02 September 2023: Clinical Research
Antibiotic-Resistant Strains of in 50 Antibiotic Treatment-Naïve Children in Northeast Poland Diagnosed by Gastric or Duodenal Biopsy Between February 2019 and May 2022
Magdalena KucharskaDOI: 10.12659/MSM.941195
Med Sci Monit 2023; 29:e941195
Abstract
BACKGROUND: In recent years, an increasing prevalence of Helicobacter pylori resistance to antibiotics has been observed. The aim of this study was to assess antibiotic resistance of Helicobacter pylori in previously untreated children from northeast Poland.
MATERIAL AND METHODS: Inclusion criteria comprised suspicion of Helicobacter pylori infection based on the presence of Helicobacter pylori antigen in the stool and/or characteristic macroscopic lesions seen on esophagogastroduodenoscopy. Samples of the gastric and/or duodenal mucosa were collected from 82 children with a median age of 13 years (range 3-17) during esophagogastroduodenoscopy between February 2019 and May 2022. The material was cultured, and positive Helicobacter pylori strains were tested for drug resistance to amoxicillin, metronidazole, and clarithromycin using the quantitative antibiotic concentration gradient stripe method E-test.
RESULTS: Based on biopsy culture, Helicobacter pylori infection was confirmed in 50 (61%) children. Helicobacter pylori resistance was most common to clarithromycin (n=19; 38%), followed by metronidazole (n=15; 30%), and the least frequent to amoxicillin (n=13; 26%). The resistance to 1 antibiotic was found in 14 children (28%). Double-drug resistance was noted in 3 children (6%) and triple drug resistance in 9 children (18%). In the whole group, 24 children (48%) were susceptible to all 3 antibiotics.
CONCLUSIONS: In this study, conducted for the first time in treatment-naïve children in northeast Poland, we found a high proportion of Helicobacter pylori strains resistant to at least 1 antibiotic. Our results may help in the appropriate choice of antibiotics for treatment of Helicobacter pylori in our region.
Keywords: Helicobacter pylori, Drug Resistance, Microbial, Gastroenterology, Child, Humans, Child, Preschool, Adolescent, Anti-Bacterial Agents, clarithromycin, metronidazole, Helicobacter Infections, Poland, Biopsy, Amoxicillin
Background
This bacterium causes many diseases, including chronic gastritis, peptic ulcer disease (PUD), gastric mucosa associated lymphoid tissue lymphoma (MALT), and gastric adenocarcinoma [7,14–17]. In children, the most common forms of
According to the latest guidelines of the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) North American Society for Pediatric Gastroenterology, Hepatology and Nutrition/NASPGHAN, the diagnosis of
There are no data on the susceptibility of
Material and Methods
ETHICS STATEMENT:
The study was approved by the local Bioethics Committee (R-I-002/599/2018). A closed-ended questionnaire that each patient completed included symptoms, the previous year’s medication history, and family history of
STUDY DESIGN:
This prospective study included 82 children from northeast Poland, aged 3–18 years old, who had undergone EGD due to GI symptoms between February 2019 and May 2022. Our department is the main pediatric endoscopic center in northeast Poland. Among the reported symptoms that were an indication for EGD were: epigastric pain, nausea, vomiting, anemia of undetermined cause, and the detection of
SAMPLING AND ANALYSIS PROCEDURE:
In patients eligible for EGD, 2 gastric mucosa biopsies for H. pylori culture were taken only if macroscopic changes according to the Kyoto classification (such as nodular gastritis, enlarged folds, and diffuse redness) were visualized [21]. EGD photos of selected patients are shown in Figure 1. The biopsies for culture were placed in cold sterile saline in an Eppendorf tube placed on ice, and were prepared for culture within 1 h. Biopsy was homogenized and plated on Helicobacter Agar modified culture medium from Becton Dickinson (BD, Germany). The bacteria were cultured at 37°C for 3–7 days under microaerophilic conditions. They were then identified based on the colony morphology pattern from the culture (small and translucent colonies with a raised bulge) and positive tests for urease, catalase, and oxidase. In addition, Gram staining of the culture material was performed to confirm the presence of spiral/curved gram-negative bacilli. Susceptibility of cultured strains to amoxicillin (AMX), metronidazole (MTZ), and clarithromycin (CL) was assessed by the E-test method (Liofilchem, Italy). Viable H. pylori colonies were suspended in Mueller-Hinton broth with 5% horse blood +20 mg/l β-NAD (BioMerieux, France) and culture suspensions with a McFarland turbidity of 3 were applied to the inoculation plates. The E-test strip of each antibiotic was placed on a plate and incubated for 3 days at 37°C under microaerophilic conditions. The minimum inhibitory concentration (MIC) was identified by the point of intersection of the inhibition zone with the E-test strip. Strains were considered resistant when the MIC value was >0.12 μg/mL for amoxicillin, >0.5 μg/mL for clarithromycin, and >8 μg/mL for metronidazole. The breakpoints are based on epidemiological cutoff values, which distinguish wild-type isolates from those with reduced susceptibility by using the European Committee on Antimicrobial Susceptibility Testing criteria (EUCAST 2020). To control the quality of drug susceptibility, the reference strain H. pylori ATCC 43504 was used.
STATISTICAL ANALYSIS:
The quantitative data are expressed as median, maximum, and minimum values, while qualitative variables are expressed as absolute frequency and percentage. Differences between groups for frequency of gender, symptoms, and endoscopic features were analyzed by the chi-squared test, and for age by the Mann-Whitney U test. If the group was smaller than 10, then no statistical calculations were made. A
Results
PATIENTS’ CHARACTERISTICS:
H. pylori strains were isolated from cultured gastric biopsy samples taken during EGD from 50 (61%) patients who had never been treated for H. pylori infection. The data on demographics, symptoms, endoscopic finding, history of medications used during the previous year, family history of H. pylori infection, and smoking habits in this group are shown in Table 1.
In the study group, there were almost equal number of boys and girls, and the median age was 11 years. However, one-third of patients (34%) were younger than 10 years (Table 2). The most frequent symptom was abdominal pain, reported by 76% of patients, followed by nausea (26%) and vomiting (18%). No children with anemia were observed among H. pylori-positive patients. In endoscopic assessment, the nodularity gastritis was the most frequent finding. Positive family history of H. pylori infection was noted in 38% of cases.
Less than half of H. pylori strains isolated from treatment-naïve children (n=24/50; 48.0%) were susceptible to all 3 antibiotics (Figure 2).
ANTIBIOTIC RESISTANCE:
The prevalence of resistance of H. pylori strains isolated from previously untreated children to individual antibacterial drugs is shown in Figure 3. Primary resistance to clarithromycin (CL) was detected in 38% (n=19/50) of the H. pylori strains (MIC range was 0.016–256.00 μg/ml) (Figure 3). Primary metronidazole (MTZ) resistance was found in 30% (n=15/50) of positive cultures (MIC range was 0.016–256.00 μg/ml). Primary resistance to amoxicillin (AMX) was confirmed in 26% (n=13/50) of the H. pylori strains (MIC range was 0.016–256.00 μg/ml). All cultured strains were positive in oxidase, catalase, and urease tests.
The prevalence of resistance to 1, 2, or 3 antibiotics among H. pylori strains isolated from previously untreated children is shown in Figure 4. Resistance to a single antibiotic was found in 14 patients (28%), with the highest rate for CL, followed by MTZ and AMX (14% vs 8% vs 6%, respectively) (Figure 4). Dual drug resistance was observed in 3 children (6%), among whom 2 (4%) had resistance to CL and MTZ, and 1 (2%) to CL and AMX. Triple drug resistance was detected in 9 patients (18%).
COMPARISON OF CHILDREN SUSCEPTIBLE TO ALL 3 ANTIBIOTICS WITH THOSE RESISTANT TO AT LEAST 1 ANTIBIOTIC:
Having realized that the study group was small, we decided to investigate if there were differences in demographics, symptoms, endoscopic finding, history of medications used during the previous year, family history of H. pylori infection, and smoking habits between children susceptible to all 3 antibiotics with those resistant to at least 1 antibiotic (Table 3). There were no significant differences in any of the evaluated parameters.
COMPARISON OF ANTIBIOTIC RESISTANCE ACCORDING TO PATIENT AGE:
We then decided to see if there were differences depending on the age of the patient with a first-diagnosed H. pylori infection in terms of H. pylori resistance to particular antibiotics, personal history of antibiotic use, family history of H. pylori infection, and family smoking habit (Table 2). Considering the age of the patients, we found that the group of children younger than 10 years was more likely to have received antibiotic therapy in the last year. We also observed a higher antibiotic resistance of H. pylori strains from older children than from children younger than 10, but this result was not statistically significant. However, we realize that the number of patients was small, so the observed differences should be interpreted with caution.
COMPARISON OF ANTIBIOTIC RESISTANCE IN THE PRE- AND POST-PANDEMIC PERIOD OF COVID-19:
Given the onset of the COVID-19 pandemic during the course of our study and the associated changes in medical care across the country, we decided to see if this affected the number of diagnosed H. pylori strains resistant to particular antibiotics, personal history of antibiotic use, family history of H. pylori infection, and family smoking habits. It is noteworthy that 46 patients were included in the study in the first year before the pandemic, while during the pandemic, only 36 EGDs were performed for another 2.5 years. Comparing results taken before or during the COVID-19 pandemic, there were no significant differences in prevalence of H. pylori resistance to antibiotics, nor between the other parameters assessed, with the exception of household smoking, which increased during the pandemic (Table 4).
Discussion
Antibiotics have become an important factor of modern healthcare [22]. However, the unnecessary use of antibiotics, especially in common viral infections is connected with development of bacterial resistance [23–26]. The increase in
In our study, the
Our study is the first report of antibiotic susceptibility of
For another antibiotic, MTZ, the primary resistance of
We found that among the tested antibiotics, resistance to AMX was the lowest and was found in 26% of the isolated bacteria. However, compared to other observations, this percentage was alarmingly high. In southern Poland, Turkey, and Israel,
Furthermore, we observed double (for MTZ and CL as well as AMX and CL) and triple resistance in 6% and 18% of
We did not find significant demographic or clinical differences between the group of patients infected by resistant strains and the group infected by susceptible strains. Similar results were noted by Ogata et al [43].
The strength of our study is that we performed the first assessment of
The limitation of the study is the small number of subjects. This could be partially explained by the COVID-19 pandemic, which resulted in a reduction in the number of EGDs performed. In addition, we could not compare our results with the adult population from our region because there are not such data. Moreover, conventional methods for antibiotic susceptibility testing include culture, which is considered the reference method for diagnosing infection. However, it has a high risk of failure due to the particular growth requirements of
Conclusions
In this study, carried out for the first time in northeast Poland to assess the antibiotic susceptibility of
Figures
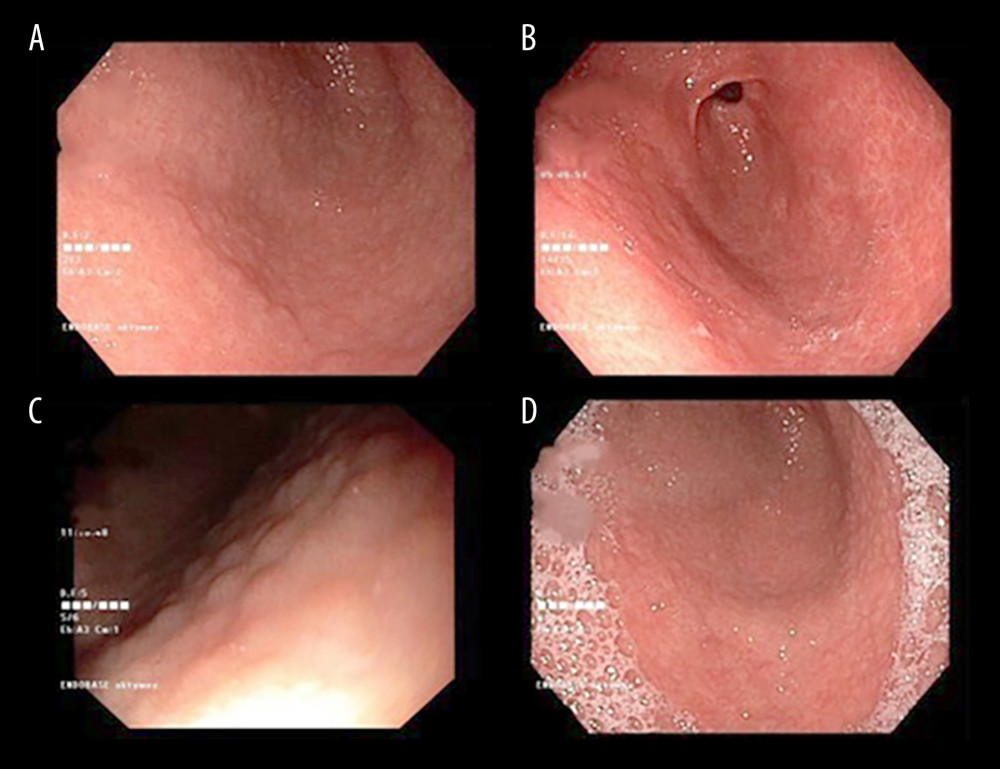 Figure 1. (A–D) Endoscopy images of the gastric mucosa of patients with H. pylori infection.
Figure 1. (A–D) Endoscopy images of the gastric mucosa of patients with H. pylori infection. 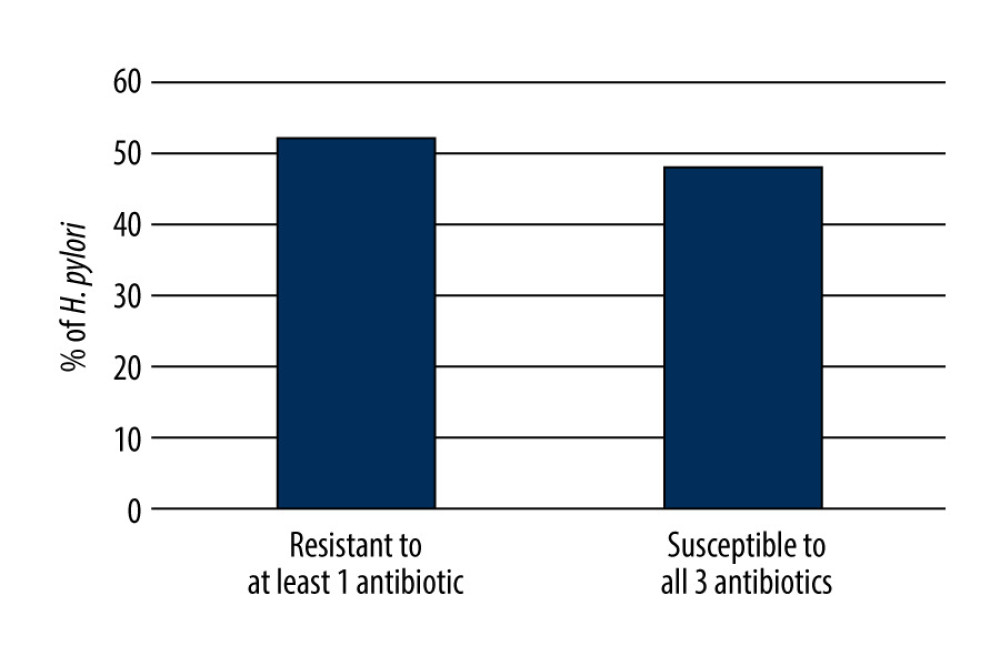 Figure 2. Resistance of H. pylori strains isolated from treatment-naïve children to at least 1 antimicrobial drug.
Figure 2. Resistance of H. pylori strains isolated from treatment-naïve children to at least 1 antimicrobial drug. 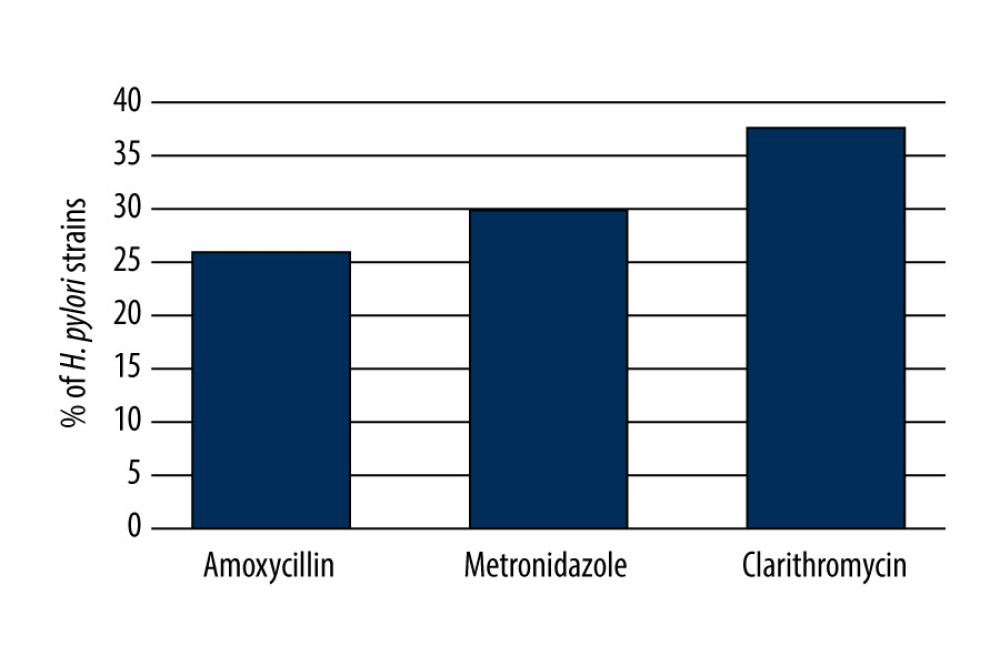 Figure 3. Resistance of H. pylori strains isolated from treatment-naïve children to each antimicrobial drug.
Figure 3. Resistance of H. pylori strains isolated from treatment-naïve children to each antimicrobial drug. 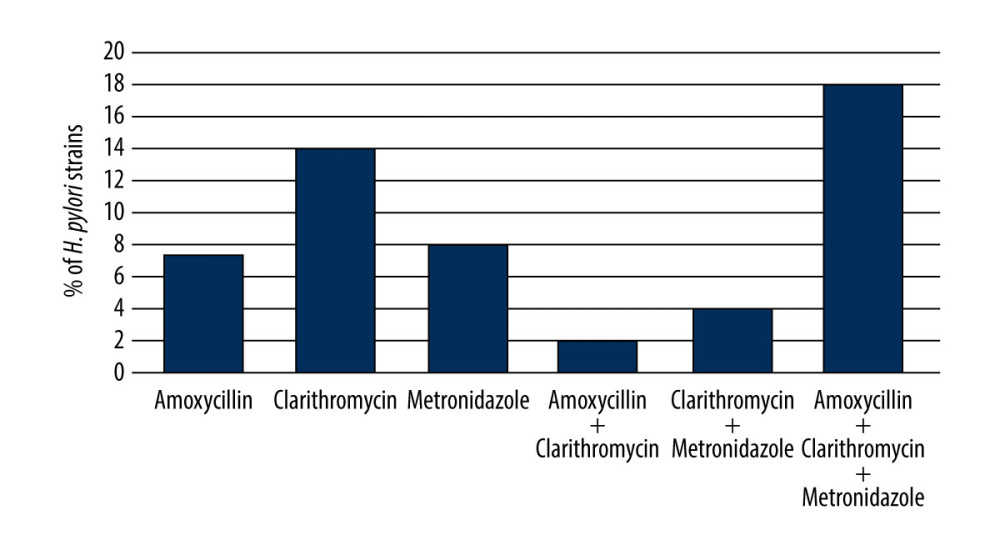 Figure 4. The frequency of resistance to 1, 2, or 3 antibiotics among H. pylori strains.
Figure 4. The frequency of resistance to 1, 2, or 3 antibiotics among H. pylori strains. Tables
Table 1. Characteristics of patients with positive H. pylori culture results (n=50).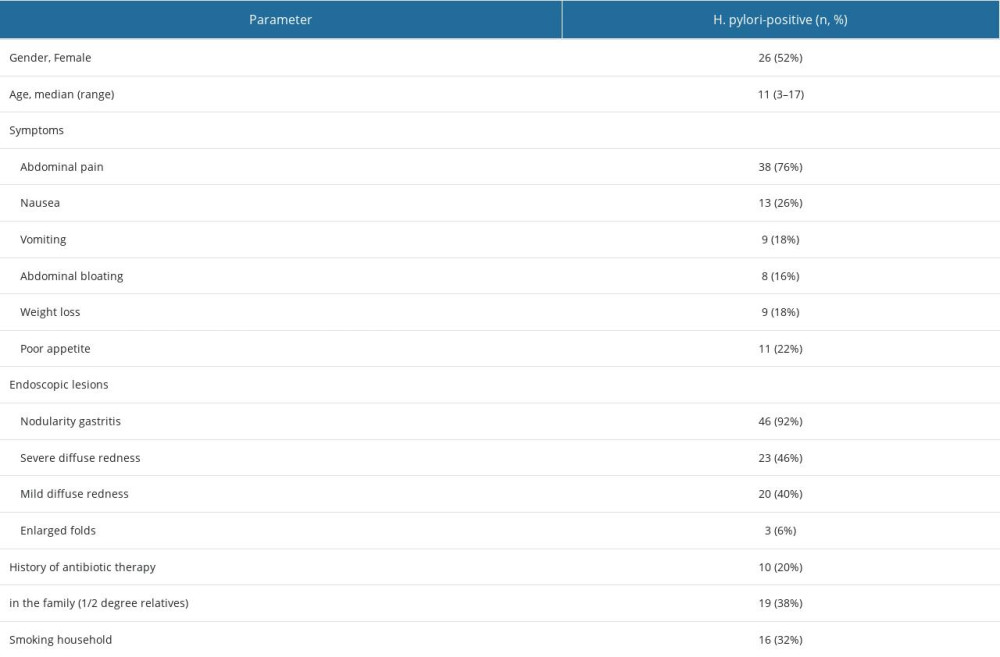 Table 2. Characteristics of patients stratified by age of patients.
Table 2. Characteristics of patients stratified by age of patients.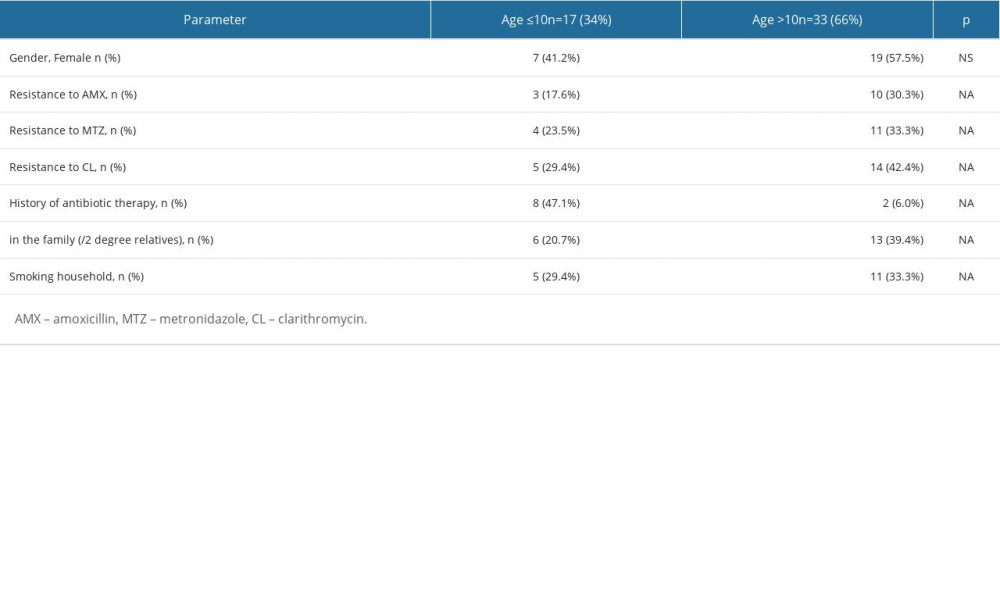 Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups.
Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups.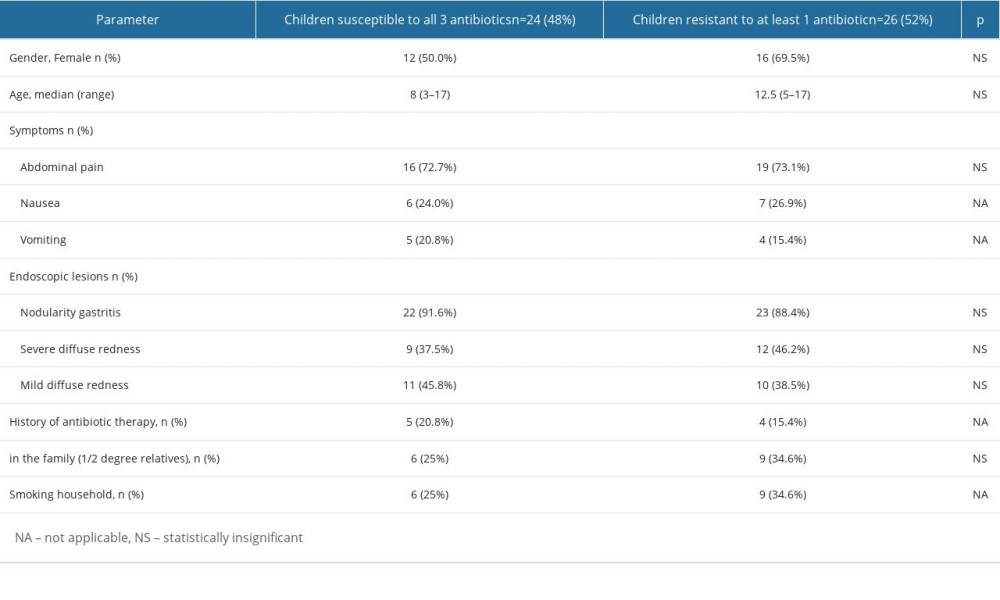 Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic).
Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic).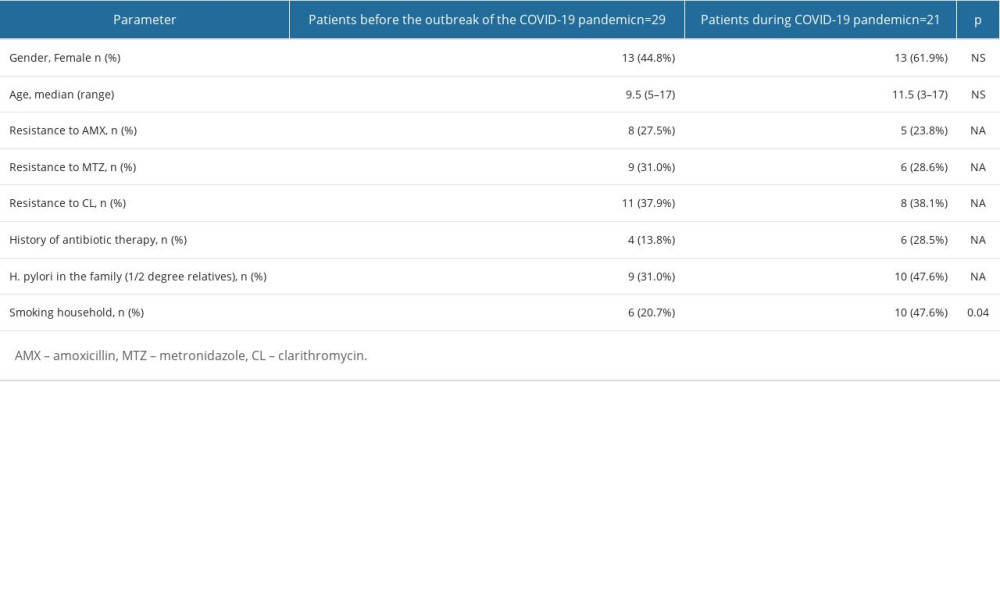
References
1. Rosu OM, Gimiga N, Stefanescu G: J Clin Med, 2022; 11(9); 2432
2. Cho J, Prashar A, Jones NL, Moss SF: Gastroenterol Clin North Am, 2021; 50(2); 261-82
3. Savoldi A, Carrara E, Graham DY: Gastroenterology, 2018; 155(5); 1372-82e17
4. Peretz A, Paritsky M, Nasser O: J Antibiot (Tokyo), 2014; 67(8); 555-57
5. Parikh NS, Ahlawat R, Helicobacter pylori: StatPearls August 8, 2022, Treasure Island (FL), StatPearls Publishing
6. Yuan C, Adeloye D, Luk TT: Lancet Child Adolesc Health, 2022; 6(3); 185-94
7. Borka Balas R, Meliţ LE, Mărginean CO: Children (Basel), 2023; 10(2); 403
8. Okuda M, Lin Y, Kikuchi S: Adv Exp Med Biol, 2019; 1149; 107-20
9. Wu W, Leja M, Tsukanov V: J Int Med Res, 2020; 48(5); 300060520926036
10. Brenner H, Rothenbacher D, Bode G, Adler G: BMJ, 1997; 315(7121); 1489-92
11. Ferro A, Morais S, Pelucchi C: Eur J Cancer Prev, 2019; 28(5); 390-96
12. Ogihara A, Kikuchi S, Hasegawa A: J Gastroenterol Hepatol, 2000; 15(3); 271-76
13. Camargo MC, Piazuelo MB, Mera RM: Acta Gastroenterol Latinoam, 2007; 37(4); 238-45
14. Liu DS, Wang YH, Zhu ZH: Antimicrob Resist Infect Control, 2019; 8; 192
15. Sabbagh P, Javanian M, Koppolu V: Eur J Clin Microbiol Infect Dis, 2019; 38(6); 1035-45
16. Chobot A, Porębska J, Krzywicka A: Acta Paediatr, 2019; 108(8); 1535-40
17. Jones NL, Koletzko S, Goodman K: J Pediatr Gastroenterol Nutr, 2017; 64(6); 991-1003
18. Manfredi M, Gismondi P, Maffini V: Gastroenterol Res Pract, 2015; 2015; 717349
19. Boyanova L, Hadzhiyski P, Gergova R, Markovska R: Antibiotics (Basel), 2023; 12(2); 332
20. Tshibangu-Kabamba E, Yamaoka Y: Nat Rev Gastroenterol Hepatol, 2021; 18(9); 613-29
21. Toyoshima O, Nishizawa T, Koike K: World J Gastroenterol, 2020; 26(5); 466-77
22. Ghosh D, Veeraraghavan B, Elangovan R, Vivekanandan P, Antibiotic resistance and epigenetics: More to it than meets the eye: Antimicrob Agents Chemother, 2020; 64(2); e02225-19
23. Bengtsson-Palme J, Kristiansson E, Larsson DGJ, Environmental factors influencing the development and spread of antibiotic resistance: FEMS Microbiol Rev, 2018; 42(1); fux053
24. Huemer M, Mairpady Shambat S, Brugger SD, Zinkernagel AS, Antibiotic resistance and persistence-Implications for human health and treatment perspectives: EMBO Rep, 2020; 21(12); e51034
25. Shrestha P, Cooper BS, Coast J, Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use: Antimicrob Resist Infect Control, 2018; 7; 98
26. Subramaniam G, Girish M, Antibiotic resistance – a cause for reemergence of infections: Indian J Pediatr, 2020; 87(11); 937-44
27. Koletzko S, Richy F, Bontems P: Gut, 2006; 55(12); 1711-16
28. Regnath T, Raecke O, Enninger A, Ignatius R: Helicobacter, 2017; 22(1), doi: 10.1111/hel.12327
29. Krzyżek P, Pawełka D, Iwańczak B: Antibiotics (Basel), 2020; 9(5); 228
30. Vécsei A, Kipet A, Innerhofer A: Helicobacter, 2010; 15(3); 214-20
31. Botija G, García Rodríguez C, Recio Linares A: An Pediatr (Engl Ed), 2021; 95(6); 431-37
32. Silva GM, Silva HM, Nascimento J: Helicobacter, 2018; 23(5); e12528
33. Butenko T, Jeverica S, Orel R, Homan M: Helicobacter, 2017; 22(5); e12400
34. Maçin S, Demir H, Özen H: Turk J Pediatr, 2015; 57(3); 254-57
35. Helmbold L, Ghebremedhin B, Bellm A: Pathogens, 2022; 11(2); 178
36. Liu DS, Wang YH, Zhu ZH: Antimicrob Resist Infect Control, 2019; 8; 192
37. Shu X, Ye D, Hu C: Sci Rep, 2022; 12(1); 17754
38. Li L, Ke Y, Yu C: Helicobacter, 2017; 22(3), doi: 10.1111/hel.12373
39. Yousefi-Avarvand A, Vaez H, Tafaghodi M: Microb Drug Resist, 2018; 24(7); 980-86
40. Kori M, Yahav J, Berdinstein R, Shmuely H: Isr Med Assoc J, 2017; 19(12); 747-50
41. Montes M, Villalon FN, Eizaguirre FJ: Helicobacter, 2015; 20(3); 169-75
42. Shu X, Yin G, Liu M: Helicobacter, 2018; 23(3); e12481
43. Ogata SK, Godoy AP, da Silva Patricio FR, Kawakami E: J Pediatr Gastroenterol Nutr, 2013; 56(6); 645-48
Figures
 Figure 1. (A–D) Endoscopy images of the gastric mucosa of patients with H. pylori infection.
Figure 1. (A–D) Endoscopy images of the gastric mucosa of patients with H. pylori infection. Figure 2. Resistance of H. pylori strains isolated from treatment-naïve children to at least 1 antimicrobial drug.
Figure 2. Resistance of H. pylori strains isolated from treatment-naïve children to at least 1 antimicrobial drug. Figure 3. Resistance of H. pylori strains isolated from treatment-naïve children to each antimicrobial drug.
Figure 3. Resistance of H. pylori strains isolated from treatment-naïve children to each antimicrobial drug. Figure 4. The frequency of resistance to 1, 2, or 3 antibiotics among H. pylori strains.
Figure 4. The frequency of resistance to 1, 2, or 3 antibiotics among H. pylori strains. Tables
 Table 1. Characteristics of patients with positive H. pylori culture results (n=50).
Table 1. Characteristics of patients with positive H. pylori culture results (n=50). Table 2. Characteristics of patients stratified by age of patients.
Table 2. Characteristics of patients stratified by age of patients. Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups.
Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups. Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic).
Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic). Table 1. Characteristics of patients with positive H. pylori culture results (n=50).
Table 1. Characteristics of patients with positive H. pylori culture results (n=50). Table 2. Characteristics of patients stratified by age of patients.
Table 2. Characteristics of patients stratified by age of patients. Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups.
Table 3. Characteristics of demographic data, laboratory parameters, endoscopic image, and clinical symptoms of the studied groups. Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic).
Table 4. Characteristics of patients based on the time of EGD performed (before outbreak of the COVID-19 pandemic vs during the COVID-19 pandemic). In Press
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








