27 April 2023: Clinical Research
A Clinical Study of 50 Partially Edentulous Patients with Fixed Partial Denture Restorations to Compare Clinical Parameters and Changes in Gingival Sulcus Width After Displacement with 2 Different Gingival Retraction Cord Materials (Cotton and Polymer)
Lakshya Kumar12ABEF*, Khurshid A. MattooDOI: 10.12659/MSM.940098
Med Sci Monit 2023; 29:e940098
Abstract
BACKGROUND: The present study aimed to compare the clinical performance and gingival sulcus width changes in partially edentulous patients using cotton and polymer gingival retraction cords.
MATERIAL AND METHODS: Fifty partially edentulous patients were divided into 2 groups (Gp C and Gp P) and were subjected to single crown/fixed partial denture treatment. Clinical parameters, including plaque index scores, placement time, and hemorrhage control scores, were assessed. Gingival sulcus width changes before and after retraction were evaluated using individual type 4 dental stone dies observed under an optical microscope. Statistical analysis was performed using dependent/independent t tests.
RESULTS: The mean placement time, hemorrhage control time, and hemorrhagic scores were lower in Gp P than in Gp C, indicating better clinical performance of polymer-based retraction cord. Both groups showed an increase in sulcus width after retraction, but Gp P had a significantly higher sulcus width (690.03±45.37) compared to Gp C (471.38±28.13). The mean difference in sulcus width between baseline and after retraction was also significantly higher in Gp P (525.84 micrometers) than in Gp C (309.11 micrometers).
CONCLUSIONS: The present study shows that polymer-based cords produce more sulcus width and have better clinical performance compared to cotton-based gingival retraction cords. These results suggest that the use of polymer-based retraction cords can improve the quality of dental impressions in partially edentulous patients.
Keywords: Dental Abutments, Dental Implants, Single-Tooth, Denture, Partial, Fixed, Resin-Bonded, Gingival Retraction Techniques, polyethylene terephthalate glycol, Humans, Polymers, Gingiva, Crowns, Hemorrhage
Background
Control of saliva in the oral cavity during various surgical and non-surgical dental procedures is both an art and a skill that comes with a thorough understanding of human biology, various dental biomaterials, clinical procedures, and techniques. Temporary deflection of marginal gingiva (lateral and vertical) is described as gingival retraction and has been traditionally achieved with retraction cords made up of absorbent cotton [1]. Accuracy of the impression for indirect restorations largely depends on a clinically sensitive marginal gingiva retraction by 0.15 to 0.2 mm, which allows material to flow and record areas of the tooth otherwise covered by gingiva [2]. An astute clinician’s gingival retraction has to be non-traumatic, comfortable, and painless while allowing impression material access to subgingival areas where it can record the non-prepared tooth contours [3]. For a clinician, the goals of preventive, therapeutic, esthetic, and clinical success depend on this small yet important clinical procedure [4]. The contact of surrounding soft tissue should be avoided by restorative materials, but at times such contact is necessary (subgingival margins). Even if these margins are chosen, careful clinical execution allows periodontal health to be maintained in such situations [5]. Depending on the clinical situation and the patient’s periodontal status, 3 primary techniques (mechanical, chemical with mechanical, and surgical) accomplish this objective [6]. Mechanical (impregnated or plain) retraction cords are customary, in which advancement since last 3 decades has mainly been focused on design (shape, size, texture and form [knitted, braided, twined)] and chemical used, with less advance in its basic composition which has traditionally been cotton-based [7]. Its action primarily involves the mechanical pushing of marginal gingiva laterally and apically while an impregnated chemical either controls bleeding or causes gingival shrinkage [3,8]. Temporarily, it can cause hemorrhage (trauma) and patient discomfort (sensitivity) and alter epithelial attachment levels [9]. In the long term, it has been associated with exacerbating gingival recession and can induce bone resorption by damaging epithelial attachment, which acts as a physical and biological barrier to microbial ingress [9,10]. The distinct advantages of cotton-based retraction cords that contribute to its clinical success over the decades are economical (low cost), ease of use and dispensing, compatibility with impregnation (vasoconstrictors or astringents, or both) [11], and ease of adaptability with varying sulcular dimensions [6,9]. The primary criticism of using retraction cord is related to the weakness of epithelial attachment, which can be injured by forces as low as 1 N/mm square [12]. Other clinically significant disadvantage includes difficult application in unfavorable periodontal conditions, leaving chemical residue, and patient inconvenience [3,9,13]. Commonly used medicaments among prosthodontists include buffered aluminum chloride (55%), followed by ferric sulfate (23%) [14]. Retraction pastes are less traumatic than gingival attachment and provide easy and quick gingival retraction [9,15]. Pressure generated during retraction paste application ranges from 300 to 500 Kpa as compared to retraction cord, which has been reported to be up to 5000 Kpa [16]. The decrease in pressure during application affects the amount of gingival retraction, which is why they have been classified as producing either mild or moderate retraction of the gingiva [3,6,9,15]. A different form of retraction system (Merocel; Merocel Co., Mystic, CT) is the polyvinyl acetate strip, a porous synthetic polymer (hydroxylated polyvinyl acetate) producing higher retraction when compared to other systems [17].
In such a competitive state, retraction cord manufacturers seem to have shifted their focus from cotton-based retraction cord to polymer-based, since the development of polymers has seen a phenomenal in the last 2 decades. Polyethylene terephthalate (PTE) is a polyester polymer produced by polymerizing glycol (ethylene) with acid (terephthalic) [18]. Its main features are its high strength (especially tensile) and stiffness despite being lightweight. Its application as a retraction cord material is primarily due to its inherent advantage of rapid absorption, high tensile strength, and a biologically inert material [19,20]. Its introduction as a gingival retraction cord material provides an alternative to traditional cotton and can overcome drawbacks of previous cord and paste retraction systems.
Therefore, this study aimed to compare the clinical parameters and changes in gingival sulcus width and lateral gingival displacement in 50 partially edentulous patients using cotton and polymer gingival retraction cord materials. We also investigated the effect of using these 2 different retraction materials on the plaque index of natural permanent teeth.
Material and Methods
ETHICS:
The study was conducted in the division of fixed Prosthodontics at a recognized postgraduate institute in northern India. The study was approved by the college and university ethics committee, which conducts all research studies (human and animal) in adherence to the ethical principles of the Helsinki Declaration. All study subjects provided written consent after being briefed about benefits, confidentiality, and cooperation.
STUDY DESIGN:
This case-control study used a comparative approach and was conducted from September 2021 to March 2022. The study collected data from a sample (purposive sampling), which in turn was selected from a cross-sectional population that reported routinely for prosthodontic treatment at the outpatient department (OPD). The study used qualitative/quantitative measures for data collection and analysis.
SAMPLE PREPARATION, SELECTION, AND GROUPING:
The invitation for participation in the study was announced at the reception of the OPD and among the postgraduate students of the department. Patients who were willing to participate had to fulfill criteria based on inclusion, exclusion, systemic and local physical health condition, and mental attitude. Inclusion criteria were age 28–50 years, good/excellent oral health condition (low dental plaque index scores), requiring a single fixed restoration (either a single crown or 3-unit fixed partial denture), good gingival health, thick gingival biotype, normal gingival probing depth (1–2 mm), normal occlusal parameters (class 1 molar and canine relations, centric occlusion coinciding with centric relation), ideal/minimum crown-to-root ratio, and being mentally competent to provide consent. Patients with systemic compromises (eg, diabetes, hypertension, cardiovascular, hyperthyroidism), unerupted teeth, high plaque index scores, high caries index, malocclusion (crowding/diastema/tipped/supraerupted/rotated/tilted/malpositioned teeth), existing prosthesis, thin gingival biotype, bleeding on probing, poor gingival and periodontal health (chronic gingivitis and periodontitis), and being mentally incompetent to provide consent. In addition to the above criteria, pregnant and lactating females and anyone with a history of allergy to th materials (nickel, beryllium) to be used were also excluded from the study [21,22]. These criteria/conditions would influence the outcome of the results.
A total of 57 patients at this stage were further screened for current periodontal health using the gingival and plaque index, as well as clinical examination (sulcus probing depth, gingival contour, periodontal pockets). This screening was done by a team of 2 periodontists with more than 10 years of clinical experience in their field. Based on their expert opinion, 7 patients had to be excluded after clinical screening as they were not deemed fit for the final study. Fifty patients (29 males and 21 females) with 60 teeth (10 maxillary incisors, 2 maxillary canines, 20 maxillary/mandibular premolars, 18 maxillary/mandibular molars) were identified and divided into 2 experimental groups: Group (Gp) C (Cotton-based retraction system – Ultrapak, Ultradent, Inc, South Jordan, Utah, USA) and Gp P (Polymer-based retraction system – Sure Cord – Plus, Suredent Corp., Korea) (Table 1). The allotment of each patient in the group was done using a block randomization method (double-blinded) while group allotment was based on tossing a coin. The naming of the group was based on the first letter of the material used in the retraction system.
CLINICAL EXPERIMENTAL PROCEDURES:
All patients were treated by the third-year postgraduate prosthodontic students working under a team of 3 experienced staff members who had no role in the current research. All students were presented with a brief demonstration and related brochure on the retraction systems. The clinical requirement that was pertaining to the study was to prepare an individual tooth as dictated by the patient’s treatment plan and following the basic principles of tooth preparation. The finish line had to be equigingival/supragingival as required by gingival condition and treatment objectives (Figure 1A). On the first day of the appointment, the plaque index was recorded for each patient, followed by gingival retraction on the unprepared planned tooth. The patient was recalled after a minimum of 7 days and the plaque index score was again recorded. The 2 comparative scores were used to evaluate the effect of the 2 different retraction systems on plaque index. Routine clinical and laboratory procedures were then followed for fabrication of either a single crown or a 3-unit fixed partial for each patient. The instruments, materials, and methods for each retraction cord were according to manufacturer’s instructions and recommendations (Table 1). For Gp C, Fisher Ultrapak packers were used to retract the gingiva along with Viscostat clear astringent as recommended. The choice of size for the thickness of retraction cord was according to the individual patient’s requirement as judged clinically (gingival biotype, gingival thickness, gingival elasticity, and the health of marginal gingiva). Gingival retraction was accomplished with correct length of the retraction cord (Figure 1B). For all patients, a standard 2-stage double-mix impression technique was used. For each impression, a 2-thickness wax spacer covered with aluminum foil was used, followed by placement of vertical stops (Figure 1C), later filled by VLC tray resin. The spacer was removed before making an impression (Figure 1D). Gingival retraction cords were removed before making impressions with addition of silicone of medium and light body (Figure 1E). All impressions were made after proper hemorrhage and fluid control. The impressions were disinfected as done routinely and later marked for the patient and the group, followed by transportation to the dental laboratory. In the laboratory, the impressions were poured with die stone and each die was prepared according to the study requirements (Figure 1F). Each die was sectioned in the center for measurements as described in previous research [22]. For all patients in both groups, the restorations were fabricated on programmed semi-adjustable articulators, using an arbitrary face bow to mount the maxillary cast and a centric interocclusal record (Poly Vinyl Siloxane, O-Bite, DMG, USA) [23]. All patients completed their clinical treatment as per the standard protocol for crown and bridge prosthodontics. Individual restorations were cemented on the final day of treatment and patients were instructed to practice correct oral hygiene [24]. All patients were provided with a free mouthwash (with antimicrobial agent 0.12% chlorhexidine gluconate) on the last treatment day and were asked to continue using it for long-term prosthesis hygiene.
MEASURES, DATA EVALUATION, COLLECTION, AND ANALYSIS:
Dental plaque scores before and after gingival retraction were measured using the dental plaque index (Score 0,1,2,3,4,5) [25]. Time taken to control hemorrhage and place the retraction cord was recorded in seconds using a stop watch. The amount of hemorrhage (hemorrhagic score) was recorded for 3 scores (score 0 – no bleeding on retraction cord removal, score 1 – bleeding controlled within 1 minute and score 2 – bleeding not controlled within 60 seconds) [9]. For all die specimens obtained in different groups, the pre-displacement and post-displacement sulcus widths were identified and observed under an optical microscope (Olympus BX43 with 50× magnification, Olympus Corporation, Tokyo, Japan) with image measurements performed using Image J software (US National Institutes of Health, Bethesda, MA, USA) using recommended guidelines [26]. The gingival sulcus width for each image was measured by a perpendicular line that was drawn from the tip of the marginal gingiva on the image to the tooth surface on its opposite side (Figure 2A, 2B). For each specimen in each group, the amount of retraction was the subtraction result of pre-gingival retraction measurement from post-gingival retraction measurement.
STATISTICAL ANALYSIS:
All measurements were calculated, statistically analyzed, and presented in microns (μm). Data distribution was first tested for normality (Kolmogorov-Smirnov normality test), and parametric or non-parametric tests, as appropriate, were employed to assess statistical significance. The independent samples
Results
DEMOGRAPHIC VARIABLES:
The present study involved selection of patients from the OPD following strict adherence to inclusion and exclusion criteria. Twenty-five patients allocated to both experimental groups had a median age of 34.5±10.32 years in Gp C and 34.7±9.82 years in Gp P. The patient’s demographic variables had no significant effect on outcomes of the research, as the differences between the 2 groups were significant. Patients in both groups completed their respective total treatment without any reporting any adverse effect during/after treatment.
CLINICAL PARAMETERS:
Clinical parameters studied for 2 different retraction cord materials were time taken for placement, control of visible hemorrhage, and hemorrhagic scores (Table 2). The mean time taken for placement in Gp C (157.1±13.13 s) was significantly higher than in Gp P (140.3±10.94 s). The mean hemorrhage control time was recorded as the time taken to completely stop bleeding from the sulcus. Hemorrhage was also scored on a scale of 0 to 2. The mean differences for both parameters in Gp C were significantly (P≤0.05) higher than in Gp P (Table 2).
DIFFERENCES IN SULCUS WIDTH:
For patients in Gp C, the mean difference in sulcus width from baseline to post-retraction was 309.11±21.16 micrometers, while in Gp P the difference was 525.84±38.32 micrometers (Table 3). Within both groups, the differences from baseline to post retraction were found to be statistically significant at a P value of <0.05. The mean change in sulcus width after retraction was lower in Gp C (471.38±28.13) as compared to Gp P (690.03±45.37) (Table 3). The differences between the 2 groups were also statistically significant (P≤0.05), showing that the change in sulcus width was greater with polymer-based retraction cord than with cotton-based retraction cord. The effect of retraction of gingiva on the dental plaque score was also investigated (Table 4). There were no statistically significant differences between the 2 groups in the dental plaque scores before and after retraction. Although the change in sulcus width was higher in polymer-based retraction cords, it did not have any influence on plaque accumulation.
Discussion
POLYETHYLENE TEREPHTHALATE AS RETRACTION CORD MATERIAL:
The last 2 decades have seen a phenomenal development of new materials, methods, and techniques, mainly due to the influence of digital technology. Patient knowledge and social influence have also witnessed changes. Besides gingival conditions, patients also demand correction of even benign conditions like hyper-pigmented gingiva for esthetic reasons [27]. Surgical tools in recent years have been modified in terms of conservation, which has given rise to electrosurgery, soft-tissue laser surgeries, and more use of rotary curettage (gingitage, gingettage) [28,29]. These conservative procedures have gained considerable clinical attention in implant-supported restorations where gingival recontouring to enhance gingival esthetics around the implant abutment is desired [30]. Compared to cotton, which is a natural fiber, the polymer-based retraction cord consists of PTE, which is a polyamide-conjugated yarn [31]. Its use in medical science has recently generated interest and has already found applications in the making of sutures, vascular grafts, tendon graft, heart valves, surgical meshes, scaffolds, and urinary and bloodstream catheters [32–34]. PTE is essentially a polyester product between ethylene glycol and dimethyl terephthalate transesterification [35]. Its applications in medical sciences are due to its advantages, which include high strength (tensile), high stiffness, strong hygroscopicity (at high temperatures), lightweight, broad temperature range usage (minus 60 degrees to 130 degrees centigrade), biostability (biological, chemical and dimensional), excellent gas and moisture barrier, bioinert (microorganism resistant), and it does not degrade biologically [33,34]. Its ability to be recyclable is due to the material liquefying at high temperature rather than burning, which is why it is considered to be a “green” plastic [35]. Its application as a gingival retraction cord material is further justified because it has been shown to be thermally safe, nontoxic if ingested, rapidly absorbed (hemostatic agents), non-leaching in the presence of chemicals, high tensile strength, light weight, and easily moldable. These features have led to its application in heart valves and vascular grafts [36]. Its chief disadvantage is associated with long-term use in contact with food in the packaging industry.
CLINICAL PARAMETERS:
Clinically, the performance of a retraction cord has traditionally been determined by its ability to control hemorrhage and gingival sulcus dilatation [37]. Dry retraction cords have been reported to cause maximum bleeding [25]. Results from our study show that the mean time for hemorrhage control in Gp P was less than with Gp C (56 s versus 80 s). Similar values for cotton-based retraction cord have been reported earlier [7,9,10]. Vag et al also reported a hemorrhagic score of 1.51 for a cotton-based retraction system, which is in agreement with the 1.57 score found in the present study. However, our results show that the polymer cord had better hemorrhage control than cotton. Sulcular hemorrhage is primarily either due to excess operator finger pressure on the cord, which pushes the epithelial attachment barrier, thereby exposing underlying capillaries or fragments of cord sticking to the dry epithelial lining, pulling it on removal of the cord. Polymers contain many long chains that are parallel to each other, which contributes to overall flexural and tensile strength [38]. PTE in a cord system allows it to disperse excess finger pressure by its own deformation and does not transfer the pressure to the underlying layers. In addition, PTE carries more astringent without being wetted by the liquid (hydrophobic), which explains its low hemorrhage scores and quick hemorrhage control upon removal. Our results also show that polymer-based cord took less time for placement (Gp C 157.1 versus Gp P 140.3). Our results for Ultrapak retraction cord (cotton) show similar placement time values as in earlier [6,12,17] and recent [39] studies.
GINGIVAL DISPLACEMENT:
Retraction of gingiva is a mandatory clinical procedure instituted in fabrication of most indirect restorations. The purpose is to provide access for impression material to record the prepared finish line and contour of the underlying natural tooth structure, which in turn allows laboratory technicians to establish a contour on the artificial crown that is biocompatible with the underlying tooth contour. This blends the 2 different surfaces, which becomes less conducive to plaque accumulation. Gingival retraction is also required if the clinician is placing subgingival margins in tooth preparation, during cementation, or if the clinician wants to assess the marginal fit of the finished restoration [40]. To achieve this, a minimal displacement of marginal gingiva has been considered, which is approximately 0.2 mm [6,22]. However, to achieve this, physical pushing of marginal gingiva is essential because the marginal gingiva shapes itself naturally to a contour that inhibits entry into the sulcus. In this study, both cords managed to significantly displace gingiva from their respective baseline values. However, in Gp P, the retraction was greater (525.84) than in Gp C (309.11). The values obtained for the cotton-based retraction cord (Ultrapak) agree with values obtained in previous studies by Al Hamad et al [12], Madaan et al [17], Chaudhari et al [20], and Gajbhiye et al [22]. Our results also are in agreement with the results obtained between the 2 investigated materials after retraction [12,17,20,22,39]. While differences of mean values are attributed to different methods and techniques (mid-section versus 4 or 6 surfaces on the tooth) of sulcus width evaluation, the outcome of the result remains same. Cotton-based retraction cords are mostly available in knitted forms, constructed like a chain with interlocking loops. This allows the cord to bend passively in multiple directions. The design of the cord (rather than capacity) to hold liquid (astringent) plays a more significant role in clinical performance of cords, which explains the reason for the production of braided, twined, or knitted forms in cotton cords [41]. When compared to cotton, the absorption of polymer-based cord is fast, which decreases the soaking time. The basic nature of the 2 fibers is physically and chemically different. Cotton is a biopolymer, absorbs water (hydrophilic), and swells causing volumetric expansion, while polymer does not absorb but holds more liquid, mainly due to its hydrophobic nature, while repelling liquid [42], but liquid is held closely by interfacial surface tension. This allows the polymer cord to more effectively increase sulcus width with a smaller cord. Cotton cord needs more bulk to produce required changes.
The width observed in this study for polymer-based retraction cord (690.0) was higher than with cotton-based cord (471.38). The values observed for retraction cord (Sure cord – plus) are slightly higher than those observed by Madaan et al [18]. The values observed in this study are closer to the values obtained for polyvinyl acetate strip (Merocel) (705.83). The differences between the 2 can be understood by the difference in technique between the 2 studies. While we followed the recommendations given by the manufacturer, they followed what is written in the textbook, which allowed them to place the cord for 10 minutes, while we maintained it for a maximum of 5 minutes. Sure Cord retraction cord is available in both cotton and polymer forms (Sure Cord – Plus). The cotton-based knitted cords absorb crevicular fluids, which exert gentle outward force on the knitted material, pushing marginal gingiva laterally, thus affecting sulcus enlargement.
The results of this study show that neither material produced any significant results in the dental plaque index. Gingiva contains abundant elastic fibers, which allows it to rebound back to its predisplaced position. The gingival fibers are also well supported by periodontal fibers, which reduces the tissue collapse once the cord is removed [43]. Wound healing with cotton gauze lined with polymer has been reported to be faster than with cotton gauze alone, due to presence of mild acid on the surface that stimulates various cells during the course of healing [42]. To enhance healing, patients were asked to brush their teeth regularly with a soft toothbrush and use mouthwash (antimicrobial) twice daily for the first week. Irrespective of the method used for gingival retraction, a certain amount of trauma to gingiva is inevitable and every living tissue responds naturally to such trauma by inflammation. Cellular response has been shown to be better when patients use mouthwash as an adjunct to other oral hygiene measures [44].
STRENGTH AND LIMITATIONS OF THE STUDY:
This study highlights the use of PTE polymer as a gingival retraction cord. Some of the existing drawbacks of other retraction cord materials like polyvinyl acetate strip, retraction pastes, and cotton-based retraction cords could be overcome with the use of PTE-based retraction cords. However, our study is limited by not considering sulcular depth variations, gingival distend ability, and various areas of sulcular width. Indirect measurements on the cast have the drawback of material expansion and contraction and various unavoidable manipulative variables that are invariably incorporated. The study also has a small sample size, which should be increased in future studies.
Conclusions
Under similar clinical circumstances, the polymer-based retraction cord took less application time, produced quicker hemorrhage control with lower hemorrhage scores, and was associated with low plaque index. The polymer-based retraction cord also produced significantly increased changes in sulcus width when compared to baseline values or to cotton-based retraction cords.
Figures
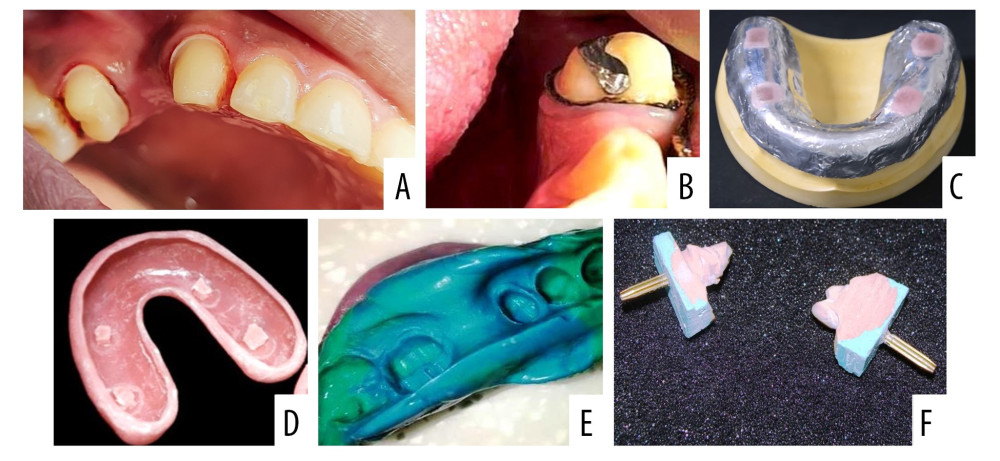 Figure 1. Sequence of clinical procedures during and after gingival retraction. (A) Gingival retraction of maxillary left canine and second premolar using cotton retraction cord. (B) Gingival retraction of a mandibular left canine and second premolar tooth using polymer retraction cord. (C) Special/custom tray fabrication showing an adapted 2 thickness modelling wax sheet with vertical stops covered with light cure resin. (D) Special tray after removal of wax spacer. (E) Definitive impression of the prepared teeth that was intended to receive a 3 unit fixed partial denture. (F) Individual dies used for fabrication of the crown and testing the sulcus depth. Photographs taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), windows 11 Pro, Microsoft corporation).
Figure 1. Sequence of clinical procedures during and after gingival retraction. (A) Gingival retraction of maxillary left canine and second premolar using cotton retraction cord. (B) Gingival retraction of a mandibular left canine and second premolar tooth using polymer retraction cord. (C) Special/custom tray fabrication showing an adapted 2 thickness modelling wax sheet with vertical stops covered with light cure resin. (D) Special tray after removal of wax spacer. (E) Definitive impression of the prepared teeth that was intended to receive a 3 unit fixed partial denture. (F) Individual dies used for fabrication of the crown and testing the sulcus depth. Photographs taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), windows 11 Pro, Microsoft corporation). 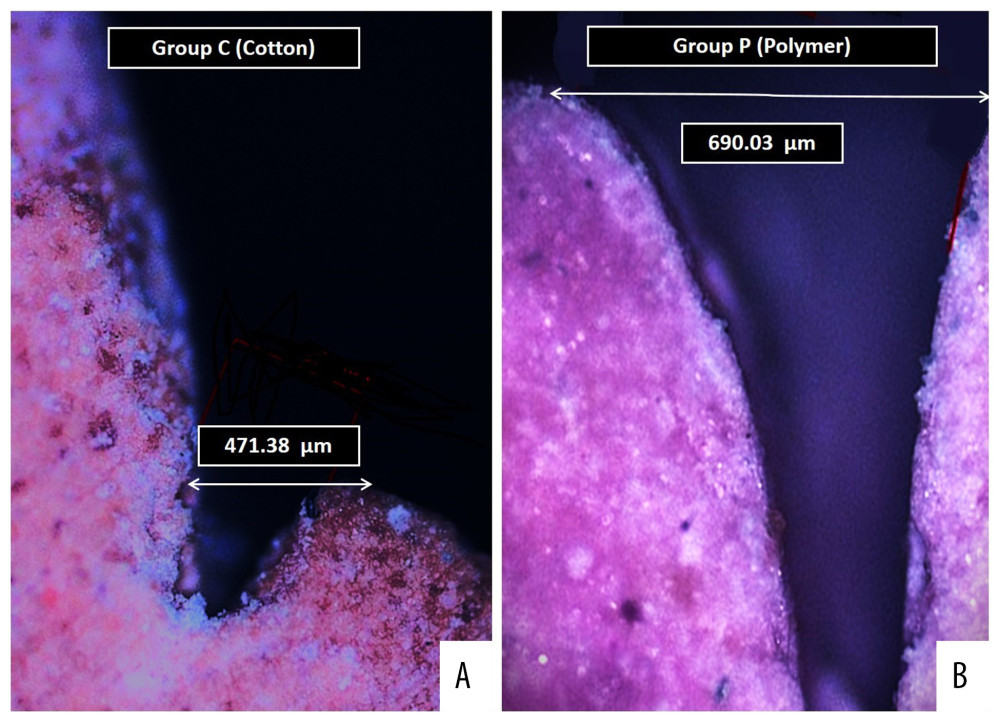 Figure 2. Digital images of the die cast observed under optical microscope showing (A) Measured sulcus width between the 2 points as perpendicular distance from the maximum height of the stone cast gingival margin on one side and tooth surface on other side for specimens in group C. (B) Measured sulcus width between the 2 same points for specimens in group P. Photographs were taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash.
Figure 2. Digital images of the die cast observed under optical microscope showing (A) Measured sulcus width between the 2 points as perpendicular distance from the maximum height of the stone cast gingival margin on one side and tooth surface on other side for specimens in group C. (B) Measured sulcus width between the 2 same points for specimens in group P. Photographs were taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Tables
Table 1. Distribution of groups according to material composition, type, and recommendations. Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure.
Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure.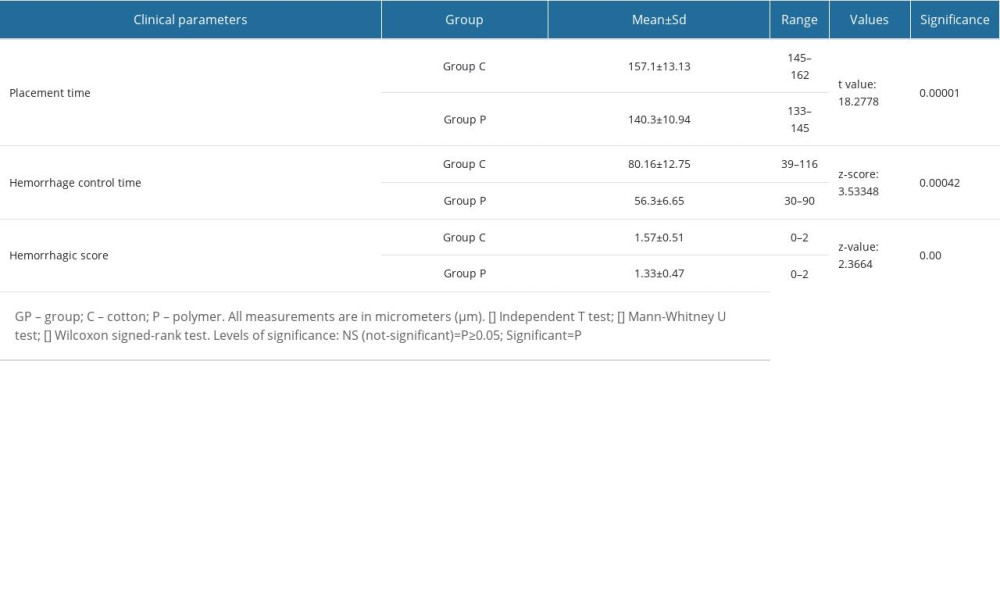 Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]).
Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]).![Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]).](https://jours.isi-science.com/imageXml.php?i=t3-medscimonit-29-e940098.jpg&idArt=940098&w=1000) Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction).
Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction).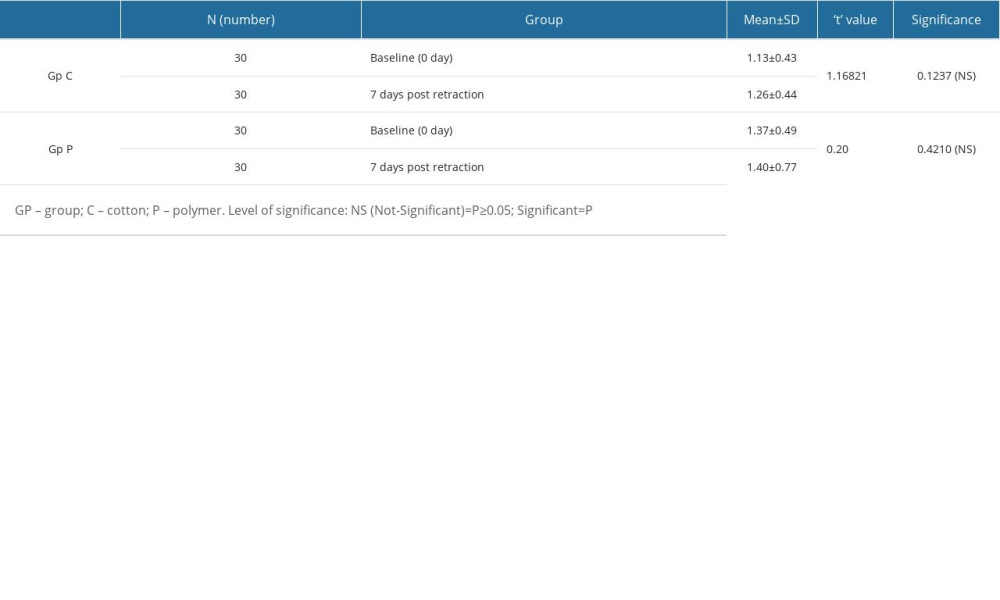
References
1. , The glossary of prosthodontic terms: J Prosthet Dent, 2005; 94; 10-92
2. Anupam P, Namratha N, Vibha S, Efficacy of two gingival retraction systems on lateral gingival displacement: A prospective clinical study: J Oral Biol Craniofac Res, 2013; 3(2); 68-72
3. Wang Y, Fan F, Li X, Influence of gingival retraction paste versus cord on periodontal health: A systematic review and meta-analysis: Quintessence Int, 2019; 50(3); 234-44
4. Safari S, Ma VS, Mi VS, Gingival retraction methods for fabrication of fixed partial denture: Literature review: J Dent Biomater, 2016; 3(2); 205-13
5. Baba NZ, Goodacre CJ, Jekki R, Won J, Gingival displacement for impression making in fixed prosthodontics: Contemporary principles, materials, and techniques: Dental Clinics, 2014; 58(1); 45-68
6. Parai P, Ojah P, Jain S, Khan N, A comparative evaluation of the efficacy of two gingival retraction systems: An in vivo study: European Journal of Molecular and Clinical Medicine, 2020; 7(8); 4870-80
7. Hadyaoui D, Daouahi N, Nouira Z, Cherif M, Gingival harmony in anterior aesthetic restorations: Dentistry Journal, 2014; 2(4); 155-62
8. Vág J, Gánti B, Mikecs B, Epinephrine penetrates through gingival sulcus unlike keratinized gingiva and evokes remote vasoconstriction in human: BMC Oral Health, 2020; 20; 305
9. Merchant A, Ganapathy DM, Maiti S, Effectiveness of local and topical anesthesia during gingival retraction: Anesthesia during cord packing: Braz Dent Sci, 2022; 25(1); e2591
10. Kannan A, Venugopalan S, A systematic review on the effect of use of impregnated retraction cords on gingiva: Research Journal of Pharmacy and Technology, 2018; 11(5); 2121-26
11. Al-Odinee NM, Al-Hamzi M, Al-Shami IZ, Evaluation of the quality of fixed prosthesis impressions in private laboratories in a sample from Yemen: BMC Oral Health, 2020; 20; 304
12. Al Hamad KQ, Azar WZ, Alwaeli HA, Said KN, A clinical study on the effects of cordless and conventional retraction techniques on the gingival and periodontal health: J Clin Periodontol, 2008; 35(12); 1053-58
13. Reddy SVG, Bharathi M, Vinod B, Gingival displacement methods used by dental professionals: A survey: J Orofac Sci, 2016; 8(2); 120-22
14. Hansen PA, Tira DE, Barlow J, Current methods of finish-line exposure by practicing Prosthodontists: J Prosthet Dent, 1999; 8; 163-70
15. Agarwal A, Lahori M, Arora S, A comparative evaluation of two contemporary cordless methods of gingival retraction – an in vivo study: Journal of Interdisciplinary Dentistry, 2019; 9(2); 51
16. Bennani V, Inger M, Aarts JM, Comparison of pressure generated by cordless gingival displacement materials: J Prosthet Dent, 2014; 112(2); 163-67
17. Madaan R, Paliwal J, Sharma V, Comparative evaluation of the clinical efficacy of four different gingival retraction systems: An in vivo study: Cureus, 2022; 14(4); e23923
18. Ghasemi A, Imani R, Yousefzadeh M, Studying the potential application of electrospun polyethylene terephthalate/graphene oxide nanofibers as electroconductive cardiac patches: Macromol Mater Eng, 2019; 304(8); 1900187
19. Silva GG, da Costa Valente ML, Bachmann L, Dos Reis AC, Use of polyethylene terephthalate as a prosthetic component in the prosthesis on an overdenture implant: Mater Sci Eng C Mater Biol Appl, 2019; 99; 1341-49
20. Chaudhari J, Prajapati P, Patel J, Comparative evaluation of the amount of gingival displacement produced by three different gingival retraction systems: An in vivo study: Contemp Clin Dent, 2015; 6; 189-95
21. Mattoo KA, Garg R, Gupta A, Jain N, Toxicology and biocompatibility of dental materials: A review: Res J Pharmac Biol Chem Sci, 2012; 3(4); 1091-99
22. Gajbhiye V, Banerjee R, Jaiswal P, Comparative evaluation of three gingival displacement materials for efficacy in tissue management and dimensional accuracy: J Indian Prosthodont Soc, 2019; 19(2); 173
23. Sharma A, Kalra T, Jain S, Comparative evaluation in linear dimensions among various interocclusal recording materials at various mounting times: An in vitro study: World J Dent, 2020; 11(6); 462-67
24. Jindal S, Mattoo KA, Arora P: Post care instructions for dental prosthesis (Fixed), 2013; 18-20, LAP Lambert Academic Publishing
25. Oliveira LM, Pazinatto J, Zanatta FB, Are oral hygiene instructions with aid of plaque-disclosing methods effective in improving self-performed dental plaque control? A systematic review of randomized controlled trials: Int J Dent Hyg, 2021; 19(3); 239-54
26. Collins TJ, ImageJ for microscopy: Biotechniques, 2007; 43(Suppl 1); S25-30
27. Garg R, Mattoo KA, Jain P, Aesthetic treatment for hyperpigmented gingiva: International Journal of Research in Medical Sciences and Technology, 2015; 1(1); 14-15
28. Sorrentino R, Ruggiero G, Zarone F, Laser systems for gingival retraction in fixed prosthodontics: A narrative review: Journal of Osseointegration, 2022; 14(1); 1-5
29. El-Ashkar A, Nabil O, Taymour M, El-Tannir A, Evaluation of zirconia crowns restoring endodontically treated posterior teeth with 2 finish line designs and 2 occlusal reduction schemes: A randomized clinical trial: J Prosthet Dent, 2022 [Online ahead of print]
30. Singh M, Mattoo KA, Jain S, Replacement of a mandibular molar with implant retained single crown: Oral Surgery, Oral Medicine, Oral Radiology, 2014; 2(2); 25-27
31. , Instructions for product: SURE CORD – Plus Available at: https://www.suredent.com/product/tissue/surecordplus
32. Cutroneo M, Havranek V, Mackova A, Overview of polyethylene terephthalate foils patterned using 10 MeV carbon ions for realization of micromembranes: Micromachines, 2023; 14(2); 284
33. Paxton NC, Allenby MC, Lewis PM, Woodruff MA, Biomedical applications of polyethylene: European Polymer Journal, 2019; 118; 412-28
34. Jafari S, Hosseini Salekdeh SS, Solouk A, Yousefzadeh M, Electrospun polyethylene terephthalate (PET) nanofibrous conduit for biomedical application: Polymers for Advanced Technologies, 2020; 31(2); 284-96
35. Suhaimi NA, Muhamad F, Abd Razak NA, Zeimaran E, Recycling of polyethylene terephthalate wastes: A review of technologies, routes, and applications: Polymer Engineering & Science, 2022; 62(8); 2355-75
36. Wang X, Chan V, Corridon PR, Acellular tissue-engineered vascular grafts from polymers: Methods, achievements, characterization, and challenges: Polymers, 2022; 14(22); 4825
37. Nowakowska D, Saczko J, Szewczyk A, In vitro effects of vasoconstrictive retraction agents on primary human gingival fibroblasts: Exp Ther Med, 2020; 19(3); 2037-44
38. Abbood IS, aldeen Odaa S, Hasan KF, Jasim MA, Properties evaluation of fiber reinforced polymers and their constituent materials used in structures – a review: Materials Today: Proceedings, 2021; 43; 1003-8
39. Beleidy M, Serag Elddien AM, Clinical comparative evaluation of different retraction systems in gingival displacement and their influence on periodontal health: A randomized clinical trial: Egyptian Dental Journal July 3, 2020; 66; 1667-78 (Fixed Prosthodontics, Removable Prosthodontics and Dental Materials)
40. Kamath R, Sarandha DL, Baid GC, Advances in gingival retraction: International Journal of Clinical Dental Science, 2011; 2(1); 110621582
41. Jokstad A, Clinical trial of gingival retraction cords: J Prosthet Dent, 1999; 81; 258-61
42. Romero-Fierro DA, Camacho-Cruz LA, Bustamante-Torres MR, Modification of cotton gauzes with poly (acrylic acid) and poly (methacrylic acid) using gamma radiation for drug loading studies: Radiation Physics and Chemistry, 2022; 190; 109787
43. Prasad KD, Agrawal G, Hegde C, Shetty M, Gingival displacement in prosthodontics: A critical review of existing methods: J Interdiscip Dent, 2011; 1(2); 80-86
44. Abullais SS, Patel SI, Asiri EA, Comparative evaluation of 3 commercial mouthwash formulations on clinical parameters of chronic gingivitis: Med Sci Monit, 2022; 28; e397111
Figures
 Figure 1. Sequence of clinical procedures during and after gingival retraction. (A) Gingival retraction of maxillary left canine and second premolar using cotton retraction cord. (B) Gingival retraction of a mandibular left canine and second premolar tooth using polymer retraction cord. (C) Special/custom tray fabrication showing an adapted 2 thickness modelling wax sheet with vertical stops covered with light cure resin. (D) Special tray after removal of wax spacer. (E) Definitive impression of the prepared teeth that was intended to receive a 3 unit fixed partial denture. (F) Individual dies used for fabrication of the crown and testing the sulcus depth. Photographs taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), windows 11 Pro, Microsoft corporation).
Figure 1. Sequence of clinical procedures during and after gingival retraction. (A) Gingival retraction of maxillary left canine and second premolar using cotton retraction cord. (B) Gingival retraction of a mandibular left canine and second premolar tooth using polymer retraction cord. (C) Special/custom tray fabrication showing an adapted 2 thickness modelling wax sheet with vertical stops covered with light cure resin. (D) Special tray after removal of wax spacer. (E) Definitive impression of the prepared teeth that was intended to receive a 3 unit fixed partial denture. (F) Individual dies used for fabrication of the crown and testing the sulcus depth. Photographs taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Figure created using MS PowerPoint, version 20H2 (OS build 19042,1466), windows 11 Pro, Microsoft corporation). Figure 2. Digital images of the die cast observed under optical microscope showing (A) Measured sulcus width between the 2 points as perpendicular distance from the maximum height of the stone cast gingival margin on one side and tooth surface on other side for specimens in group C. (B) Measured sulcus width between the 2 same points for specimens in group P. Photographs were taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash.
Figure 2. Digital images of the die cast observed under optical microscope showing (A) Measured sulcus width between the 2 points as perpendicular distance from the maximum height of the stone cast gingival margin on one side and tooth surface on other side for specimens in group C. (B) Measured sulcus width between the 2 same points for specimens in group P. Photographs were taken using a digital single-lens reflex (DSLR) camera (Canon EOS 700D) with 100 mm macro lens) with/without ring flash. Tables
 Table 1. Distribution of groups according to material composition, type, and recommendations.
Table 1. Distribution of groups according to material composition, type, and recommendations. Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure.
Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure.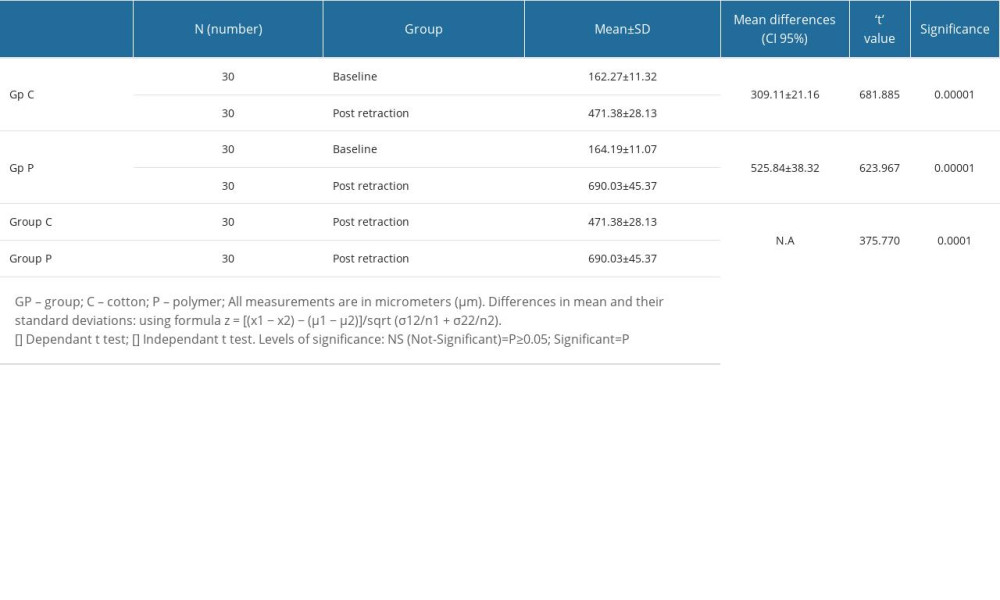 Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]).
Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]). Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction).
Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction). Table 1. Distribution of groups according to material composition, type, and recommendations.
Table 1. Distribution of groups according to material composition, type, and recommendations. Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure.
Table 2. Mean scores of various clinical parameters among patients of both groups investigated during clinical procedure. Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]).
Table 3. Differences in gingival retraction measurements of studied groups (within groups [baseline to post-retraction] and between groups [post-retraction]). Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction).
Table 4. Differences in plaque index scores among patients in both groups at 2 different time intervals (before retraction and 7 days after retraction). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








