03 October 2023: Clinical Research
Evaluating the Efficacy of Platelet-Rich Fibrin Matrix versus Subepithelial Connective Tissue Grafts in Dental Root Coverage: A Comparative Study Using Modified Ruben’s Technique
Justina P. Louis1DEF, Jasmine M. CrenaDOI: 10.12659/MSM.941473
Med Sci Monit 2023; 29:e941473
Abstract
BACKGROUND: Dental root coverage, crucial in managing gingival recessions, traditionally utilizes subepithelial connective tissue grafts. However, this approach has limitations such as donor site morbidity and graft availability. Recent studies have introduced platelet-rich fibrin (PRF) as an alternative, leveraging its regenerative potential and growth factors. Despite the promise, comparative assessments between PRF and conventional grafts remain limited. This research probes whether PRF, when used beneath a modified Ruben's mixed flap, could provide comparable or superior dental root coverage than a subepithelial connective tissue graft.
MATERIAL AND METHODS: We enrolled 30 patients exhibiting Miller's class I and II recession in this comparative case series. Patients were randomly assigned to receive either a connective tissue graft (15 patients) or a PRF matrix (15 patients), both covered by a modified Ruben's mixed flap.
RESULTS: Clinical parameters, including full mouth plaque scores, bleeding scores, probing sulcus depth, clinical attachment level, gingival position assessment, width, and thickness of attached gingiva, were assessed in both the control and test groups at baseline, 6 months, and 12 months post-surgery. Significant differences were observed at all intervals.At the 12-month mark, the control group (connective tissue graft) achieved 91% complete root coverage, while the test group (PRF matrix) achieved 86%. However, this difference was not statistically significant.
CONCLUSIONS: The study outcomes suggest comparable gains in root coverage and attached gingiva between the connective tissue graft and PRF matrix groups. Thus, the results support our hypothesis that a subepithelial PRF matrix can serve as a viable alternative to a subepithelial connective tissue graft for treating dental root coverage.
Keywords: autografts, Blood Platelets, Connective Tissue, Fibrin, gingival recession, Humans, Platelet-Rich Fibrin, Gingiva, Treatment Outcome, Tooth Root
Background
Tissue engineering requires an intricate interplay between native cells, growth factors, and signaling molecules, as well as the matrix, which serves as a framework for regeneration [1]. The key to tissue engineering is stimulating a series of events that cascade in a temporal sequence, which results in a coordinated cellular migration into the defect from the surrounding tissues. One of the challenges of both hard and soft tissue regeneration is to provide a bioactive matrix, so that, even while providing the framework for cellular migration, the matrix would be able to leach certain growth factors and signaling molecules required for cellular migration into the matrix. The ideal scaffold therefore serves as a mesh for the extracellular cells and growth factors, while serving as a matrix that facilitates and regulates cellular processes like mitosis, cell synthesis, and migration [2]. Gingival recession is defined as exposure of the root surface, due to the apical displacement of the gingival margin from the cemento-enamel junction [3–5]. Although gingival recession frequently occurs without any symptoms, patient-related concerns due to gingival recession are dentinal hypersensitivity from exposed root surfaces, apprehension regarding tooth loss, poor esthetics, inability to maintain proper oral hygiene, and bleeding gums.
Procedures used to correct gingival recession are broadly categorized into free gingival autograft and its variants, pedicle grafts, and matrices for soft tissue augmentation. The sub-pedicle connective tissue graft is often considered as the criterion standard, owing to the high percentage of predictable root coverage reported by different studies [6,7]. Studies with subepithelial connective tissue graft [8] and acellular dermal matrix allograft have shown predictable root coverages of 96% and 89.1%, respectively. Another study by Sedon et al conducted in 2 groups of patients treated with subepithelial connective tissue graft and an acellular dermal matrix-guided tissue regeneration membrane revealed predictable root coverage of 98.9% and 92.3%, respectively [9].
The pedicle graft technique, which involves a bilaminar technique, uses dual blood supply to the soft tissue graft, which increases the potential for optimal healing using this technique. Matrices have been used for root coverage with a high degree of success. The techniques commonly use either a guided tissue regeneration membrane [10] or an acellular dermal matrix [11] beneath a coronally or a laterally displaced flap to ensure predictable periodontal tissue regeneration, along with management of the esthetic deficit. The techniques that use these matrices also demonstrated a high level of success, which was comparable to the level of root coverage with a sub-pedicle connective tissue graft. There is also a much higher potential for new attachment, as evaluated in a few histological studies [12]. A histological study performed by Harris et al in their study on gingival recession coverage done with connective tissue graft and partial thickness flap showed new bone, cementum, and connective tissue attachment after 5 months of healing [13]. Also, a study by Bruno et al evaluated root coverage using a subepithelial connective tissue graft after 1 year, which histologically suggested connective tissue attachment [14]. A study has also revealed healing by the long junctional epithelium [15].
Concerns with the matrix technique, however, involve the additional cost for the matrix, despite it having a similar healing potential as connective tissue grafts. Platelet rich fibrin (PRF), a second generation platelet concentrate, is a rich source of growth factors that stimulates wound healing and thereby improves clinical success in various procedures, including root coverage procedures [16,17]. PRF has many of the characteristics that are required for an ideal matrix, including enhancing soft and hard tissue healing, so as to engineer the regeneration of the lost periodontal tissues. PRF also contains physiologically available thrombin, which results in slow polymerization of fibrinogen to fibrin, leading to a physiologic architecture that will serve as a favorable bioactive matrix for periodontal wound healing [18]. The cytokines that are present in the platelet concentrates play an important role in wound healing, with the natural polymerization of PRF resulting in incorporation of platelet-derived cytokines in a fibrin mesh, which allows the progressive release of growth factors over time (7–11 days) as the network of fibrin disintegrates. These intrinsic cytokines have an increased span of activity, as they will be released and used only at the time of matrix remodeling, which creates a long-term effect. The added advantage of PRF is the presence of a natural fibrin network that prevents growth factors from proteolysis [19].
The use of PRF as a tissue engineering scaffold has been investigated by many researchers [20], who have reported that PRF is a superior scaffold, compared with collagen, for human periosteal cell proliferation [19], bone tissue engineering, and for development of micro-revascularization, resulting in a more efficient matrix which causes cell migration and cell adhesion. PRF also has angiogenic properties, which help in the formation of new blood vessels and allow migration, division, and phenotype change of the endothelial cells. The fibrin matrix also acts like a barrier membrane, which prevents epithelial down-growth and modulates the expression of growth factors and fibroblastic cells, facilitating their migration inside the wound. The existing data validate that the PRF membrane can be considered as a 3-dimensional mesh polymerized in a specific structure, with incorporation of platelets, leukocytes, and growth factors and the presence of circulating stem cells, which enable optimal healing. Studies on PRF have long shown its success in periodontal defects, including socket augmentation [21] and gingival recession [22]. In a recent study by Chekurthi et al [22], advanced PRF was used for RT2 (recession type 2) gingival recession, demonstrating esthetic advantages. Recently, Jankovic et al performed a coronally advanced flap-PRF matrix as part of a randomized controlled trial [23]. They reported complete root coverage of 75.85% in the PRF group and 79.56% in the coronally advanced flap group. Their study highlights the potential to use PRF as a matrix for root coverage, although they did not report a high percentage of complete root coverage. A few case series and case reports suggest a high degree of root coverage with the sub-pedicle PRF matrix, especially with a laterally displaced flap, to increase the predictability of the pedicle flaps. Gautam et al showed an improvement in clinical attachment level after 6 months, resulting in an observed recession coverage of up to 80% [24]. In 2013, Singh et al assessed the modified laterally sliding flap along with PRF for class II gingival recession, and the 6-month follow-up showed 80% root coverage [25]. Oncu et al suggested the use of PRF in multiple recessions, stating that PRF is a viable alternative to the subepithelial connective tissue graft [26]. It is therefore clear that PRF has the potential to be the perfect matrix for root coverage when used with a pedicle, which has all the features of the lost periodontal tissues.
The Ruben mixed pedicle flap technique uses a partial full-thickness and a partial split-thickness pedicle flap [27] and a modification of the lateral sliding flap technique, which was originally described by Harvey in 1970 [28,29]. It is therefore proposed that the partial full-thickness segment, which is dissected from the area just adjacent to the recession site, will serve as a rich reservoir of progenitor cells, from the cambium layer of the periosteum, which could migrate into the PRF matrix. Further, when a PRF matrix is overlaid by a Ruben’s mixed-pedicle flap, we expect the transient release of growth factor from the matrix, which would initiate the growth of connective tissue elements from the periodontal ligament and periosteum into the recession site. Further keratinized tissue from the adjacent tooth could significantly increase the width of the attached gingiva, which could together secure a comprehensive regeneration of the lost periodontal tissues. The probable outcome based on a systematic review and meta-analysis by Rodas et al would be to equate the subepithelial connective tissue graft with PRF so that PRF could be used as a viable alternative to subepithelial connective tissue graft to bypass the use of morbid autografts [29].
To date, no prospective randomized controlled clinical trials have compared the efficacy of a sub-pedicle PRF matrix with a sub-pedicle connective tissue graft underlying a modified mixed Ruben’s flap technique; therefore, in the present study, we aimed to compare dental root coverage following the use of a PRF matrix or subepithelial connective tissue graft combined with the modified laterally positioned pedicle flap-revised technique (modified Ruben’s technique) in 30 patients with Millers class I and II gingival recession.
Material and Methods
SAMPLE SIZE CALCULATION:
The formula for the sample size required comparing 2 population means μ0 and μ1, with common variance, σ2, was
where
The resulting estimated sample size was 15 study participants in each group, with a power of 95%. SPSS software was used.
INCLUSION CRITERIA:
To participate in the study, patients had to present gingival recession of Miller Class I or II, age between 18 and 45 years, full mouth plaque scores ≤10%, full mouth bleeding scores v10%, and facial surface gingival recession depth ≥2 mm.
EXCLUSION CRITERIA:
Patients with the following were excluded from the study: cervical abrasion, root caries, smoking, trauma from occlusion, bruxism, non-vital teeth, pregnancy or lactation, any systemic disease with a potential influence on periodontal tissues, wound healing processes, or the immune response action, periodontal disease, sites adjacent to dental implants/edentulous sites, sites at teeth with prosthetic crowns, presence of adjacent recessions, and previous periodontal surgery.
EVALUATION OF CLINICAL PARAMETERS:
Prior to intervention, all patients underwent phase I therapy, including scaling and root planing, followed by polishing using a rubber cup to ensure optimal gingival health and minimize the inflammation prior to surgery. The patients were instructed to follow oral hygiene instructions, including correction of brushing habit (modified Stillman brushing technique).
Percentage of root coverage was calculated using the formula
The following clinical parameters were assessed at baseline, 6 months and 12 months after surgery: Full mouth plaque scores [30] were assessed as a dichotomous index, using plaque disclosing agent.
Full mouth bleeding scores [31] were assessed using gentle probing of the orifice of the gingival crevice.
MODIFIED RUBEN’S MIXED PEDICLE FLAP TECHNIQUE: After proper isolation of the surgical field, the operative site was anesthetized using 2% lignocaine hydrochloride, with adrenaline at 1: 80 000 concentration. A collar of tissue was removed around the recession by 2 vertical incisions joining at 1 point apical to the base of the recession [27].
PREPARATION OF DONOR SITE: The donor site was prepared by placing a vertical incision from the gingival margin to the oral mucosa up to the level of the tooth, which was prepared for root coverage with a No.15 surgical blade, after which a thorough root planing was done, as depicted in Figures 1 and 2.
PREPARATION OF CONNECTIVE TISSUE GRAFT: The connective tissue graft was harvested from the palate using a trap door technique, and the desired amount of connective tissue graft harvested was placed on the denuded root surface (recipient bed) and sutured using simple interrupted sutures at the coronal most portion and at the lateral portions. The flap was then slid to completely cover the sub-pedicle connective tissue graft and secured using sling sutures. The periodontal dressing (Coe-pack) was placed to cover the surgical area as depicted in Figure 1.
PRF SITE: An amount of 5 mL of blood was withdrawn in a 10-mL autoclaved test tube without an anticoagulant and was centrifuged immediately. Blood was centrifuged in a single step using a centrifugation machine (R-8C) for 10 min at 3000 rpm. The resultant product consisted of the following 3 layers: a top layer consisting of platelet-poor plasma, bottom layer consisting of red blood cells, and the middle portion consisting of a PRF clot. The PRF membrane was obtained by squeezing the fibrin clot between sheets of sterile gauze to obtain a fibrin membrane. The harvested PRF matrix was placed over the denuded root and stabilized using simple interrupted sutures at the coronal most portion and at the lateral portions. The flap was then slid to completely cover the sub-pedicle PRF matrix and secured using sling sutures, as depicted in Figure 2.
POSTSURGICAL CARE AND MEASUREMENTS: After the surgery, a periodontal dressing (Coe pack) was placed to cover the surgical area. The pre- and postoperative evaluation is shown in Figures 3 and 4. The patients were advised to abstain from mechanical oral hygiene procedures in the surgical area and also were advised to avoid pulling of lips. Chlorhexidine mouth rinse (0.12%) was used to support local plaque control for 2 weeks. Dressing and sutures were removed 12 days after surgery. Patients were recommended to follow all normal postoperative oral hygiene instructions and were prescribed analgesic medication (ibuprofen 400 mg every 8 h) for 5 days. All clinical parameters were assessed at 6 and 12 months postoperatively. All patients were instructed to maintain adequate plaque control during the course of the study. Professional maintenance care was performed at each recall visit (6 and 12 months), and the post-surgical measurements were performed as percentage of root coverage, full mouth plaque scores, full mouth bleeding scores, probing sulcus depth, clinical attachment level at the surgical site, assessment of gingival position, assessment of width of attached gingiva at the surgical site, and attachment of thickness of attached gingiva at the surgical site. No postoperative complications were encountered. Figures 3 and 4 depict the pre- and postoperative comparative photographs of the control group (connective tissue graft) and test group (PRF).
STATISTICAL ANALYSIS:
Sample size was calculated using SPSS software based on proportionate methodology, with type II β error set at 95% and type I α error set at 5%. The data were analyzed using ANOVA to analyze the mean, standard deviation, and test of significance of mean values for various parameters for the descriptive statistics for the control and test groups. Paired
Results
The descriptive statistics including age and sex were equal for the control group (28.2±4.91) and test group (26.70±5.17), with a sex distribution of male: female of 7: 8 in the control group and 9: 6 in test group; the variables were evaluated at different time intervals.
The intergroup comparisons of test and control groups showed no statistical significance when the different parameters were compared at baseline, 6 months, and 1 year (Table 1): full mouth plaque scores,
Intragroup comparisons in the control group among all clinical parameters at baseline, 6 months, and 1 year revealed statistically significant differences
Intragroup comparisons in the test group among all clinical parameters at baseline, 6 months, and 1 year revealed statistically significant differences (
Percentage of root coverage assessed by Fischer’s exact test at the 1-year time point revealed that both the control group and test group had significant increases in root coverage (91% and 86%, respectively). The intergroup comparison revealed no statistical significance (
Discussion
The results of the present study revealed that both techniques, a connective tissue graft and a PRF membrane covered by a lateral pedicle graft, are effective in the treatment of gingival recession defects, with significant root coverage of 91% and 86%, respectively, and a clinical attachment gain at 1 year of 4 mm in the connective tissue graft group and of 3.74 mm in the PRF group. In this study, we aimed to adopt a modified Ruben’s laterally displaced pedicle flap overlying a bioactive matrix PRF, which accelerates the predictability of root coverage and increases the thickness of attached gingiva, and to compare it with a sub-pedicle connective tissue graft.
Regenerative periodontal therapy has shown substantial improvement over the last 3 decades, owing to our understanding of the principles that govern periodontal wound healing, which is thanks to the prominent researchers who have helped us understand the nuances of biological wound repair [32]. This progress has taken us from the use of autogenous grafts to the use of membranes for regeneration of periodontal tissues and bioactive matrices that actively modulate postoperative periodontal wound healing. This improvement can also be explained by stringent patient and surgical site selection, and through the evolution of newer surgical techniques that result in optimal periodontal wound healing. Periodontal wound healing aims at healing with minimal or no tissue loss in which matrices help promote wound stability and wound maturation, which in turn, facilitates the release of growth factors and signaling molecules for potential regeneration of the periodontal tissues [33]. The platelet concentrate enmeshed in a fibrin network is rich in growth factors, such as platelet-derived growth factor AB, transforming growth factor, and vascular endothelial growth factor, which are released in a sustained manner for over 1 week and up to 28 days. Recent studies have also shown that PRF can stimulate the proliferation of periodontal ligament fibroblasts and osteoblasts and increase the secretion of osteoprotegerin, which is probably due to its ability to upregulate the signaling molecule, extracellular signal-regulated protein kinase (ERK).
In the present study, complete root coverage was obtained in 93% of cases in the control group and 87% of cases in the test group. In the literature, reports of mean root coverage for connective tissue graft in combination with a coronally advanced flap range from 70% to 98%. Zucchelli et al reported an 80% root coverage of treatment with a modified laterally repositioned flap along with PRF [33]. Another case report, by Bharti et al, on the application of PRF with a lateral pedicle graft, confirmed 80% root coverage [25]. In accordance with the above-mentioned studies, in the present study, we gave a higher significance of root coverage with both sub-pedicle connective tissue grafts and the sub-pedicle PRF matrix.
Out of 15 patients in the control group, 1 patient had minimal root coverage of 60%, and of 15 patients in the test group, 2 patients had minimal root coverage of 50% and 33%, respectively. The possible reason for minimal root coverage in the patients in both groups could have been inadequate maintenance of oral hygiene by the patients; as seen in Table 1, full mouth bleeding scores showed a statistically significant difference between the control and test groups at baseline. The possibility of gingival biotype playing an important factor in determining root coverage cannot be ignored. Our study used a laterally repositioned flap, thus warranting the presence of adequate attached gingiva in the site neighboring the surgical site, and all patients had adequate width of attached gingiva. Therefore, inflammation at the surgical site could have been the reason for the minimal overall difference in predictability of root coverage between the control and test groups. The possible causes of failure for the control group could be inadequate blood supply from surrounding tissues due to inadequate recipient site preparation. Also, possible failures of the test group could be PRF positioning at the cemento-enamel junction resulting in initial root exposure, which has been reported in 53% of single recessions treated, with other reasons being PRF consistency and platelet concentration.
This study demonstrated that there was no statistically significant difference in probing sulcus depth reduction and clinical attachment level gain between the 2 groups. In the test group, the mean gain of clinical attachment level was 3.74 mm, whereas it was 4 mm in the control group. This non-significant change in clinical attachment level gain was similar to that found by Cheung et al, who compared coronally advanced flap with platelet concentrates [34]. Jankovic et al presented a randomized controlled trial on PRF and connective tissue graft with an coronally advanced flap, in which they observed an attachment gain of 2.96 mm for the connective tissue graft group and an attachment gain of 2.87 mm in the test group. In comparison with the Jankovic study, the present study had a higher clinical attachment gain [23].
In the present study, the increased width of keratinized tissue in the connective tissue graft group was related to the ability of connective tissue of palatal graft to induce keratinization of epithelium. Notably, gain in width of keratinized tissue obtained in the PRF group could be explained because of a tissue manifestation of the proliferation of gingival or periodontal fibroblast as a result of the influence of the growth factors from platelets entrapped in the fibrin mesh. However, other studies showed only modest gain in the width of keratinized gingiva when similar defects were treated with membranes [35]. The present study agrees with previous studies, which emphasize that utilizing connective tissue graft increases the width of attached gingiva.
Aroca et al reported inferior root coverage of about 80.7% at the test site (coronally advanced flap+ PRF) as compared with approximately 91.5% at the control site (coronally advanced flap), but an additional gain in gingival mucosal thickness was achieved, when compared to conventional therapy [36].The initial thickness of the flap and the type of dissection at the recipient site will alter the connective tissue microcirculation and the diffusion of tissue fluid into the inter-positioned soft tissue graft. The importance of soft tissue thickness for root coverage with a coronally advanced flap has been stressed by Hwang et al in a systematic review on factors governing root coverage [37]. The soft tissue increase in the present study may be explained as action of concentrated platelet derived growth factor, vascular endothelial growth factor, and tumor-derived growth factor, the main growth factors in PRF. These growth factors might enhance soft tissue healing by increasing the angiogenesis and matrix biosynthesis during wound healing. Regardless of the fact that growth factors trapped in PRF mesh are slowly released and able to accelerate the regenerative potential, the structure of the fibrin network is the key element of the improved PRF healing process. An increase in thickness of the keratinized tissues reported in the above-mentioned studies both studies may contribute to a long-term stable clinical outcome, as keratinized tissue width acts as a predictor for the stability of the gingival margin in the long term, as mentioned by Barootchi et al [38], with reduced probability of recurrence of recession. Our study has shown equivalent results with respect to esthetic coverage as a previous study on treatment of gingival recession [39]. Considering other factors, such as cost to the patient, surgical time, patient morbidity, healing period, pain, and patient satisfaction, better esthetic outcomes have been achieved when using PRF.
The present study had a few limitations. First, the evaluators of the study were not blinded to the group the patients belong to, which could have resulted in bias at the time of evaluation. Second, the point of measurement for the thickness of the attached gingiva could have been standardized with the stent, as done in other studies [40,41]. Third, the patient selection criteria did not differentiate between those patients with a thin or thick gingival biotype. Despite these few limitations, this study has shown comparable levels of root coverage, clinical attachment gain, and gain in width and thickness of attached gingiva between the groups, which indicates that a sub-pedicle PRF matrix could be a definitive alternative to the criterion standard sub-pedicle connective tissue graft for root coverage.
Conclusions
The present study has demonstrated a significant increase in root coverage for the control and test groups, with complete root coverage of 93% for the control group and 87% for the test group. A significant gain in clinical attachment level for the control and test group was observed. At 12 months after surgery, a predictable increase in thickness and width of attached gingiva was also noted. We therefore conclude that PRF, when used beneath a modified Ruben’s mixed flap, can be considered as a definitive alternative to the sub-pedicle connective tissue graft.
Figures
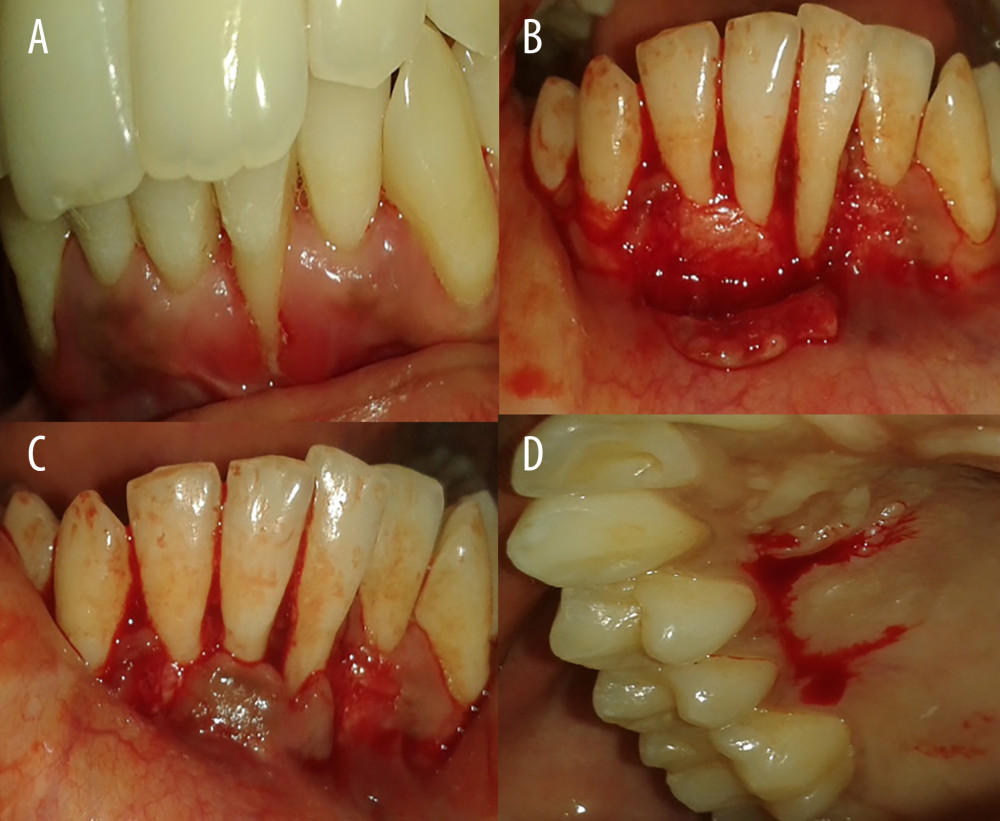 Figure 1. Gingival recession coverage with connective tissue graft. (A) Preoperative photograph of left mandibular lateral incisors (31); (B) incision for laterally repositioned flap; (C) checking flap approximation; and (D) harvesting connective tissue graft.
Figure 1. Gingival recession coverage with connective tissue graft. (A) Preoperative photograph of left mandibular lateral incisors (31); (B) incision for laterally repositioned flap; (C) checking flap approximation; and (D) harvesting connective tissue graft. 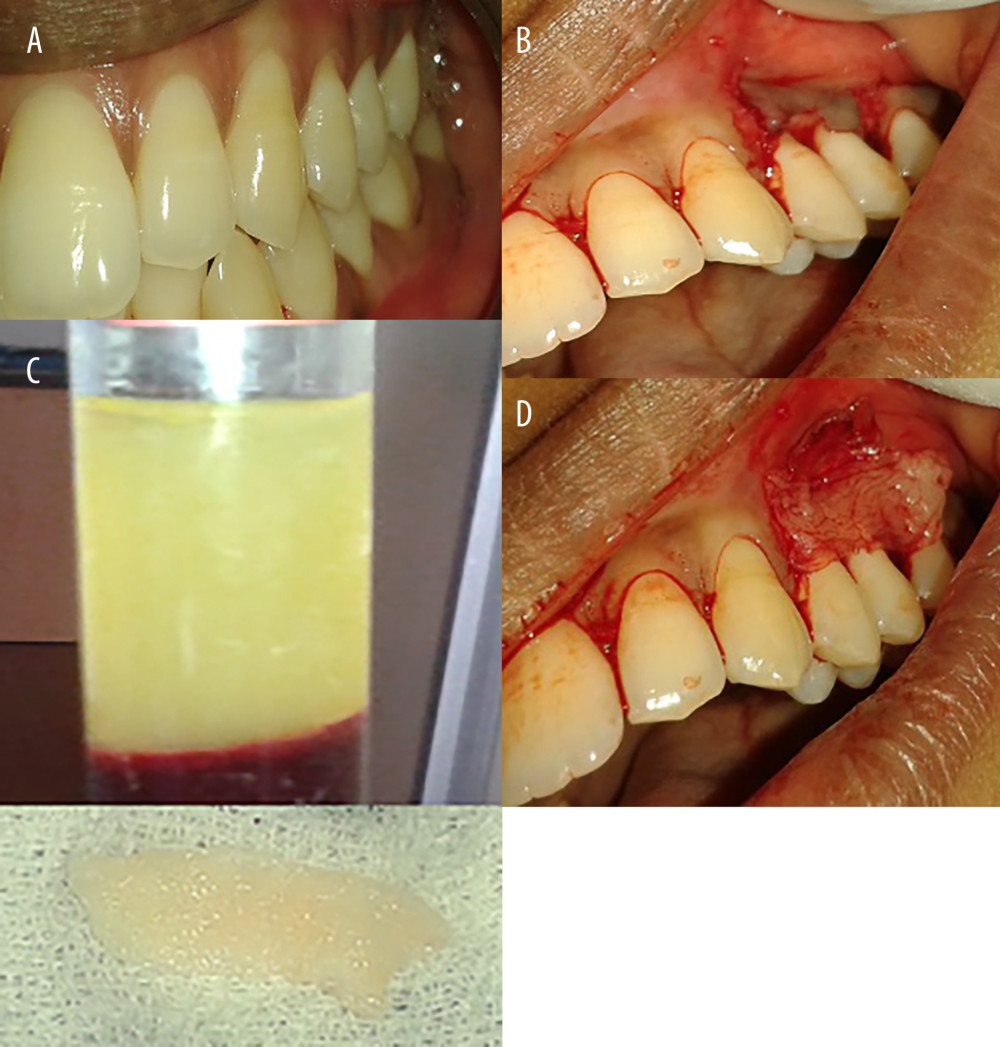 Figure 2. Gingival recession coverage with platelet-rich fibrin. (A) Preoperative photograph of left maxillary premolar, 24,25; (B) platelet-rich fibrin; (C) incision for laterally repositioned flap; and (D) placement of platelet rich fibrin on recipient bed.
Figure 2. Gingival recession coverage with platelet-rich fibrin. (A) Preoperative photograph of left maxillary premolar, 24,25; (B) platelet-rich fibrin; (C) incision for laterally repositioned flap; and (D) placement of platelet rich fibrin on recipient bed. 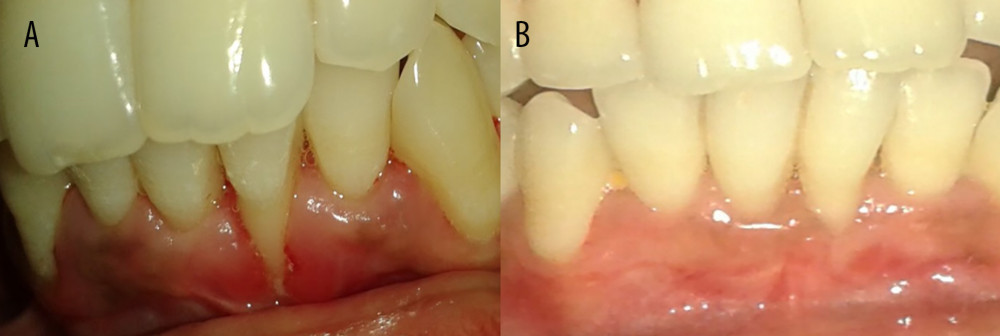 Figure 3. Preoperative and postoperative photographs of control group (connective tissue graft). (A) Preoperative photograph of left mandibular lateral incisor 31; (B) 1 year after surgery-31.
Figure 3. Preoperative and postoperative photographs of control group (connective tissue graft). (A) Preoperative photograph of left mandibular lateral incisor 31; (B) 1 year after surgery-31. 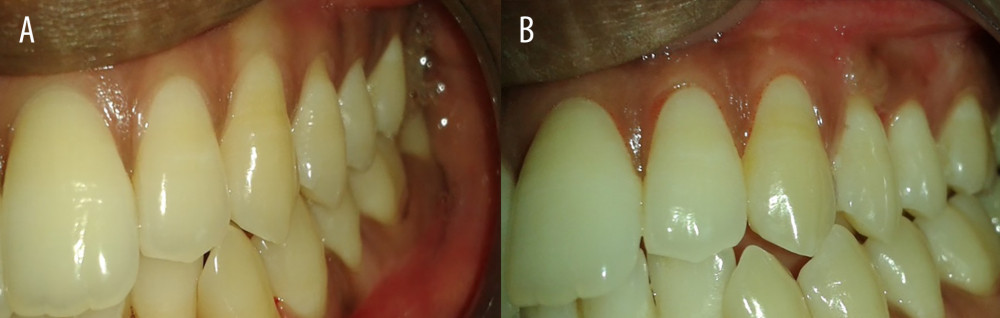 Figure 4. Preoperative and postoperative photographs of test group (PRF). (A) Preoperative photograph of left maxillary premolar −24,25; (B) 1 year after surgery of left maxillary premolar −24,25.
Figure 4. Preoperative and postoperative photographs of test group (PRF). (A) Preoperative photograph of left maxillary premolar −24,25; (B) 1 year after surgery of left maxillary premolar −24,25. 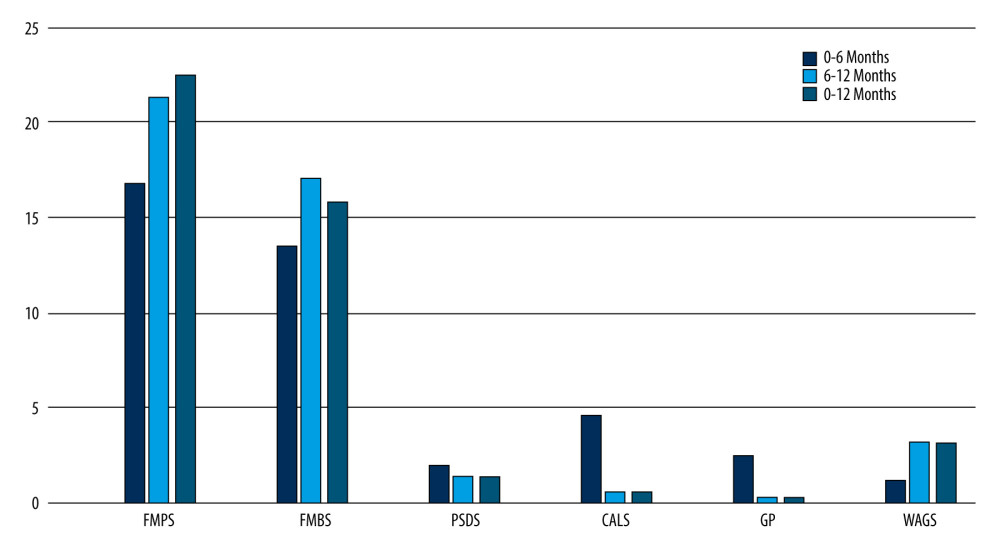 Figure 5. Intragroup comparison of clinical parameters in the control group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site.
Figure 5. Intragroup comparison of clinical parameters in the control group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site. 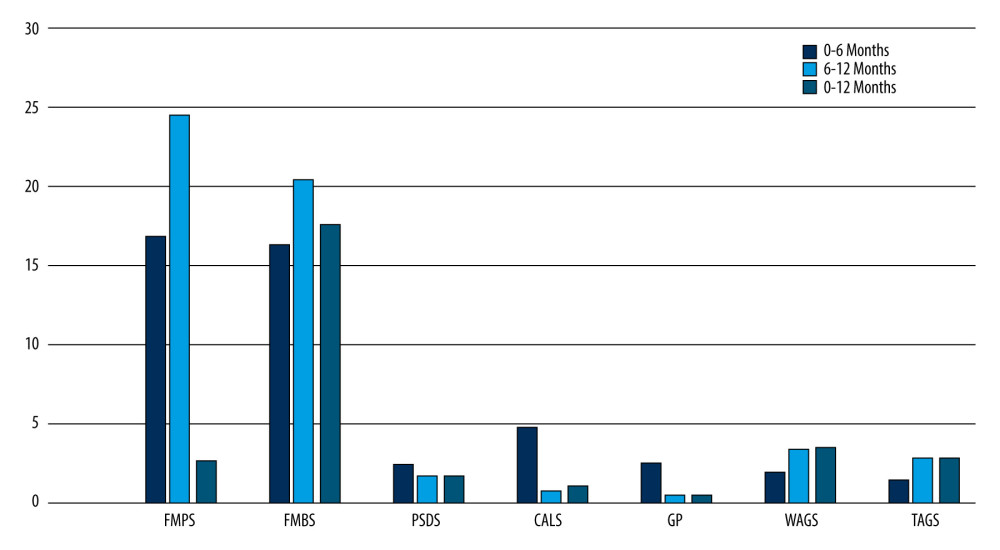 Figure 6. Intragroup comparison of clinical parameters in the test group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site.
Figure 6. Intragroup comparison of clinical parameters in the test group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site. Tables
Table 1. Intergroup comparison of clinical parameters at baseline, 6 months, and 12 months after surgery: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).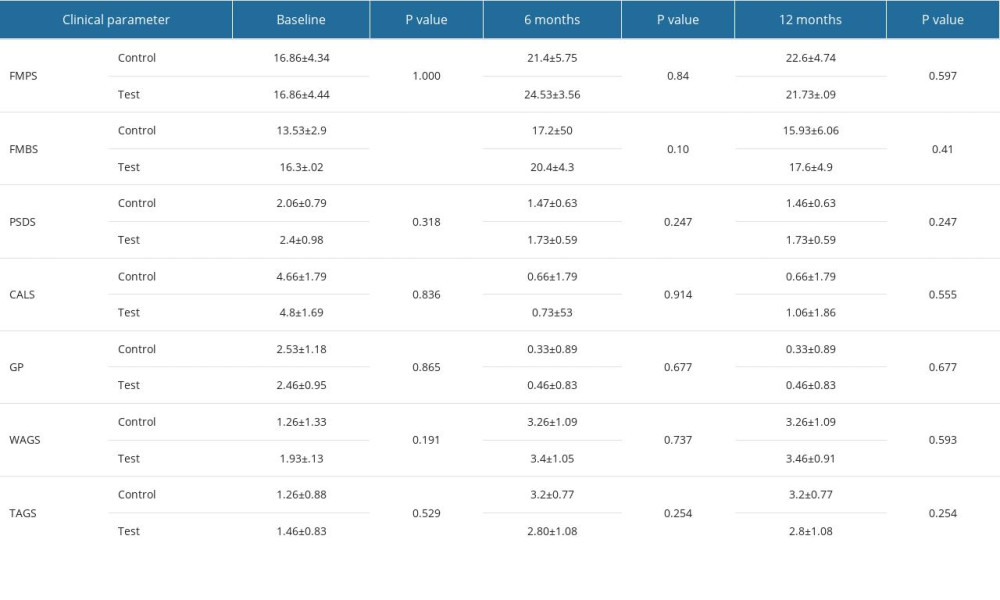 Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).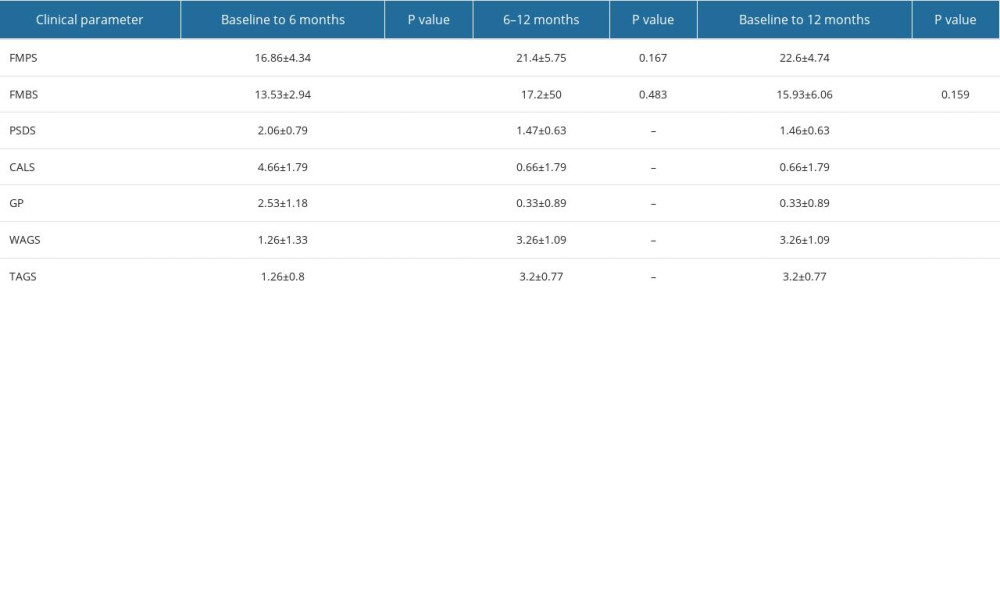 Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).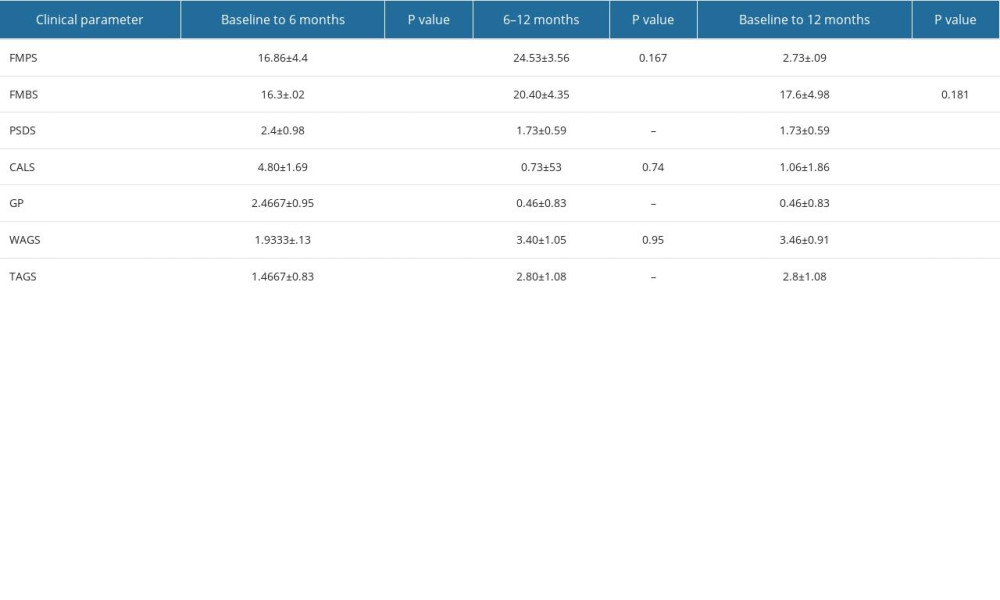 Table 4. Percentage of root coverage in the control group and test group.
Table 4. Percentage of root coverage in the control group and test group.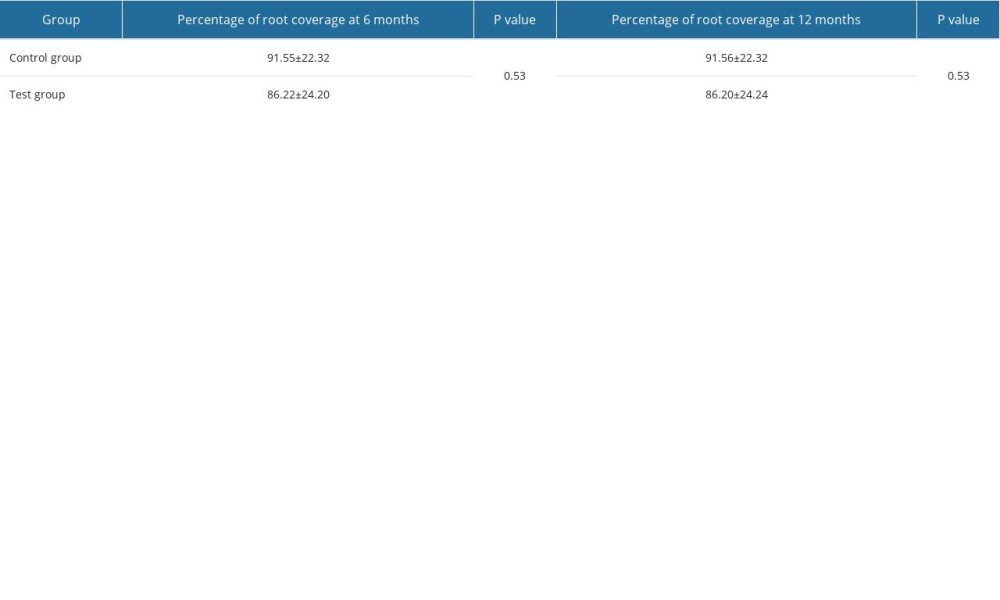
References
1. Ripamonti U, Reddi AH, Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins: Crit Rev Oral Biol Med, 1997; 8(2); 154-63
2. Martin I, Wendt D, Heberer M, The role of bioreactors in tissue engineering: Trends Biotechnol, 2004; 22(2); 80-86
3. Miller PD, A classification of marginal tissue recession: Int J Periodontics Restorative Dent, 1985; 5(2); 8-13
4. Wennström JL, Mucogingival therapy: Ann Periodontol, 1996; 1(1); 671-701
5. Armitage GC, Development of a classification system for periodontal diseases and conditions: Ann Periodontol, 1999; 4(1); 1-6
6. Langer B, Langer L, Subepithelial connective tissue graft technique for root coverage: J Periodontol, 1985; 56(12); 715-20
7. Chambrone L, Ortega MAS, Sukekava F, Root coverage procedures for treating single and multiple recession-type defects: An updated Cochrane systematic review: J Periodontol, 2019; 90(12); 1399-422
8. Karam PS, Sant’Ana AC, de Rezende ML, Root surface modifiers and subepithelial connective tissue graft for treatment of gingival recessions: A systematic review: J Periodontal Res, 2016; 51(2); 175-85
9. Sedon CL, Breault LG, Covington LL, Bishop BG, The subepithelial connective tissue graft: Part II. Histologic healing and clinical root coverage: J Contemp Dent Pract, 2005; 6(2); 139-50
10. Harris RJ, Root coverage with connective tissue grafts: An evaluation of short- and long-term results: J Periodontol, 2002; 73(9); 1054-59
11. Novaes AB, Grisi DC, Molina GO, Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession: J Periodontol, 2001; 72(11); 1477-84
12. Karring T, Nyman S, Gottlow J, Laurell L, Development of the biological concept of guided tissue regeneration – animal and human studies: Periodontol 2000, 1993; 1; 26-35
13. Harris RJ, Successful root coverage: A human histologic evaluation of a case: Int J Periodontics Restorative Dent, 1999; 19(5); 439-47
14. Bruno JF, Bowers GM, Histology of a human biopsy section following the placement of a subepithelial connective tissue graft: Int J Periodontics Restorative Dent, 2000; 20(3); 225-31
15. Stähli A, Párkányi L, Aroca S, The effect of connective tissue graft or a collagen matrix on epithelial differentiation around teeth and implants: A preclinical study in minipigs: Clin Oral Investig, 2023; 27(8); 4553-66
16. Choukroun J, Adda F, Schoeffler CVA, Vervelle A, PRF: An opportunity in Perioimplantology: Implantodontie, 2001; 42; 55-62
17. Pavlovic V, Ciric M, Jovanovic V, Platelet-rich fibrin: Basics of biological actions and protocol modifications: Open Med (Wars), 2021; 16(1); 446-54
18. Lynch SE, de Castilla GR, Williams RC, The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing: J Periodontol, 1991; 62(7); 458-67
19. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T, Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF): Trends Biotechnol, 2009; 27(3); 158-67
20. Gassling V, Douglas T, Warnke PH, Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering: Clin Oral Implants Res, 2010; 21(5); 543-49
21. Ibrahim TM, Prakash PS, Appukutan D, Subramanian S, Comparative evaluation of bone dimensional changes in extraction sockets preserved with calcium phosphosilicate bone substitutes with and without platelet-rich fibrin: A randomized controlled clinical trial: Int J Periodontics Restorative Dent, 2022; 42(2); 261-67
22. Chekurthi S, Tadepalli A, Parthasarathy H, Evaluation of clinical efficacy of advanced platelet-rich fibrin in the management of gingival recession defects: Case series: Clin Adv Periodontics, 2022; 12(2); 88-93
23. Jankovic S, Aleksic Z, Klokkevold P, Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial: Int J Periodontics Restorative Dent, 2012; 32(2); e41-50
24. Gautam A, Advantage of platelet-rich fibrin and lateral position pedicle flap technique for the treatment of gingival recession: Int J Exp Dent Sci, 2020; 9; 13-15
25. Singh J, Bharti V, Laterally positioned flap-revised technique along with platelet rich fibrin in the management of Miller class II gingival recession: Dent Res J (Isfahan), 2013; 10(2); 268-73
26. Öncü E, The use of platelet-rich fibrin versus subepithelial connective tissue graft in treatment of multiple gingival recessions: A randomized clinical trial: Int J Periodontics Restorative Dent, 2017; 37(2); 265-71
27. Ruben MP, Goldmon HM, Janson W, Biological considerations fundamental to successful employment of laterally repositioned pedicle flaps and free autogenous gingival grafts in periodontal therapy: Periodontal Surgery, 1976; Ch.9, Illinois, Springfield
28. Harvey PM, Surgical reconstruction of the gingiva. II. Procedures: N Z Dent J, 1970; 66(303); 42-52
29. Rodas MAR, Paula BL, Pazmiño VFC, Platelet-rich fibrin in coverage of gingival recession: a systematic review and meta-analysis: Eur J Dent, 2020; 14(2); 315-26
30. O’Leary TJ, Drake RB, Naylor JE, The plaque control record: J Periodontol, 1972; 43(1); 38
31. Ainamo J, Bay I, Problems and proposals for recording gingivitis and plaque: Int Dent J, 1975; 25(4); 229-35
32. Sculean A, Chapple IL, Giannobile WV, Wound models for periodontal and bone regeneration: the role of biologic research: Periodontol 2000, 2015; 68(1); 7-20
33. Zucchelli G, Amore C, Sforza NM, Bilaminar techniques for the treatment of recession-type defects. A comparative clinical study: J Clin Periodontol, 2003; 30(10); 862-70
34. Cheung WS, Griffin TJ, A comparative study of root coverage with connective tissue and platelet concentrate grafts: 8-month results: J Periodontol, 2004; 75(12); 1678-87
35. Trombelli L, Scabbia A, Wikesjö UM, Calura G, Fibrin glue application in conjunction with tetracycline root conditioning and coronally positioned flap procedure in the treatment of human gingival recession defects: J Clin Periodontol, 1996; 23(9); 861-67
36. Aroca S, Keglevich T, Barbieri B, Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study: J Periodontol, 2009; 80(2); 244-52
37. Hwang D, Wang HL, Flap thickness as a predictor of root coverage: A systematic review: J Periodontol, 2006; 77(10); 1625-34
38. Barootchi S, Tavelli L, Di Gianfilippo R, Long term assessment of root coverage stability using connective tissue graft with or without an epithelial collar for gingival recession treatment. A 12-year follow-up from a randomized clinical trial: J Clin Periodontol, 2019; 46(11); 1124-33
39. Abirami T, Subramanian S, Prakash PSG, Comparison of connective tissue graft and platelet rich fibrin as matrices in a novel papillary augmentation access: A randomized controlled clinical trial: Eur J Dent, 2019; 13(4); 607-12
40. Borghetti A, Glise JM, Monnet-Corti V, Dejou J, Comparative clinical study of a bioabsorbable membrane and subepithelial connective tissue graft in the treatment of human gingival recession: J Periodontol, 1999; 70(2); 123-30
41. Schinstine M, Iacovitti L, 5-Azacytidine and BDNF enhance the maturation of neurons derived from EGF-generated neural stem cells: Exp Neurol, 1997; 144(2); 315-25
Figures
 Figure 1. Gingival recession coverage with connective tissue graft. (A) Preoperative photograph of left mandibular lateral incisors (31); (B) incision for laterally repositioned flap; (C) checking flap approximation; and (D) harvesting connective tissue graft.
Figure 1. Gingival recession coverage with connective tissue graft. (A) Preoperative photograph of left mandibular lateral incisors (31); (B) incision for laterally repositioned flap; (C) checking flap approximation; and (D) harvesting connective tissue graft. Figure 2. Gingival recession coverage with platelet-rich fibrin. (A) Preoperative photograph of left maxillary premolar, 24,25; (B) platelet-rich fibrin; (C) incision for laterally repositioned flap; and (D) placement of platelet rich fibrin on recipient bed.
Figure 2. Gingival recession coverage with platelet-rich fibrin. (A) Preoperative photograph of left maxillary premolar, 24,25; (B) platelet-rich fibrin; (C) incision for laterally repositioned flap; and (D) placement of platelet rich fibrin on recipient bed. Figure 3. Preoperative and postoperative photographs of control group (connective tissue graft). (A) Preoperative photograph of left mandibular lateral incisor 31; (B) 1 year after surgery-31.
Figure 3. Preoperative and postoperative photographs of control group (connective tissue graft). (A) Preoperative photograph of left mandibular lateral incisor 31; (B) 1 year after surgery-31. Figure 4. Preoperative and postoperative photographs of test group (PRF). (A) Preoperative photograph of left maxillary premolar −24,25; (B) 1 year after surgery of left maxillary premolar −24,25.
Figure 4. Preoperative and postoperative photographs of test group (PRF). (A) Preoperative photograph of left maxillary premolar −24,25; (B) 1 year after surgery of left maxillary premolar −24,25. Figure 5. Intragroup comparison of clinical parameters in the control group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site.
Figure 5. Intragroup comparison of clinical parameters in the control group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site. Figure 6. Intragroup comparison of clinical parameters in the test group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site.
Figure 6. Intragroup comparison of clinical parameters in the test group. FMPS – full mouth plaque score; FMBS – full mouth bleeding score; PSDS – probing sulcus depth at surgical site; CALS – clinical attachment level at surgical site; GP – gingival position (recession height); WAGS – width of attached gingiva at surgical site. Tables
 Table 1. Intergroup comparison of clinical parameters at baseline, 6 months, and 12 months after surgery: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 1. Intergroup comparison of clinical parameters at baseline, 6 months, and 12 months after surgery: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 4. Percentage of root coverage in the control group and test group.
Table 4. Percentage of root coverage in the control group and test group. Table 1. Intergroup comparison of clinical parameters at baseline, 6 months, and 12 months after surgery: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 1. Intergroup comparison of clinical parameters at baseline, 6 months, and 12 months after surgery: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 2. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the control group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS).
Table 3. Intragroup comparison of clinical parameters at baseline, 6 months, and 12 months in the test group: full mouth plaque scores (FMPS), full mouth bleeding scores (FMBS), probing sulcus depth at surgical site (PSDS), clinical attachment level at the surgical site (CALS), gingival position/recession height (GP), width of attached gingiva (WAGS), and thickness of attached gingiva (TAGS). Table 4. Percentage of root coverage in the control group and test group.
Table 4. Percentage of root coverage in the control group and test group. In Press
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








