07 December 2023: Clinical Research
Prognostic Factors for Uterine Sarcoma and Carcinosarcoma: Insights from a 10-Year Follow-Up Study
Beata KotowiczDOI: 10.12659/MSM.941562
Med Sci Monit 2023; 29:e941562
Abstract
BACKGROUND: Uterine sarcomas and carcinomas are rare tumors and treatment outcomes are far from expected. We investigated the prognostic significance of selected serum biomarkers and the impact of some clinical and tissue factors on overall survival (OS) at 10-year follow-up.
MATERIAL AND METHODS: The material for analysis was a group of 34 patients with uterine sarcomas and 18 with carcinomas. Immunohistochemistry was performed to determine Ki 67, p53 and ER and PR. Concentrations: CA 125, IL8, VEGF, SFTL1, VEGF R2, sTNFRI and MMP-9 were determined in the serum of patients before treatment and in the control group.
RESULTS: The most frequently elevated levels observed of sTNF RI in 94% and VEGF in 62%. On the ROC curve analysis, sTNF RI and VEGF concentrations showed the highest sensitivity. Patients with striated cell sarcoma, smooth cell sarcoma and high-grade rhabdomyosarcoma had the worst prognosis. Patient age, FIGO stage and expression of Ki67, p53, ER and PR, CA 125 (p<0.038) and IL-8 (p<0.024) were statistical prognostic factors for OS. However, in multivariate analysis, serum levels of: CA 125 concentration (p<0.045), age (p<0.010) and p53 expression (p<0.014) were found to be significant independent prognostic factors.
CONCLUSIONS: A 10-year follow-up of patients with uterine sarcoma indicates that age above 60 years at diagnosis and high p53 expression and elevated CA125 levels before treatment can be independent prognostic factors. The high diagnostic sensitivity of sTNF RI and VEGF suggests the possibility of using these biomarkers in the early diagnosis of uterine sarcomas.
Keywords: Prognosis, Sarcoma, Uterine Neoplasms
Background
Uterine sarcomas and carcinosarcomas are rare and aggressive cancers. They account for less than 10% of cancers originating in the uterine corpus. [1,2] Uterine sarcomas are divided into homologous and heterologous types. In the homologous form, the tumor contains only elements of tissues found in the uterus. In heterologous tumors, we recognize cells that are not characteristic of the uterus, such as cartilage or bone elements. Carcinosarcoma was separated from this group of tumors a few years ago and classified as a metaplastic carcinoma with carcinomatous and sarcomatous elements arising from single malignant epithelial clone. The etiology of these tumors is unclear. Heterogenicity of this malignancy is caused by chromosomal translocations inside tumors. The rarity of both uterine sarcomas and carcinosarcoma means that most clinical observations come from retrospective analyses. However, the clinical course of carcinosarcoma is like aggressive uterine sarcomas and this is the reason why we decided to admit this type of tumors in presented analysis.
Among the most cited prognostic factors in uterine sarcomas and carcinosarcomas are clinical stage and tumor type. Certainly, other factors such as tumor proliferative activity, the presence of estrogen and progesterone receptors, and tumor necrosis are also considered prognostic factors [2–5].
From a clinical point of view, it is very important to determine the histological type and degree of maturity of the tumor in the postoperative material. Tumors of myometrial and subperitoneal origin, originating from the endometrium, with a typical morphological picture, do not cause diagnostic problems and their histopathological diagnosis is usually based on routine hematoxylin and eosin staining. Endometrial sarcomas with an atypical or mixed microscopic picture require confirmation of the diagnosis by immunohistochemical staining using a broader panel of antibodies [6,7].
In clinical practice, immunohistochemical assessment of tumor proliferation activity expressed as the mitotic index Ki-67 is used. The histological type and degree of maturity of endometrial mesenchymal tumors, supported by molecular tests and immunohistochemical cell markers, are prognostic factors that allow patients to be divided into groups according to prognosis of survival and choice of treatment. The majority (approximately 80%) of endometrial stromal sarcoma (ESS) show the presence of steroid receptors, estrogen receptor (ER), and progesterone receptor (PR), and a similar percentage expresses aromatase, the content of which increases with the stage of ESS [5,8–10].
Overexpression of p53 protein, p16 protein and TGFβ, among others, is also observed in the course of uterine sarcomas. The factors presented here have both direct and indirect effects on the nature of the cancers in question and can serve as prognostic indicators [11].
Significant prognostic factors in several cancers are levels of cytokines and carcinoma antigen 125 (CA 125) [12–13]. Of the serum markers, CA 125, a key marker in gynecological oncology, has relatively low sensitivity in this tumor location. In recent years, the study of cytokines, particularly proangiogenic cytokines, has been the subject of much study. Within this group of cytokines, a key role is attributed to vascular endothelial growth factor (VEGF), which is a ligand for 2 receptors: VEGFR1 (sFLT1) and VEGFR2. Under physiological conditions, expression of these receptors occurs in endothelial cells and their overexpression is observed in many types of cancer, which may indicate involvement in pathological angiogenesis [14,15]. Interleukin 8 (IL-8) is also involved in tumor angiogenesis and, as a chemotactic factor, stimulates neutrophils [12,14–16].
In the context of cancer, metalloproteinases may also be important. The role of soluble metalloproteinases (MMPs) and their soluble tissue inhibitors (TIMPs), which are involved in the regulation of cancer cell proliferation, angiogenesis, and apoptosis, is ambiguous. In uterine sarcomas, an important role is particularly attributed to MMP-9, overexpression of which may be a potential predictor of poor clinical progression and prognosis in endometrial cancer [17,18].
In the work presented here, we undertook an analysis of the selected prognostic factors and evaluated their significance in the 10-year follow-up of patients. An important value was the fact that none of the patients were lost to follow-up and all clinical data were kept up to date.
The aim of this study was to assess the prognostic value of selected serum biomarkers: CA 125, IL8, VEGF, SFTL1, VEGF R2, soluble tumor necrosis factor receptor (sTNFRI) and MMP-9, and to analyse the impact of recognized clinical and tissue factors on the distant survival of patients with uterine sarcomas under more than 10-year follow-up.
Material and Methods
ETHICS STATEMENT:
The protocol of research was approved by the Bioethics Committee at the Maria Skłodowska-Curie National Research Institute of Oncology in Warsaw, Poland; date 03.02.2009 (authorization No. 7/2009). We performed a single center, prospective, observational study according to the ethical standards of the Declaration of Helsinki. The samples were taken after informed consent from all the study participants.
PATIENTS:
The material for analysis was a group of 52 patients with uterine sarcomas who were treated between 2009 and 2011 at the Maria Skłodowska-Curie National Research Institute of Oncology in Warsaw. In this group there were 38 patients initially treated at our center, while 14 women were admitted from other hospitals for treatment due to tumor recurrence. All 38 patients underwent hysterectomy with adnexa and according to histopathological result the form of follow-up was proposed. Seven patients with carcinosarcoma and one with rhabdomyosarcoma received postoperative adjuvant radiotherapy. Radiotherapy was applied to the pelvic fields in the form of teletherapy using the box technique up to a dose of 50.4 Gy. All patients with ESS low-grade and adenosarcoma received oral megestrol acetate at a dose of 160mg/day after surgery for a minimum of 12 months. Ten patients with newly diagnosed tumors received chemotherapy. Six were treated by using doxorubicin with ifosfamide and 4 received paclitaxel with carboplatin. All received 5 to 6 cycles of chemotherapy. Eleven patients received no adjuvant treatment including 2 women with leiomyosarcoma in clinical stage IA and 5 in clinical stage IB. Three patients with high-grade ESS in clinical stage IA and one with the same histopathological diagnosis in clinical stage IB were only followed up without any adjuvant treatment. In the group of 14 patients with recurrent tumor, complete surgically excision was performed. All 14 patients received complementary chemotherapy in case of leiomyosarcoma with doxorubicin and ifosfamide and in case of carcinosarcoma and high-grade stromal sarcoma with paclitaxel and carboplatin. Five patients with leiomyosarcoma second line of chemotherapy – docetaxel and gemcitabine, have been received due to next progression.
DETERMINATION OF TISSUE BIOMARKERS EXPRESSION: The expression of tissue markers was assessed in all patients, immunohistochemistry was performed to determine Ki 67, p53 and ER and PR. For immunohistochemical analysis, 4 μm thick sections were evaporated with xylene and washed with ethanol. To quench endogenous peroxidase activity, the sections were cooled for 15 minutes and then incubated with 3% H2O2. Blocking was performed using a serum-free protein block, DakoCytomation (Carpenteria, California, USA), for 30 minutes. Ki67, p53, ER and PR antibodies were used. All preparatory work was done automatically: ER ABCAM (SP1) at a dilution of 1: 100, PR NOVOCASTRA at a dilution of 1: 200 and Ki 67 DACO at a dilution of 1: 10. In case of Ki 67, the percentage expression of this protein was evaluated: 0–30% low; 31–60% medium, and 61–100% high and p53 intensity as absent (1), weak (2) means low, moderate (3) and strong (4) means high. ER and PR were determined on a 5-grade scale meaning 1–3 low expression and 4 and 5 meaning high expression (Figure 1A–1D).
DETERMINATION OF SERUM BIOMARKER:
Concentrations of selected serum biomarkers were determined in the same patients: CA 125, IL8, VEGF, sFTL1, VEGF R2, sTNFRI, and MMP-9 prior to treatment in both primary and recurrent tumors. In addition, the control group consisted of 40 healthy women, aged from 32 to 63 a median of 55 years, in whom the same biomarkers were measured. Individuals in the control group gave verbal consent to participate in the study; they were healthy female colleagues at the Institute. Blood was collected during periodic screening with the occupational doctor, who analyzed the baseline laboratory tests and extended the work permit. The median age of the women in the control group was similar to the patients’ group.
All sera were stored in a low-temperature freezer until assays were performed. CA125 and sFLT1 serum concentration was determined using the electrochemiluminescence method in the Cobas 6000 system by ROCHE Diagnostics, Mannheim, Germany, and other parameters were determined using the enzyme-linked immunosorbent assay (ELISA) method with R&D Systems, Abingdon, UK kits. A cut-off point of biomarkers were established based on its concentration in the control group of healthy volunteers, taking the 95th percentile; the concentration of CA125 as a standard tumor marker, according to manufacturer’s recommendations (35 IU/mL).
Survival time was calculated from the date of first treatment, including patients treated for recurrence, to the date of death or date of last follow-up. Patients were followed up regularly every 3–6 months for the first 5 years and then once a year. None of the patients were lost to follow-up and information on death and date was obtained from the patients’ families. This observational study had a follow-up time of 10 years [19].
STATISTICAL ANALYSIS:
Data were analyzed using STATISTICA 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Absolute counts (n) and relative counts (%) were estimated for categorical variables, while median with range (minimum-maximum) for numerical variables. The Mann-Whitney U test was used to analyze the differences in variables within and between groups. The impact of clinicopathological features and soluble biomarkers on disease-free survival (DFS) and overall survival (OS) was examined in univariate and multivariate analyzes using the log rank test and Cox proportional hazards model, respectively. The assumption of proportionality was tested graphically. The results were illustrated with survival curves obtained by the Kaplan-Meier method.
The diagnostic power of determined parameters was analysed using the statistical MedCalc program, which included the definition of the areas under the receiver operating characteristic (ROC) curves
Results
CLINICAL CHARACTERISTICS OF THE STUDY GROUP:
The data of 52 patients with sarcoma were analyzed. Tables 1 and 2 shows the clinical characteristics of the study group. The patients’ age at the time of the disease onset was from 23 to 93 years, and was 56 years on average. The most common histopathological type of tumor in the analyzed group was carcinosarcoma. The next most frequent tumor was leiomyosarcoma, and 90% of the patients were in low stages of progression (FIGO IA–IIA).
THE CONCENTRATIONS OF BIOMARKERS:
To estimate the diagnostic sensitivity of the parameters measured, concentrations of all biomarkers were compared, including the ovarian cancer standard marker CA 125, in the group of patients vs the control group. The concentration values (cut-off) differentiating healthy individuals from patients with uterine sarcoma, at low stages, were determined.
Based on concentrations in the control group, 95th percentile cut-off points were established: IL-8 – 12.5 pg/mL, VEGF – 25 pg/mL, VEGF R1 – 482 pg/mL, VEGF R2 – 11 832 pg/mL, sTNF RI – 175 pg/mL, and MMP-9 – 734 ng/mL. Using these cut-off points in the study group of patients, the most frequently elevated levels of sTNF RI were observed in 94% and VEGF in 62%; while the CA 125 concentration was elevated in 28%. Based on the ROC curve analysis for the determined biomarkers, while maintaining optimal sensitivity and specificity of the test, for patients vs controls, the concentration of sTNF RI and VEGF revealed higher sensitivity than other biomarkers, with areas under receiver operating characteristic curve. Significant differences were observed between the AUC for VEGF and for CA125 (P=0.011), as well as for VEGF receptors (P=0.018). The area under the ROC curve for sTNF RI was significantly greater when compared to the ROC for CA125, IL-8, and MMP-9 (P=0.001) (Table 3, Figure 2).
SURVIVAL ANALYSIS:
The longest survival time was 165 months, when the longest-observed patient was censored. Among a total of 33 patients, 63% died during follow-up, 25% died within 10 months, and 50% survived more than 33 months.
Survival differed significantly between the 5 histopathological types – carcinosarcoma, leiomyosarcoma, high-grade endometrial stromal sarcoma, adenosarcoma, and low-grade endometrial stromal sarcoma (P=0.006), but did not differ significantly between carcinosarcoma, leiomyosarcoma, and high-grade endometrial stromal sarcoma (P=0.306) (Figure 3). By 165 months, when the longest-observed patient was censored, 16 patients had died due to carcinosarcoma, 9 patients had died due to leiomyosarcoma, 6 patients had died due to high-grade endometrial stromal sarcoma, and 1 patient had died due to adenosarcoma. Table 4 shows the number of deaths, percentages, and survival time of patients according to the histopathological type of sarcoma.
When analyzing the clinical FIGO stage together without separating into clinical stages, only stage IA had a good prognosis, and in this stage all patients were still alive. In patients at stages IB, IIA, and IIB–IIIA, 70%, 92%, and 100% of patients had died, respectively, and the median survival times were 25, 19, and 16 months for stages IB, IIA, and IIB, respectively. The difference in survival between stage IA and other clinical FIGO stages was significant (P<0.001) (Figure 4). Univariate analysis, followed by Cox analysis, was performed to determine the relationship between biomarker levels and overall survival. Log rank testing of the biomarkers studied showed a significant relationship for CA 125 (P<0.038) and IL-8 levels (P<0.024), while Cox multivariate analysis showed the marker CA 125 (P<0.045) was an independent prognostic factor associated with OS; elevated levels before treatment were correlated with shorter survival of patients with uterine sarcomas (Table 5, Figure 5).
However, expression of immunohistochemistry markers was prognostically significant for survival and was confirmed in the univariate analysis: Ki 67 (P=0.003), p53 (P<0.001), ER (P=0.003), and PR (P=0.005). Figure 6 shows overall survival according to p53 expression, which proved to be the strongest factor in Cox analysis. Median survival (months) was 134 for low expression (3 of 12 patients died - 25%), 105 for medium expression (4 of 8 patients died – 50%) and 21 for high expression (26 of 32 patients died – 81%).
Older women had a significantly shorter survival (P=0.016) (Figure 7); as many as 85% of patients aged 60 and older died during observance (median survival time was12 months). While 50% of patients younger than 60 died, and survival time was significantly longer, with a median of 96 months.
In multivariate analysis, of the selected factors, only the age of the patients at diagnosis, the expression of p53, and CA125 levels were found to be significant (Table 5).
TUMOR RECURRENCE:
Among patients initially treated at the National Research Institute of Oncology in Warsaw, relapse was diagnosed in 14 patients (26.92%). The tumor recurrences were observed after 5–39 months, with a mean time of 14 months.
Tumor recurrence was most common in patients with leiomyosarcoma (8 recurrences in the group of 13 patients [62%]), followed by low-grade endometrial stromal sarcoma (1 recurrence in a group of 4 patients [25%]), high-grade endometrial stromal sarcoma (2 recurrences in a group of 10 patients [20%]), and carcinosarcoma (3 recurrences in a group of 18 patients [17%]). There were no recurrences in the other histopathological types of the tumor: adenosarcoma, rhabdomyosarcoma, and solitary malignant fibrous tumor.
The survival time after tumor recurrence was up to 130 months, when the longest-observed patient was censored. A total of 10 patients with tumor recurrence (71%) had died by that time, 25% of patients with tumor recurrence died within 7 months after recurrence, and 50% of patients with recurrence survived more than 12 months after the recurrence.
In the group of the 10 patients who died after recurrence, 1 patient died on the 6th day after surgery, 1 died in the 5th month, 2 died in the 7th month, 2 died in the 9th month, one died in the 12th month, 1 died in the 13th month, and 1 died in the 7th year after recurrence. Four patients were still alive at the time when they were being observed (102, 122, 123, and 130 months after recurrence).
Discussion
Uterine sarcomas are a heterogeneous group of tumors and are classified as rare tumors [1,2]. Gathering sufficient knowledge about them requires time and often the formation of international research groups. Therapeutic management depends on the type of sarcoma, but most of them require individualization and have a poor prognosis. The size of our study group was small; however, given the rarity of this tumor, a group of patients treated at a single center is significant, which formed the basis for statistical analyses and summary of the results obtained. An additional strength of this study was the more than 10-year follow-up of patients. In the available literature, we have not noted analyses of survival at 10 years after treatment. Works published in recent years present PFS and OS results over a 5-year period and only 10-year experience in the management of patients with uterine sarcomas [20]. p53 protein expression, CA 125 concentrations, and age are very important independent prognostic factors in uterine sarcoma and carcinosarcoma patients. Based on the collected clinical data and analyses of selected markers in blood serum and immunohistochemical studies, we tried to identify significant prognostic factors in the analyzed group of patients. Bear in mind that carcinosarcoma is currently defined as metaplastic carcinoma, but at the time when the material was collected, carcinosarcoma was classified in the group of uterine sarcomas. Because of its similar prognosis to aggressive sarcomas, we decided to analyze this group of tumors. Gathering different types of uterine sarcomas into a single group obviously has its own interpretative limitations. However, the small numbers of some sarcoma types did not allow separate analysis by histological subtype. A similar pooled analysis of the results of treatment of uterine sarcomas has also been done by other authors [21–23]. In our study, of the histopathological types, carcinosarcoma was the most common, followed by leiomyosarcoma, and they accounted for almost 60% of diagnoses, and these 2 cancers accounted for about 70% of the population analyzed. If we remove carcinosarcoma from the group, leiomyosarcoma becomes the most common cancer. Similar opinions are held by other authors [2,21]. Ramirez states that the number of leiomyosarcoma diagnoses with such compiled statistic increases to 63% [2]. Most treatment failures were observed in the carcinosarcoma group, where 89% of patients died. In second place was leiomyosarcoma, which killed 69% of women, and in third place was high-grade sarcoma, which killed 60%. These results are confirmed by other authors. In the study by Burle De Siqueira Ferroni Plentz et al, the chance of 5-year survival for carcinosarcoma or leiomyosarcoma was 5.5 times lower than for other forms of sarcomas [23]. However, it should be emphasized that the treatment of patients with sarcomas between 2009 and 2011 and today differs if only using targeted therapy in some types of these tumors. New therapies such as Pazopanib oral tyrosine kinase inhibitor or Trabectedin could increase the effectiveness of the therapy. Hence, the survival outcomes of patients currently treated may be improving [24,25]. In our study, 37% of patients (more than one-third of the cohort,) lived for more than 10 years, which suggests that, despite the significant aggressiveness of sarcomas, some patients respond well to treatment, which is even more reason to look for prognostic factors.
In the analysis performed, all patients in stage IA survived, as against patients in higher stages, according to the FIGO classification. In this stage, in addition to cases of adenosarcoma or low-grade stromal sarcoma, there were 2 cases each of leiomyosarcoma and high-grade stromal sarcoma. In previous studies by numerous authors, FIGO stage I was not separated into sub-stages; therefore, survival in this stage was 53-74% [2,21,26]. Therefore, it should be strongly emphasized that patients with uterine sarcomas in FIGO IA stage according to the 2009 classification have good survival
Our analysis shows that the expression of p53, Ki67, and ER and PR has a high prognostic significance, and these factors significantly differentiate malignant forms of tumors of muscular origin from benign forms [8,27]. Another study found a correlation between Ki 67 expression and rapid growth of leiomyosarcoma [3,28]. Rapid tumor growth implies the possibility of faster progression and thus higher tumor stage, which was confirmed in our study.
Also important is the expression of p53 protein as a significant prognostic factor, which confirmed its importance in both univariate and multivariate analysis. The tumor suppressor protein p53 functions as the ‘guardian of the genome’ by inducing cell cycle arrest and apoptosis in response to oncogene activation, DNA damage, and other stress signals [28,29]. A significant association has been described between mutated p53 gene expression and higher mortality in patients with leiomyosarcoma [30]. In smooth-muscle tumors of the gynecological glands, a significant association with disease-free survival was found for p53 but not for ki67 [31]. In addition, Nakamura M et al. described overexpression of p53 in immunohistochemistry is a significant prognostic factor only in endometrial cancer [32].
There are also publications in which this factor was found to have no prognostic significance [8,33]. ER and PR expression assays had a very important role in our study, as high expression of both receptors improved prognosis in patients with these tumors. This is in accordance with opinions expressed by other authors, who emphasize the importance of hormonal therapy in the case of uterine sarcomas with intense expression of hormone receptors. This applies not only to low-grade stromal sarcoma or angiosarcoma, but also to leiomyosarcoma [34–36]. In our study, all patients received megestrol acetate 160 mg/day as adjuvant treatment, with good tolerance. In other studies, other gestagens or GnRH analogs or aromatase inhibitors were used. The effect of disease stabilization or partial regression was achieved in up to half of the patients [36]. In the diagnosis of uterine sarcomas, imaging studies are imperfect, and we can only confirm the disease in postoperative material, so it was important to investigate whether serum biomarkers could be a helpful tool in the initial diagnosis of patients. In most cases, a definitive diagnosis of sarcoma was established only after surgery. In this study, we analysed the concentrations of selected serum biomarkers associated with both tumor angiogenesis and associated inflammation. We demonstrated significantly higher concentrations of all biomarkers in patients with myoma compared with a group of healthy women. We showed the highest diagnostic sensitivity for sTNFRI and VEGF, which may be useful in the early diagnosis of uterine sarcoma. Angiogenesis, which is the formation of new blood vessels from pre-existing capillaries, is one of the essential steps in the development of solid tumors. VEGF are all well-known promoters or mediators of cancer angiogenesis. There are many publications on the diagnostic utility of angiogenesis markers, VEGF in gynecological tumors and especially in uterine cancer. and endometrial cancer [12,15,16]. When investigating the importance of biomarkers as potential prognostic factors, we found that the markers CA125 and IL-8, determined before treatment, have prognostic value for overall survival time. However, only CA125 in addition to age and p53 expression is an independent factor for OS. Matsuo et al found that the CA-125 classification remained an independent prognostic factor for PFS in patients with uterine carcinosarcoma, and the abnormal CA-125 level was associated with a 35% increased risk for recurrence or progression compared to the normal CA-125 level [13]. Ross et al showed that elevated tumor antigen-125 after treatment was associated with poorer overall survival in patients with uterine carcinosarcoma and indicates that tumor antigen-125 levels may predict disease recurrence and provide prognostic information regarding survival [37].
A limitation of the present study is its observational nature, as the methodology did not include some analyses of molecular factors that are now quite commonly used. Another limitation is that some of the patients were not treated in our center from the beginning, and came only at the time of diagnosis of cancer recurrence. However, the fact that no patient was lost to follow-up deserves to be emphasized. The inclusion in the analysis of patients with carcinosarcomas, a tumor that now belongs to the group of metaplastic carcinomas, was appropriate because the clinical course is similar to other tumors in the group of mesenchymal neoplasms.
Conclusions
This retrospective study of the results of treatment of uterine sarcomas and analysis of selected prognostic factors shows that patients in FIGO IA stage live more than 10 years. Both age of patients at diagnosis below 60 years and high expression of estrogen and progesterone receptors in the tumor are prognostically favorable factors. In contrast, high expression of p53 or Ki67 and high levels of CA125 and IL-8 are associated with shorter patient survival. The high diagnostic sensitivity of sTNF RI and VEGF suggests the possibility of using this biomarker in early diagnosis in these patients.
Figures
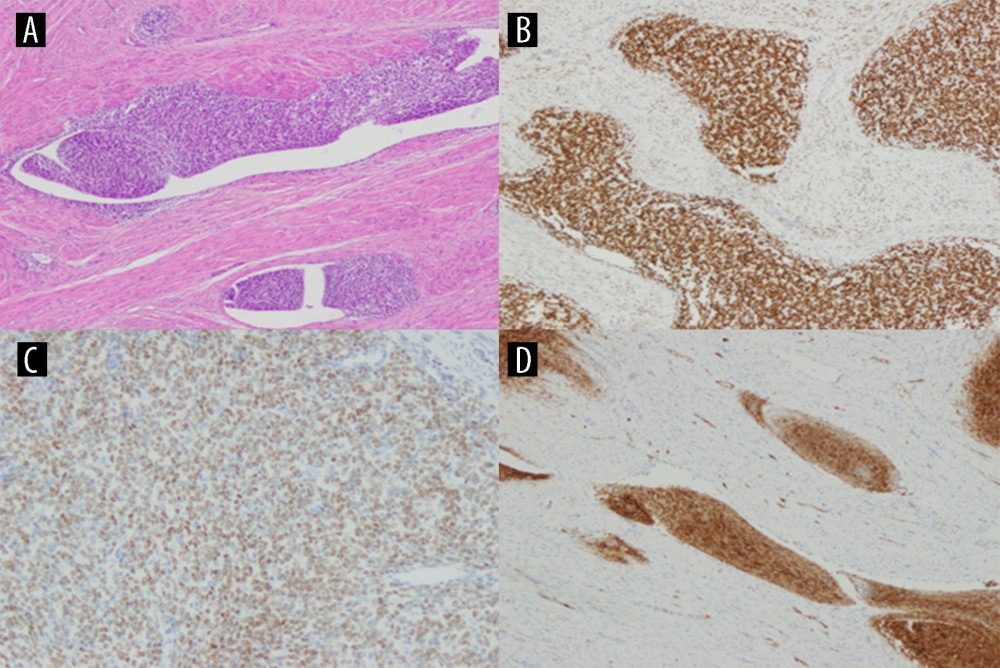 Figure 1. Low-grade endometrial stroma sarcoma (LG ESS) staining with hematoxylin-eosin (A), high expression of ER (B), high expression of PR (C), high expression of Ki67 (D). Created using Windows 10Pro, PowerPoint.
Figure 1. Low-grade endometrial stroma sarcoma (LG ESS) staining with hematoxylin-eosin (A), high expression of ER (B), high expression of PR (C), high expression of Ki67 (D). Created using Windows 10Pro, PowerPoint. 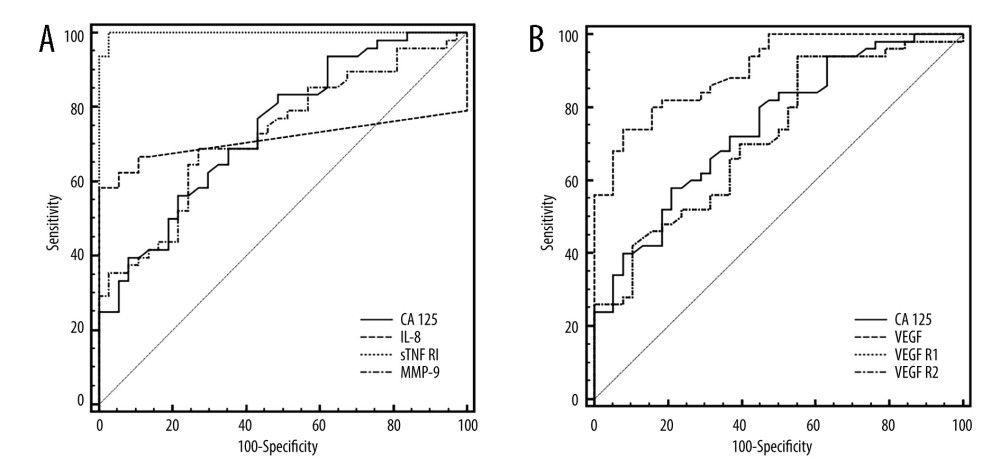 Figure 2. ROC curves of CA125 and inflammatory biomarkers (A) and VEGF and their receptors, (B) assays in uterine sarcoma patients vs control group. Created using MedCalc statistical software.
Figure 2. ROC curves of CA125 and inflammatory biomarkers (A) and VEGF and their receptors, (B) assays in uterine sarcoma patients vs control group. Created using MedCalc statistical software. 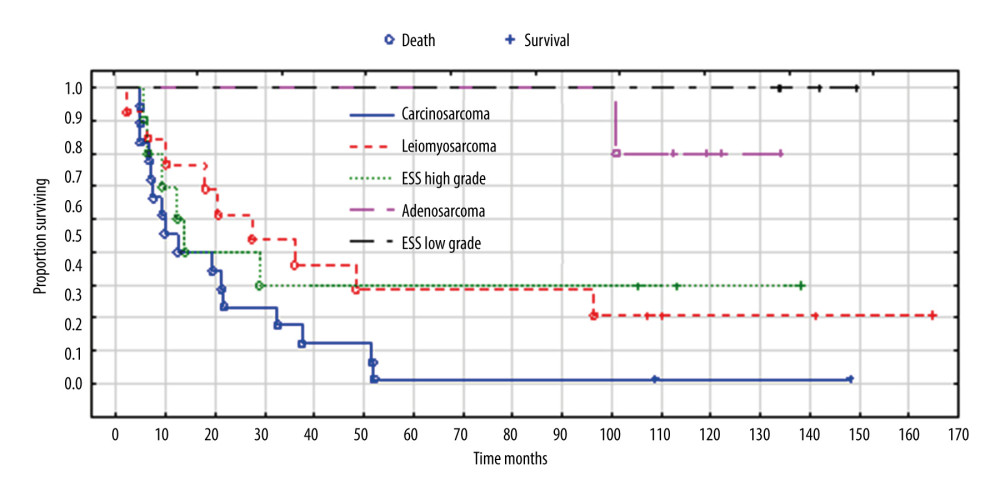 Figure 3. Overall survival according to histopathological types of sarcoma. Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis.
Figure 3. Overall survival according to histopathological types of sarcoma. Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis. 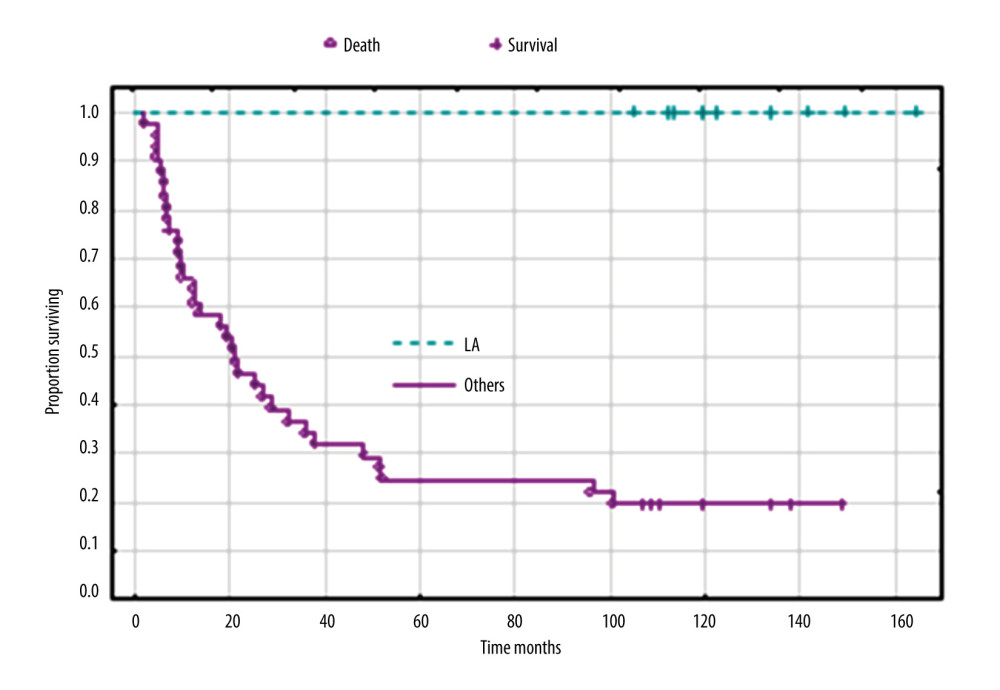 Figure 4. Overall survival according to clinical FIGO stage IA versus others (IB, IIA, IIB, and IIIA). Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis.
Figure 4. Overall survival according to clinical FIGO stage IA versus others (IB, IIA, IIB, and IIIA). Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis. 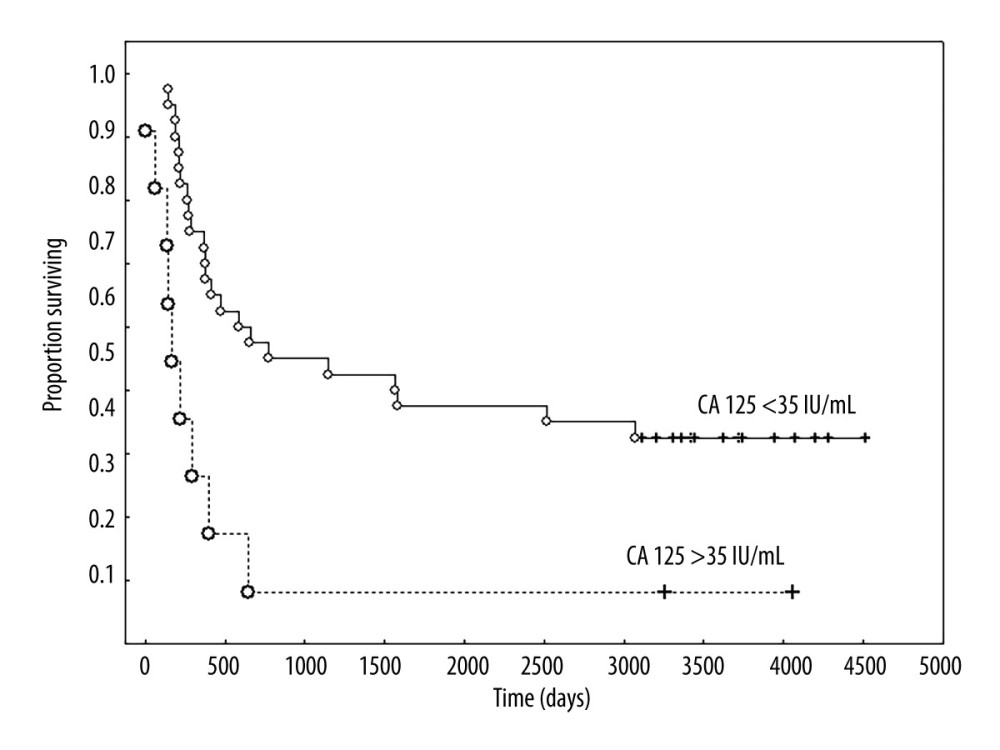 Figure 5. Overall survival according to the concentration of CA 125 IU/mL. Created using Statistica 6.0 software STATSOFT, Kaplan-Meier survival analysis.
Figure 5. Overall survival according to the concentration of CA 125 IU/mL. Created using Statistica 6.0 software STATSOFT, Kaplan-Meier survival analysis. 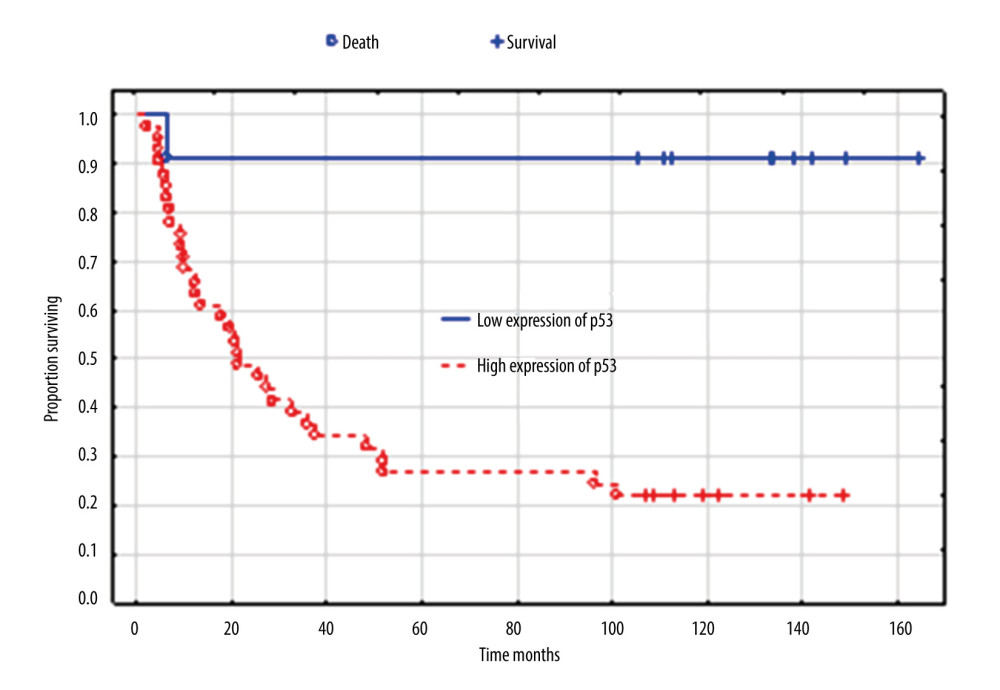 Figure 6. Overall survival according to expression of p53. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Kaplan-Meier survival analysis.
Figure 6. Overall survival according to expression of p53. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Kaplan-Meier survival analysis. 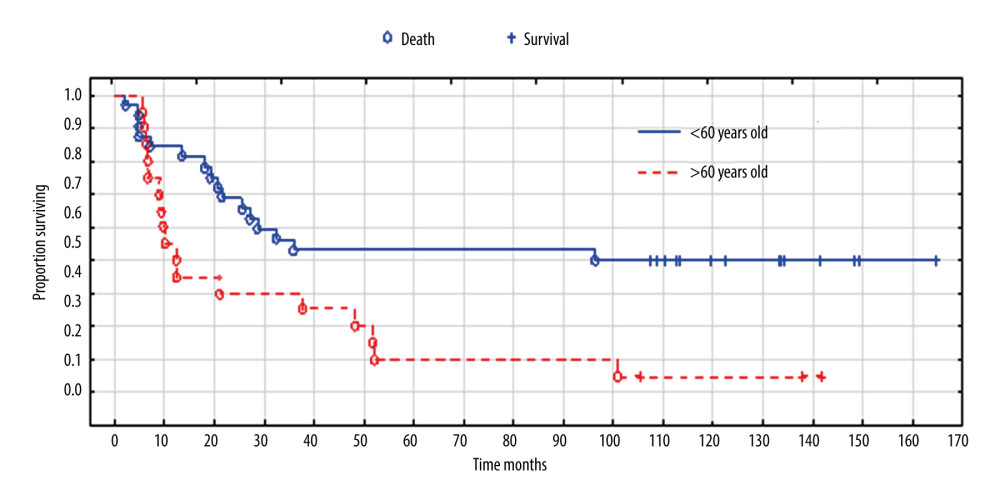 Figure 7. Overall survival according to age. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Survival analysis.
Figure 7. Overall survival according to age. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Survival analysis. Tables
Table 1. Patients’ characteristics.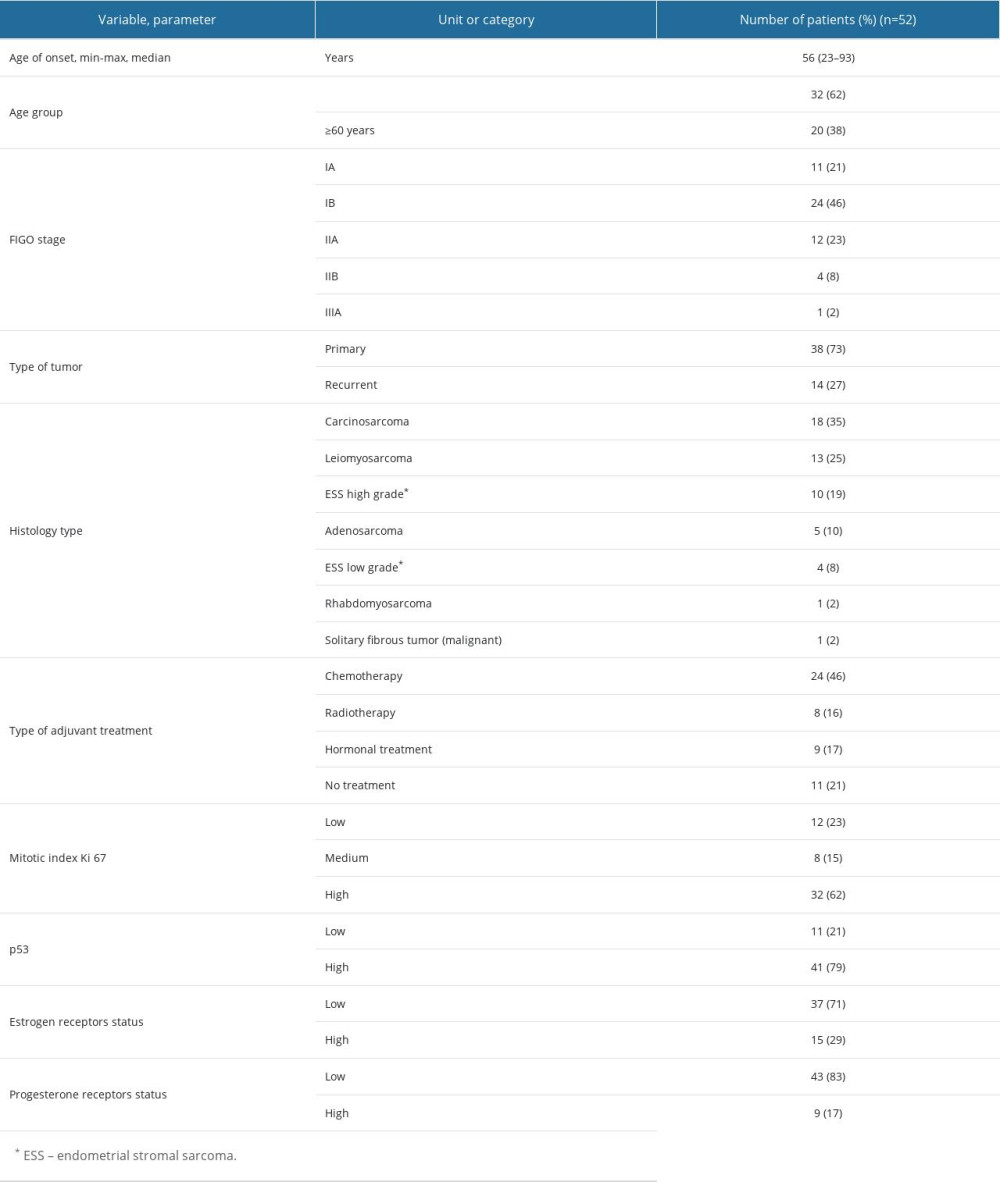 Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas.
Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas.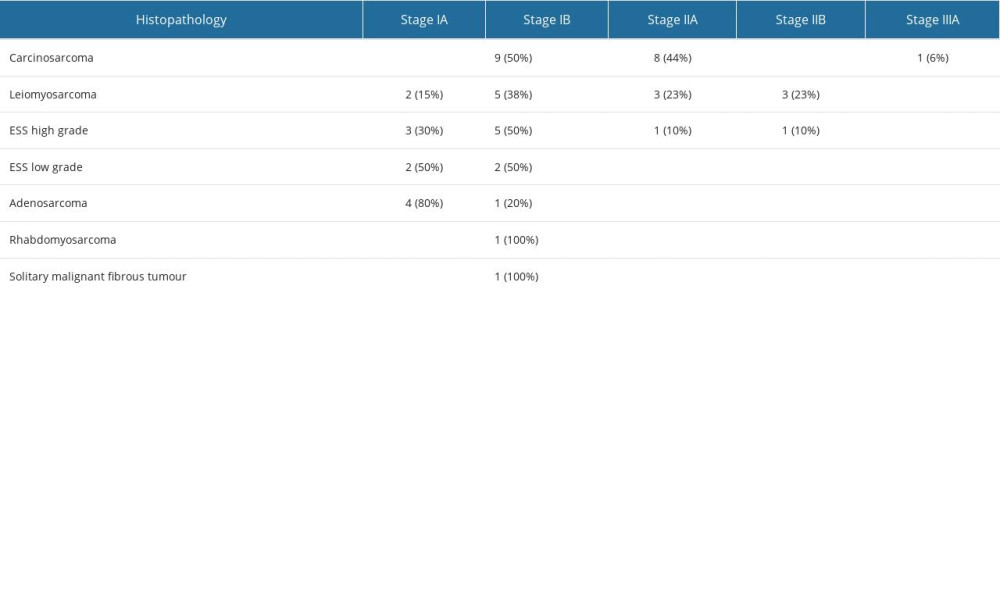 Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients.
Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients.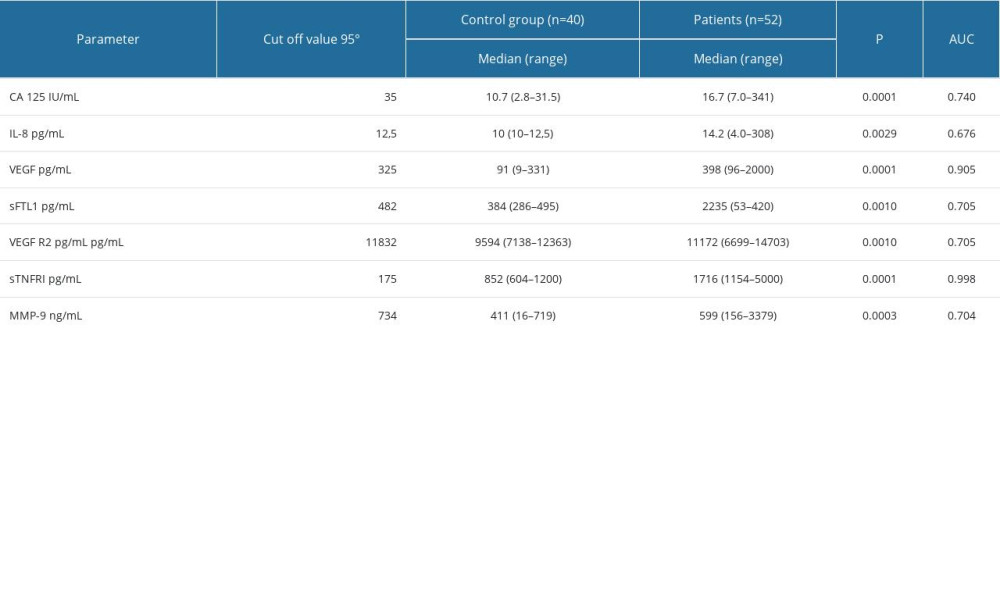 Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months.
Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months.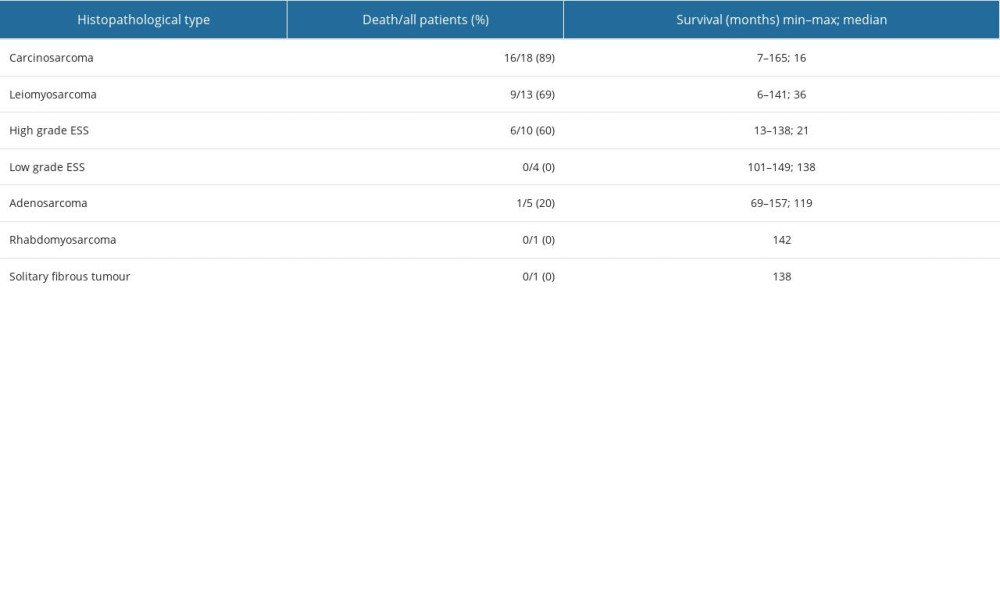 Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients.
Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients.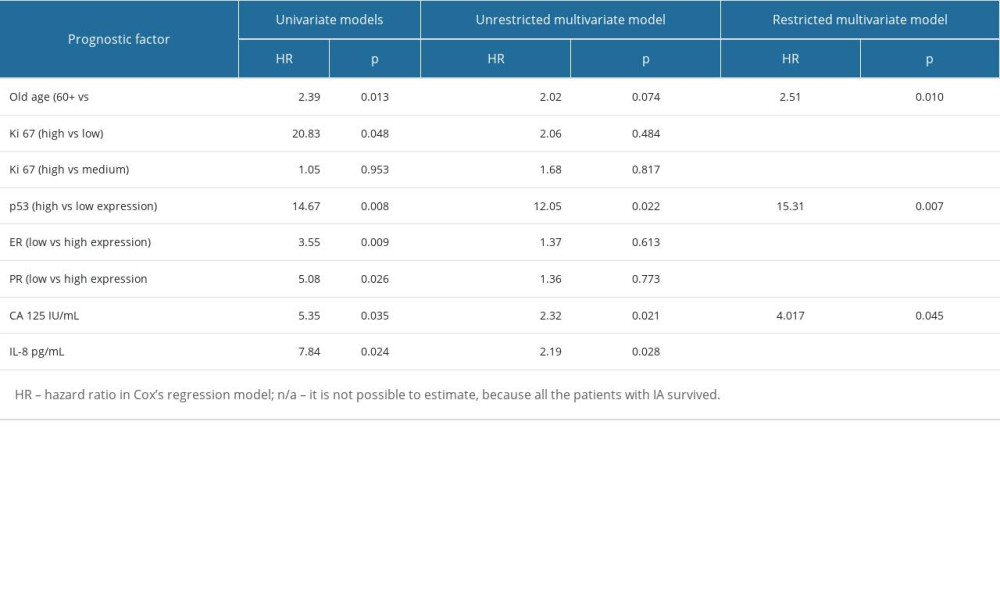
References
1. Tropé CG, Abeler VM, Kristensen GB, Diagnosis and treatment of sarcoma of the uterus. A review: Acta Oncol, 2012; 51(6); 694-705
2. Ramirez PT, Salvo G, Uterine sarcomas MSD Manual: Professional version, 2020
3. Travaglino A, Raffone A, Catena U, Ki67 as a prognostic marker in uterine leiomyosarcoma: A quantitative systematic review: Eur J Obstet Gynecol Reprod Biol, 2021; 266; 119-24
4. Davidson B, Kjaereng ML, Forsund M, Progesterone receptor expression is an independent prognosticator in FIGO stage I uterine leiomyosarcoma: Am J Clin Pathol, 2016; 145; 449-58
5. Hinchcliff E, Rumpf J, Ratan R, Hormone receptor status and the role of oophorectomy in uterine leiomyosarcoma: Gynecol Oncol, 2022; 167(3); 490-95
6. Croce S, Devouassoux-Shisheboran M, Pautier P, Uterine sarcomas and rare uterine mesenchymal tumors with malignant potential. Diagnostic guidelines of the French Sarcoma Group and the Rare Gynecological Tumors Group: Gynecol Oncol, 2022; 167(2); 373-89
7. Gadducci A, Multinu F, De Vitis LA, Endometrial stromal tumors of the uterus: Epidemiology, pathological and biological features, treatment options and clinical outcomes: Gynecol Oncol, 2023; 171; 95-105
8. Petrovic D, Babic D, Forko JI, Martinac I, Expression of Ki-67, p53 and progesterone receptors in uterine smooth muscle tumors. Diagnostic value: Coll Antropol, 2010; 34(1); 93-97
9. Hoang L, Chiang S, Lee CH, Endometrial stromal sarcomas and related neoplasms: New developments and diagnostic considerations: Pathology, 2018; 50; 162-77
10. Roy M, Musa F, Taylor SE, Huang M, Uterine sarcomas: How to navigate an ever-growing list of subtypes: Am Soc Clin Oncol Educ Book, 2022; 42; 1-10
11. Kobayashi H, Uekuri C, Akasaka J, The biology of uterine sarcomas: A review and update: Mol Clin Oncol, 2013; 1(4); 599-60
12. Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Clinical significance of pretreatment serum levels of VEGF and its receptors, IL-8, and their prognostic value in type I and II endometrial cancer patients: PLoS One, 2017; 12(10); e01845761
13. Matsuo K, Ross MS, Yunokawa M, Clinical utility of CA-125 in the management of uterine carcinosarcoma: J Gynecol Oncol, 2018; 29(6); e88
14. Dziobek K, Oplawski M, Grabarek BO, Changes in the expression profile of VEGF-A, VEGF-B, VEGFR-1, VEGFR-2 in different grades of endometrial cancer: Curr Pharm Biotechnol, 2019; 20(11); 955-63
15. Yetkin-Arik B, Kastelein AW, Klaassen I, Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy: Biochim Biophys Acta Rev Cancer, 2021; 1875(1); 188446
16. Roškar L, Roškar I, Rižner L, Diagnostic and therapeutic values of angiogenic factors in endometrial cancer: Biomolecules, 2022; 12(1); 7
17. Huang H, Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances: Sensors (Basel), 2018; 18(10); 3249
18. Li X, Li Zha, Li B, Clinical significance of MMP-9 overexpression in endometrial cancer: A PRISMA-compliant meta-analysis: Front Oncol, 2022; 12; 925424
19. Bi Q, Miao Z, Shen J, Detecting the research trends and hot spots in external irradiation therapy for rectal cancer: J Cancer, 2022; 13(7); 2179-88
20. Khan SR, Soomar SM, Asghari T, Prognostic factors, oncological treatment and outcomes of uterine sarcoma: 10 years’ clinical experience from a tertiary care center in Pakistan: BMC Cancer, 2023; 23; 510
21. Kyriazoglou A, Liontos M, Ziogas DC, Management of uterine sarcomas and prognostic indicators: Real world data from a single institution: BMC Cancer, 2018; 18; 1247-55
22. Wang F, Dai X, Chen H, Clinical characteristics and prognosis analysis of uterine sarcoma: A single-institution retrospective study: BMC Cancer, 2022; 22(1); 1050
23. Burle de Siqueira Ferroni Plentz T, Candido EC, Dias LF, Diagnosis, treatment of uterine sarcoma: A retrospective cohort study of 122 cases: Mol Clin Oncol, 2020; 13; 81
24. Pautier P, Floquet A, Chevreau C, Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): A non-randomised, multicentre, phase 2 trial: Lancet Oncol, 2015; 16(4); 457
25. Sunar V, Korkmaz V, Akin S, Efficacy of Pazopanib in patients with metastatic uterine sarcoma: A multi-institutional study: J BUON, 2019; 24(6); 2327-32
26. Kapp DS, Shin JY, Chan JK, Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy: Cancer, 2008; 112; 820-30
27. Mayhofer K, Lozanov P, Bodner K, Ki-67 expression in patients with uterine leiomyomas, uterine smooth muscle tumors of uncertain malignant potential (STUMP) and uterine leiomyosarcomas (LMS): Acta Obstet Gynecologica Scand, 2004; 83(11); 1085-88
28. Muller PA, Vousden KH, P53 mutations in cancer: Nat Cell Biol, 2013; 15; 2-8
29. Bosoteanu M, Deacu M, Aschie M, The role of pathogenesis associated with the tumor microclimate in the differential diagnosis of uterine myocytic tumors: J Clin Med, 2023; 12(12); 4161
30. Bosoteanu M, Deacu M, Voda RI, Five-year retrospective study of uterine STUMP and leiomyosarcoma: Clin Pract, 2022; 12(6); 897-907
31. Travaglino A, Raffone A, Gencarelli A, p53, p16 and ki67 as immunohistochemical prognostic markers in uterine smooth muscle tumors of uncertain malignant potential (STUMP): Pathol Res Pract, 2021; 226; 153592
32. Nakamura M, Obata T, Daikoku T, Fujiwara H, The association and significance of p53 in gynecologic cancers: The potential of targeted therapy: Int J Mol Sci, 2019; 20(21); 5482
33. Nissreen M, Stewart Colin JR, Sarah Ch, p53 immunohistochemical analysis of fusion-positive uterine sarcomas: Histopatology, 2020; 78(6); 805-13
34. Zang Y, Dong M, Zhang K, Hormonal therapy in uterine sarcomas: Cancer Med, 2019; 8(4); 1339-49
35. Wen KC, Horng HC, Wang PH, Uterine sarcoma Part I – Uterine leiomyosarcoma: The Topic Advisory Group systematic review: Taiwan J Obstet Gynecol, 2016; 55(4); 463-71
36. Maccaroni E, Lunerti V, Agostinelli V, New insights into hormonal therapies in uterine sarcomas: Cancers (Basel), 2022; 14(4); 921
37. Ross MS, Chandler CK, Matsuo K, Cancer antigen 125 is associated with disease status in uterine carcinosarcoma: Rare Tumors, 2019; 11; 2036361319884159
Figures
 Figure 1. Low-grade endometrial stroma sarcoma (LG ESS) staining with hematoxylin-eosin (A), high expression of ER (B), high expression of PR (C), high expression of Ki67 (D). Created using Windows 10Pro, PowerPoint.
Figure 1. Low-grade endometrial stroma sarcoma (LG ESS) staining with hematoxylin-eosin (A), high expression of ER (B), high expression of PR (C), high expression of Ki67 (D). Created using Windows 10Pro, PowerPoint. Figure 2. ROC curves of CA125 and inflammatory biomarkers (A) and VEGF and their receptors, (B) assays in uterine sarcoma patients vs control group. Created using MedCalc statistical software.
Figure 2. ROC curves of CA125 and inflammatory biomarkers (A) and VEGF and their receptors, (B) assays in uterine sarcoma patients vs control group. Created using MedCalc statistical software. Figure 3. Overall survival according to histopathological types of sarcoma. Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis.
Figure 3. Overall survival according to histopathological types of sarcoma. Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis. Figure 4. Overall survival according to clinical FIGO stage IA versus others (IB, IIA, IIB, and IIIA). Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis.
Figure 4. Overall survival according to clinical FIGO stage IA versus others (IB, IIA, IIB, and IIIA). Created using Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA), Kaplan-Meier survival analysis. Figure 5. Overall survival according to the concentration of CA 125 IU/mL. Created using Statistica 6.0 software STATSOFT, Kaplan-Meier survival analysis.
Figure 5. Overall survival according to the concentration of CA 125 IU/mL. Created using Statistica 6.0 software STATSOFT, Kaplan-Meier survival analysis. Figure 6. Overall survival according to expression of p53. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Kaplan-Meier survival analysis.
Figure 6. Overall survival according to expression of p53. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Kaplan-Meier survival analysis. Figure 7. Overall survival according to age. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Survival analysis.
Figure 7. Overall survival according to age. Created using the Statistica 13.1 software (STATSOFT; Statistica, Tulsa, OK, USA). Survival analysis. Tables
 Table 1. Patients’ characteristics.
Table 1. Patients’ characteristics. Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas.
Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas. Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients.
Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients. Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months.
Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months. Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients.
Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients. Table 1. Patients’ characteristics.
Table 1. Patients’ characteristics. Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas.
Table 2. Comparison of FIGO stage (2009) with histological types of sarcomas. Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients.
Table 3. Serum concentrations of biomarkers in healthy subjects as controls and in uterine sarcoma patients. Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months.
Table 4. The histopathological type of tumor, number of patients who died/number of all patients, the percentage of deaths, and the survival time in months. Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients.
Table 5. Significant prognostic factors for survival in univariate and multivariate analysis in the analyzed group of patients. In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








