11 November 2023: Clinical Research
Comparative Analysis of Symptomatology in Hospitalized Children with RSV, COVID-19, and Influenza Infections
Weronika M. Balas1BCDEF, Andrzej ŚliwczyńskiDOI: 10.12659/MSM.941229
Med Sci Monit 2023; 29:e941229
Abstract
BACKGROUND: The clinical course of respiratory syncytial virus (RSV), SARS-CoV-2, and influenza infections comprises many non-specific symptoms, which makes diagnosis difficult. The aim of this study was to retrospectively analyze the symptomatology of these infections in children and to search for correlations between them.
MATERIAL AND METHODS: A total of 121 children with a positive RSV (n=61), influenza (n=31), or SARS-CoV-2 (n=29) antigen test were enrolled in this retrospective analysis. Children were aged up to 71 months (median, 8 months). The collected data were collated by performing statistical analysis using the chi-square test and comparing the results using OR (odds ratio) and 95%CI (confidence interval).
RESULTS: There was a higher risk of fever in children with influenza than in those with RSV. Patients infected with RSV had a higher risk of nasal blockage than those with SARS-CoV-2. Dyspnea was more common in RSV infection than in influenza. Severe, sleep-awakening cough was more frequent in children with RSV than in those with COVID-19. Influenza was more prevalent in children aged >24 months than in those aged 7-24 months. RSV-infected children had a higher risk of numerous auscultatory changes compared to those with SARS-CoV-2. In the case of RSV infection, symptoms requiring hospitalization occurred later than in SARS-CoV-2 infection.
CONCLUSIONS: Children aged >24 months were at higher risk of contracting influenza. Numerous auscultatory changes, nasal blockage, and dyspnea were more common in children with RSV. There was a higher risk of dyspnea in children with RSV. Fever was more frequent in children with influenza. However, none of the symptoms clearly indicated the etiology of the infection.
Keywords: COVID-19, Respiratory Syncytial Virus Infections, Influenza, Human, Signs and Symptoms, Child, Hospitalized
Background
The World Health Organization (WHO) declared the COVID-19 pandemic in March 2020 [1]. Because of this, a number of restrictions were introduced in many countries [2], which led to significant changes in the seasonal patterns of the circulation of respiratory viral infections, including influenza and respiratory syncytial virus (RSV) [3]. The decline in these infections was most notable at the onset of the COVID-19 pandemic [4,5]. However, this pattern changed in 2021, when a so-called ‘compensatory epidemic’ was noticed. At that time, the incidence of RSV- and influenza-associated diseases increased dramatically and far exceeded the levels observed in previous autumn and winter seasons [3]. In addition, a change in the median age of patients, more severe courses of infection, and atypical RSV and influenza seasons were observed in Japan, Australia, and America [6–9]. The classic epidemiology and symptomatology of these infections therefore needs updating and further observation.
Respiratory syncytial virus is a highly infectious pathogen, transmitted by droplets and through direct contact. At 2 years of age, approximately 95% of children have antibodies indicative of contact with the virus [10]. Symptomatology of the infection varies from mild upper respiratory tract infections to severe lower respiratory tract infections, including bronchiolitis [4].
Influenza infection is contracted by droplets and through direct contact. The infection progresses with fever, headache, cough, runny nose, pharyngitis, and general malaise. The most severely affected group of pediatric patients are children under 5 years of age, with 1000 out of 100 000 children requiring hospitalization each year [11]. Influenza poses a serious health risk for many patients, not only because of the severe primary infection, but also because of the high rate of complications associated with it [12].
The aim of the present study was to retrospectively analyze the incidence and symptomatology of RSV, influenza, and SARS-CoV-2 infection in children hospitalized at the Department of Pediatric and Neonatal Medicine, Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw from September 2022 to January 2023.
Material and Methods
For this retrospective study, were enrolled 121 patients with confirmed RSV, influenza, or SARS-CoV-2 infection at the Department of Pediatric and Neonatal Medicine of the Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw. The study included participants who were aged up to 71 months, had been diagnosed with RSV, SARS-CoV-2, or influenza infection, and had been hospitalized in the unit. Those above 71 months of age or receiving patient care only in a hospital emergency department or outpatient clinic were excluded from the ongoing study. The observation period lasted 153 days and included children hospitalized between 1 September 2022 and 31 January 2023. Indications for hospitalization of the child included: restlessness, impaired consciousness, febrile convulsions, occurrence of apnea, tachypnoea, oxygen saturation levels below 92%, age less than 3 months old, difficulty in feeding and maintaining hydration, and complications of infection including pneumonia, comorbidities, and poor economic conditions of the family [4,37]. After discharge from the hospital, patients were no longer under observation. The Rapid-VIDITEST RSV test, or a colorimetric immunochromatographic assay that detects RSV virus antigens in nasal secretion samples, was used to confirm RSV infection [13]. The sensitivity of the test was 95% and the specificity was >99%. SARS-CoV-2 virus was identified by Abbott Panbio™ rapid antigen test by nasal swab collection (test sensitivity 93%, specificity 99%) [14] and by detection of genetic material by RT-PCR assay using the Roche cobas Liat analyzer [15]. The swab collection process was conducted as follows: Initially, a specific amount of buffer was inserted into the extraction tube. Subsequently, a nasopharyngeal swab was acquired, using a twisting motion of at least 5 turns. This swab was then introduced into the buffer-filled tube, and the contents were thorough mixed. Then, a few drops from this mixture were carefully applied onto the designated test plate. Following waiting 15–20 min, the resulting test outcome became discernible [38]. During RT-PCR, 30 or fewer cycles (Ct ≤30) were required to amplify viral RNA to reach detectable levels. Ct values are inversely proportional to viral load: a lower Ct value indicates a higher concentration of viral RNA, while a higher Ct value suggests less viral RNA in the sample [38]. Influenza virus type A and B antigens were detected using an immunochromatographic test, a qualitative Rapid test manufactured by HUMASIS (test specificity, 100%). Clinical symptoms, laboratory findings (eg, CRP, leukocytosis, and oxygen saturation), and the course of the disease in each patient were evaluated during the study. Correlations, or lack of them, were sought between symptoms and type of infection as well as age groups (0–6 months, 7–24 months, >24 months) and the presence of symptoms. The study protocol was approved by the Bioethics Committee of the Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw. Informed consent was not required for retrospective analysis of medical records of hospitalized patients. The collected data were collated by performing statistical analysis using the chi-square test for independence.
Results
GENERAL CHARACTERISTICS:
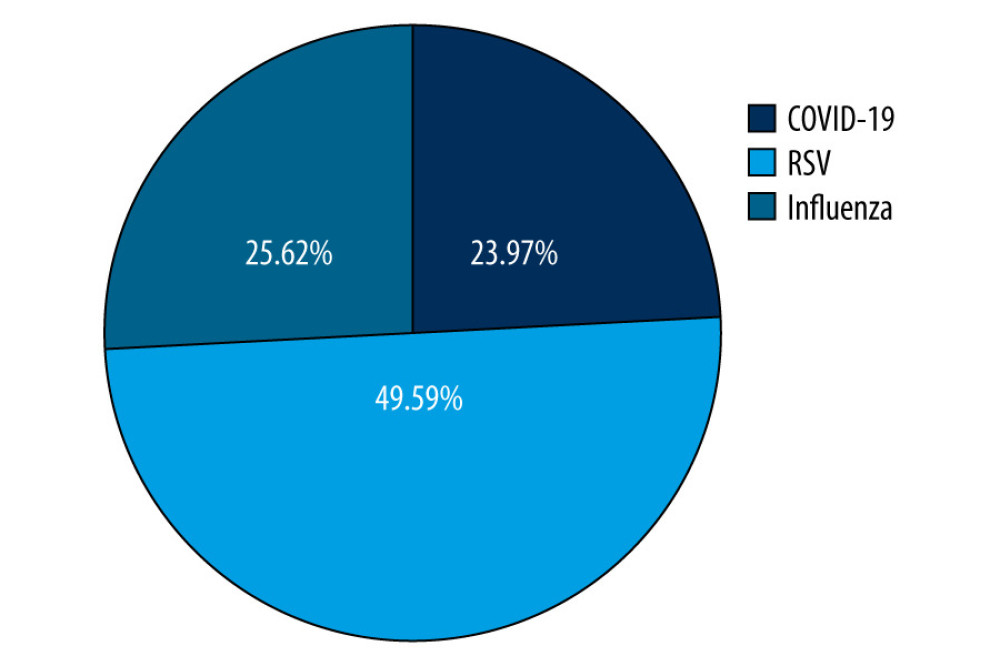
We included 71 (58.68%) boys and 50 (41.32%) girls. Patients were aged up to 71 months, with a median age of 8 months and a mean age of 18 months. Children were divided into 3 age groups: 0–6 months. [51/121; (42.1%)], 7–24 months of age [39/121; (32.2%)], and >24 months [31/121; (25.7%)]. The separate age ranges served to further investigate differences in symptomatology at successive developmental stages. The age-based grouping aimed to segregate younger children requiring more intensive attention from older children, particularly in the context of RSV infection. There were 61 patients with a positive RSV test, 31 with influenza, and 29 infected with SARS-CoV-2 (Figure 1).
COMPARISON BETWEEN AGE GROUPS AND DIAGNOSES:
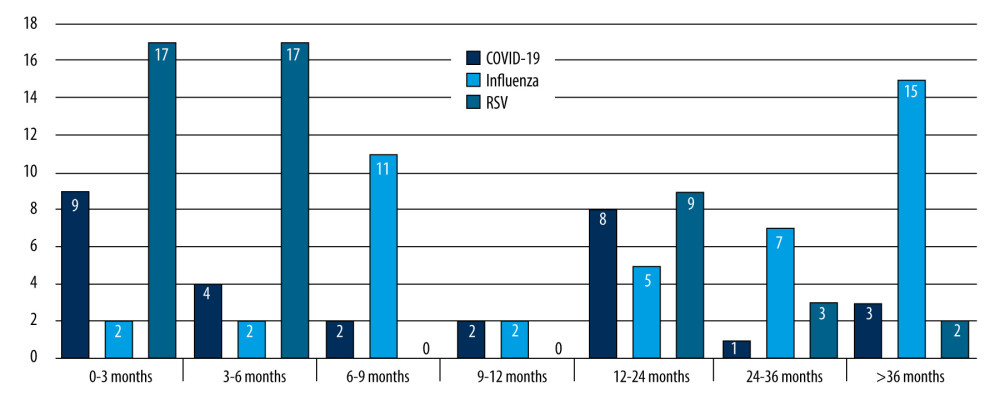
Tables 1–3 present a comparative analysis of symptoms with the etiology of infection and age group with observed symptoms. The analysis showed a correlation between the etiology of infection and the age of the patient (P<0.05, χ2) (Figure 2). Influenza infection was more frequent in children aged >24 months than in those aged 7–24 months [70% vs 16%, OR=16.62; 95% CI (4.92–56.17)]. Fever was more frequent in children with influenza than in those with RSV [97% vs 51%, OR=7.37; 95% CI (2.74–19.81)]. A correlation was observed between age group and duration of fever in RSV infections (p=0.0039, χ2). In RSV-infected patients, fever lasted longest in the 7–24 months group and shortest in children 0–6 months. A correlation was noted between cough severity and type of infection (P<0.00001, χ2). Severe, sleep-awakening cough was more prevalent in children with RSV than in those with COVID-19 [56% vs 20%, OR=5.01; 95% CI (1.78–14.09)]. Dyspnea was more common in RSV infection than in influenza [67% vs 19%, OR=8.99; 95% CI (3.17–25.54)]. Children infected with RSV had a higher risk of developing numerous auscultatory changes than children with COVID-19 [72% vs 10%, OR=78; 95% CI (18.06–336.8)]. There was a significant correlation between the presence of nasal discharge and disease in children (P<0.01, χ2). Patients infected with RSV had a higher probability of having nasal discharge than those with COVID-19 [98% vs 73%, OR=19.09; 95% CI (2.22–164.14)]. There was a correlation between the day the patient required hospitalization and the etiology of the infection (P<0.01, χ2). In the case of RSV infection, symptoms requiring hospitalization appeared later than in the case of SARS-CoV-2 infection [OR=5.87; 95% CI (1.97–17.44)]. A red, inflamed throat had a greater likelihood of occurring in children with influenza than in those with RSV [12% vs 5%, OR=8.39; 95% CI (1.82–38.61)]. The statistical analyses did not reveal a sex-dependent association with the type of disease and its severity (COVID-19 [χ2=0.6822], RSV [χ2=0.1205], influenza [χ2=0.1551]). There were no statistically significant differences between patient groups in sex distribution, hydration status, leukocytosis, decreases in saturation, type of auscultatory changes, or conjunctivitis (P<0.05, χ2).
Discussion
The COVID-19 pandemic has had a very large impact worldwide. According to WHO data, more than 6 million people died from the disease between December 2019 and the end of January 2021 [16]. Thanks to the collected data, we know that although COVID-19 initially affected mainly the respiratory tract, it also affects many organs and systems, causing a number of complications, such as multi-system inflammatory syndrome in children (MIS-C) [17]. Influenza and RSV infection also cause a significant disease burden for children worldwide due to high rates of hospitalization, morbidity, and mortality [18]. Collectively, Ujiie et al, Karlsson et al, and Li et al have noted a shift in the median age of onset due to the pandemic, along with more severe courses and atypical periods of RSV and influenza [6–9]. The classic epidemiology and symptomatology of these infections therefore needs updating and further observation. Our aim in the present study was to analyze the similarities and differences between clinical and laboratory characteristics of these 3 viral infections.
Fever and cough are very common symptoms of influenza infection and occurred in 74% of patients. Nayak et al’s research confirms this hypothesis and estimates the frequency of co-occurrence of these symptoms at 60–90% in influenza-infected patients [18]. According to the literature, the presence of nasal discharge is also a very common symptom, found in 60–90% of children [18]; in our patients, the frequency was 90%. Symptoms such as decreases in oxygen saturation, shortness of breath, and nasal blockage were rarely observed in patients. Influenza was more common in patients aged >24 months than in younger patients. We did not find confirmation of this result in many studies, as this age group was not specified. Many articles highlighted a higher risk of hospitalization of children with influenza under 5 years of age compared to older children [18,30].
The results of a systematic review of COVID-19 symptomatology clearly indicate that fever and cough are the predominant symptoms, present in about 40–60% of SARS-CoV-2-infected patients, with all other symptoms occurring much less frequently (10–20%) [21]. Typical symptoms such as nasal discharge or a reddened, inflamed throat, headache, and fatigue are sporadic in COVID-19 [21]. Furthermore, dyspnea in SARS-CoV-2 infection is described as occurring in less than 10–20% of cases [19,21]. The literature data are consistent with our results.
RSV infection was associated with a high prevalence of dyspnea and pathologic auscultatory findings of the lungs. These symptoms were similarly common in the group of patients studied by Friedman and Attia [20]; more than half of the patients experienced a severe, sleep-awakening cough, but fever was less common. This is consistent with the results of an American study [20]. In multicenter retrospective studies, a shift in the median age of RSV cases after the pandemic was evident. A study by Pruccoli et al shows that before the pandemic the median age was 4.1 months and after it 6.4 months [29]. The post-pandemic data are consistent with ours.
Our results showed that fever (ie, ≥38.6°C) was more common in patients with influenza than in COVID-19, which is supported by data presented by Yilmaz et al [19]. Fever was also more frequent in patients with influenza than in those with RSV. A prospective analysis by Friedman and Attia found a statistically significant difference in the distribution of fever between influenza and RSV (
During hospitalization, the treatment was primarily aimed at relieving symptoms, using only supportive care [31]. Fever was managed with acetaminophen, ibuprofen, or a combination of acetaminophen and ibuprofen [33]. Oxygen therapy was provided for patients experiencing respiratory failure. Neuraminidase inhibitors are recommended for influenza infection diagnosed within 48 h after symptom onset [32]. During hospitalization of a patient infected with SARS-CoV2, various treatments are available depending on the virus variant, the patient’s condition, and the presence of risk factors for progression [34]. The numerous treatment options are the result of an evolution driven by a combination of clinical observations and research findings. Overall, COVID-19 treatment has evolved from a focus on symptom relief to more targeted and evidence-based approaches. Patients at high risk of progression to severe COVID-19 are recommended to receive monoclonal antibodies (eg, casirivimab with imdevimab, or sotrovimab) or antiviral drugs (eg, remdesivir, nirmatrelvir with ritonavir, or molnupiravir) [31,35]. For the treatment of children with COVID-19 who receive remdesivir and oxygen therapy, dexamethasone is recommended. The addition of steroids to treatment is a critical advance in managing the cytokine storm associated with severe cases. Other medications for use in severely ill children requiring oxygen therapy include a JAK-1 inhibitor (baricitinib) and an IL-6 interleukin inhibitor (tocilizumab) [31]. Additionally, the administration of thromboprophylaxis should be considered unless contraindicated [36]. Each patient should be approached individually depending on age, the clinical course of the disease, and factors that increase the risk of COVID-19 progression. The rapid development of vaccines highlights the significance of prevention rather than focusing only on treatment.
Our study has had some limitations. The study data were obtained retrospectively from the medical records of patients hospitalized at 1 center. The availability of data was limited to what has been documented in the past, which might not cover all the relevant outcomes of interest, thus limiting the scope of the study. Additionally, the data collected may suffer from information error if there were inaccuracies in the records used for the analysis. Another limitation of the study is that there were fewer patients with influenza and COVID-19 than patients with RSV. The last limitation of the analysis was that patient follow-up was confined to only 1 infectious season.
Conclusions
Children aged >24 months had a higher risk of contracting influenza than did younger children.
Numerous auscultatory changes, dyspnea, nasal blockage, and severe cough were more frequent in children with RSV than in those with COVID-19. Influenza was associated with a higher prevalence of fever compared to RSV. However, none of the symptoms clearly indicated the etiology of the infection. After the pandemic, there was a shift in the median age at RSV infection.
Tables
Table 1. Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values.![Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values.](https://jours.isi-science.com/imageXml.php?i=t1-medscimonit-29-e941229.jpg&idArt=941229&w=1000) Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values.
Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values.![Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values.](https://jours.isi-science.com/imageXml.php?i=t2-medscimonit-29-e941229.jpg&idArt=941229&w=1000) Table 3. Comparison of symptom prevalence between different diagnoses.
Table 3. Comparison of symptom prevalence between different diagnoses.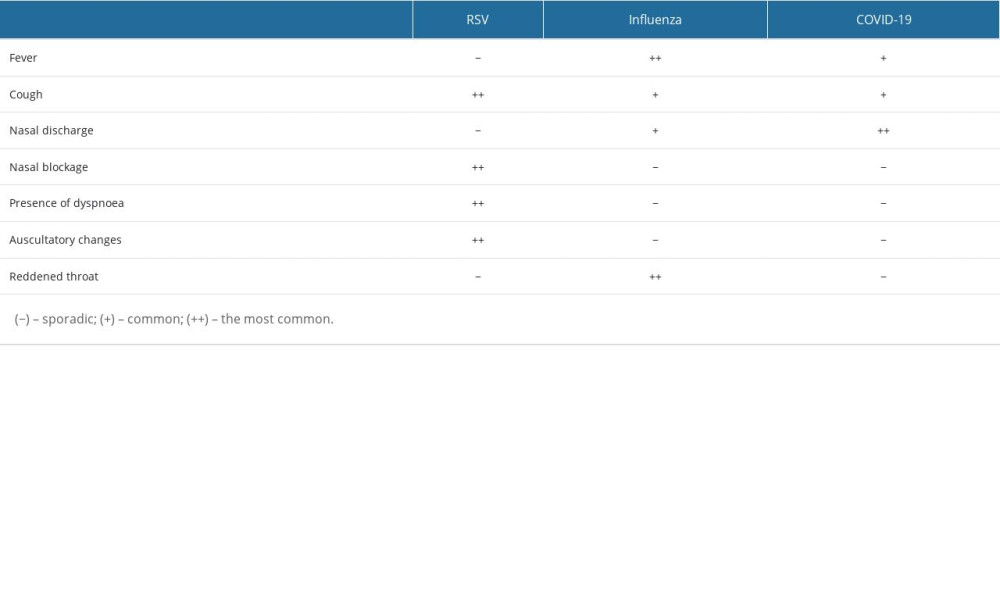
References
1. Sybilski AJ, Dylematy pediatry w dobie pandemii: Medycyna Faktów, 2020; 13(3); 344-49 [in Polish]
2. Toba N, Gupta S, Ali AY, COVID-19 under 19: A meta-analysis: Pediatr Pulmonol, 2021; 56; 1332-41
3. Chow EJ, Uyeki TM, Chu HY, The effects of the COVID-19 pandemic on community respiratory virus activity: Nat Rev Microbiol, 2023; 21(3); 195-210
4. Pogonowska M, Guzek A, Gościńska A, Compensatory epidemic of RSV infections during the COVID-19 pandemic. An analysis of infections in children hospitalised in the Department of Paediatrics, Paediatric Nephrology and Allergology of the Military Medical Institute in Warsaw in 2020–2021: Pediatr Med Rodz, 2022; 18; 52-57
5. Li Y, Wang X, Blau DM, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis: Lancet, 2022; 399; 2047-64
6. Karlsson EA, Mook PAN, Vandemaele K, Review of global Influenza circulation, late 2019 to 2020, and the impact of the COVID-19 pandemic on Influenza circulation: Wkly Epidemiol Rec, 2021; 96; 241-64
7. Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N, Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan: Emerg Infect Dis, 2021; 27; 2969-70
8. von Hammerstein AL, Aebi C, Barbey F, Interseasonal RSV infections in Switzerland – rapid establishment of a clinician-led national reporting system (RSV EpiCH): Swiss Med Wkly, 2021; 151; w30057
9. Foley DA, Yeoh DK, Minney-Smith CA, The interseasonal resurgence of respiratory syncytial virus in australian children following the reduction of coronavirus disease 2019 – related public health measures: Clin Infect Dis, 2021; 73; e2829-e30
10. Glezen WP, Taber LH, Frank AL, Kasel JA, Risk of primary infection and reinfection with respiratory syncytial virus: Am J Dis Child, 1986; 140; 543-46
11. Caini S, Spreeuwenberg P, Kusznierz GF, Distribution of Influenza virus types by age using case-based global surveillance data from twenty-nine countries, 1999–2014: BMC Infect Dis, 2018; 18; 269
12. Bennet R, Hamrin J, Wirgart BZ, Influenza epidemiology among hospitalized children in Stockholm, Sweden 1998–2014: Vaccine, 2016; 34; 3298-302
13. : Rapid-VIDITEST RSV Available from: https://www.listarfish.it/product_files/30103/VID-ODZ-065%20EN_RSV_card_10-2014-A.pdf
14. : In vitro diagnostic rapid test for qualitative detection of SARS-CoV-2 antigen (Ag) Available from: https://dam.abbott.com/en-gb/panbio/120007883-v1-Panbio-COVID-19-Ag-Nasal-AsymptomaticSe.pdf
15. : Letter of Authorization- ROCHE SARS-CoV-2 Nucleic acid test for use on the cobas Liat System Available from:https://www.fda.gov/media/150274/download
16. World Health Organization: Coronavirus (COVID-19) Dashboard. Daily counts of COVID-19 cases, deaths and vaccinations May 17, 2023 Available from: https://covid19.who.int/
17. Dufort EM, Koumans EH, Chow EJ, Multisystem inflammatory syndrome in children in New York State: N Engl J Med, 2020; 383; 347-58
18. Nayak J, Hoy G, Gordon A, Influenza in children: Cold Spring Harb Perspect Med, 2021; 11; a038430
19. Yılmaz K, Şen V, Aktar F, Does COVID-19 in children have a milder course than Influenza?: Int J Clin Pract, 2021; 75; e14466
20. Friedman MJ, Attia MW, Influenza A in young children with suspected respiratory syncytial virus infection: Acad Emerg Med, 2003; 10; 1400-3
21. Viner RM, Ward JL, Hudson LD, Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents: Arch Dis Child, 2021; 106; 802-7
22. Howard-Jones AR, Burgner DP, Crawford NW, COVID-19 in children. II: Pathogenesis, disease spectrum and management: J Paediatr Child Health, 2022; 58; 46-53
23. Henry BM, Benoit SW, de Oliveira MHS, Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review: Clin Biochem, 2020; 81; 1-8
24. Cui X, Zhao Z, Zhang T, A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19): J Med Virol, 2021; 93; 1057-69
25. Prais D, Schonfeld T, Amir JIsraeli Respiratory Syncytial Virus Monitoring Group, Admission to the Intensive Care Unit for respiratory syncytial virus bronchiolitis: a national survey before palivizumab use: Pediatrics, 2003; 112; 548-52
26. Dougherty NN, Meissner HC, Respiratory syncytial virus immunoprophylaxis: Impact on epidemiology: Paediatr Drugs, 2000; 2; 127-32
27. Moler FW, Steinhart CM, Ohmit SE, Stidham GL, Effectiveness of ribavirin in otherwise well infants with respiratory syncytial virus-associated respiratory failure. Pediatric Critical Study Group: J Pediatr, 1996; 128; 422-28
28. Pormohammad A, Ghorbani S, Khatami A, Comparison of Influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings: Rev Med Virol, 2021; 31; e2179
29. Pruccoli G, Castagno E, Raffaldi I, The importance of RSV epidemiological surveillance: A multicenter observational study of RSV infection during the COVID-19 pandemic: Viruses, 2023; 15; 280
30. Poehling KA, Edwards KM, Weinberg GA, The underrecognized burden of Influenza in young children: N Engl J Med, 2006; 355; 31-40
31. Marczyńska M, Pokorska-Śpiewak M, Talarek EUpdated recommendations for the prevention, diagnosis and treatment of COVID-19 in children in Poland. Recommendations for pediatricians and general practitioners (18.02.22): Przegląd Pediatryczny, 2022; 51(1); 7-17 [in Polish]
32. Uyeki TM, Bernstein HH, Bradley JS, Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza [published erratum appears in Clin Infect Dis. 2019;68(10):1790]: Clin Infect Dis, 2019; 68(6); e1-e47
33. Sobolewska-Pilarczyk M, Pokorska-Śpiewak M, Stachowiak A, COVID-19 infections in infants: Sci Rep, 2022; 12(1); 7765
34. Nittari G, Pallotta G, Amenta F, Tayebati SK, Current pharmacological treatments for SARS-COV-2: A narrative review: Eur J Pharmacol, 2020; 882; 173328
35. Ahmed A, Rojo P, Agwu A, Remdesivir treatment for COVID-19 in hospitalized children: CARAVAN interim results: Virtual Feb 12–16, 2022
36. Sochet AA, Morrison JM, Jaffray J, Enoxaparin thromboprophylaxis in children hospitalized for COVID-19: A phase 2 trial: Pediatrics, 2022; 150(1); e2022056726
37. Makowiec-Dyrda M, Tomasik T, Windak A, Profilaktyka i leczenie grypy: Wytyczne Kolegium Lekarzy Rodzinnych w Polsce, 2016 [in Polish]
38. https://borpol.com.pl/wp-content/uploads/2021/11/120007864-v1-Panbio-COVID-19-Ag-Nasal-Asympt-Self-Swa-2.pdf
Figures
Tables
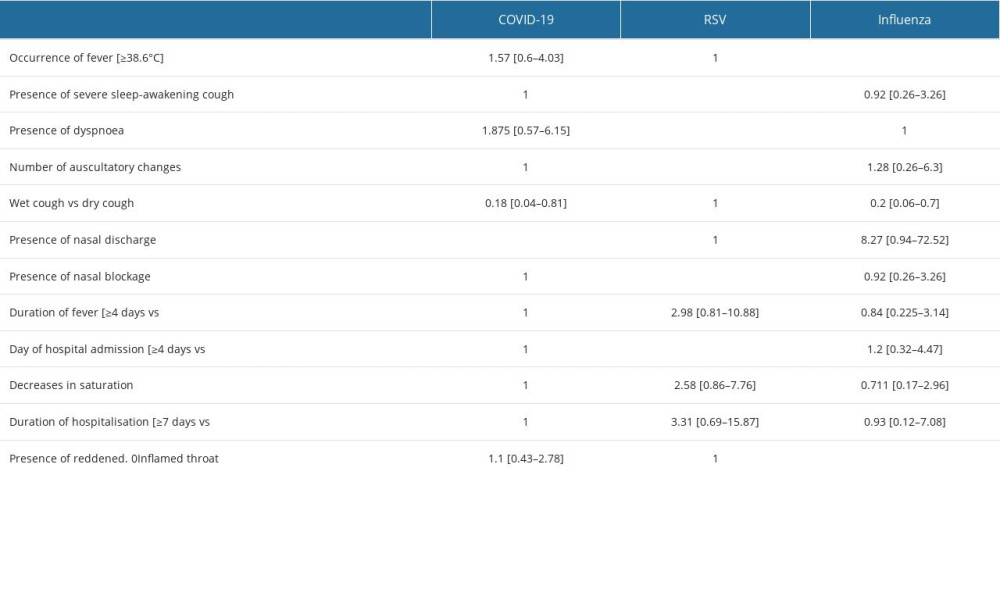 Table 1. Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values.
Table 1. Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values.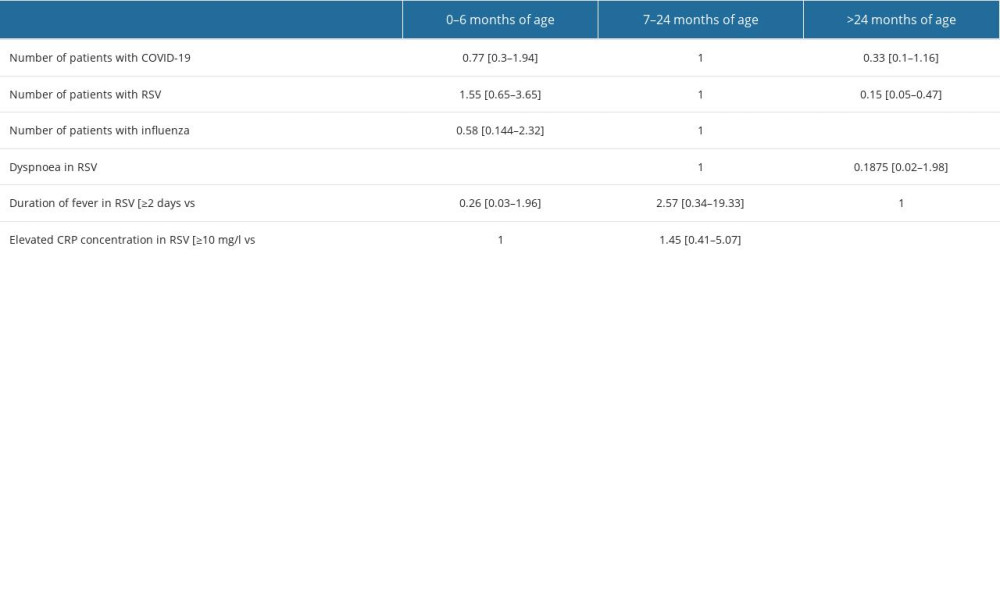 Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values.
Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values. Table 3. Comparison of symptom prevalence between different diagnoses.
Table 3. Comparison of symptom prevalence between different diagnoses. Table 1. Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values.
Table 1. Risk of the occurrence of disease symptoms depending on the diagnosis (OR; [95%CI]) – in bold statistically significant values. Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values.
Table 2. Occurrence of symptoms depending on the age of the child diagnosis (OR; [95%CI]) – in bold statistically significant values. Table 3. Comparison of symptom prevalence between different diagnoses.
Table 3. Comparison of symptom prevalence between different diagnoses. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952