02 July 2023: Review Articles
Advancements in Zebrafish Models for Breast Cancer Research: Unveiling Biomarkers, Targeted Therapies, and Personalized Medicine
Anna WawruszakDOI: 10.12659/MSM.940550
Med Sci Monit 2023; 29:e940550
Abstract
ABSTRACT: Breast cancer (BC) is the most frequently diagnosed malignancy in women worldwide. Despite the wide variety of therapeutic methods for BC, their results are not satisfying, especially in triple-negative breast cancer (TNBC) patients. One of the main challenges in efficient oncology is achieving optimal conditions to evaluate a molecular genotype and phenotype of a tumor. Therefore, new therapeutic strategies are urgently needed. Animal models are an important tool for the molecular and functional characterization of BC, and for the development of targeted BC therapies. Zebrafish, as a promising screening model organism, has been widely applied in the development of patient-derived xenografts (PDX) for the discovery of novel potential antineoplastic drugs. Moreover, the generation of BC xenografts in zebrafish embryos/larvae allows for a description of the tumor growth, cell invasion, and systemic interaction between tumor and host in vivo without immunogenic rejection of transplanted cancer cells. Interestingly, zebrafish can be genetically manipulated and their genome has been fully sequenced. Genetic studies in zebrafish have described new genes and molecular pathways involved in BC carcinogenesis. Thus, the zebrafish in vivo model is becoming an exquisite alternative for metastatic research and for discovering new active agents for BC therapy. Herein, we systematically reviewed the recent cutting-edge advances in zebrafish BC models for carcinogenesis, metastasis, and drug screening. This article aims to review the current status of the role of the zebrafish (Danio reiro) in preclinical and clinical models of biomarker identification and drug targeting, and developments in personalized medicine in BC.
Keywords: Breast Neoplasms, Drug Screening Assays, Antitumor, Neoplasm Metastasis, Zebrafish Proteins, Female, Humans, Animals, Precision Medicine, Zebrafish, Triple Negative Breast Neoplasms, biomarkers, carcinogenesis
Background
Breast cancer (BC) is a heterogeneous disease with different molecular subtypes [1]. Approximately 70% of breast tumors exhibit estrogen receptor (ER) expression; therefore, endocrine therapy is widely used for these patients. However, there is a high risk of recurrence in this therapy regimen [1]. Triple-negative breast cancer (TNBC) is the most aggressive subtype of BC, with associated chemoresistance. Due to the very heterogeneous character of TNBC, response to the existing therapeutic options is very limited. Therefore, effective personalized therapies with relatively low adverse effects are urgently required [2].
The use of animals in preclinical studies has been crucial in biomedical science development for decades [3]. In vivo models enable a better understanding of the pathological mechanisms driving disease and testing new therapy approaches (eg, drugs or their combinations, vaccination, irradiation) [4]. Zebrafish (
The functional and structural homology of around 76–82% of genes involved in human diseases is shared between humans and zebrafish (ZF) [12]. Compared to mice or rats, fish brings numerous advantages like fast development, a large number of offspring, low costs, and the possibility to carry out the experiment on a large scale (Figure 1) [5].
Numerous transgenic lines of ZF are commonly available, which significantly contributes to easier experimentation [4]. Among them, there are lines with labeled organism components (macrophages [13], neutrophils [14], or vascular system [11]), with immune system deficiency [15,16], or without pigmentation [17]. Especially the last species, called the Casper line, is a very powerful model in cancer research as its transparency combined with labeled cancer cells or proteins enables observation of tumor development, cell invasion, metastasis, or drug response [5] (Figure 2).
Research studies describe almost every type of cancer that has been already tested in the ZF model, including BC [18], gastric cancer [19], colorectal cancer [20], non-small cell lung adenocarcinoma [21], pancreatic ductal adenocarcinoma [22], melanoma [23], glioblastoma [24], and others [3,5].
This article aims to review the current status of the role of the ZF (
Zebrafish Breast Cancer In Vivo Models
There are 2 directions to achieve a pathological condition in animal models: by introducing gene(s) to induce the disease (oncogenes) or to inhibit expression of targeted genes using genetic engineering methods (Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) or morpholino for knockdown), or by tumor transplantation (xenografts) [5]. In the case of BC, creating an environment similar to the patient using genetic engineering is difficult because of the lack of
Missing the mammary organs where BC develops is compensated for by xenotransplantation. Xenografts can be created from cell lines (CDX - cell lines-derived xenografts) or directly from surgery or biopsy of the patient (PDX - patient-derived xenografts). Numerous different BC cell lines have been successfully transplanted into ZF, eg, MCF-7 [28], MDA-MB-231 [16,29], MDA-MB-435 [30], MDA-MB-468 [31], and BT-474 [30]. Generally, 2 days after fertilization (dpf), fish are injected with ~800–1500 cells suspension. After 1 day, the tumoral mass is formed [32].
Some studies used three-dimensional (3D) spheroids to better mimic the initial stages of tumor angiogenesis and metastasis [33]. MCF7 and mammary adipose tissue-derived mesenchymal stromal/stem cells (MAT-MSCs) co-cultures created the spheroids that were transplanted into ZF. Injection of co-cultures showed higher tumor invasiveness compared to MCF7 monoculture. Another study presented a complex model based on the co-culture of MCF-7 or MDA-MB-231 human mammary tumor cells mixed with human preadipocytes (hMADS, human multipotent adipose-derived stem cells) to generate organoids [28].
For a better understanding of the molecular mechanisms in cancer development, mutated cell lines were transplanted. BC cells with silenced
Breast Cancer Patient-Derived Xenografts
Cell lines are easily available and cheap. However, for medical reasons, using CDX is connected with some limitations. Multiple passaging leads to clonal selection and loss of tumor heterogeneity [39]. Differences in gene expression can be noticed even among the same cell line. An analysis of a commonly used BC cell line (MCF-7) indicated a parental cell line and its 3 subclones showed significant differences at the genomic and gene expression levels [40]. That might cause a different response for drug treatment of different strains among the same cell line. That effect was described in testes of 27 MCF7 strains when some of them indicated a drug response and some were non-responsive [41]. Moreover, some studies have found that ZF xenografts have poor predictive value in response to therapeutics. Tests of 39 compounds applied in both CDX models and phase II clinical trials at the National Cancer Institute’s Developmental Therapeutics Program did not indicate any close correlation [42]. A comprehensive comparison of molecular profiles in copy number variation (CNV), mutation, mRNA, and protein expression between 68 cell lines and 1375 primary breast tumors from The Cancer Genome Atlas (TCGA) showed that cell lines can mirror some molecular properties but not all of them. Among tested lines, T47D, BT483, and MDA-MB-453 showed the highest similarity with tumors [43].
Using cell lines in vitro or in vivo generally does not mimic the patient-specific environment and leads to differences in progression and treatment outcome. To get the most reliable results, using cells derived from patients is necessary. Patient-derived xenografts (PDXs), also called cancer “Avatars” [4], are generated by the implantation of human primary tumor cells or tissues, obtained from surgery or biopsy, into a host animal. For the first time, ZF was used as a PDX for leukemia [44]. Nowadays, PDXs are considered a very important tool in cancer therapy. Thanks to that, the most efficient treatment can be applied for an individual patient, which promotes the development of personalized medicine. For BC, mice have been described as the most effective PDX model [39,45–47]. Although much research has been done on BC CDX using ZF, the number of zPDX (zebrafish PDX) is still, surprisingly, limited [16,48,49].
Studies show that ZF is a good model to test patient breast tumors and is also a promising alternative to mice. The effective implantation success rate for zPDX can be very high (95%). Moreover, transplanted cancer cells were spread in ZF and correlated with clinical data on advancing tumor stage from stage 1 to stage 3 disease. Importantly, the response to drug treatment (docetaxel and trastuzumab) observed in fish was the same in 14 of 18 cases as in the mouse PDX models, clearly showing that the ZF model can be used successfully, like the mouse model [49]. Similar results were obtained by Yan et al in 2018 when they compared rates of proliferation and apoptosis of MDA-MB-231 TNBC cells between fish and mice [17]. Another study showed that ZF might be a promising model in preclinical BC therapy to identify prognostic markers and predict response to treatment. The behavior of zPDX with bone metastasis derived from BC patients has been compared to established BC cell lines. Primary cells from patients were extravasated from vessels and invaded the CHT (caudal hematopoietic tissues) of ZF. It was found that the patient’s primary cells can spread out, throwing blood vessels and invading the hematopoietic tissue of the ZF tail [50].
Troubleshooting In Vivo Models
One of the biggest problems, generally, with animal models is that their immune systems can cause rejection of transplanted cells; therefore, intensive studies have been conducted to develop a mutant with deficient immunity. A variety of immunocompromised ZF strains have been created using
Epithelial-Mesenchymal Transition (EMT) in Tumor Metastasis
Metastasis is a complex and multi-stage process consisting of the spreading of cancer cells, infiltration, and extravasation, and finally colonization of metastatic cells in distant organs, which in turn leads to secondary lesions [54,55]. Epithelial-mesenchymal transition (EMT) is a major phenomenon that contributes to cancer metastasis and drug resistance [56]. During EMT, epithelial cells lose intercellular connections and gain the ability to migrate, becoming highly invasive mesenchymal cells. EMT is characterized by a loss of cellular cohesion mediated by the downregulation of adhesion molecules such as E-cadherin, β-catenin, occludin, and claudin-1. E-cadherin expression is regulated by numerous transcriptional factors, such as
Modeling Breast Cancer Metastasis with Zebrafish
Efforts to find therapeutic agents that target cancer metastases have often failed in clinical trials. Clinical failures are frequently due to the lack of modern, available, and easy-to-maintain preclinical models for metastasis research [55].
The transparent embryos make the ZF an ideal in vivo model to visualize migration of tumor cells [58]. Although ZF have an exceptionally strong innate immune system, they do not develop a mature immune system in the first 2 weeks. Therefore, the use of ZF in cancer research has recently expanded with the establishment of human cancer cell xenograft models [59]. Another advantage of using ZF xenografts in metastasis research is the fact that implanted cancer cells can be easily labeled with fluorescent dyes, making it possible to quickly identify neoplastic vs normal cells. As a result, studies on dynamic microtumor formation, cell invasion, and angiogenesis can be easily visualized in the body of a live fish [59].
A primary culture originating from a female patient with bone metastases from BC was cultured and then injected into ZF embryos to test its metastatic potential [50]. In the first step, the ZF model was validated by examining the colonization of 2 BC cell lines into 2-dpf ZF embryos. Experiments were performed on MDA-MB-231, a TNBC cell line, and MCF7, a hormone receptor-positive BC cell line. Interestingly, the MCF7 cells did not survive in the ZF embryo. In contrast, MDA-MB-231 cells spread and colonized various parts of the ZF, including tail hematopoietic tissue (CHT), indicating a strong migratory phenotype. Finally, the cells with the same hormonal receptor status as MCF7 cells were taken from the patient’s bone lesion and implanted in ZF [50]. The cells demonstrated an aggressive phenotype as they were extravasated from the vessels occupying various parts of the embryos [50].
In another study, ZF embryos were used to identify invasive subpopulations of MDA-MB-231 BC cells [55]. A subpopulation consistent with metastatic cells circulating in the human body was first created described by injecting MDA-MB-231 cells into the yolk sac 2 days after embryo fertilization and then collecting the cells that migrated to the tail [55]. Analysis of differential gene expression in an invasive subpopulation with the parental total population showed activation of EMT in circulating cells. It has been demonstrated that knockdown of the
Importance of TGF-β in the Process of Metastasis
Transforming growth factor beta (TGF-β) plays an important role in controlling the development of BC [60]. It has been demonstrated that ZF xenografts are a good in vivo model for the pharmacological modulators and genetic perturbation of TGF-β signaling studies in human BC cells [60]. ZF embryo xenograft assays were described to investigate the mode of TGF-β signaling and its role in extravasation of BC cells, as well as regulation of angiogenesis. Tumor cells were fluorescently labeled with mCherry and then injected into a ZF embryo into Cuvier’s duct or perivitelline space. The invasive and migrating human cancer cells and the induction of new blood vessel formation were observed [60]. A ZF xenograft was generated by injecting MDA-MB-231 cells into the circulation of the embryo, and their migration to the avascular collagenous tail was observed. It was noted that BC cells dispersed very quickly and formed micrometastases in just 6 days. Blocking the TGF-β pathway through inhibition of TGF-β receptor kinase or knockdown of
Role of PTEN in Invasion and Migration of Cancer Cells
Another analysis that was done in the BC metastasis context in the ZF model concerned the insufficiency of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) protein, as a common marker in patients with malignant BC [62]. It was shown that PTEN deficiency induced an increase in MCF-7 cell invasion and migration by EMT induction. In addition, the knockdown of
Role of CXCR4-CXCL12 Chemokine Signaling Axis in Cancer Cell Migration
The CXCR4-CXCL12 chemokine signaling axis is a driver of cell migration in physiological and pathological conditions, including BC metastasis. Although CXCR4-CXCL12 axis-targeted therapies are in clinical trials, there still are no effective therapeutic approaches established for blocking CXCR4 in TNBC [36]. The CXCR4-CXCL12 axis acts across both ZF and human organisms, and drives the formation of tumor micrometastases of TNBC BC cells in zebrafish. IT1t is a small-molecule potent CXCR4 antagonist and orthosteric competitor of the CXCL12 N-terminal signaling peptide. IT1t impairs signaling activation by interfering with the docking of the ligand domain to the receptor. Treatment of MDA-MB-231 TNBC cells with IT1t as well as genetic silencing of CXCR4 significantly inhibited early metastasis xenotransplantation ZF model in vivo [36]
Metastatic Capacity of Circulating Tumor Cells
The metastatic competency of BC CTCs (circulating tumor cells) and CTC clusters in ZF embryos were analyzed [63]. CTCs are cancer cells that have sloughed off of the primary tumor and circulated in the blood [64]. Interestingly, CTC clusters derived from MDA-MB-231 and MCF7 cells exhibited more diverse behavior than single CTCs in in vitro metastasis analyses. In this study, CTC clusters were shown to have an advantage in terms of survival and proliferation over single CTCs, better adapting them to survival in circulation. However, the CTC clusters in the ZF experiments had less ability to invade and spread. Interestingly, the grouping of even a few cells increased their survival. Comparable results were obtained in a mouse model [63].
Study of Effect of the Microenvironment on Breast Cancer Cells
The newest research concerns environmental pollution, specifically persistent organic pollutants (POP), which, according to previous results, contributes to development of BC. Recent clinical studies have shown that increased POP concentration is associated with an increased risk of BC progression and metastases [28]. To study the effect of the microenvironment on BC cells, a co-culture model containing MCF-7 or MDA-MB-231 BC cells and hMADS preadipocytes as well as TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxins) was used. Using the ZF larvae model, BC cells were shown to become metastatic when cultured with pre-adipocytes and exposed to a persistent organic pollutant, TCDD [28].
Zebrafish as a Preclinical Model for Drug Screening
Over the last decade, ZF have played a significant role in human diseases modeling and in finding novel therapeutic options for them [65,66]. For this purpose, ZF have been widely used in the creation of PDX cancer models to identify patient-specific responses, and in phenotypic drug screening of different antineoplastic agents [67,68]. Numerous therapeutic approaches that recently entered into clinical trials and clinics have their genesis in ZF [69]. Interestingly, ZF respond to different drugs and active agents at physiologically relevant dose ranges. In addition, drug activity can be studied at a single-cell resolution within the complexity of a whole animal after combination with cell- or tissue-specific reporters and gene editing technologies [69]. The unique opportunity to use ZF for drug screening is possible thanks to the many advantages of this model, like a fully sequenced genome, rapid embryonic development, short generation interval, transparent embryos (which make them amenable to microscopy-based screens usually restricted to cell cultures), low maintenance, and the ability to use large numbers due to cost-effectiveness (Figure 2) [68,70]. These advantages have recently helped to popularize ZF as a great model system for pharmaceutical and biomedical studies, including BC drug screening (Figure 3).
Monoclonal Antibodies Studies in Zebrafish Model
Olaparib is a poly ADP ribose polymerase (PARP) inhibitor used in the therapy of metastatic HER2-negative BC with an inherited
Bevacizumab is a recombinant humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF). The overall average response for bevacizumab as metastatic BC monotherapy is not satisfactory at only 9.3%. Additionally, bevacizumab treatment is linked with serious adverse effects such as renal toxicity, bleeding, and cardiovascular complications [71]. Depending on the individual tumor character, anti-VEGF therapy can either block or stimulate metastasis. Therefore, an assay for the prediction of individual response to therapy would have great value to guide treatment options. Zebrafish BC xenografts were used for determining the response to bevacizumab and its impact on angiogenesis and micrometastasis. Hs578T primary breast carcinoma and MDA-MB-468 pleural effusion metastasis-originated BC cells were injected into the perivitelline space of ZF larvae 2 days after fertilization and analyzed at 4 days after injection for testing the antiangiogenic and metastatic impact of bevacizumab in ZF xenografts. Cell death analysis demonstrated a significant induction of apoptosis in Hs578T TNBC xenografts accompanied by a 40% reduction in tumor size. Additionally, the Hs578T TNBC tumors that exhibited the highest angiogenic potential were also the most affected by bevacizumab treatment, with 20% reduction in vessel density and vessel infiltration (Table 1) [71].
Synthetic Drugs Studies in Zebrafish Model
The ZF model has also been used extensively in the study of synthetic drugs. Actein is a cycloartane triterpenoid that exhibits anti-cancer and anti-angiogenic activities [72]. A metastatic human BC cell xenograft model was established in transparent ZF embryos to determine the anti-migration effect of actein in vivo. Injection of MDA-MB-231 cells into the yolk sac was applied to test the activity of acetin. After quantification, obtained results demonstrated that the number of migratory cells in embryos treated with 60 μM of acetin significantly decreased (75%), confirming the anti-proliferation and anti-migration activity of actein in the ZF xenograft model. The survival rate of ZF embryos was not affected by actein, suggesting that actein at tested doses did not induce observable toxicity to the ZF embryos. The findings reveal that actein can be a potential anti-metastatic candidate drug for the treatment of advanced human BC (Table 1) [72].
1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine (edelfosine, ET) is an alkyl-lysophospholipid tested in phase II clinical trials in patients with inoperable brain tumors (or previously treated with other therapies with no positive results) [73]. Nanometric emulsion containing edelfosine (ET-NE) was prepared for the TNBC treatment to determine the biodistribution, toxicity, and anti-cancer efficacy in ZF embryos. In vivo results demonstrated that the ET-NE nanosystems can penetrate through the skin biological barrier into the yolk sac and significantly decrease highly aggressive and invasive tumoral cells’ proliferation in ZF embryos bearing MDA-MB-231 xenograft tumor, leading to its regression. Overall, the obtained results indicate that the developed ET-NEs formulation can be a promising therapy for treatment of TNBC (Table 1) [73].
Another drug, 5k, new N-2(5H)-furanonyl sulfonyl hydrazone derivative 2(5H)-furanone derivative significantly inhibited the proliferation of MCF7 BC cells and angiogenesis in the MCF7 ZF xenograft model as reflected in decreased tumor area and volume, and the number and length of ectopic vessels were reduced in a dose-dependent pattern. These results suggest that N-2(5H)-furanonyl sulfonyl hydrazine derivatives can be potent drugs for BC treatment (Table 1) [74].
The anti-cancer potential of [Ru(bipy)2(C12H8N6-N,N)][CF3SO3]2 Ru1 and [{Ru(bipy)2}2(μ-C12H8N6-N,N)][CF3SO3]4 Ru2 ruthenium complexes was studied in an MCF7 ZF xenograft model [75]. Both newly developed active compounds effectively prevented MCF7 proliferation and inhibited tumor growth compared to control in MCF7 BC cell ZF xenografts, with no significant signs of lethality observed. Therefore, both compounds show potential for further in vivo mouse studies, as well as for their use as scaffolds for further chemical modifications to improve their drug-like properties (Table 1) [75].
Natural Products Studies in Zebrafish Model
Natural products have been extensively studied in the last decade [76]. Fangjihuangqi decoction is a classic prescription in Chinese medicine that has been clinically used in therapy of fatigue, upper-extremity edema, and enhancement of immunity to prevent cancer recurrence in postoperative BC patients [76]. Fangjihuangqi decoction significantly inhibits tumor growth and reduces the number of cancer cells in the abdominal cavity and improves the abnormal morphology of tumor cells in the TNBC xenograft ZF model. To establish a human TNBC xenograft tumor in a ZF model, TGFβ1-treated MDA-MB-231 cells were microinjected into a 2dpf ZF yolk sac. TGFβ1-treated MDA-MB-231 cells presented a strong tumor formation ability. Fangjihuangqi decoction reduced the length of tumor neoplastic lymphatics and restrained the sprouts’ number of tumor neovascularization via the reverse EMT process. Western blotting analysis demonstrated that Fangjihuangqi decoction significantly increased E-cadherin and decreased Snail2, TWIST1, zinc finger E-box binding homeobox 2 (ZEB2), and TGFβ1 protein expression. All these results using the ZF model suggest that the Fangjihuangqi decoction could prevent TNBC metastasis through significant inhibition of tumor lymphangiogenesis and neovascularization (Table 1) [76].
Accumulating evidence suggests that breast cancer stem cells (CSCs) are related to tumor recurrence and drug resistance. Therefore, targeting CSCs can provide a useful approach to BC therapy [77]. It has been revealed that Chinese medicines with their natural active compounds can be involved in stem cell properties regulation. Gomisin M2 is a natural product isolated from the Schisandra viridis A. C. Smith herb that is used as an anti-cancer agent. Gomisin M2 reduced MDA-MB-231 and HCC1806 TNBC progenitor cell growth and proliferation in ZF embryos. Interestingly and unexpectedly, gomisin M2 blocked the CSCs growth in vivo in approximately 2-fold lower concentrations than these suppressing formation of tumor spheres, suggesting that gomisin M2 effectively targets cancer cells in the in vivo tumor microenvironment with lower doses than in vitro. Additionally, HCC1806 SCs underwent tail migration, although any fluorescent particles had migrated to the tail after gomisin M2 treatment. These findings suggest that gomisin M2 may be potentially used as a therapeutic active agent in targeting breast CSCs (Table 1) [77].
Bakuchiol is a meroterpene found in the Psoralea corylifolia L. dried ripe fruit and widely used in traditional Chinese herbal medicine [78]. The metastatic MCF7 breast CSCs were injected into 48-hpf (hours post-fertilization) ZF embryos and were exposed to 0.5 and 1 μg/ml of bakuchiol. Bakuchiol induced apoptosis and inhibited BCSCs metastasis in ZF embryos. The percentage of embryos without metastasis increased and the number of fluorescent particles decreased dose-dependently after bakuchiol treatment. Interestingly, the doses used for in vivo metastasis experiments were lower than for in vitro studies. These findings suggest that bakuchiol has great potential as anti-breast cancer therapy; however, further experiments are needed (Table 1) [78].
Furanodiene, a natural terpenoid isolated from Rhizoma Curcumae herb, demonstrates antineoplastic activity against different types of cancer cells [79]. ZF xenograft models with MCF7 and MDA-MB-231 human cancer cell lines were established and validated with anti-cancer agents used for treatment of cancer patients in clinics. It has been demonstrated that furanodiene is therapeutically effective for MCF7 BC cells xenotransplanted into ZF. Moreover, furanodiene showed a strong synergistic anti-cancer effect in combination with 5-fluorouracil (5-FU) in an MDA-MB-231 transplanted model. Additionally, ZF injected with drug-resistance cancer cells were used to determine the anti-multidrug resistance effects of furanodiene. Furanodiene reversed multiple drug resistance (MDR) in the ZF xenotransplanted with adriamycin-resistant MDA-MB-231 cells. The caspases 8, 9, and 3/7 activity were notably increased in the BC cell xenotransplanted ZF treated with furanodiene, suggesting that furanodiene-induced ZF apoptosis are caspase 8- and caspase 9- dependent. Additionally, furanodiene demonstrated its anti-neoplasm effects via inhibition of angiogenesis and induction of reactive oxygen species (ROS) production and DNA strand breaks. Furanodiene caused suppression of efflux transporter P-glycoprotein Pgb function and reduction of Pgp protein level, but not MDR1-Pgp-related gene expression [79]. All these results suggest that furanodiene is a potential sensitizing agent to chemotherapeutic drugs, probably mainly through blocking Pgp efflux transportation; however, further cellular, biochemical, and molecular mechanisms studies are needed to confirm furanodiene pharmacology in mammalian models (Table 1).
Jadomycin B is a secondary metabolite biosynthesized by the soil bacterium Streptomyces venezuelae ISP5230 under stress conditions [80]. It has been demonstrated that jadomycin B reduces MDA-MB-231 cell proliferation in a dose-dependent fashion 48 h after transplantation into the yolk sacks of ZF larvae; 60 μmol/l and 80 μmol/l of jadomycin B significantly decreased ZF larval viability after 120 h and 96 h, respectively. The MTD (the highest dose of a drug that does not cause unacceptable adverse effects) of jadomycin B was determined as 40 μmol/l in ZF larvae, which is approximately 10–20-fold higher than its IC50 in in vitro BC cell cytotoxicity experiments (Table 1) [80]
In summary, all these findings suggest that ZF has become an important vertebrate model for assessing BC drug effects.
Future Directions
The advantage of using ZF in BC therapy is its potential to provide a platform for drug discovery and testing. Researchers can use ZF to screen large numbers of compounds and identify those that are effective in treating BC. This can help to accelerate the drug discovery process and bring new therapies to patients. It is commonly known that the perfect therapy plan should be highly individualized, especially in the treatment of BC, where a huge heterogeneity of the tumor is observed. Using low-cost and ease-of-maintenance animal models has recently become a hot topic in medicine and genetics. Here, we summarized approaches like ZF genome editing, creating conditional immune compromised models, and selecting the best therapy that together have a significant impact on development of personalized medicine. There are still open questions, including the viability of immunodeficiency models or tumor rejection, but we believe that intensive research will solve these issues very soon. Overall, the use of ZF in BC research and therapy has tremendous potential to advance our understanding of this disease and to develop new, more effective treatments. Although ZF PDX still needs to be validated, we believe that this model gives information that is highly translatable to humans.
Conclusions
Cancer studies have rapidly moved away from autonomous tumor cells to a complex systemic approach. The systemic response plays an important role in tumor cell behavior, as well as affecting therapy and patient outcomes. Recently, ZF have been increasingly applied as a good in vivo model for human disease research with a particular focus on cancers. ZF BC models are currently widely used in carcinogenesis studies, monitoring of disease progression, and testing new standard or alternative therapies [5]. Here, we presented a rationale for using ZF as powerful in vivo models for BC mechanisms studies and testing new potential BC therapy agents. This review has presented the current status of the role of the ZF (
Figures
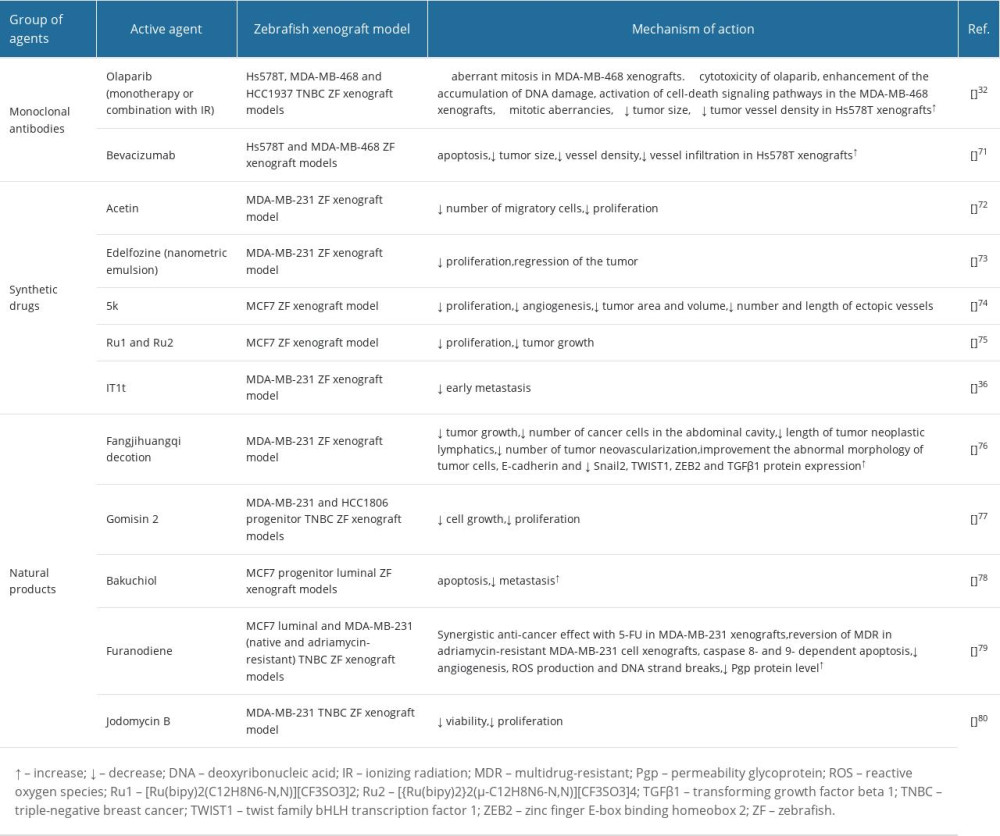
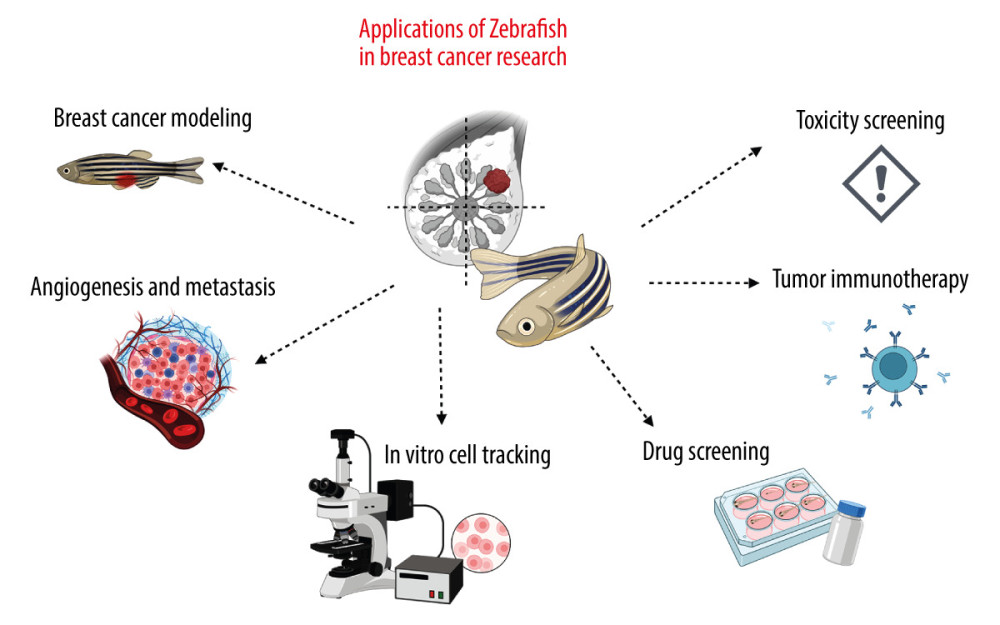 Figure 1. Examples of applications of the zebrafish (ZF) (Danio reiro) model in the field of breast cancer (BC) research include BC modeling, angiogenesis and metastasis studies, in vivo cell tracking, drug and toxicity screening, and tumor immunotherapy. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 1. Examples of applications of the zebrafish (ZF) (Danio reiro) model in the field of breast cancer (BC) research include BC modeling, angiogenesis and metastasis studies, in vivo cell tracking, drug and toxicity screening, and tumor immunotherapy. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). 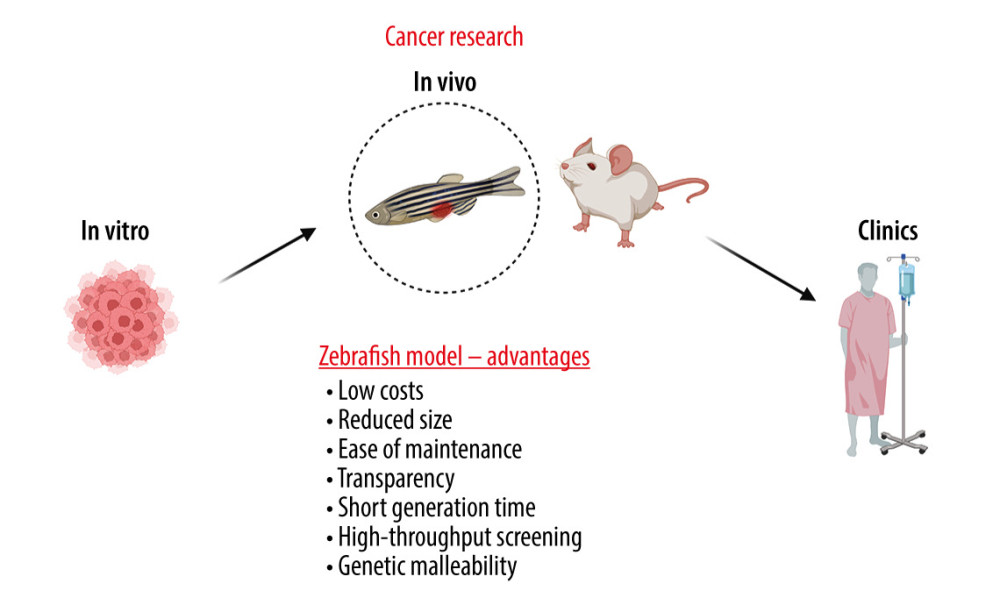 Figure 2. The advantages of zebrafish (ZF) (Danio reiro) as a vertebrate preclinical animal model include low cost and ease of maintenance, short generation time, small size, intensive external development, transparent embryos, and convenient genetic manipulations and analysis. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 2. The advantages of zebrafish (ZF) (Danio reiro) as a vertebrate preclinical animal model include low cost and ease of maintenance, short generation time, small size, intensive external development, transparent embryos, and convenient genetic manipulations and analysis. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). 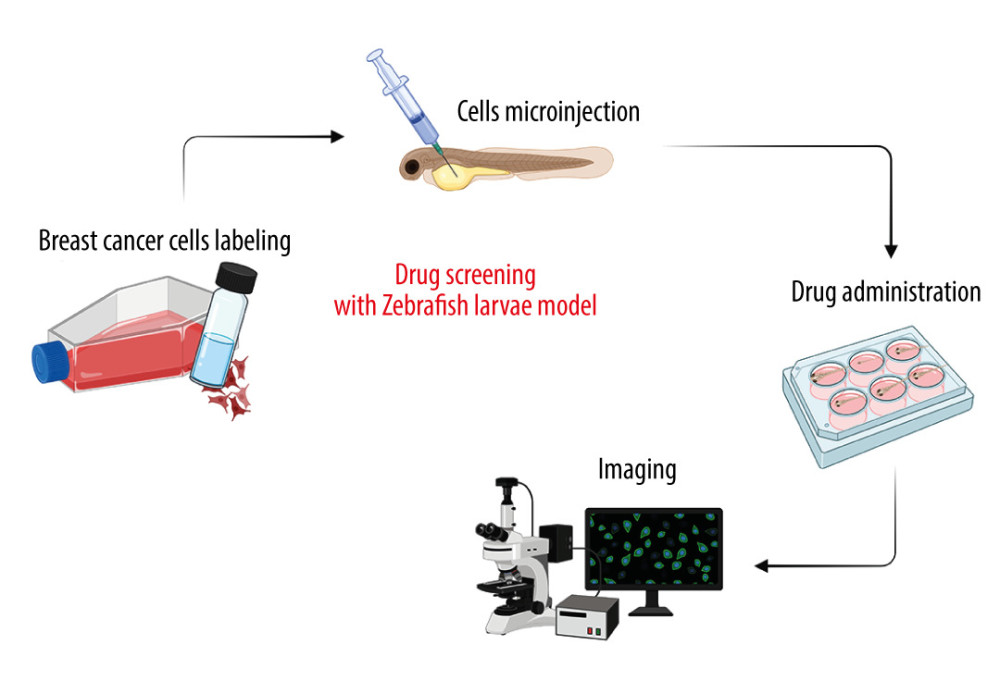 Figure 3. Drug screening scheme with zebrafish (ZF) (Danio reiro) model. Breast cancer (BC)-labeled cells were microinjected into yolk-sac zebrafish larvae, treated with the drugs, and visualized microscopically. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 3. Drug screening scheme with zebrafish (ZF) (Danio reiro) model. Breast cancer (BC)-labeled cells were microinjected into yolk-sac zebrafish larvae, treated with the drugs, and visualized microscopically. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). References
1. Aalhate M, Mahajan S, Singh H, Nanomedicine in therapeutic warfront against estrogen receptor-positive breast cancer: Drug Deliv Transl Res, 2023; 13; 1621-53
2. Kumar H, Gupta NV, Jain R, A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer: J Adv Res, 2023; 23; 2090-132
3. Facchinello N, Schiavone MG, Al-Hamaly MA, Zebrafish cancer avatars: A translational platform for analyzing tumor heterogeneity and predicting patient outcomes: Int J Mol Sci, 2023; 24; 2288
4. Costa B, Estrada MF, Mendes RV, Fior R, Zebrafish avatars towards personalized medicine – a comparative review between avatar models: Cells, 2020; 9; 293
5. Dudziak K, Nowak M, Sozoniuk M: Int J Mol Sci, 2022; 23; 10255
6. Carnovali M, Banfi G, Mariotti M, Zebrafish models of human skeletal disorders: Embryo and adult swimming together: Biomed Res Int, 2019; 2019; 1253710
7. Bowley G, Kugler E, Wilkinson R, Zebrafish as a tractable model of human cardiovascular disease: Br J Pharmacol, 2022; 179; 900-17
8. Robea MA, Balmus IM, Ciobica A, Parkinson’s disease-induced zebrafish models: Focussing on oxidative stress implications and sleep processes: Oxid Med Cell Longev, 2020; 2020; 1370837
9. Saleem S, Kannan RR, Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery: Cell Death Discov, 2018; 4; 1-13
10. Kim HG, Kim HT, Leach NT, Translocations disrupting PHF21A in the Potocki-Shaffer-syndrome region are associated with intellectual disability and craniofacial anomalies: Am J Hum Genet, 2012; 91; 56-72
11. Chávez MN, Aedo G, Fierro FA, Zebrafish as an emerging model organism to study angiogenesis in development and regeneration: Front Physiol, 2016; 7; 56
12. Cabezas-Sáinz P, Pensado-López A, Sáinz B, Sánchez L, Modeling cancer using zebrafish xenografts: Drawbacks for mimicking the human microenvironment: Cells, 2020; 9; 1978
13. Ferrero G, Gomez E, Lyer S, The macrophage-expressed gene (mpeg) 1 identifies a subpopulation of B cells in the adult zebrafish: J Leukoc Biol, 2020; 107; 431-43
14. Hall C, Flores M, Storm T, The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish: BMC Dev Biol, 2007; 7; 42
15. Jung IH, Chung YY, Jung DE, Impaired lymphocytes development and xenotransplantation of gastrointestinal tumor cells in Prkdc-null SCID zebrafish model: Neoplasia, 2016; 18; 468-79
16. Yan C, Brunson DC, Tang Q, Visualizing engrafted human cancer and therapy responses in immunodeficient zebrafish: Cell, 2019; 177; 1903-14
17. White RM, Sessa A, Burke C, Transparent adult zebrafish as a tool for in vivo transplantation analysis: Cell Stem Cell, 2008; 2; 183-89
18. Gopal U, Monroe JD, Marudamuthu AS, Development of a triple-negative breast cancer leptomeningeal disease model in zebrafish: Cells, 2023; 7; 995
19. Nakonieczna S, Grabarska A, Gawel K: Int J Mol Sci, 2022; 23; 10330
20. Maradonna F, Fontana CM, Sella F, A zebrafish HCT116 xenograft model to predict anandamide outcomes on colorectal cancer: Cell Death Dis, 2022; 13; 1060
21. Wang Q, Wang W, Pan W, Case report: Two patients With EGFR Exon 20 insertion mutanted non-small cell lung cancer precision treatment using patient-derived xenografts in zebrafish embryos: Front Oncol, 2022; 12; 884798
22. Wang X, Li W, Jiang H, Zebrafish xenograft model for studying pancreatic cancer-instructed innate immune microenvironment: Int J Mol Sci, 2022; 23; 6442
23. Kazi MA, Sahito R, Abbas Q, the inhibitory effect of polyphenon 60 from green tea on melanin and tyrosinase in zebrafish and A375 human melanoma cells: Evid Based Complement Alternat Med, 2022; 2022; 7739023
24. Ai X, Ye Z, Xiao C, Clinically relevant orthotopic xenograft models of patient-derived glioblastoma in zebrafish: Dis Model Mech, 2022; 15; dmm049109
25. Cayuela ML, Claes KBM, Ferreira MG, The zebrafish as an emerging model to study DNA damage in aging, cancer and other diseases: Front Cell Dev Biol, 2019; 6; 178
26. Kuchenbaecker K, Hopper J, Barnes D, Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers: JAMA, 2017; 317; 2402-16
27. Vierstraete J, Willaert A, Vermassen P, Accurate quantification of homologous recombination in zebrafish: brca2 deficiency as a paradigm: Sci Rep, 2017; 7; 1-10
28. Koual M, Tomkiewicz C, Guerrera IC, Aggressiveness and metastatic potential of breast cancer cells co-cultured with preadipocytes and exposed to an environmental pollutant dioxin: an in vitro and in vivo zebrafish study: Environ Health Perspect, 2021; 129; 37002
29. Britto DD, Wyroba B, Chen W, Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model: Dis Model Mech, 2018; 11; dmm035998
30. Eguiara A, Holgado O, Beloqui I, Xenografts in zebrafish embryos as a rapid functional assay for breast cancer stem-like cell identification: Cell Cycle, 2011; 10; 3751-57
31. Schwarz-Cruz Y, Celis A, Ceballos-Cancino G, Vazquez-Santillan K, Basal-type breast cancer stem cells over-express chromosomal passenger complex proteins: Cells, 2020; 9; 709
32. Varanda AB, Martins-Logrado A, Ferreira MG, Fior R, Zebrafish xenografts unveil sensitivity to olaparib beyond BRCA status: Cancers (Basel), 2020; 12; 1769
33. Ambrosio MR, Mosca G, Migliaccio T, Glucose enhances pro-tumorigenic functions of mammary adipose-derived mesenchymal stromal/stem cells on breast cancer cell lines: Cancers (Basel), 2022; 14; 5421
34. Pruszko M, Milano E, Zylicz A, Zebrafish as experimental model to establish the contribution of mutant p53 and ID4 to breast cancer angiogenesis in vivo: J Thorac Dis, 2018; 10; 231-33
35. Sharma P, Yadav P, Sundaram S, HMGB3 inhibition by miR-142-3p/sh-RNA modulates autophagy and induces apoptosis via ROS accumulation and mitochondrial dysfunction and reduces the tumorigenic potential of human breast cancer cells: Life Sci, 2022; 304; 120727
36. Tulotta C, Stefanescu C, Beletkaia E, Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model: Dis Model Mech, 2016; 9; 141-53
37. Sharif GM, Campbell MJ, Nasir A, An AIB1 isoform alters enhancer access and enables progression of early-stage triple-negative breast cancer: Cancer Res, 2021; 81; 4230-41
38. Peperstraete E, Lecerf C, Collette J, Enhancement of breast cancer cell aggressiveness by lncRNA H19 and its Mir-675 derivative: insight into shared and different actions: Cancers (Basel), 2020; 12; 1730
39. Souto EP, Dobrolecki LE, Villanueva H, In vivo modeling of human breast cancer using cell line and patient-derived xenografts: J Mammary Gland Biol Neoplasia, 2022; 27; 211-30
40. Nugoli M, Chucana P, Vendrell J, Genetic variability in MCF-7 sublines: evidence of rapid genomic and RNA expression profile modifications: BMC Cancer, 2003; 3; 13
41. Ben-David U, Siranosian B, Ha G, Genetic and transcriptional evolution alters cancer cell line drug response: Nature, 2018; 560; 325-30
42. Johnson JI, Decker S, Zaharevitz D, Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials: Br J Cancer, 2001; 84; 1424-31
43. Jiang G, Zhang S, Yazdanparast A, Comprehensive comparison of molecular portraits between cell lines and tumors in breast cancer: BMC Genomics, 2016; 17; 525
44. Bentley VL, Veinotte CJ, Corkery DP, Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia: Haematologica, 2015; 100; 70-76
45. Guillen KP, Fujita M, Butterfield AJ, A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology: Nat Cancer, 2022; 3; 232-50
46. Derose YS, Wang G, Lin YC, Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes: Nat Med, 2011; 17; 1514-20
47. Ma D, Hernandez GA, Lefebvre AEYT, Patient-derived xenograft culture-transplant system for investigation of human breast cancer metastasis: Commun Biol, 2021; 4; 1268
48. Rodriguez GV, Ali Z, Erkstam A, Zebrafish tumor-derived xenografts established from breast cancer pdx models predict treatment outcome and dissemination risk with superior sensitivity and specificity: Cancer Res, 2021; 81; 2995
49. Corsinovi D, Usai A, De Sarlo M, Zebrafish avatar to develop precision breast cancer therapies: Anticancer Agents Med Chem, 2022; 22; 748-59
50. Mercatali L, La Manna F, Groenewoud A, Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model: Int J Mol Sci, 2016; 17; 1375
51. Wienholds E, Schulte-Merker S, Walderich B, Plasterk RHA, Target-selected inactivation of the zebrafish rag1 gene: Science, 2002; 297; 99-102
52. Tang Q, Abdelfattah NS, Blackburn JS, Optimized cell transplantation using adult rag2 mutant zebrafish: Nat Methods, 2014; 11; 821-24
53. Moore JC, Tang Q, Yordán NT, Single-cell imaging of normal and malignant cell engraftment into optically clear prkdc-null SCID zebrafish: J Exp Med, 2016; 213; 2575-89
54. Redig AJ, Mcallister SS, Breast cancer as a systemic disease: A view of metastasis: J Intern Med, 2013; 274; 113-26
55. Xiao J, McGill JR, Nasir A, Identifying drivers of breast cancer metastasis in progressively invasive subpopulations of zebrafish-xenografted MDA-MB-231: Mol Biomed, 2022; 3; 16
56. Mokhamatam RB, Irlapati VK, Dravida S, Targeting epithelial-mesenchymal transition – an ongoing wild goose chase: J Cancer Metastasis Treat, 2020; 6; 28
57. Thomas C, Rajapaksa G, Nikolos F, ERβ1 represses basal-like breast cancer epithelial to mesenchymal transition by destabilizing EGFR: Breast Cancer Res, 2012; 14; 148
58. Tulotta C, Groenewoud A, Snaar-Jagalska BE, Ottewell P, Animal models of breast cancer bone metastasis: Methods Mol Biol, 2019; 1914; 309-30
59. Ren J, Liu S, Cui C, Ten Dijke P, Invasive behavior of human breast cancer cells in embryonic zebrafish: J Vis Exp, 2017; 2017; 55459
60. Li C, Ma J, Groenewoud A, Establishment of embryonic zebrafish xenograft assays to investigate TGF-β family signaling in human breast cancer progression: Methods Mol Biol, 2022; 2488; 67-80
61. Drabsch Y, He S, Zhang L, Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model: Breast Cancer Res, 2013; 15; 106
62. Chiang KC, Hsu SY, Lin SJ, PTEN insufficiency increases breast cancer cell metastasis in vitro and in vivo in a xenograft zebrafish model: Anticancer Res, 2016; 36; 3997-4005
63. Martínez-Pena I, Hurtado P, Carmona-Ule N, Dissecting breast cancer circulating tumor cells competence via modelling metastasis in zebrafish: Int J Mol Sci, 2021; 22; 9279
64. Lin D, Shen L, Luo M, Circulating tumor cells: Biology and clinical significance: Signal Transduct Target Ther, 2021; 6; 404
65. Sukardi H, Chng HT, Chan ECY, Zebrafish for drug toxicity screening: bridging the in vitro cell-based models and in vivo mammalian models: Expert Opin Drug Metab Toxicol, 2011; 7; 579-89
66. Wiley DS, Redfield SE, Zon LI, Chemical screening in zebrafish for novel biological and therapeutic discovery: Methods Cell Biol, 2017; 138; 651-79
67. Li X, Li M, The application of zebrafish patient-derived xenograft tumor models in the development of antitumor agents: Med Res Rev, 2023; 43; 212-36
68. Wiley DS, Redfield SE, Zon LI, Chemical screening in zebrafish for novel biological and therapeutic discovery: Methods Cell Biol, 2017; 138; 651-79
69. Patton EE, Zon LI, Langenau DM, Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials: Nat Rev Drug Discov, 2021; 20; 611-28
70. Lee HC, Lin CY, Tsai HJ, Zebrafish, an in vivo platform to screen drugs and proteins for biomedical use: Pharmaceuticals (Basel), 2021; 14; 500
71. Rebelo de Almeida C, Mendes RV, Pezzarossa A, Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy: Commun Biol, 2020; 3; 299
72. Wu XX, Yue GGL, Dong JR, Actein inhibits the proliferation and adhesion of human breast cancer cells and suppresses migration in vivo: Front Pharmacol, 2018; 9; 1466
73. Saraiva SM, Gutiérrez-Lovera C, Martínez-Val J, Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model: Sci Rep, 2021; 11; 9873
74. Yang K, Yang JQ, Luo SH, Synthesis of N-2(5H)-furanonyl sulfonyl hydrazone derivatives and their biological evaluation in vitro and in vivo activity against MCF-7 breast cancer cells: Bioorg Chem, 2021; 107; 104518
75. Lenis-Rojas OA, Roma-Rodrigues C, Fernandes AR, Evaluation of the in vitro and in vivo efficacy of ruthenium polypyridyl compounds against breast cancer: Int J Mol Sci, 2021; 22; 8916
76. Guo Y, Fan Y, Pei X, Fangjihuangqi decoction inhibits MDA-MB-231 cell invasion in vitro and decreases tumor growth and metastasis in triple-negative breast cancer xenografts tumor zebrafish model: Cancer Med, 2020; 9; 2564-78
77. Yang Y, Hao E, Pan X, Gomisin M2 from Baizuan suppresses breast cancer stem cell proliferation in a zebrafish xenograft model: Aging (Albany NY), 2019; 11; 8347-61
78. Li L, Liu CC, Chen X, Mechanistic study of bakuchiol-induced anti-breast cancer stem cell and in vivo anti-metastasis effects: Front Pharmacol, 2017; 8; 746
79. Zhu XY, Guo DW, Lao QC, Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models: Sci Rep, 2019; 9; 4541
80. McKeown BT, Relja NJ, Hall SR, Pilot study of jadomycin B pharmacokinetics and anti-tumoral effects in zebrafish larvae and mouse breast cancer xenograft models: Can J Physiol Pharmacol, 2022; 100; 1065-76
Figures
 Figure 1. Examples of applications of the zebrafish (ZF) (Danio reiro) model in the field of breast cancer (BC) research include BC modeling, angiogenesis and metastasis studies, in vivo cell tracking, drug and toxicity screening, and tumor immunotherapy. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 1. Examples of applications of the zebrafish (ZF) (Danio reiro) model in the field of breast cancer (BC) research include BC modeling, angiogenesis and metastasis studies, in vivo cell tracking, drug and toxicity screening, and tumor immunotherapy. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). Figure 2. The advantages of zebrafish (ZF) (Danio reiro) as a vertebrate preclinical animal model include low cost and ease of maintenance, short generation time, small size, intensive external development, transparent embryos, and convenient genetic manipulations and analysis. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 2. The advantages of zebrafish (ZF) (Danio reiro) as a vertebrate preclinical animal model include low cost and ease of maintenance, short generation time, small size, intensive external development, transparent embryos, and convenient genetic manipulations and analysis. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). Figure 3. Drug screening scheme with zebrafish (ZF) (Danio reiro) model. Breast cancer (BC)-labeled cells were microinjected into yolk-sac zebrafish larvae, treated with the drugs, and visualized microscopically. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023).
Figure 3. Drug screening scheme with zebrafish (ZF) (Danio reiro) model. Breast cancer (BC)-labeled cells were microinjected into yolk-sac zebrafish larvae, treated with the drugs, and visualized microscopically. The figure was created with BioRender.com (Toronto, Canada) (accessed on 23 April 2023). In Press
15 Mar 2024 : Clinical Research
Impact of Cluster Nursing Intervention on ICU Patients' Psychological Well-Being and Complications Associat...Med Sci Monit In Press; DOI: 10.12659/MSM.942855
26 Mar 2024 : Clinical Research
New Computerized Planning Algorithm and Clinical Testing of Optimized Nuss Bar Design for Patients with Pec...Med Sci Monit In Press; DOI: 10.12659/MSM.943705
07 May 2024 : Clinical Research
Treatment of AVN-Induced Proximal Pole Scaphoid Nonunion Using a Fifth and Fourth Extensor Compartmental Ar...Med Sci Monit In Press; DOI: 10.12659/MSM.944553
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952