14 April 2023: Clinical Research
Efficacy and Safety of Low-Dose versus High-Dose Postoperative Intrathecal Morphine in 62 Women Undergoing Elective Cesarean Section Delivery at Full Term
Liu Fei1ADE, Hao Shuai2ABE, Zhou Chen3CD, Yao Jie4BD, Quan Zhefeng2BE*DOI: 10.12659/MSM.939567
Med Sci Monit 2023; 29:e939567
Abstract
BACKGROUND: Adequate pain control is desired in women undergoing cesarean section. This study aimed to compare the efficacy and safety of low- and high-dose postoperative intrathecal morphine in 62 women undergoing elective cesarean section delivery at full term.
MATERIAL AND METHODS: We performed a prospective, randomized, controlled, multicenter clinical study from April to November 2022. Full-term, 22-38-year-old pregnant women who were singleton pregnancies, weighing 55-80 kg, scheduled for elective cesarean section, were enrolled. A total of 62 patients were randomly assigned into either the low-dose (60 μg morphine, N=32) or high-dose (100 μg morphine, N=30) group. Post-cesarean pain intensity was recorded at 4, 12, and 24 hours. Patients requiring additional rescue analgesics or with adverse effects were documented.
RESULTS: There were no differences in age, weight, height, gestational age, or operating time between the 2 groups (all P>0.05). The 2 groups also had no statistically significant differences in the resting and exercise pain intensities at 4, 12, and 24 hours after cesarean section (P>0.05). Most patients (53 patients) did not require additional analgesics, suggesting an overall successful analgesic rate of 85.5%. The low-dose group had a lower incidence of pruritus than the high-dose group (13% vs 40%, P=0.029). The 2 groups had no differences in the other adverse effects.
CONCLUSIONS: A single dose of intrathecal 60 μg morphine could provide adequate analgesia comparable with 100 μg morphine, with a lower incidence of pruritus, in Chinese women after cesarean delivery.
Keywords: Drug-Related Side Effects and Adverse Reactions, Normorphine, Pain, Postoperative, Female, Humans, Pregnancy, young adult, Adult, Morphine, Analgesics, Opioid, Cesarean Section, Prospective Studies, Analgesics, Injections, Spinal, Pruritus, Double-Blind Method
Background
The global cesarean section rate has constantly risen in the past few decades [1]. It was estimated that Eastern Asia could have the world’s highest cesarean section rate at 63% in 2030 [1]. Postoperative pain is a major concern in women after a cesarean section [2]. Intrathecal opioids are often considered the criterion standard to provide spinal analgesia during the post-cesarean period [3]. Morphine is the most frequently used opioid due to its well-known analgesic effects, wide availability, and long duration of action, up to 36 hours [4,5]. However, the optimal dose of intrathecal morphine during post-cesarean analgesia still requires further investigation [6].
Most studies on post-cesarean analgesia reported a single dose of 100–200 μg intrathecal morphine administration after the cesarean delivery [7–10]. A high dose of morphine could provide better and long-term pain relief but at the expense of the increased risk of adverse effects, such as nausea, vomiting, pruritus, or even respiratory distress. It was reported that a single dose of intrathecal 50 μg morphine could provide less effective post-cesarean analgesia, whereas a single dose of more than 100 μg of intrathecal morphine could significantly increase the incidence of post-cesarean adverse effects [11,12]. Adequate postoperative analgesia is strongly desired by pregnant women, but the increased incidence of postoperative opioid adverse effects could be associated with poor patient satisfaction, more complications, prolonged hospital stay, and higher medical expenses [13–15]. In addition, current evidence-based recommendations on the analgesia after the elective cesarean section suggest to apply a multimodal analgesic approach to maximize the pain relief and minimize the adverse effects [16,17]. A study reported adequate analgesia with intrathecal morphine injection and oral acetaminophen after cesarean section [18]. However, an optimal dose of intrathecal morphine to provide safe and effective postoperative analgesia still requires further investigations.
Compared with Whites, Chinese have a different body habitus [19,20]. The opioid pharmacokinetics and pharmacodynamics are also different [21]. Chinese patients might require a small dose of opioids and are less tolerant of the adverse effects of opioids [22]. There were very limited studies on the optimal dose of intrathecal morphine for post-cesarean analgesia in China. We performed a prospective, randomized, controlled, multicenter clinical study and studied different doses of intrathecal morphine in Chinese pregnant women after cesarean section delivery. We aimed to compare the efficacy and safety of low-dose vs high-dose postoperative intrathecal morphine in women undergoing elective cesarean section delivery at full term. Our study results could provide a reference for improving postpartum care in these patient populations.
Material and Methods
ETHICS STATEMENT:
This study protocol was approved by the Institutional Review Board of Beijing Youan Hospital, Capital Medical University, China (approval No. LL-201410) and was prospectively registered at the Chinese Clinical Trial Registry (registration No. 16008276) before initiation of the study. All study participants signed the written informed consent document.
STUDY DESIGN AND PARTICIPANT SELECTION:
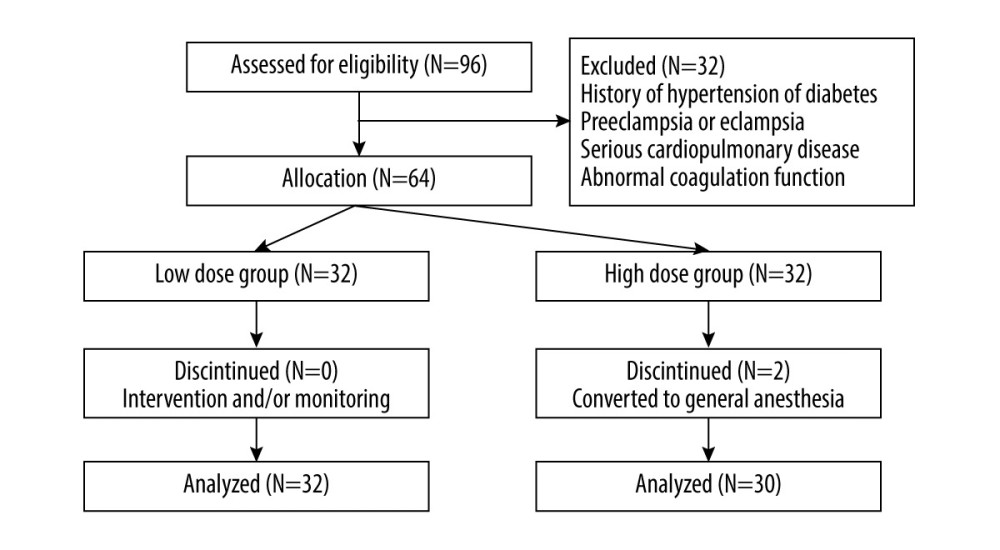
We performed a prospective, randomized, controlled, multicenter clinical study in pregnant women who were scheduled for elective cesarean section at the First Hospital Affiliated with the Hebei North University and the Kunyu People’s Hospital, China, from April to November 2022. The participant inclusion criteria were: 1) age 22–38 years old, full-term, singleton pregnant women scheduled for elective cesarean section; 2) American Society of Anesthesiologists grade II; and 3) normal antenatal examinations, including normal liver and renal functions. Exclusion criteria were: 1) medical history of hypertension, diabetes, or with preeclampsia or eclampsia during the pregnancy; 2) serious cardiopulmonary disease; 3) abnormal coagulation function; or 4) previous spinal surgery or trauma.
STUDY PROTOCOL:
Based on a random-number table, participating pregnant women were assigned to either the low-dose (intrathecal 60 μg morphine) or high-dose (intrathecal 100 μg morphine) group. The low-dose morphine solution was prepared by adding 10 mg morphine into 250 ml normal saline (40 μg/mL). The high-dose morphine solution was prepared by adding 10 mg morphine into 100 ml normal saline (100 μg/mL).
In the operating room, these women were routinely monitored by their blood pressure, heart rate, respiratory rate, and peripheral oxygen saturation (SpO2). After peripheral venous access was established, intravenous lactate Ringer’s solution was given. With the pregnant woman in the right lateral decubitus position, a lumbar puncture was performed in the L3–4 space [23]. The patients in the low-dose group received a solution comprising 1.5 ml 40 μg/mL (60 μg) low-dose morphine solution and 1.5 ml 1% ropivacaine. The patients in the high-dose group received a solution comprising 1 ml 100 μg/mL (100 μg) high-dose morphine solution with 1.5 ml 1% ropivacaine and 0.5 ml normal saline [24]. After the injection, an epidural catheter was placed and fixed. Patients were turned to the supine position with a slight 15° tilt to the left side. Ephedrine (6 mg) was given if the systolic blood pressure was lower than 90 mm Hg. Atropine (0.3 mg) was given if the heart rate was lower than 55 beats/min. Five minutes after initiating the spinal anesthesia, a pinprick was used to test the level of sensory block. The operation was started after the level reached T6. After the operation, all pregnant women were returned to the obstetric ward for close observation. There was no analgesic pump provided. These patients were told to inform the staff about any unbearable pain that required more analgesics.
DATA COLLECTION AND OUTCOME MEASUREMENTS:
Participant baseline demographics, weight, height, and gestational age were documented. The duration of the operating time was recorded. Those patients with an operating time >60 min or who received additional epidural analgesics or turned to general anesthesia were excluded from the final analysis.
The primary outcomes included the resting and exercise pain intensity rated by a visual analog scale (VAS) at 4, 12, and 24 hours after cesarean delivery. The secondary outcomes included the number of additional rescue analgesic administrations. Adverse effect evaluations included assessments of nausea, vomiting, respiratory distress, pruritus, dizziness, chills, and agitations.
STATISTICAL ANALYSIS:
According to previous studies, the incidence of pruritus in the 60-ug morphine group was 12%, while the incidence of pruritus in the 100-ug morphine group was 45%, power (1-β)=0.8 and α=0.05. The total sample size was 26 patients. Assuming a 20% attrition rate, the preferred sample size was determined as 32 patients for each group.
Data analysis was performed with Minitab software (version 19.0, Minitab LLC, State College, Pennsylvania, United States) and RStudio software (version 1.1.456, Rstudio, Inc., Boston, Massachusetts, United States). Continuous variables are presented as mean±standard deviation (SD) or median with interquartile range and compared by
Results
PARTICIPANT ENROLLMENT AND BASELINE CHARACTERISTIC COMPARISONS:
There were 64 pregnant women approached for the study. Finally, 62 pregnant women (32 in the low-dose group and 30 in the high-dose group) completed the study and were analyzed (Figure 1).
There were no statistically significant differences in the age, weight, height, gestational age, or operating time between the 2 groups (P>0.05) (Table 1).
COMPARISONS OF ANALGESIC EFFICACY BETWEEN HIGH-DOSE AND LOW-DOSE GROUPS:
There were no statistically significant differences in the resting pain and exercise pain VAS at 4, 12, and 24 hours after the cesarean section between the low-dose and high-dose groups (P>0.05) (Table 2).
Most patients (53 patients) did not require additional analgesics, which suggested an overall successful analgesic rate of 85.5%. There were 4 and 5 patients in the low-dose and high-dose groups, respectively, who required additional analgesics. Each of them received 0.3 g ibuprofen once, and no more analgesic requirement was reported.
COMPARISONS OF ADVERSE EFFECTS BETWEEN 2 GROUPS:
The low-dose group had fewer incidences of pruritus than the high-dose group (P=0.029) (Table 3). All patients with pruritus had mild symptoms, which were resolved spontaneously without special treatments. The 2 groups had no statistically significant differences in the incidences of nausea, vomiting, respiratory distress, or dizziness (P>0.05). There were no reports of chills or agitation in any study participants.
Discussions
In our present study, a single low-dose 60 μg morphine provided adequate post-cesarean analgesic effects, comparable to a single high-dose 100 μg morphine in intrathecal analgesia in Chinese women. Our primary outcome was VAS pain scores with movement at 24 hours, which had no statistically significant difference between the 2 groups. Compared with the high-dose 100 μg morphine group, the low-dose 60 μg morphine group had significantly lower incidence of pruritus.
Our analysis of the adverse effects of intrathecal morphine injection showed that the overall incidence of adverse effects was lower than in a previous report (up to 60%), which might be due to the different sample sizes and study populations [6]. The 60 μg morphine group had a statistically significantly low incidence of pruritus than the 100 μg morphine group. This was consistent with earlier studies showing that a high dose of morphine was more likely to have adverse effects [11]. Considering the high overall success rate of analgesics, equivalent analgesic effects between 60 μg and 100 μg morphine up to 24 hours after cesarean section, and high incidence of adverse effects from the 100 μg morphine, we recommend that a single dose of 60 μg intrathecal morphine should be given to women for post-cesarean analgesia. The other reason for selecting 60 μg morphine was its easy preparation. Using the method described in the methods section, 60 μg morphine (1.5 ml of 40 μg/mL morphine solution) could be easily prepared and administered in clinical practice.
Effective and safe postoperative pain relief is essential to ensure successful recovery after surgery [25]. Postoperative pain is the greatest concern in pregnant women undergoing cesarean section [12,13]. Poor and inadequate pain control after cesarean section can delay surgical recovery, impact the maternal-newborn bonding, and even lead to persistent postoperative chronic pain [26–29]. Opioids are the most commonly used analgesics during post-cesarean pain management [26]. The American Society of Anesthesiologists’ obstetric anesthesia guidelines and the American Pain Society’s clinical practice guidelines recommend intrathecal administration of small amounts of opioids after a cesarean section to achieve adequate postoperative analgesia [30]. Morphine is a hydrophilic opioid and can be retained in the cerebrospinal fluid after an intrathecal injection, with analgesic effects lasting up to 90 min [31]. A single-dose intrathecal injection of morphine can meet the analgesic requirements during the perioperative and postpartum periods. A previous study reported that a multimodal analgesic approach (intrathecal 300 μg morphine injection and 1 g oral acetaminophen) could provide adequate pain relief within 24 hours after cesarean section [18]. The adverse effects from a high-dose morphine, such as nausea, vomiting, pruritus, and dizziness, could decrease patient satisfaction and are a significant undesirable experience reported by patients. High doses of opioids can even cause respiratory distress, which could seriously endanger postpartum women’s health. It is interesting to determine whether a multimodal analgesic approach could further decrease the required morphine dosage during intrathecal injection.
The strength of our study is its prospective randomized multicenter design. It was the first study on Chinese pregnant women to determine the appropriate intrathecal morphine dose for post-cesarean analgesia. Our study results showed an overall successful analgesic rate of 85.5% (53/62), with only 14.5% (9 patients) requiring additional analgesics. Our successful analgesia rate was higher than in previous studies, which reported more patients requiring additional post-cesarean analgesics [20]. In addition, a previous study showed that the 50 μg morphine dose was less effective than the 100 μg morphine dose when considering the post-cesarean resting pain intensity, time to first additional analgesic request, and the total amount of post-cesarean morphine requirement [32]. In our present study, intrathecal injection of 60 μg and 100 μg morphine produced comparable analgesia after cesarean delivery. The differences between the present study and those previous studies might be due to racial disparities in body habitus, morphine pharmacokinetics and pharmacodynamics, and pain tolerance and sensitivity. This highlights the importance of our present study to provide a reference standard for using intrathecal morphine for postoperative pain control in the Chinese population.
The main limitation of our study was its small sample size. The analgesia was provided by intrathecal morphine injection. However, the technique of intrathecal injection could vary among different providers, which might affect the outcome evaluations. In addition, we administrated morphine together with ropivacaine. Patients might also receive other anesthetic or sedative medications during the perioperative period. All of these medications could affect the outcome evaluations and limit the generalizability of our study results in hospitals using other anesthetic or sedative medications. We also did not evaluate the outcomes of neonates in the present study. Obviously, some patients with certain medical conditions, such as previous spinal surgery or trauma, might have difficulty performing intrathecal injection, and our analgesic approach might have limitations in these patients. Future studies should address these questions.
Conclusions
In conclusion, a single dose of intrathecal 60 μg morphine injection could provide adequate analgesia comparable to a 100-μg morphine injection, but with significantly lower incidence of pruritus, in Chinese women undergoing cesarean section delivery at full term.
Tables
Table 1. Baseline characteristic comparisons between the low- and high-dose morphine groups.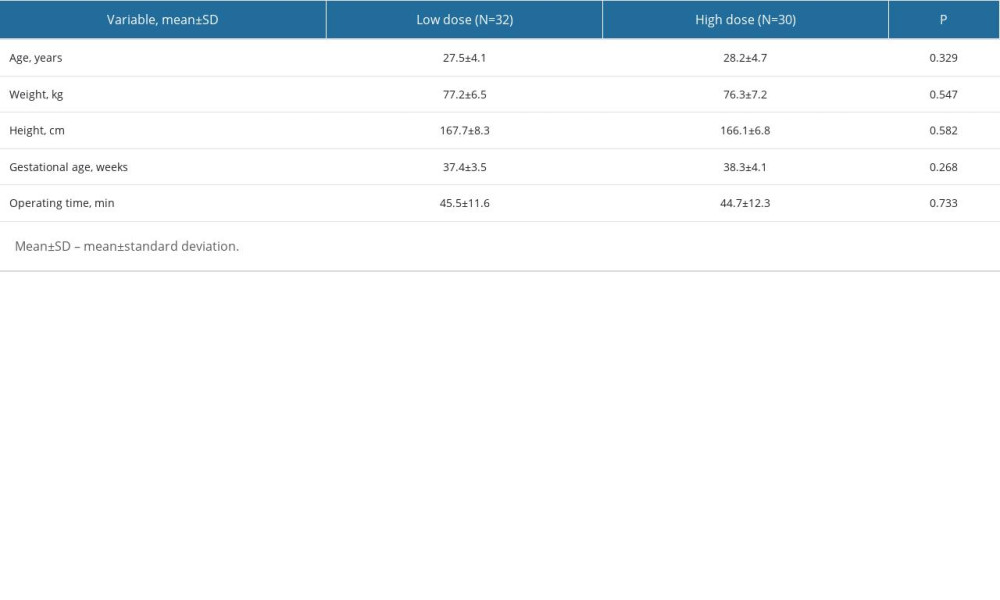 Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups.
Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups.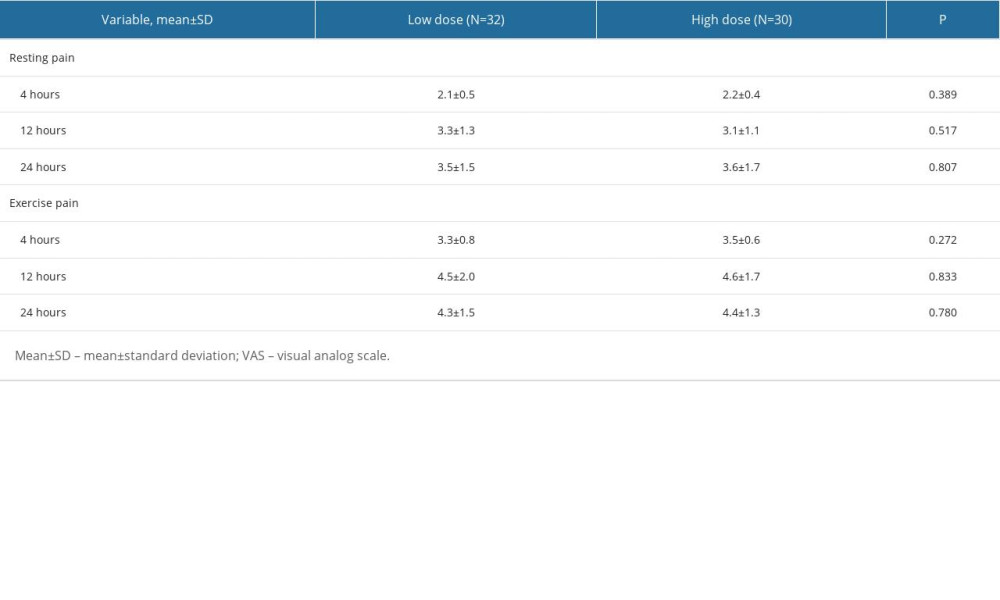 Table 3. Adverse effects comparisons between the low- and high-dose morphine groups.
Table 3. Adverse effects comparisons between the low- and high-dose morphine groups.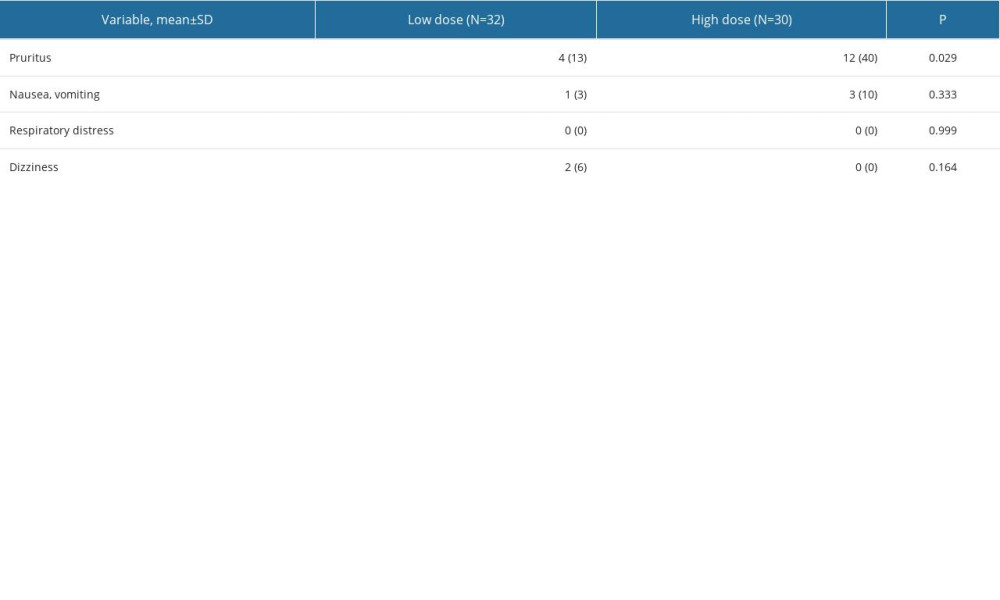
References
1. Betran AP, Ye J, Moller AB, Trends and projections of caesarean section rates: Global and regional estimates: BMJ Global Health, 2021; 6(6); e005671
2. Bjørnstad J, Ræder J, Post-operative pain after caesarean section: Tidsskr Nor Laegeforen, 2020; 140(7); 5
3. Armstrong S, Fernando R, Side effects and efficacy of neuraxial opioids in pregnant patients at delivery: A comprehensive review: Drug Safety, 2016; 39(5); 381-99
4. Bujedo BM, Santos SG, Azpiazu AU, A review of epidural and intrathecal opioids used in the management of postoperative pain: J Opioid Manag, 2012; 8(3); 177-92
5. Mugabure Bujedo B, A clinical approach to neuraxial morphine for the treatment of postoperative pain: Pain Res Treat, 2012; 2012; 612145
6. Sharpe EE, Molitor RJ, Arendt KW, Intrathecal morphine versus intrathecal hydromorphone for analgesia after cesarean delivery: A randomized clinical trial: Anesthesiology, 2020; 132(6); 1382-91
7. Irwin R, Stanescu S, Buzaianu C: Anaesthesia, 2020; 75(1); 89-95
8. Highland KB, Robertson I, Lutgendorf M, Variation by default: Cesarean section discharge opioid prescription patterns and outcomes in Military Health System hospitals: A retrospective longitudinal cohort study: BMC Anesthesiology, 2022; 22(1); 218
9. Dahlquist K, Stuart A, Kallen K, Planned cesarean section vs planned vaginal delivery among women without formal medical indication for planned cesarean section: A retrospective cohort study of maternal short-term complications: Acta Obstet Gynecol Scand, 2022; 101(9); 1026-32
10. Pangthipampai P, Dejarkom S, Poolsuppasit S: BMC Anesthesiology, 2021; 21(1); 90
11. Sultan P, Halpern SH, Pushpanathan E, The effect of intrathecal morphine dose on outcomes after elective cesarean delivery: A meta-analysis: Anesth Analg, 2016; 123(1); 154-64
12. Neall G, Bampoe S, Sultan P, Analgesia for Caesarean section: BJA Educ, 2022; 22(5); 197-203
13. Carvalho B, Cohen SE, Lipman SS, Patient preferences for anesthesia outcomes associated with cesarean delivery: Anesth Analg, 2005; 101(4); 1182-87
14. Liu ZQ, Du WJ, Yao SL, Enhanced recovery after cesarean delivery: A challenge for anesthesiologists: Chin Med J, 2020; 133(5); 590-96
15. Yang CC, Chuang YF, Chen PE, Effect of postoperative adverse events on hospitalization expenditures and length of stay among surgery patients in Taiwan: A nationwide population-based case-control study: Front Med, 2021; 8; 599843
16. Bollag L, Lim G, Sultan P, Society for obstetric anesthesia and perinatology: Consensus statement and recommendations for enhanced recovery after cesarean: Anesth Analg, 2021; 132(5); 1362-77
17. Zaslansky R, Baumbach P, Edry R, Following evidence-based recommendations for perioperative pain management after cesarean section is associated with better pain-related outcomes: analysis of registry data: J Clin Med, 2023; 12(2); 676
18. Booth JL, Harris LC, Eisenach JC, A randomized controlled trial comparing two multimodal analgesic techniques in patients predicted to have severe pain after cesarean delivery: Anesth Analg, 2016; 122(4); 1114-19
19. Fan X, Dai J, He J, Optimal gestational weight gain in Chinese pregnant women with gestational diabetes mellitus: A large retrospective cohort study: J Obstet Gynaecol Res, 2023; 49(1); 182-93
20. Butwick AJ, Wong CA, Guo N, Maternal body mass index and use of labor neuraxial analgesia: A population-based retrospective cohort study: Anesthesiology, 2018; 129(3); 448-58
21. Zhou HH, Sheller JR, Nu H, Ethnic differences in response to morphine: Clin Pharmacol Ther, 1993; 54(5); 507-13
22. Lee A, Gin T, Oh TE, Opioid requirements and responses in Asians: Anaesth Intensive Care, 1997; 25(6); 665-70
23. Weigl W, Bieryło A, Wielgus M, Perioperative analgesia after intrathecal fentanyl and morphine or morphine alone for cesarean section: A randomized controlled study: Medicine (Baltimore), 2017; 96(48); e8892
24. Chen LK, Lin PL, Lin CJ, Huang CH, Patient-controlled epidural ropivacaine as a post-cesarean analgesia: A comparison with epidural morphine: Taiwan J Obstet Gynecol, 2011; 50(4); 441-46
25. Lovich-Sapola J, Smith CE, Brandt CP, Postoperative pain control: Surg Clin North Am, 2015; 95(2); 301-18
26. Carvalho B, Butwick AJ, Postcesarean delivery analgesia: Best Pract Res Clin Anaesthesiol, 2017; 31(1); 69-79
27. Sato I, Iwasaki H, Luthe SK, Comparison of intrathecal morphine with continuous patient-controlled epidural anesthesia versus intrathecal morphine alone for post-cesarean section analgesia: A randomized controlled trial: BMC Anesthesiology, 2020; 20(1); 138
28. Borges NC, de Deus JM, Guimarães RA, The incidence of chronic pain following cesarean section and associated risk factors: A cohort of women followed up for three months: PLoS One, 2020; 15(9); e0238634
29. Molgora S, Fenaroli V, Cracolici E, Antenatal fear of childbirth and emergency cesarean section delivery: A systematic narrative review: J Reprod Infant Psychol, 2020; 38(4); 436-54
30. , Practice guidelines for obstetric anesthesia: An updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology: Anesthesiology, 2016; 124(2); 270-300 No authors listed
31. Dupoiron D, Intrathecal therapy for pain in cancer patients: Curr Opin Support Palliat Care, 2019; 13(2); 75-80
32. Sultan Pervez, Halpern H Stephen, Pushpanathan Ellile, The effect of intrathecal morphine dose on outcomes after elective cesarean delivery: A meta-analysis: Anesth Analg, 2016; 123(1); 154-64
Tables
 Table 1. Baseline characteristic comparisons between the low- and high-dose morphine groups.
Table 1. Baseline characteristic comparisons between the low- and high-dose morphine groups. Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups.
Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups. Table 3. Adverse effects comparisons between the low- and high-dose morphine groups.
Table 3. Adverse effects comparisons between the low- and high-dose morphine groups. Table 1. Baseline characteristic comparisons between the low- and high-dose morphine groups.
Table 1. Baseline characteristic comparisons between the low- and high-dose morphine groups. Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups.
Table 2. Pain intensity (VAS) comparisons at 4, 12, and 24 hours after the cesarean section between the low- and high-dose morphine groups. Table 3. Adverse effects comparisons between the low- and high-dose morphine groups.
Table 3. Adverse effects comparisons between the low- and high-dose morphine groups. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952