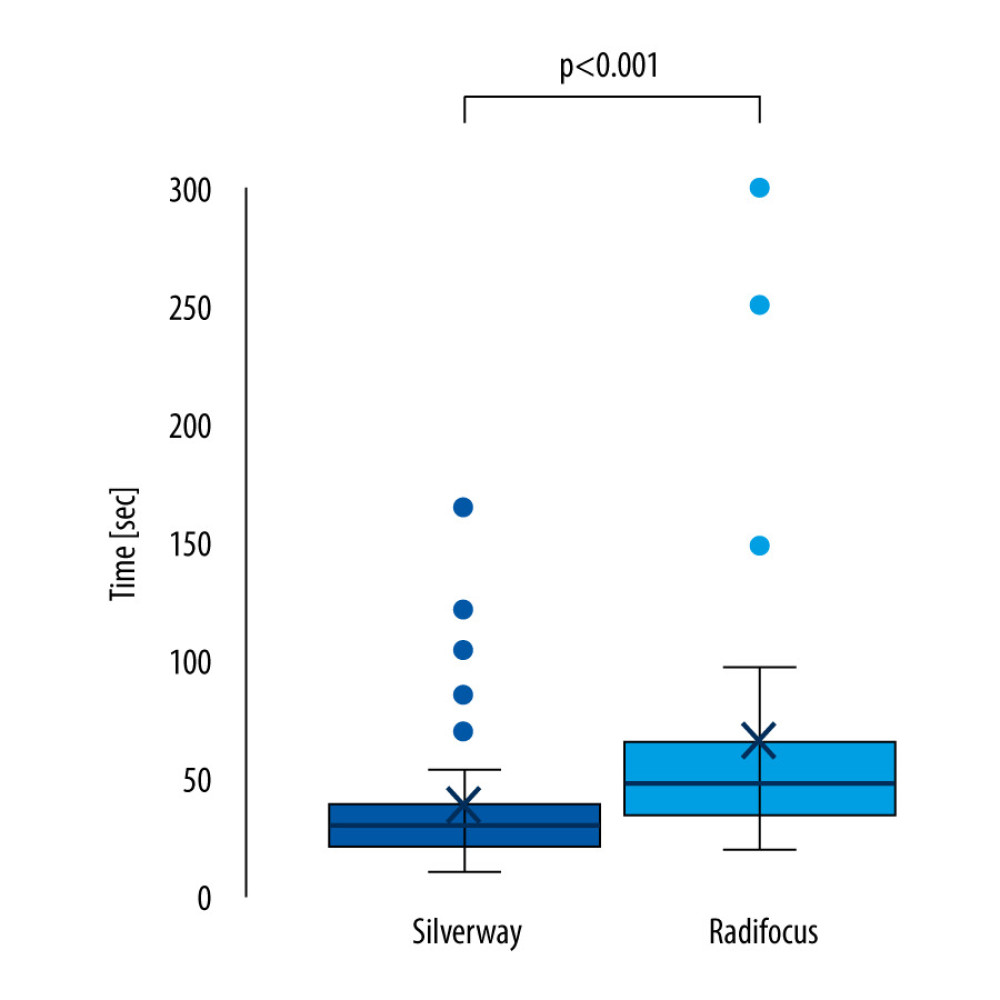 Figure 2. Boxplot chart showing the primary endpoint (time in seconds).
Figure 2. Boxplot chart showing the primary endpoint (time in seconds). 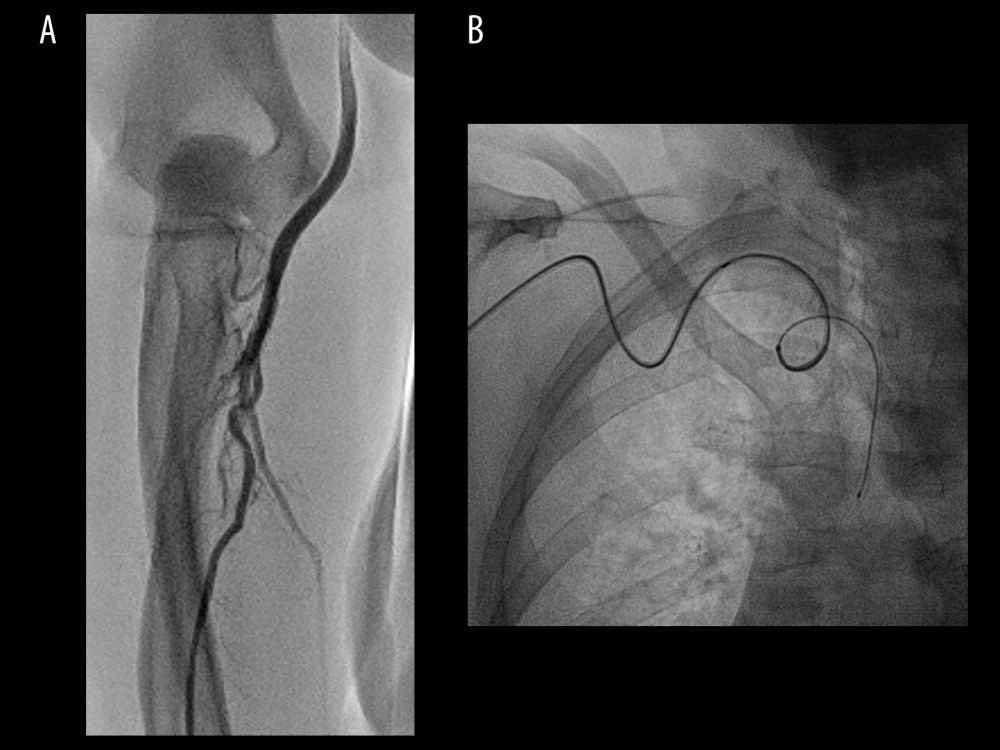 Figure 3. Tortuosity at radial artery level (A) and subclavian/brachiocephalic level (B) in 1 patient. The Silverway wire was able to cross both anomalies and deliver the catheter to the aortic valve in 127 seconds.
Figure 3. Tortuosity at radial artery level (A) and subclavian/brachiocephalic level (B) in 1 patient. The Silverway wire was able to cross both anomalies and deliver the catheter to the aortic valve in 127 seconds. References
1. Neumann FJ, Sousa-Uva M, Ahlsson A, 2018 ESC/EACTS Guidelines on myocardial revascularization: EuroIntervention, 2019; 14; 1435-534
2. Valgimigli M, Frigoli E, Leonardi S, Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): Final 1-year results of a multicentre, randomised controlled trial: Lancet, 2018; 392; 835-48
3. Jolly SS, Yusuf S, Cairns J, Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial: Lancet, 2011; 377; 1409-20
4. Mason PJ, Shah B, Tamis-Holland JE, An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: A scientific statement from the American Heart Association: Circ Cardiovasc Interv, 2018; 11; e000035
5. Lo TS, Nolan J, Fountzopoulos E, Radial artery anomaly and its influence on transradial coronary procedural outcome: Heart, 2009; 95; 410-15
6. Kanei Y, Kwan T, Nakra NC, Transradial cardiac catheterization: A review of access site complications: Catheter Cardiovasc Interv, 2011; 78; 840-46
7. Sandoval Y, Bell MR, Gulati R, Transradial artery access complications: Circ Cardiovasc Interv, 2019; 12; e007386
8. Valsecchi O, Vassileva A, Musumeci G, Failure of transradial approach during coronary interventions: anatomic considerations: Catheter Cardiovasc Interv, 2006; 67; 870-78
9. vanden Buijs D, Poels E, Ferdinande B, The silverway guidewire: An observational study on successful crossing when a conventional J-tip wire has failed: J Clin Cardiol Cardiovasc Interv, 2022 2641-0419/239
10. Bertrand OF, Rao SV, Pancholy S, Transradial approach for coronary angiography and interventions: Results of the first international transradial practice survey: J Clin Cardiol Cardiovasc Interv, 2010; 3; 1022-31
11. Avdikos G, Karatasakis A, Tsoumeleas A, Radial artery occlusion after transradial coronary catheterization: Cardiovasc Diagn Ther, 2017; 7; 305-16
12. Zankl AR, Andrassy M, Volz C, Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins: Clin Res Cardiol, 2010; 99; 841-47
13. Ruiz-Salmeron RJ, Mora R, Velez-Gimon MRadial artery spasm in transradial cardiac catheterization. Assessment of factors related to its occurrence, and of its consequences during follow-up: Rev Esp Cardiol, 2005; 58; 504-11 [in Spanihs]
14. Tuncez A, Kaya Z, Aras D, Incidence and predictors of radial artery occlusion associated transradial catheterization: Int J Med Sci, 2013; 10; 1715-19
15. Ho HH, Jafary FH, Ong PJ, Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: Incidence, predisposing factors, prevention, and management: Cardiovasc Revasc Med, 2012; 13; 193-95
16. Rashid M, Kwok CS, Pancholy S, Radial artery occlusion after transradial interventions: A systematic review and meta-analysis: J Am Heart Assoc, 2016; 5; e002686
Figures
 Figure 1. A case of tortuosity in the radial artery which could not be passed with the standard J-tip guidewire (A). The Radifocus wire had low steerability and torque transmission; therefore, it always chose the distal path (B). The Silverway wire was able to cross the tortuosity and reached the aortic valve in 19 seconds (no image available).
Figure 1. A case of tortuosity in the radial artery which could not be passed with the standard J-tip guidewire (A). The Radifocus wire had low steerability and torque transmission; therefore, it always chose the distal path (B). The Silverway wire was able to cross the tortuosity and reached the aortic valve in 19 seconds (no image available). Figure 2. Boxplot chart showing the primary endpoint (time in seconds).
Figure 2. Boxplot chart showing the primary endpoint (time in seconds). Figure 3. Tortuosity at radial artery level (A) and subclavian/brachiocephalic level (B) in 1 patient. The Silverway wire was able to cross both anomalies and deliver the catheter to the aortic valve in 127 seconds.
Figure 3. Tortuosity at radial artery level (A) and subclavian/brachiocephalic level (B) in 1 patient. The Silverway wire was able to cross both anomalies and deliver the catheter to the aortic valve in 127 seconds. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952