05 August 2023: Clinical Research
Assessing Automatic Plethysmographic Ankle-Brachial Index Devices in Peripheral Artery Disease Detection: A Comparative Study with Doppler Ankle-Brachial Index Measurements
Aleksandra DanielukDOI: 10.12659/MSM.940829
Med Sci Monit 2023; 29:e940829
Abstract
BACKGROUND: The ankle-brachial index (ABI) is a critical diagnostic test for peripheral artery disease (PAD), albeit requiring technical expertise and dedicated resources. The advent of automatic ABI devices proposes a more accessible approach, necessitating fewer resources and less expertise. This study was conducted to gather data on PAD prevalence and to evaluate the correlation and efficacy of automatic ABI measurements vs traditional Doppler ABI measurements to understand their potential role in primary care settings.
MATERIAL AND METHODS: ABI measurements were obtained using both the Doppler method and an automatic plethysmographic device (Dopplex ABility, Huntleigh Healthcare).
RESULTS: Of the 290 participants (mean age 67.6±7.4 years), Doppler ABI method identified 16.8% with abnormal results (<0.9), while the automatic method identified only 5.9%. The mean Doppler ABI was 1.05±0.15, and the mean automatic ABI was 1.12±0.13. The sensitivity of the automatic ABI measurements was 22.2%, and the specificity was 96.8%, with a positive predictive value of 57.1%, and a negative predictive value of 86.9%. Adjustments in the automatic assessment and inclusion of pulse wave velocity enhanced the diagnostic capabilities of the automatic ABI device.
CONCLUSIONS: While the automatic plethysmographic ABI device may lack the necessary diagnostic capabilities to replace the traditional Doppler ABI device as a standalone test in PAD diagnosis, it could still offer significant value in primary care settings if integrated with adjusted cut-off points and pulse wave velocity analysis.
Keywords: peripheral arterial disease, Lower Extremity, Physicians, Primary Care, Humans, Middle Aged, Aged, Ankle Brachial Index, Pulse Wave Analysis, Predictive Value of Tests, Ultrasonography, Doppler
Background
Peripheral artery disease (PAD) is an atherosclerotic disease that most commonly affects the arteries of the lower extremities. In 2015, the estimated total number of cases of PAD worldwide reached 236 million, with the numbers continuing to rise [1]. PAD in the lower extremities is referred to as lower extremity arterial disease (LEAD). Symptoms can range from claudication to critical limb ischemia, sometimes leading to limb loss and even death [2]. Approximately 20% to 50% of patients with PAD are asymptomatic [2]. Due to nonspecific early symptoms and commonly asymptomatic presentation, the disease often remains undiagnosed or misdiagnosed [3]. Still, even in the asymptomatic stage, PAD increases cardiovascular risk and overall morbidity [4,5].
The ankle-brachial index (ABI) is recommended as the first-line noninvasive test for PAD in the lower extremities [6,7]. However, the traditional method of ABI measurement using a Doppler probe requires training and is time-consuming [6,8,9]. To address these challenges, automatic devices for ABI measurement have been developed to simplify the technique and provide consistent results [10–13]. Although there is promising evidence for oscillometric automatic devices, studies on plethysmographic devices are limited [11–13].
Automatic measurements have certain limitations, such as reduced accuracy in diabetic patients and overall higher values than those obtained with the Doppler ABI device [14,15]. Current guidelines recommend the use of traditional Doppler methods for ABI measurement over the use of automatic devices [6,16]. Nevertheless, research continues to assess the accuracy of new devices. This study aimed to evaluate the accuracy of automatic ABI measurements performed with the method of air plethysmography compared with those of Doppler ABI measurements.
Material and Methods
STUDY POPULATION:
Patients aged 50 years or older from a primary care clinic were invited to participate in the study. The inclusion criteria were an appropriate age and the ability to provide consent for study participation. Exclusion criteria included post-limb amputation, marked edema in either foot, skin lesions preventing the use of a blood pressure cuff, and the inability to remain supine during the examination.
METHODS OF EXAMINATION:
Informed consent was obtained from all study participants. The examiner gathered the patients’ history, including information on PAD history, smoking, hypertension, diabetes, atrial fibrillation, coronary disease, chronic kidney disease, and medication usage. The researcher administered the Edinburgh questionnaire, a standardized tool, to assess participants for PAD symptoms. The Edinburgh questionnaire aims to identify typical claudication symptoms of PAD by asking participants about the presence of pain in the lower limbs and the situations in which the pain occurs and resolves. While the diagnostic value of the Edinburgh questionnaire has been previously assessed as weak [17], in this study, it was used to assess the presence of typical symptoms rather than as a diagnostic modality.
The examiner palpated the femoral, posterior tibial, and dorsalis pedis pulses, which are typically examined when suspecting LEAD. Carotid and femoral arteries, as well as the abdominal aorta, were auscultated to search for murmurs and physical examination signs of atherosclerosis in vascular beds. The patients’ weight and body mass index (BMI) were assessed using the Tanita MC780-MA device. Any BMI above 25.0 was categorized as abnormal.
For Doppler ABI assessment, the patients’ arm blood pressure was manually measured with a sphygmomanometer, on both arms. Subsequently, ankle-level blood pressure on both legs was measured using a Doppler probe (Dopplex DMX Digital Doppler, Huntleigh Healthcare) to detect a signal on the posterior tibial artery. The higher of the 2 arm blood pressure values was used for ABI calculation in each leg. An automatic ABI measurement was then performed using the Dopplex ABility Automatic ABI System (Huntleigh Healthcare) device. ABI values below 0.9 were considered abnormal, and values above 1.3 were considered non-diagnostic. The Dopplex ABility device also provided a pulse volume waveform (PVW) graph, which was assessed by the researchers as normal or abnormal. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for the automatic device using Doppler ABI results as the reference standard. These parameters were first assessed using the standard 0.9 ABI cut-off point and subsequently recalculated for other cut-off points from the literature. The sensitivity, specificity, PPV, and NPV were also recalculated for a joint analysis of automatic ABI and PVW, again using Doppler ABI as the reference standard.
The study received approval from the Bioethical Committee of the Medical University of Bialystok, Poland.
STATISTICAL ANALYSIS:
Statistical analysis was performed using IBM SPSS Statistics 25 software. Data normality was assessed using the Shapiro-Wilk test, and most ABI results showed an abnormal distribution, except for Doppler ABI results on the right legs. The correlation between abnormal ABI and patient characteristics and the correlation between the 2 methods of ABI measurements were assessed. For assessing the agreement between automatic ABI measurements and Doppler ABI measurements, the Wilcoxon test was used, owing to the lack of normality in the assessed data. The Pearson correlation coefficient was used to assess the correlation between automatic and Doppler measurements. The level of statistical significance was set at α=0.05, with
Results
DEMOGRAPHIC DATA:
The demographic data of the participants in the study are presented in Table 1. The study included 290 patients, with a mean age of 67.6±7.4 years; 24.7% were men and 75.3% were women; and 71.7% of patients were current or past smokers. A total of 28 patients (9.7%) had coronary heart disease, 185 (63.8%) had hypertension, 46 (15.9%) had diabetes, 4 (1.4%) had chronic kidney disease, and 28 (9.7%) had atrial fibrillation. Additionally, 13 patients (4.5%) had experienced a previous acute coronary incident, 3 (1%) had a transient ischemic attack, and 6 (2.1%) had a history of cerebral stroke. Among the patients, 50 (17.2%) reported regularly taking glucose-lowering medication, 57 (19.7%) reported taking antiplatelet medication, 18 (6.2%) reported taking anticoagulants, 178 (61.4%) reported taking blood pressure-lowering medication, and 109 (37.6%) reported taking statins. Six patients (2.1%) reported being previously diagnosed and treated for PAD. To assess the presence of PAD symptoms, the Edinburgh questionnaire was administered, yielding positive results in 30 patients (10.3%). We found a significant correlation between a positive Edinburgh questionnaire score and an abnormal automatic ABI (Fisher’s exact test, P=0.02). However, there was no statistically significant correlation between a positive Edinburgh questionnaire and abnormal Doppler ABI (Fisher’s exact test, P=0.299). We did not find a statistically significant correlation between the use of PAD treatment medication and ABI values, for both automatic (χ2 test, P=0.68 for statins and Fisher’s exact test, P=0.16 for antiplatelet medication) and traditional (χ2 test, P=0.98 for statins and χ2 test, P=0.11 for antiplatelet medication) devices.
ABI MEASUREMENTS:
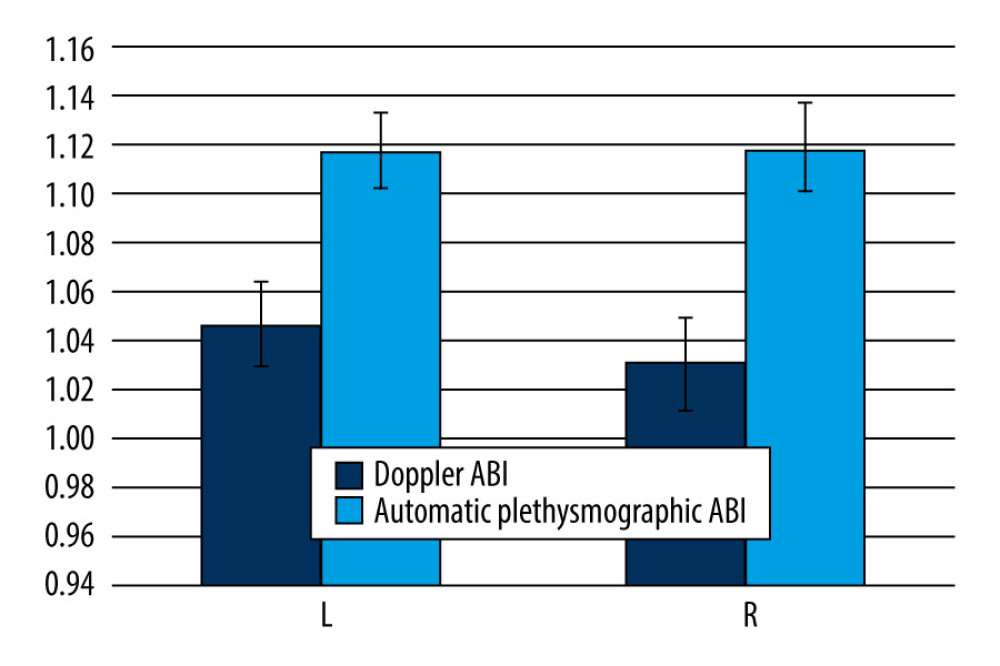
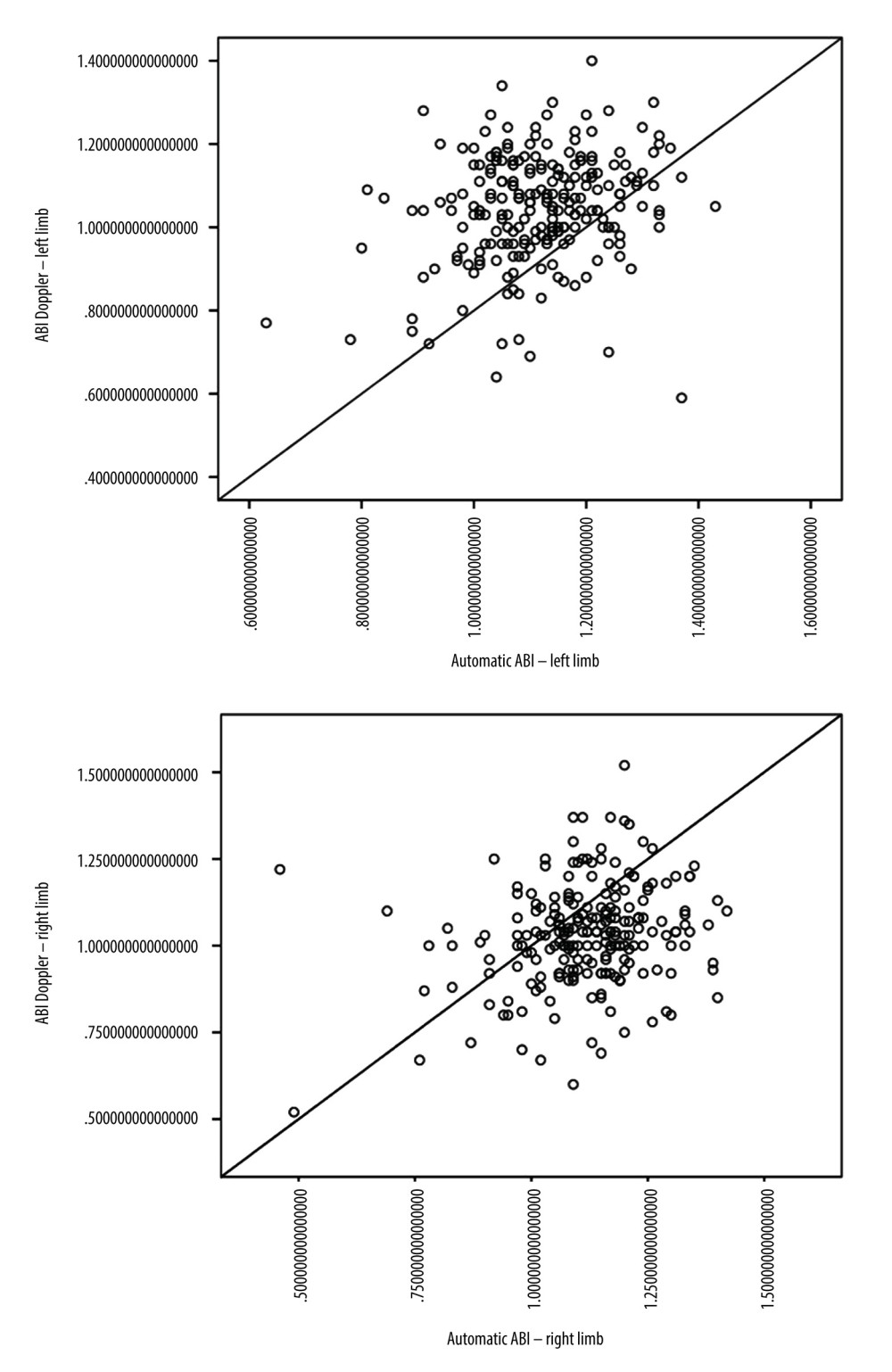
Doppler ABI measurements could not be obtained for 11 of the 580 limbs, mainly due to patient discomfort during cuff inflation. Automatic ABI measurements were not obtained for 131 of 580 limbs, mostly due to equipment error messages or patient discomfort during the examination. The mean automatic ABI for both legs was 1.12±0.13, while the mean Doppler ABI for both legs was 1.05±0.15. Abnormal Doppler ABI was found in 16.8% of the study population. In the 50–65 year age group, 13.5% of the population had abnormal ABI, while in the 65–80 year age group, 19% had abnormal ABI, and in the >80 year age group, 20% had abnormal ABI. For automatic ABI, 5.9% had abnormal ABI, comprising 4.1% of the 50–65 year age subgroup, 6.8% of the 65–80 year age subgroup, and 1.25% of the over 80 year age subgroup. The mean level of ABI in automatic measurements was higher than the mean level of ABI measured by Doppler (Figure 1), and the difference was statistically significant according to the Wilcoxon test. The level of effect, as indicated by the r value, was classified as moderate (Table 2). Using the standard cut-off ABI level of 0.9, the sensitivity of automatic measurements was 22.2%, and the specificity of automatic measurements was 96.8%. The positive predictive value (PPV) was 57.1%, and the negative predictive value (NPV) was 86.9%. ABI results above 1.3 were considered non-diagnostic and were omitted for the calculations of sensitivity, specificity, PPV, and NPV. The correlation between automatic and Doppler measurements was assessed using a Pearson correlation coefficient (Figure 2), and a low positive correlation was observed between the measurements, with r=0.23 for both limbs. We took into account previous publications that suggested different cut-off levels for PAD diagnosis when using automatic ABI measurements. In previous studies by Babaei et al and Davies et al, the optimal ABI cut-off levels were set at 1.2 and 1.04, respectively [18,19]. We conducted a simulation and assessed sensitivity, specificity, PPV, and NPV at the previously suggested levels. At the level of 1.2, we observed an improved sensitivity of 91.9%, but at the expense of considerably lower specificity, which decreased to 18.5%. The PPV and NPV for an automatic ABI cut-off level of 1.2 were 17.3% and 92.5%, respectively. The automatic ABI cut-off level of 1.04 resulted in a sensitivity of 54%, specificity of 78.9%, PPV of 32.2%, and NPV of 90.2%.
Furthermore, previous studies have shown that the diagnostic quality of automatic plethysmographic devices can be improved when the pulse volume waveform (PVW) is also considered during diagnosis. We performed an analysis of sensitivity, specificity, PPV, and NPV for a joint result of ABI and PVW measured by the plethysmographic device. Whenever either the ABI or PVW were abnormal, the result was classified as positive. For an abnormal automatic ABI, we used the 0.9 cut-off point. In the ABI and PVW analysis, we found that the sensitivity of the device was 67.6%, specificity was 51.5%, PPV was 20.5%, and NPV was 89.6%. When adjusting the cut-off points, we observed a sensitivity of 97.3%, specificity of 10%, PPV of 16.6%, and NPV of 95.2% with the 1.2 cut-off, and a sensitivity of 78.4%, specificity of 69.5%, PPV of 19.7%, and NPV of 91.1% with the 1.04 cut-off for the automatic ABI device (Table 3).
Discussion
STUDY LIMITATIONS:
A potential limitation of our study was the use of Doppler ABI as the reference standard to assess automatic ABI accuracy in LEAD diagnosis. While Doppler ABI is considered a first-line tool in LEAD diagnosis, its diagnostic performance is not considered perfect, and using a more sensitive diagnostic modality might have proved more beneficial for the study [26].
Conclusions
Overall, we found that nearly 20% of our primary care patients over 50 years of age had abnormal Doppler ABI values, consistent with the diagnosis of LEAD. In most of our patients, the disease was asymptomatic and undiagnosed. We observed that the diagnostic capability of automatic plethysmographic ABI, especially the low sensitivity, is insufficient to introduce it as a standalone test in LEAD diagnosis. However, adjusting the cut-off point to a higher level and considering PVW may improve its diagnostic applicability, especially in a non-specialized setting.
Tables
Table 1. Demographics of the study participants.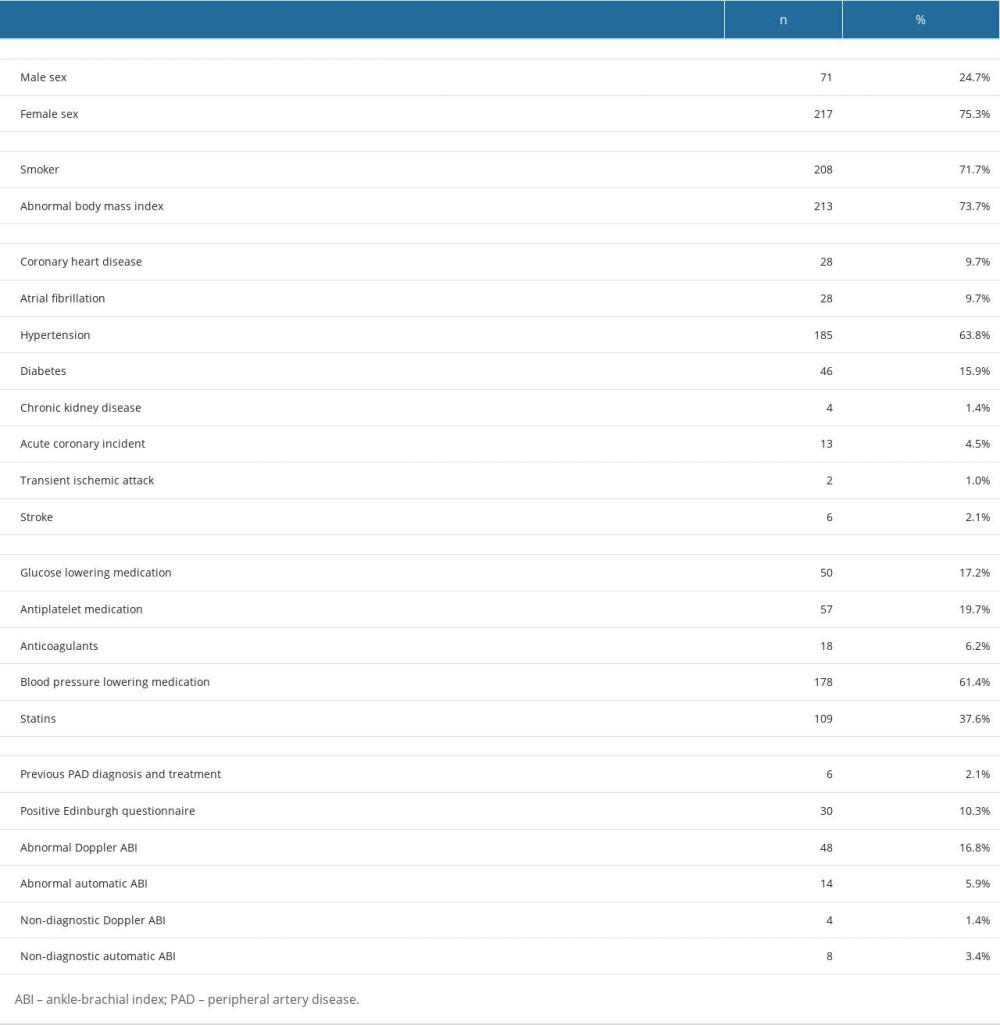 Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test.
Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test.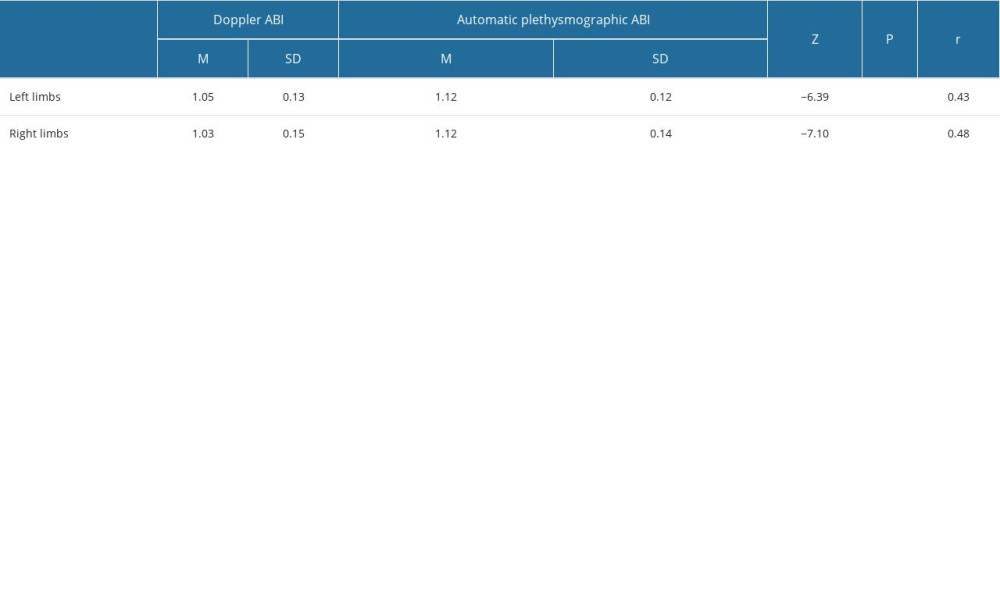 Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis.
Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis.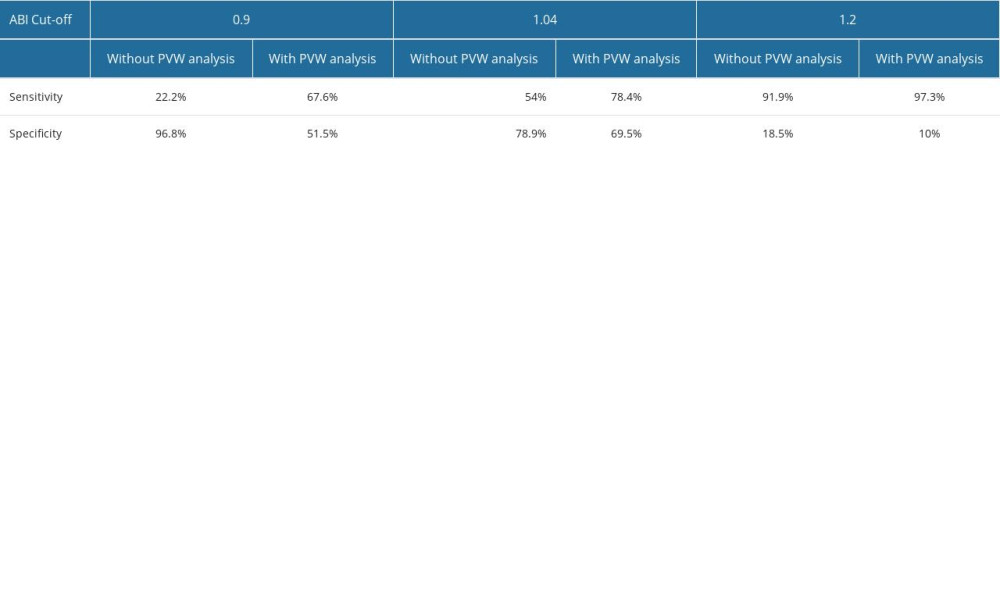
References
1. Horváth L, Németh N, Fehér G, Epidemiology of peripheral artery disease: Narrative review: Life (Basel), 2022; 12(7); 1041
2. McDermott MM, Guralnik JM, Ferrucci L, Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication: Circulation, 2008; 117(19); 2484-91
3. Schorr EN, Peden-McAlpine C, Treat-Jacobson D, Lindquist R, Characterization of the peripheral artery disease symptom experience: Geriatr Nurs, 2015; 36(4); 293-300
4. Behroozian AA, Beckman JA, Asymptomatic peripheral artery disease: Silent but deadly: Prog Cardiovasc Dis, 2021; 65; 2-8
5. Aday AW, Matsushita K, Epidemiology of peripheral artery disease and polyvascular disease: Circ Res, 2021; 128(12); 1818-32
6. Abramson BL, Al-Omran M, Anand SSPrimary Panel, Canadian Cardiovascular Society 2022 guidelines for peripheral arterial disease: Can J Cardiol, 2022; 38(5); 560-87
7. Aboyans V, Ricco JB, Bartelink MELESC Scientific Document Group, 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS): Eur Heart J, 2018; 39(9); 763-816
8. Nexøe J, Damsbo B, Lund JO, Munck A, Measurement of blood pressure, ankle blood pressure and calculation of ankle brachial index in general practice: Fam Pract, 2012; 29(3); 345-51
9. Yap Kannan R, Dattani N, Sayers RD, Bown MJ, Survey of ankle-brachial pressure index use and its perceived barriers by general practitioners in the UK: Postgrad Med J, 2016; 92(1088); 322-27
10. Vega J, Romaní S, Garcipérez FJ, Vicente LPeripheral arterial disease: Efficacy of the oscillometric method: Rev Esp Cardiol, 2011; 64(7); 619-21 [in Spanish]
11. Khan SZ, Awn-Bin-Zafar , Waris N, Comparison of ankle-brachial index (ABI) measured by an automated oscillometric apparatus with that by standard hand-held doppler in patients with Type-2 diabetes: Pak J Med Sci, 2019; 35(4); 1167-72
12. Hageman D, van den Houten MML, Pesser N, Diagnostic accuracy of automated oscillometric determination of the ankle-brachial index in peripheral artery disease: J Vasc Surg, 2021; 73(2); 652-60
13. Ichihashi S, Desormais I, Hashimoto T, Accuracy and reliability of the ankle brachial index measurement using a multicuff oscillometric device versus the Doppler method: Eur J Vasc Endovasc Surg, 2020; 60(3); 462-68
14. Ichihashi S, Hashimoto T, Iwakoshi S, Kichikawa K, Validation study of automated oscillometric measurement of the ankle-brachial index for lower arterial occlusive disease by comparison with computed tomography angiography: Hypertens Res, 2014; 37(6); 591-94
15. Diehm N, Dick F, Czuprin C, Oscillometric measurement of ankle-brachial index in patients with suspected peripheral disease: Comparison with Doppler method: Swiss Med Wkly, 2009; 139(25–26); 357-63
16. National Institute for Health and Care Excellence, Recommendations. Peripheral Arterial Disease: Diagnosis and Management: Guidance, NICE Available from: https://www.nice.org.uk/guidance/cg147/chapter/Recommendations#diagnosis
17. Boylan L, Nesbitt C, Wilson LInvestigators OBOTN, Reliability of the Edinburgh Claudication Questionnaire for identifying symptomatic PAD in general practice: Angiology, 2021; 72(5); 474-79
18. Babaei MR, Malek M, Rostami FT, Non-invasive vascular assessment in people with type 2 diabetes: Diagnostic performance of Plethysmographic-and-Doppler derived ankle brachial index, toe brachial index, and pulse volume wave analysis for detection of peripheral arterial disease: Prim Care Diabetes, 2020; 14(3); 282-89
19. Davies JH, Williams EM, Automated plethysmographic measurement of the ankle-brachial index: A comparison with the Doppler ultrasound method: Hypertens Res, 2016; 39(2); 100-6
20. Danieluk A, Chlabicz S, Automated measurements of ankle-brachial index: A narrative review: J Clin Med, 2021; 10(21); 5161
21. Lewis JE, Williams P, Davies JH, Non-invasive assessment of peripheral arterial disease: Automated ankle brachial index measurement and pulse volume analysis compared to duplex scan: SAGE Open Med, 2016; 4; 2050312116659088
22. Lewis J, Hawkins M, Barree P, A comparison between a new automatic system and Doppler method for obtaining ankle brachial pressures: J Foot Ankle Res, 2010; 3; O15
23. van der Slegt J, Verbogt NP, Mulder PG, The clinical applicability of an automated plethysmographic determination of the ankle-brachial index after vascular surgery: Vascular, 2016; 24(5); 545-48
24. Millen RN, Thomas KN, Majumder A, Accuracy and repeatability of the Dopplex ability: Expert Rev Med Devices, 2018; 15(3); 247-51
25. Danieluk A, Niemcunowicz-Janica A, Windak A, Chlabicz S, Diagnosis and treatment of lower extremity arterial disease – a survey among family medicine trainees in Poland: Int J Environ Res Public Health, 2023; 20(2); 1392
26. Alagha M, Aherne TM, Hassanin A, Diagnostic performance of ankle-brachial pressure index in lower extremity arterial disease: Surg J (N Y), 2021; 7(3); e132-e37
Figures
Tables
 Table 1. Demographics of the study participants.
Table 1. Demographics of the study participants. Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test.
Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test. Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis.
Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis. Table 1. Demographics of the study participants.
Table 1. Demographics of the study participants. Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test.
Table 2. Ankle-brachial index (ABI) level comparison in automatic and Doppler measurements by Wilcoxon test. Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis.
Table 3. Sensitivity and specificity of automatic plethysmographic ankle-brachial index (ABI) at different cut-off levels, with and without pulse volume waveform (PVW) analysis. In Press
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952