01 September 2023: Clinical Research
Complex Blood Supply Patterns in Cesarean Scar Pregnancy: Insights from Digital Subtraction Angiography Imaging
Feng Gao1BCEF, Yu Lu2BD, Xiaoqing Guo3C, Jie Gao4B, Wenjing Wang4BC, Jiejun Cheng4EFG*, Le Fu4AFDOI: 10.12659/MSM.940133
Med Sci Monit 2023; 29:e940133
Abstract
BACKGROUND: Understanding the blood supply pattern of cesarean scar pregnancy (CSP) can effectively help to determine the best choice of treatment. The aim of this study was to investigate the blood supply pattern and outcomes of patients with CSP through digital subtraction angiography (DSA) imaging.
MATERIAL AND METHODS: This was a retrospective cohort study. Patients were divided into 2 groups according to the type of CSP. The DSA images of these patients were reviewed, including the type of blood supply, dominant vessel, and collateral blood supply to the gestational sac. The clinical outcomes were analyzed between the 2 groups.
RESULTS: Thirty-seven patients with type I and 29 patients with type II CSP were enrolled in this study. Type II CSP showed a higher proportion of rich blood supply than type I (44.83% vs 29.72%, P>0.05). Compared with type II CSP, type I CSP tended to have bilateral dominant blood supply predominance (67.57% vs 41.38%, P<0.05). The incidence of collateral blood supply was 5.41% in the type I CSP group and 31.03% in the type II CSP group (P<0.05). In the type II CSP group, multiple collateral blood vessels were found in 4 patients. The superior vesicle artery was the most common source of collateral blood supply in both groups. Two patients with type II CSP suffered massive bleeding during surgery after uterine artery embolization (UAE). None of the patients received a hysterectomy.
CONCLUSIONS: UAE is safe and effective for both types of CSP. The blood supply pattern is more complex and abnormal in type II CSP. More attention should be paid to the collateral blood supply to achieve complete embolization during the UAE procedure in the case of type II CSP.
Keywords: Pregnancy, uterine artery embolization, Female, Humans, Angiography, Digital Subtraction, Cicatrix, Retrospective Studies, Arteries
Background
Cesarean scar pregnancy (CSP) is a special type of ectopic pregnancy in which a gestational sac is implanted in, or in close contact with, the niche left from a previous cesarean section [1]. In recent years, rising rates of cesarean delivery have resulted in increasing numbers of CSPs [2,3]. CSP patients may suffer serious complications, including life-threatening hemorrhage, uterine rupture, hysterectomy, and even maternal mortality [4]. Thus, timely diagnosis and termination of pregnancy is the standard management for CSP [5]. To improve the understanding and management of CSP, CSP is divided into type I (endogenous type) and type II (exogenous type) according to the growth progress of the gestational sac [6,7]. The endogenous type of CSP may result in a viable pregnancy, but the risk of bleeding from placental sites is high. The exogenous type may be complicated by uterine rupture and massive bleeding in the first trimester [6]. The risk of massive bleeding and treatment challenge is different for different types of CSP [8,9].
The management of CSP varies, and there has been no consensus regarding the optimal treatment [10]. It has been reported that more than a half of all CSPs treated with dilation and curettage (D&C) alone required additional treatment [10,11]. However, in another study, treatment with uterine curettage had a 95% success rate in cases of type I CSP, while this treatment only showed 27% success in cases of type II CSP [12]. The blood supply characteristics of different types of CSP may be different, resulting in different bleeding risks.
Uterine artery embolization (UAE) can minimize bleeding associated with D&C and accelerate the resolution of the gestational sac [13,14]. Concomitant use of UAE increases the success rate of the primary treatment for CSP [15,16]. Furthermore, UAE integrates imaging diagnosis and treatment. It can not only occlude blood flow of bilateral uterine arteries, but also reveal the blood supply characteristic of CSPs [13,17]. However, research thus far has mainly focused on the efficacy and safety of UAE in the treatment of CSP, while research on digital subtraction angiography (DSA) image features of CSP is rare.
Our hypothesis was that different types of CSP have different bleeding risks because of their different blood supply patterns. Understanding the blood supply characteristics of patients with different types of CSP can help them choose the most appropriate treatment. Therefore, in this study, we aimed to explore the blood supply patterns through DSA images of 2 CSP types to improve clinical awareness and optimize treatment strategies.
Material and Methods
This study was approved by the Ethics Committee of the Shanghai First Maternity and Infant Hospital (registration number: KS22281). Patients with diagnosed CSP were reviewed from November 2018 to June 2022. All patients signed an informed consent form before hospitalization. The inclusion criteria were as follows: (1) the diagnosis of cesarean scar pregnancy was confirmed by surgery and pathological analysis; (2) patients were treated with bilateral UAE before surgery; (3) the types of CSP were confirmed by ultrasound; (4) the gestational age was <12 weeks according to the time of last menstrual period. The exclusion criteria were as follows: (1) incomplete clinical data; (2) comorbidity with fibroids or adenomyosis; (3) followup data were incomplete (Figure 1).
CSP was divided into 2 types according to the ultrasonography results [18]. Type I CSP was defined as gestational sac partially located in the niche, with progression to the cervico-isthmic space or uterine cavity (endogenous type). Type II was defined as gestational sac mostly or completely located in the niche, with deep invasion of the scar defect, and progression toward the bladder and abdominal cavity. Once the diagnosis and classification of CSP was made, the patient was treated within 3 days.
Clinical characteristics were collected, including age, number of cesarean sections, gravidity, parity, D&Cs, time interval from type classification to treatment, time interval since last cesarean, gestational age, diameter of the gestational sac, anterior myometrium thickness, preoperative serum b-hCG level, the presence of fetal heartbeat, and the presence of vaginal bleeding and/or abdominal pain. Blood loss during the surgery was estimated by suction and weighing of swabs. The surgeon, anesthetist, and nurse confirmed the estimated blood loss.
UAE was performed by an experienced radiologist (Feng Gao, 15 years of experience in interventional radiology). The right femoral artery was used for access by the Seldinger technique. The bilateral uterine artery was super-selectively catheterized with a 5-F Roberts uterine catheter (APT Medical, Hunan, China). UAE was performed in all procedures with the use of standard gelatin sponge particles (560–710 μm/710–1000 μm) (Alicon, HangZhou, China). The embolization endpoint was defined as stasis of flow in the uterine artery. Ultrasound-guided D&C was performed within 48 hours after embolization. Clinical outcomes were recorded according to the medical records. The technical success of UAE was defined as complete occlusion of the uterine artery and no gestational sac staining upon angiography. The clinical success of the UAE was defined as minor estimated blood loss (<50 ml) during the subsequent procedure and followup.
The DSA imaging analysis features were evaluated by 2 interventionists (with 15 years and 6 years of experience, respectively) in obstetric intervention. Controversial results were analyzed by a third interventional physician’s observation, and concordant opinion was later adopted as the final diagnostic findings. The content of the imaging analysis included type of blood supply, dominant vessel, and collateral blood supply to the gestational sac.
Gestational sac blood supply was classified into 3 grades according to the level of richness: 1=minimal blood flow; 2=moderate blood flow; 3=high vascularity. The specific DSA manifestations were defined as follows: minimal blood flow type with only shallow gestational sac staining in parenchymal phase (Figure 2A); moderate blood flow type with clear gestational sac staining (Figure 2B); high vascularity type with significant patchy, clumped vessels on imaging (resembling contrast extravasation), significantly faster blood flow, or a combination of uterine arteriovenous malformations (Figure 2C). Dominant vessels were divided into unilateral predominance and bilateral predominance (Figure 3).
The patients were divided into 2 groups according the type of CSP: type I or type II. The characteristics of the blood supply, and the general laboratory and clinical data regarding outcomes after UAE were compared between the 2 groups.
Statistical Package for the Social Sciences (SPSS, version 16.0) was used for statistical analysis. Pearson chi-square tests were performed to compare the differences in clinical data and MRI characteristics between the case group and control group, and Mann-Whitney U tests were performed for quantitative variables. Multivariate analysis was performed to investigate the influences of each factor on each blood supply, including: (1) bilateral uterine arteries, and (2) collateral blood supply. A
Results
A total of 344 patients with CSP were enrolled in our hospital from November 2018 to June 2022. Among these were 66 cases of patients treated with bilateral UAE before D&C, who met the inclusion criteria for enrollment in this study. According to their US records, 37 patients (56.1%) were classified as type I CSP and were assigned to Group I, and the other 29 (43.9%) were classified as type II CSP and were assigned to Group II (Figure 1). In the type II CSP group, 3 cases showed mixed cystic solid masses on ultrasound.
The baseline clinical characteristics of different types of CSP are shown in Table 1. There were no significant differences between the 2 groups in terms of age before treatment, number of cesarean deliveries, time interval from last cesarean section, gestational age, serum β-hCG, the presence of fetal heartbeat, and the presence of vaginal bleeding and/or abdominal pain. Myometrial thickness and gestational sac maximum diameter were measured by ultrasound, and the differences were not significant.
Table 2 shows the characteristics of the blood supply between the 2 groups. Although Group II had a higher proportion of rich blood supply (Group I vs Group II: 29.72% vs 44.83%), the difference between the 2 groups was not statistically significant. Compared with Group II, Group I tended to have bilateral dominant blood supply predominance (Group I vs Group II: 67.57% vs 41.38%,
The technical success rate of UAE was 100% in both groups of patients. All the patients in Group I acquired clinical success. In Group II, 2 patients experienced massive vaginal bleeding during surgery. One of the 2 had an estimated blood loss of 200 ml during surgery, and received laparoscopic repair afterwards; the other patient suffered retained products of conception after surgery and was treated conservatively. Both of these patients had multiple collateral blood vessels. In other patients, no serious adverse events occurred. There were no significant differences in blood loss during surgery, duration of hospital stay, or menstrual recovery time between the 2 groups (Table 6). Although the superior vesicle artery was embolized due to collateral blood supply in 11 patients, there were no adverse effects on bladder function after embolization.
Discussion
CSP has received considerable attention because of its increasing incidence, as well as its life-threatening risks [19,20]. Type II CSP warrants more attention because of a greater risk of catastrophic complications [21]. In the present study, we found that type II CSP had a higher incidence and more complicated collateral blood supply compared with type I CSP, which may have adverse impacts on the efficacy of UAE and patient safety.
In our center, once CSP was diagnosed, multi-disciplinary treatment was arranged to provide optimal treatment choice. UAE was performed only in the patients with high bleeding risk. The UAE indication was as follows: (1) rich blood supply on ultrasonography or MRI, (2) longer pregnancy duration (>8 weeks), and 3) a distance of <2 mm between the gestational mass and the bladder. These indications were similar to those mentioned in previous works [22].
Blood loss was minimal in our study compared with other recent reports [23,24]. We have achieved this minimal intraoperative blood loss because all the patients underwent pre-surgery UAE. UAE is a method of blocking the blood supply and vascular plexus of the gestational sac through the embolization of the bilateral uterine arteries, thereby reducing the risk of major bleeding from the CSP [25]. All 66 patients in this study underwent pre-surgery UAE, and as a result, only 2 patients suffered massive vaginal bleeding during the operation.
The clinical manifestations of CSP present a wide range of variations, and the classification of CSP type is yet to be standardized. Kaelin Agten et al classified 2 types of CSP as ‘on the scar’ (partially or fully on top of a well-healed scar) and ‘in the niche’ (within a deficient or dehiscent scar). Their results demonstrated that patients with CSP implanted “on the scar” had a better outcome than those with CSP implanted “in the niche” [26]. In the new CSP classification, CSP can be used as a collective term that includes all pregnancies (gestational sac and/or placenta) with implantation in, or in close contact with, the niche. A CSP can occur only when a niche is present and not in relation to a healed cesarean section scar (on the scar) [1]. According to the new definition, we excluded ‘on the scar CSP’ from the study.
Balci and Ercan described type 1 and type 2 CSPs according to the growth progress of the gestational sac [18]. We chose this classification because it is simple and there is no need to define the implantation depth. In this retrospective study, we were able to quickly classify patients based on their ultrasound reports. Based on the Balci and Ercan proposal, experts added some additional items from other classification systems to refine the types of CSP in 2022 [1]. The new proposal is more comprehensive and reproducible. However, it needs to use and record the terminology recommended in their study, which was not available in previous records. We will evaluate the relevance of the proposed new CSP type in future research.
The main blood supply to the uterus in early pregnancy comes from the uterine artery, but as gestational weeks progress, many collateral arteries become involved [27–29]. Complete embolization of all arteries supplying blood to the uterus is a major factor affecting hemostasis [17]. The total incidence of collateral blood supply in our study was 16.67%, which is similar to that reported in previous studies [30]. Our result showed that, in early pregnancy, type II CSP had a much higher incidence of collateral blood supply than type I CSP. Tian reported that the incidence of multivessel blood supply in CSP was 5.35% [17]. Similarly, 4 patients (4/66, 6.1%) were found with multiple collateral blood vessels in our study. However, all 4 patients had type II CSP. The incidence is as high as 13.79% (4/29) in type II CSP. Our present results indicate that more attention should be paid to the existence and embolism of collateral blood supply during surgery to ensure complete embolization of the gestational sac.
The most common collateral blood vessel seen in the present study was the superior vesicle artery. Considering that the bladder is adjacent to the cesarean scar, collateral blood supply may develop during the progress of the CSP. Relatively large particles should be used for superior vesicle artery embolization to avoid bladder necrosis. In addition, close attention should be paid to the possibility of invasion of the bladder during the operation. If necessary, a urologist should be asked to assist in the operation.
In our study, the technical and clinical success rate was 100%, and this outcome was attributed to our procedure strategy. Before UAE, abdominal aorta angiography was performed to characterize the uterine blood supply. During the procedure, we used the stratification method to embolize uterine arteries, using a range of gelatin particle sizes, from small (560–710 μm) to large (710–1000 μm). After UAE, we performed abdominal aortic angiography again to confirm that all gestational blood vessels had been embolized, especially the ovarian artery. If the ovarian artery was involved in the gestational blood supply, we used the large size of gelatin particles (710–1000 μm) to embolize the ovarian artery. In contrast to uterine adenomyosis, CSP confers a risk of basilar artery embolization leading to uterine ischemia, if too-small sizes of gelatin particles (<300 μm) are used to embolize the uterine artery [31]. Therefore, we did not use these small-size gelatin particles (<300 μm) to embolize the uterine artery.
Previous reports have documented significant maternal complications, such as insular edema and bladder necrosis [32,33]. However, in our study, no complications were seen. We believe this is attributable to the participation of an experienced interventional radiology team from the beginning to the end of the procedure. Gelatin sponge, a temporary embolizing agent, is the preferred agent for occlusion in the UAE procedure [34]. We only used standardized gelatin sponge particles with a diameter of more than 560 μm for embolization. Besides, occluded arteries larger than 700 μm tend to ensure distal perfusion via anastomoses with other pelvic arteries [35].
Although the risk of placenta accreta spectrum is high, type I CSP has the potential to survive [6,36,37]. In normal pregnancy, the blood supply of the bilateral uterine arteries is generally balanced. We found that type I CSP tended to show bilateral predominance, while type II CSP favored unilateral predominance. These results suggest that type II CSP may achieve more collateral blood supply, which necessitates more concern and early intervention.
It would be interesting to correlate DSA and color Doppler imaging findings, and we have tried to reviewed color Doppler imaging. However, as this is a retrospective study, this was not possible as only a few patients saved the images. Besides, due to inconsistent imaging conditions for color Doppler imaging and varying sensitivity of machines to blood flow, it is difficult to fully attain the blood flow features of CSP from these saved images. In the future, we plan to conduct a prospective 3-dimensional power Doppler ultrasound assessment of blood flow and calculate the vascularization index of the CSP, to better compare with DSA imaging features.
It is acknowledged that this study has several limitations. First, this is a retrospective study, which may result in a selection bias. Second, the sample size is relatively small and all samples come from a single center. Future research should expand the number of cases to carry out multi-center observation with large sample size to reduce the impact of regional factors.
Conclusions
In conclusion, our results demonstrate that prophylactic UAE is an effective and safe method to prevent hemorrhage in both types of CSP. The blood supply pattern of type II CSP is more complex and abnormal than type I. Therefore, in type II CSP, more attention should be paid to the collateral blood supply, to achieve complete embolization during the procedure of UAE.
Figures
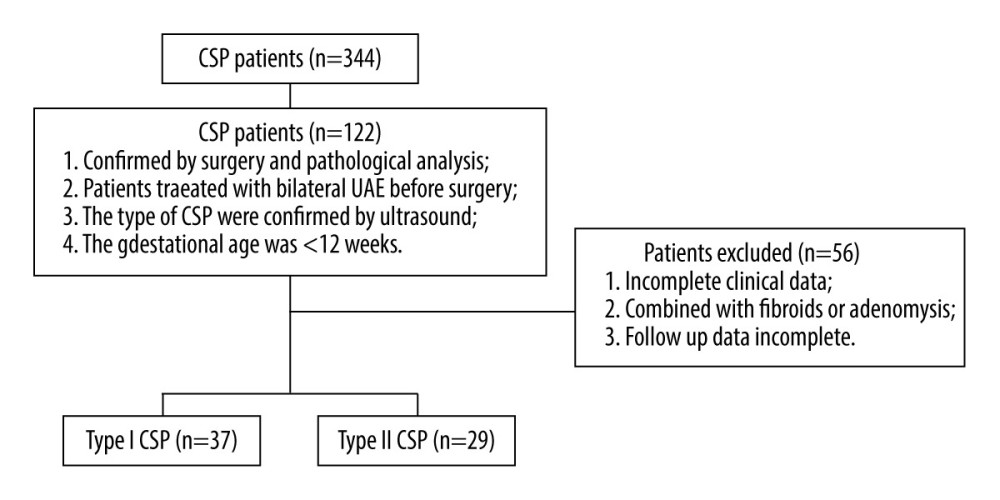 Figure 1. Flowchart of patient selection. The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 1. Flowchart of patient selection. The figure was created with PowerPoint software (Microsoft 365 Office). 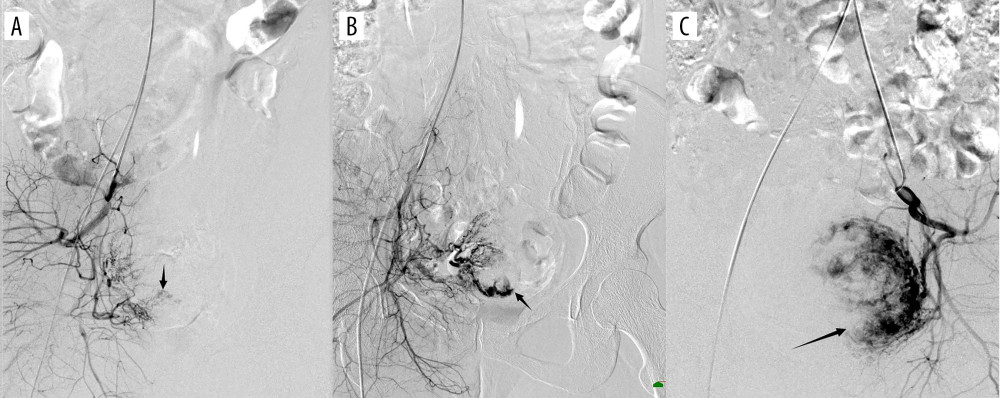 Figure 2. (A) Digital subtraction angiography imaging showing sparse gestational sac staining (black arrow); (B) general gestational sac staining (black arrow); (C) obvious gestational sac staining (black arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 2. (A) Digital subtraction angiography imaging showing sparse gestational sac staining (black arrow); (B) general gestational sac staining (black arrow); (C) obvious gestational sac staining (black arrow). The figure was created with PowerPoint software (Microsoft 365 Office). 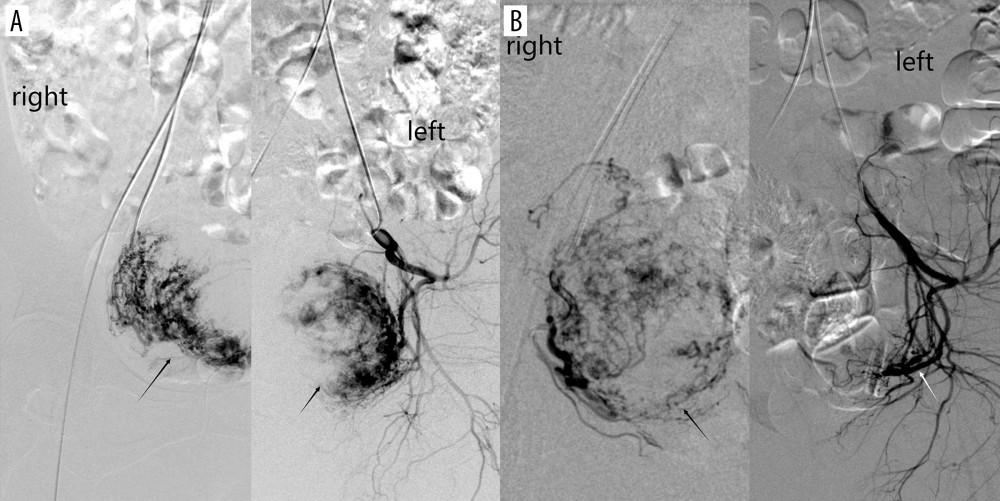 Figure 3. (A) Digital subtraction angiography imaging showing bilateral uterine artery supply to the gestational sac (black arrow); (B) right uterine artery supply to the gestational sac (black arrow), with no gestational sac blood flow from the left uterine artery (white arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 3. (A) Digital subtraction angiography imaging showing bilateral uterine artery supply to the gestational sac (black arrow); (B) right uterine artery supply to the gestational sac (black arrow), with no gestational sac blood flow from the left uterine artery (white arrow). The figure was created with PowerPoint software (Microsoft 365 Office). 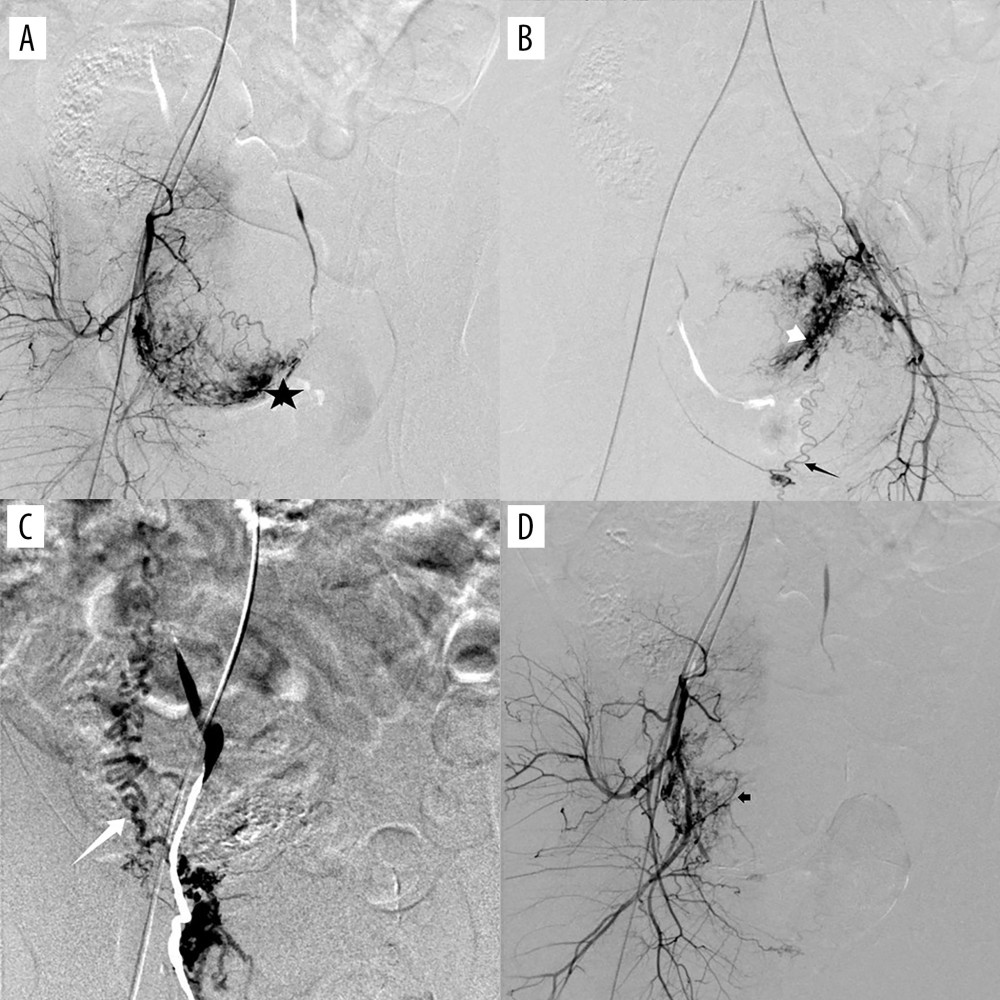 Figure 4. (A) Digital subtraction angiography imaging showing uterine artery (star) and multiple collateral blood supplies to the gestational sac; (B) superior vesicle arteries (white short arrow); (B) internal pudendal artery (black long arrow); (C) ovarian artery (white long arrow); and (D) other branches from the internal iliac artery (black short arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 4. (A) Digital subtraction angiography imaging showing uterine artery (star) and multiple collateral blood supplies to the gestational sac; (B) superior vesicle arteries (white short arrow); (B) internal pudendal artery (black long arrow); (C) ovarian artery (white long arrow); and (D) other branches from the internal iliac artery (black short arrow). The figure was created with PowerPoint software (Microsoft 365 Office). Tables
Table 1. Baseline characteristics.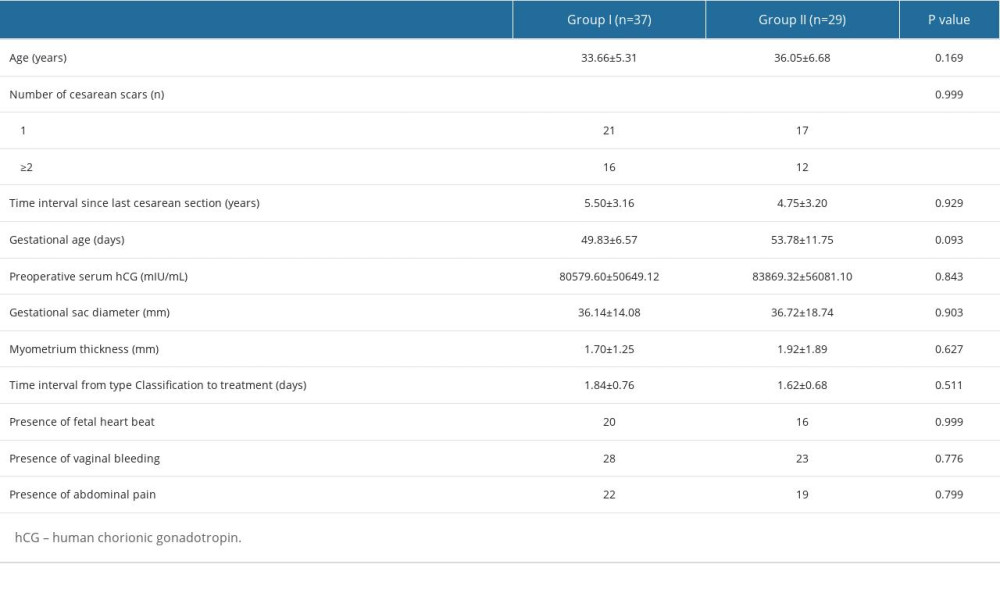 Table 2. Characteristics of the blood supply.
Table 2. Characteristics of the blood supply.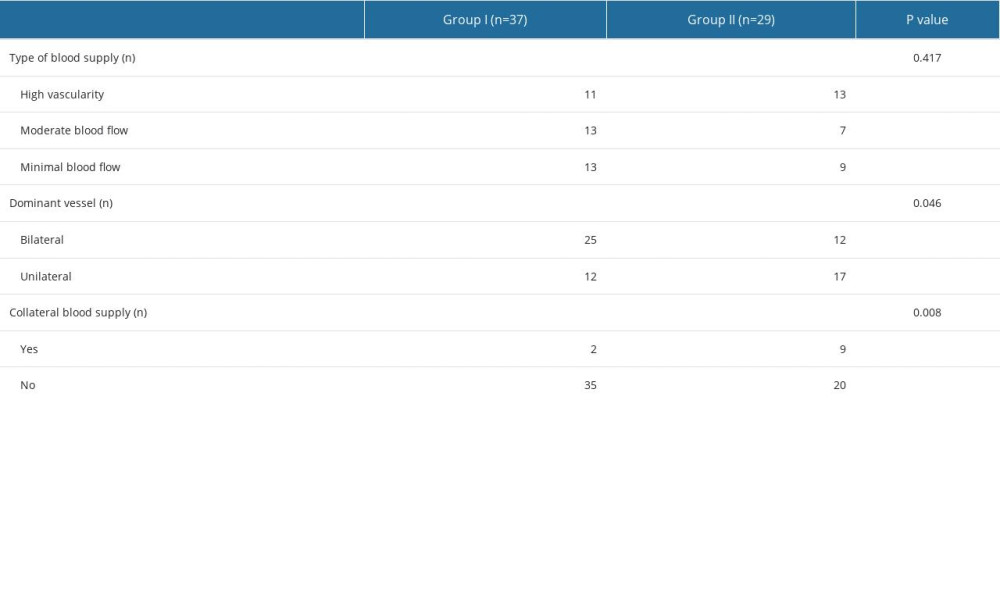 Table 3. Characteristics of collateral blood supply.
Table 3. Characteristics of collateral blood supply.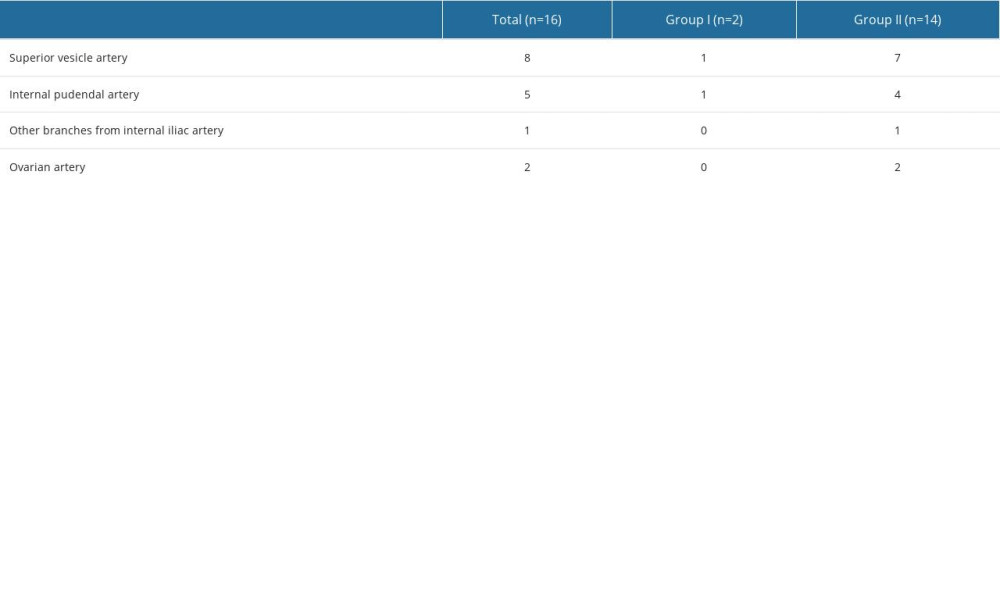 Table 4. Multivariate logistic regression analysis for dominant vessel.
Table 4. Multivariate logistic regression analysis for dominant vessel.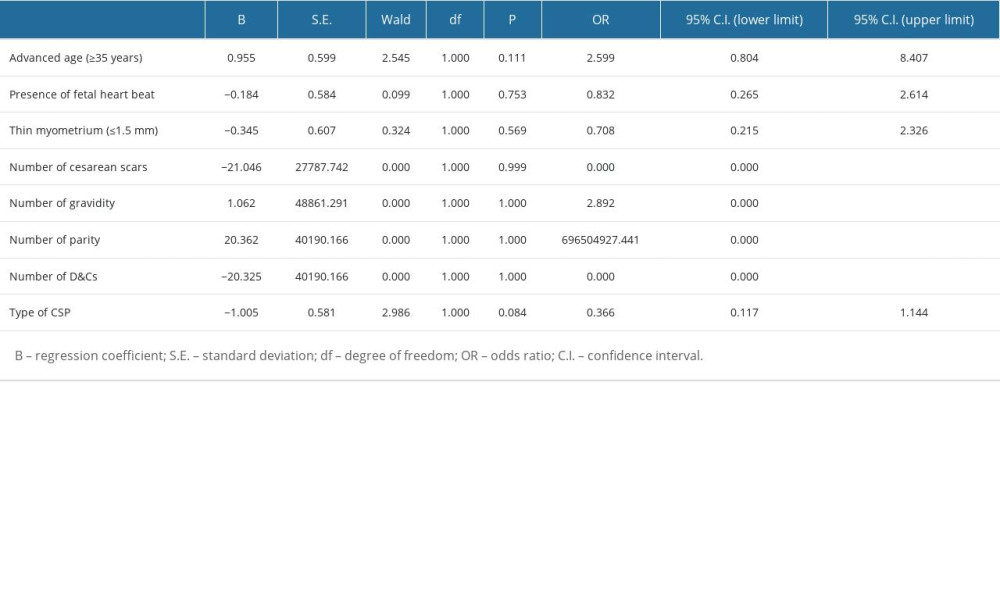 Table 5. Multivariate logistic regression analysis for collateral blood supply.
Table 5. Multivariate logistic regression analysis for collateral blood supply.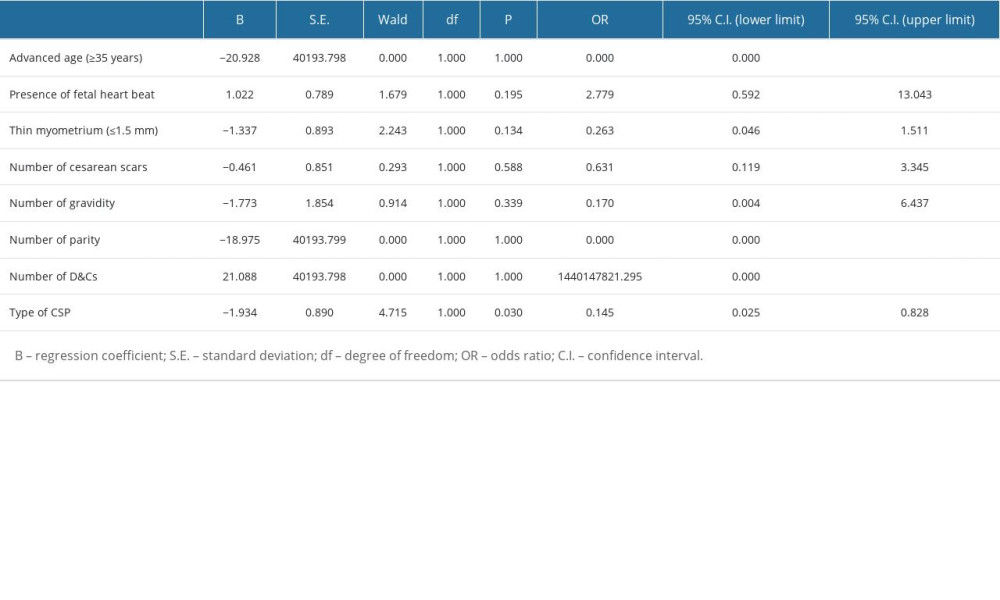 Table 6. Clinical outcomes.
Table 6. Clinical outcomes.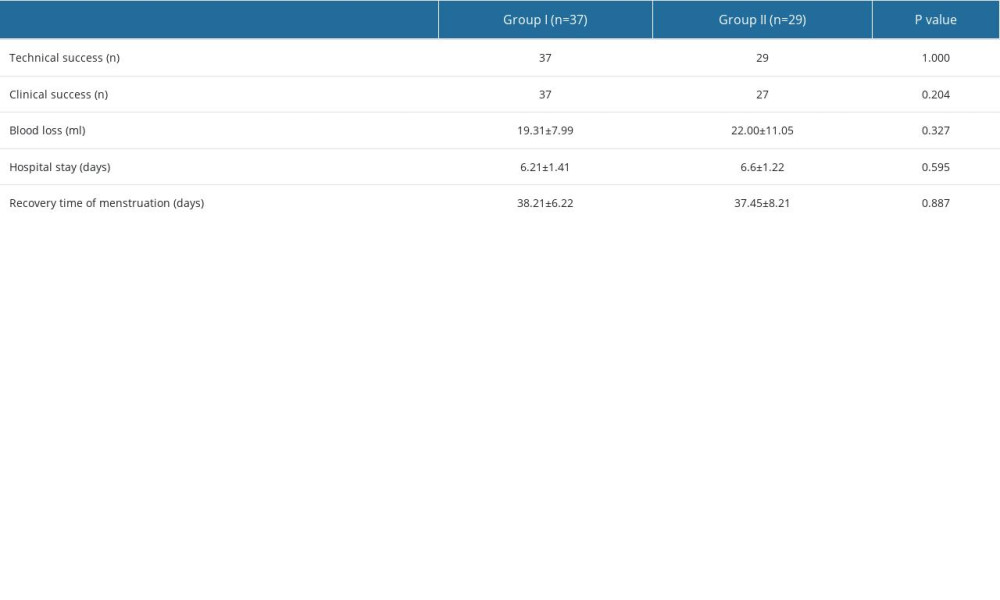
References
1. Jordans IPM, Verberkt C, De Leeuw RA, Definition and sonographic reporting system for Cesarean scar pregnancy in early gestation: modified Delphi method: Ultrasound Obstet Gynecol, 2022; 59; 437-49
2. Jurkovic D, Hillaby K, Woelfer B, First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar: Ultrasound Obstet Gynecol, 2003; 21; 220-27
3. Huang L, Zhao L, Shi H, Clinical efficacy of combined hysteroscopic and laparoscopic surgery and reversible ligation of the uterine artery for excision and repair of uterine scar in patients with type II and III cesarean scar pregnancy: Med Sci Monit, 2020; 26; e924076
4. Timor-Tritsch IE, Monteagudo A, Cali G, Easy sonographic differential diagnosis between intrauterine pregnancy and cesarean delivery scar pregnancy in the early first trimester: Am J Obstet Gynecol, 2016; 215; 225e1-7
5. Society for Maternal-Fetal Medicine (SMFM); Russell Miller, Cynthia Gyamfi-Bannerman; Publications Committe, Society for maternal-fetal medicine consult series #63: Cesarean scar ectopic pregnancy: Am J Obstet Gynecol, 2022; 227; B9-B20
6. Gonzalez N, Tulandi T, Cesarean scar pregnancy: A systematic review: J Minim Invasive Gynecol, 2017; 24; 731-38
7. Cao S, Qiu G, Zhang P, A comparison of transvaginal removal and repair of uterine defect for type II cesarean scar pregnancy and uterine artery embolization combined with curettage: Front Med (Lausanne), 2021; 8; 654956
8. Peng Y, Dai Y, Yu G, Jin P, Analysis of the type of cesarean scar pregnancy impacted on the effectiveness and safety of high intensity focused ultrasound combined with ultrasound-guided suction curettage treatment: Int J Hyperthermia, 2022; 39; 1449-57
9. Lin Y, Xiong C, Dong C, Yu J, Approaches in the treatment of cesarean scar pregnancy and risk factors for intraoperative hemorrhage: A retrospective study: Front Med (Lausanne), 2021; 8; 682368
10. Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, Svarre Nielsen H, Cesarean scar pregnancy: A systematic review of treatment studies: Fertil Steril, 2016; 105; 958-67
11. Liu L, Ross WT, Chu AL, Deimling TA, An updated guide to the diagnosis and management of cesarean scar pregnancies: Curr Opin Obstet Gynecol, 2020; 32; 255-62
12. Shen F, Lv H, Wang L, A comparison of treatment options for type 1 and type 2 caesarean scar pregnancy: A retrospective case series study: Front Med (Lausanne), 2021; 8; 671035
13. Zhang G, Li J, Tang J, Role of collateral embolization in addition to uterine artery embolization followed by hysteroscopic curettage for the management of cesarean scar pregnancy: BMC Pregnancy Childbirth, 2019; 19; 502
14. Qiao B, Zhang Z, Li Y, Uterine artery embolization versus methotrexate for cesarean scar pregnancy in a Chinese population: A meta-analysis: J Minim Invasive Gynecol, 2016; 23; 1040-48
15. An X, Ming X, Li K, Wang J, The analysis of efficacy and failure factors of uterine artery methotrexate infusion and embolization in treatment of cesarean scar pregnancy: ScientificWorldJournal, 2013; 2013; 213603
16. Zhang B, Jiang ZB, Huang MS, Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: Results of a case series and review of the literature: J Vasc Interv Radiol, 2012; 23; 1582-88
17. Tian H, Li S, Jia W, Risk factors for poor hemostasis of prophylactic uterine artery embolization before curettage in cesarean scar pregnancy: J Int Med Res, 2020; 48; 300060520964379
18. Timor-Tritsch IE, Monteagudo A, Bennett TA, A new minimally invasive treatment for cesarean scar pregnancy and cervical pregnancy: Am J Obstet Gynecol, 2016; 215; 351e1-8
19. Timor-Tritsch IE, A Cesarean scar pregnancy is not an ectopic pregnancy: Ultrasound Obstet Gynecol, 2022; 59; 424-27
20. Jurkovic D, Tellum T, Kirk E, Cesarean scar pregnancy IS an ectopic pregnancy: Ultrasound Obstet Gynecol, 2022; 59; 831-32
21. Qi F, Chai ZY, Liu MM, Type 2 cesarean scar pregnancy successfully treated via hysteroscopy-assisted laparoscopy: J Minim Invasive Gynecol, 2019; 26; 1273-81
22. Yang X, Zheng W, Wei X, Management of cesarean scar pregnancy: Importance of gestational age at diagnosis and disease type-A single center’s 5 years of experience involving 223 cases: Front Surg, 2023; 10; 1055245
23. Timor-Tritsch I, Buca D, Di Mascio D, Outcome of cesarean scar pregnancy according to gestational age at diagnosis: A systematic review and meta-analysis: Eur J Obstet Gynecol Reprod Biol, 2021; 258; 53-59
24. Ban Y, Shen J, Wang X, Cesarean scar ectopic pregnancy clinical classification system with recommended surgical strategy: Obstet Gynecol, 2023; 141; 927-36
25. Li C, Li C, Feng D, Transcatheter arterial chemoembolization versus systemic methotrexate for the management of cesarean scar pregnancy: Int J Gynaecol Obstet, 2011; 113; 178-82
26. Kaelin Agten A, Cali G, Monteagudo A, The clinical outcome of cesarean scar pregnancies implanted “on the scar” versus “in the niche”: Am J Obstet Gynecol, 2017; 216; 510e1-e6
27. Leleup G, Fohlen A, Dohan A, Value of round ligament artery embolization in the management of postpartum hemorrhage: J Vasc Interv Radiol, 2017; 28; 696-701
28. Tokue H, Tokue A, Tsushima Y, Kameda T, Risk factors for massive bleeding based on angiographic findings in patients with placenta previa and accreta who underwent balloon occlusion of the internal iliac artery during cesarean section: Br J Radiol, 2019; 92; 20190127
29. Pelage JP, Le Dref O, Soyer P, Arterial anatomy of the female genital tract: Variations and relevance to transcatheter embolization of the uterus: Am J Roentgenol, 1999; 172; 989-94
30. Zhang W, Li G, Hua CFEffect factors of collateral blood supply of patients with early trimester cesarean scar pregnancy: Zhonghua Yi Xue Za Zhi, 2022; 102; 130-35 [in Chinese]
31. Allerkamp HH, Clark AR, Lee TC, Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation: Hum Reprod, 2021; 36; 571-86
32. Zhou M, Chen T, Li Y, Modified sandwich embolization technique for postpartum hemorrhage caused by uterine artery pseudoaneurysm: A case series: Arch Gynecol Obstet, 2020; 302; 1469-77
33. Porcu G, Roger V, Jacquier A, Uterus and bladder necrosis after uterine artery embolisation for postpartum haemorrhage: BJOG, 2005; 112; 122-23
34. Camacho A, Ahn EH, Appel E, Uterine artery embolization with gelfoam for acquired symptomatic uterine arteriovenous shunting: J Vasc Interv Radiol, 2019; 30; 1750-58
35. Deux JF, Bazot M, Le Blanche AF, Is selective embolization of uterine arteries a safe alternative to hysterectomy in patients with postpartum hemorrhage?: Am J Roentgenol, 2001; 177; 145-49
36. Timor-Tritsch IE, Monteagudo A, Cali G, Cesarean scar pregnancy and early placenta accreta share common histology: Ultrasound Obstet Gynecol, 2014; 43; 383-95
37. Jauniaux E, Zosmer N, De Braud LV, Development of the utero-placental circulation in cesarean scar pregnancies: A case-control study: Am J Obstet Gynecol, 2022; 226; 399e1-e10
Figures
 Figure 1. Flowchart of patient selection. The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 1. Flowchart of patient selection. The figure was created with PowerPoint software (Microsoft 365 Office). Figure 2. (A) Digital subtraction angiography imaging showing sparse gestational sac staining (black arrow); (B) general gestational sac staining (black arrow); (C) obvious gestational sac staining (black arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 2. (A) Digital subtraction angiography imaging showing sparse gestational sac staining (black arrow); (B) general gestational sac staining (black arrow); (C) obvious gestational sac staining (black arrow). The figure was created with PowerPoint software (Microsoft 365 Office). Figure 3. (A) Digital subtraction angiography imaging showing bilateral uterine artery supply to the gestational sac (black arrow); (B) right uterine artery supply to the gestational sac (black arrow), with no gestational sac blood flow from the left uterine artery (white arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 3. (A) Digital subtraction angiography imaging showing bilateral uterine artery supply to the gestational sac (black arrow); (B) right uterine artery supply to the gestational sac (black arrow), with no gestational sac blood flow from the left uterine artery (white arrow). The figure was created with PowerPoint software (Microsoft 365 Office). Figure 4. (A) Digital subtraction angiography imaging showing uterine artery (star) and multiple collateral blood supplies to the gestational sac; (B) superior vesicle arteries (white short arrow); (B) internal pudendal artery (black long arrow); (C) ovarian artery (white long arrow); and (D) other branches from the internal iliac artery (black short arrow). The figure was created with PowerPoint software (Microsoft 365 Office).
Figure 4. (A) Digital subtraction angiography imaging showing uterine artery (star) and multiple collateral blood supplies to the gestational sac; (B) superior vesicle arteries (white short arrow); (B) internal pudendal artery (black long arrow); (C) ovarian artery (white long arrow); and (D) other branches from the internal iliac artery (black short arrow). The figure was created with PowerPoint software (Microsoft 365 Office). Tables
 Table 1. Baseline characteristics.
Table 1. Baseline characteristics. Table 2. Characteristics of the blood supply.
Table 2. Characteristics of the blood supply. Table 3. Characteristics of collateral blood supply.
Table 3. Characteristics of collateral blood supply. Table 4. Multivariate logistic regression analysis for dominant vessel.
Table 4. Multivariate logistic regression analysis for dominant vessel. Table 5. Multivariate logistic regression analysis for collateral blood supply.
Table 5. Multivariate logistic regression analysis for collateral blood supply. Table 6. Clinical outcomes.
Table 6. Clinical outcomes. Table 1. Baseline characteristics.
Table 1. Baseline characteristics. Table 2. Characteristics of the blood supply.
Table 2. Characteristics of the blood supply. Table 3. Characteristics of collateral blood supply.
Table 3. Characteristics of collateral blood supply. Table 4. Multivariate logistic regression analysis for dominant vessel.
Table 4. Multivariate logistic regression analysis for dominant vessel. Table 5. Multivariate logistic regression analysis for collateral blood supply.
Table 5. Multivariate logistic regression analysis for collateral blood supply. Table 6. Clinical outcomes.
Table 6. Clinical outcomes. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








