22 December 2023: Clinical Research
Efficacy of Continuous-Wave Transscleral Cyclophotocoagulation Post-Pars Plana Vitrectomy in Glaucoma Patients: A Retrospective Study from Poland
Izabela Kuciel-PolczakDOI: 10.12659/MSM.941770
Med Sci Monit 2023; 29:e941770
Abstract
BACKGROUND: Glaucoma, a vision-threatening condition, results from optic nerve damage and affects millions of people worldwide. Often asymptomatic, it is hereditary, with risk factors like hypertension, diabetes, and steroid use. Despite its link with intraocular pressure (IOP), not everyone with high IOP develops glaucoma. After pars plana vitrectomy (PPV), patients face increased IOP risks. Traditional treatment includes pharmacotherapy, and, when ineffective, surgical interventions. Continuous-wave transscleral cyclophotocoagulation (CW-TSCPC) is an alternative for refractory glaucoma but can have complications. Our study compares the efficacy and safety of CW-TSCPC after PPV.
MATERIAL AND METHODS: The study group consisted of 18 patients diagnosed with glaucoma who underwent the CW-TSCP procedure as the first-choice therapy after conservative treatment of glaucoma proved ineffective. The comparison group consisted of 12 patients who underwent the CW-TSCP procedure after conservative drug treatment and in whom surgical treatment of glaucoma had been unsuccessful. All patients had inadequate control of IOP after PPV.
RESULTS: Study and comparison group patients showed a decrease in IOP during the follow-up, independent of the type of endotamponade used (P<0.05). When the indication for PPV was retinal detachment hemorrhage into the vitreous chamber, a significant decrease in IOP between 0 days and 180 days was only found in the study group (P<0.05). In contrast, when the indication for PPV was the state after uveitis or proliferative diabetic retinopathy, a significant decrease in IOP was found at 180 days in the study and comparison groups (P<0.05).
CONCLUSIONS: The analysis showed that the CW-TSCPC procedure can be recommended as the first-choice invasive treatment in patients with increased IOP after PPV.
Keywords: Glaucoma, Intraocular Pressure, Ocular Hypertension, Vitrectomy
Background
Glaucoma is caused by chronic, progressive damage to the optic nerve, resulting in a gradual deterioration of vision until it is completely lost [1–3]. The most common type of glaucoma is open-angle glaucoma, which affects nearly 57.5 million people worldwide, and, unfortunately, this is an increasing trend [4,5]. Particularly worrisome is the fact that the disease is asymptomatic for a long time, and 1 in 2 people with the disease are unaware of it [6,7]. Notably, the disease is 4 times more common in siblings and 2 times more common in the offspring of sufferers compared to the healthy population [6,7]. Important risk factors for the development of glaucoma include hypertension or hypotension, diabetes, migraine, myopia >4 diopters (D), and the use of steroid therapy [5,8–11]. While glaucoma can develop even when intraocular pressure (IOP) is low, it is the IOP value and its diurnal fluctuations that favor development of the disease [12–14]. Therefore, the main goal of glaucoma treatment is to lower the IOP, individually for each patient, to a level at which no disease progression is observed [12–14]. Nevertheless, even people with normal IOP (11–21 mmHg) can experience progressive damage to the optic nerve, resulting in a diagnosis of glaucoma. Moreover, elevated IOP does not always equate to glaucoma [12–14]; in a portion of the population, IOP above 21 mmHg does not cause optic nerve damage [12–14]. This condition is referred to as ocular hypertension, and only individuals at high risk for glaucoma require treatment [15,16].
The management of glaucoma begins with non-invasive methods (eg, pharmacotherapy) [2,15,17]. This involves the use of drugs from 5 different drug classes, which work by targeting the production of aqueous fluid and improving its drainage through conventional, choroidal–scleral, or both pathways [2,15,17]. Subsequently, in the absence of improvement, invasive treatment is used, including surgery of the exophthalmos angle as well as cyclodestructive procedures involving permanent damage to the ciliary body’s production of watery fluid [18,19]. This includes transcorneal cyclophotocoagulation with a 1064-nm infrared Nd-YAG laser as well as transcorneal cyclophotocoagulation with an 810-nm diode laser [18,19]. Currently, traditional continuous-wave transcleral cyclophotocoagulation (CW-TSCPC) is only used when other methods are ineffective [20,21]. This is because CW-TSCPC involves many serious adverse effects, such as vision loss, hyphema, macular edema, hypotony, persistent inflammation, phthisis bulbi, and sympathetic ophthalmia [13,22,23].
Patients who have undergone vitrectomy through the planar part of the ciliary body (PPV) may experience an elevation in IOP pressure [24–27]. This increase in IOP can be attributed to various factors, including the specific indication for the procedure, the type of endotamponade utilized, improper laser photocoagulation of the retina, postoperative inflammatory responses, and the administration of steroid medications [24–27]. These factors can potentially affect the structure and function of the retinal angle, thereby contributing to the observed rise in IOP [24–27].
One of the most common complications after PPV is an increase in IOP [28–31]. It is believed that this is, among other things, the result of oxidative stress on the trabecular meshwork, caused by diffusion of oxygen from the vitreous chamber to the anterior chamber [28–31]. There are several causes of secondary glaucoma after vitrectomy [28–31]. Application of oil endotamponade silicone can lead to various pathophysiological processes, namely pupillary block, inflammatory angle closure by adhesions, iris rubeosis, post-inflammatory and caused glaucoma steroids and the migration of emulsified and non-emulsified silicone oil into the chamber anterior, which causes infiltration and occlusion of the trabecular meshwork [32–34]. Use of endotamponade gas (sulfur hexafluoride, perfluoroethane, perfluoropropane) is also associated with the risk of increasing IOP after surgery [32–34]. The causes of these complications include, but are not limited to, the development of inflammation, trabecular damage from gas particles, infusion of fluids, cells, cytokines, proteins, and solid particles [32–34]. If the gas concentration is too high, it overexpands, and because of low extensibility of the sclera, intraocular pressure increases [32–35]. Silicone oils and perfluoro propane gases have a density less than water and rise to the top of the eyeball, leading to development of trabecular glaucoma [32–34]. Very rarely, we observe the development of glaucoma months or even years after surgery [32–34].
With the development of surgical techniques, PPV is becoming one of the most common ophthalmic surgeries and is used to treat many vitreous diseases and the eyeball [24,31,36]. The incidence of secondary glaucoma after uncomplicated PPV ranges from 15% to 20% [24,31,36]. Unfortunately, it is sometimes resistant to pharmacological treatment due to the changed trabecular structure after PPV (eg, clogging by oil particles, gas particles, development of fibrous tissue, vascular system filtration angle) [24,31,36]. Therefore, intraocular pressure is normalized in patients after PPV it can be problematic [24,31,36]. Surgical procedures also show lower effectiveness than in eyes that have not previously undergone posterior vitrectomy [24,31,36]. Trabeculectomy with mitomycin C is a widely performed glaucoma surgery, but success depends on properly tightened filter vesicles, and conjunctival scarring after eye surgery is the main risk factor for surgical failure [37]. In turn, treatment of secondary glaucoma using drainage implants (eg, Ahmed valves) is associated with a greater risk of corneal decompensation due to contact with the tube, implant migration, tube puncture through the conjunctiva, excessive fibrosis of the capsule around the plate, double vision, and strabismus, or, very rarely, endophthalmitis [38,39]. Complications related to valve implantation surgery or trabeculectomy also include early and late postoperative hypotension [38,39]. Due to need for repeated intraocular procedures in patients after PPV, CW-TSCPC seems to be a safe and effective method of treating glaucoma, without exposing the patient to subsequent procedures that violate continuity of the eye tissues [40].
In cases where filtration with trabeculectomy or express valve is limited in its effectiveness for treating glaucoma after PPV due to conjunctival scarring or internal orifice overgrowth, alternate approaches may be considered [41,42]. One such approach is cyclophotocoagulation, which involves use of a diode laser that emits a beam with a wavelength of 800–850 nm [41,42]. This laser energy is targeted at the pigment epithelium of the ciliary body, where it is absorbed by melanin, generating heat and inducing a coagulative effect [41,42]. This process leads to destruction of the ciliary body and subsequently reduces production of aqueous fluid, thereby lowering IOP [41,42].
The validity of using CW-TSCPC in patients with refractory glaucoma (ie, glaucoma that is not controlled despite prior treatment involving filtering surgery or pharmacotherapy alone) has been established for more than 3 decades [41,43]. It has been shown that 63–89% of these patients experience a reduction of IOP to less than 22 mmHg [41,43]. However, it should be kept in mind that there is considerable variability in the percentage of patients who achieve the intended effects of CW-TSCPC treatment due to different patient populations and laser parameters [44]. Murphy et al observed that the CW-TSCPC procedure was most effective in patients diagnosed with chronic angle-closed glaucoma (CACG) compared to other types of glaucoma [44].
It has also been shown that IOP prior to the CW-TSCPC procedure affects the effectiveness of the procedure itself [40]. Vernon et al found that 94% of patients whose preoperative IOP was higher than 30 mmHg achieved an IOP reduction of at least 30%, while if preoperative IOP was below 30 mmHg, an IOP reduction of 30% was found in only 74% of cases [40].
Studies have indicated the utility of using CW-TSCPC in patients who had increased IOP after post-penetrating keratoplasty (PK) [45]. Shah et al reported a decrease in IOP from 33 mmHg (median) to 16 mmHg (median) after CW-TSCPC when the IOP increase occurred after PK, although the majority of participants in the study required a repeat CW-TSCPC procedure [45].
Nevertheless, the CW-TSCPC procedure is associated with postoperative complications, including postoperative pupillary abnormalities, hyphema, inflammation, hypotony, retreatments, reduced visual acuity, pain, lens subluxation, staphyloma formation, scleral perforation, and sympathetic ophthalmia [46,47].
The literature on CW-TSCPC indicates that this invasive technique is an excellent option for IOP reduction in patients with glaucoma refractory to current treatment when other invasive methods prove insufficient [18,20,21,30,33].
Therefore, the purpose of our study was to evaluate and compare the efficacy and safety of CW-TSCPC in 30 patients diagnosed with glaucoma after PPV, for whom CW-TSCP was the primary glaucoma treatment, compared to patients after PPV who underwent surgical glaucoma treatment as the primary treatment without expected clinical improvement, and for whom CW-TSCPC was performed as the next step.
Material and Methods
ETHICS:
We obtained a permission from the Institution of the Ethical Committee operating at the Academy of Silesia in Katowice, Poland. No. 03/KEBN/2021. Medical records from 2017 to 2022 were analyzed.
CHARACTERISTICS OF THE STUDY GROUP:
The study group consisted of 18 patients diagnosed with glaucoma (7 females, 11 males) who underwent CW-TSCP as the first-choice procedure after conservative treatment of glaucoma proved ineffective. The inclusion and exclusion criteria for the study group are shown in Table 1. The PPV was performed for the following reasons: 1) retinal detachment (8 patients received silicone oil as an endotamponade); 2) hemorrhage into the vitreous chamber (6 patients received silicone oil as an endotamponade); SF6 endotamponade was used (2 patients received silicone oil as an endotamponade); and 3) state after uveitis/PDR (BSS; 2 patients received balanced salt solution endotamponade).
CHARACTERISTICS OF THE COMPARISON GROUP:
The comparison group consisted of 12 patients (7 women, 5 men) for whom the CW-TSCP procedure was carried out after unsuccessful conservative drug treatment and surgical treatment of glaucoma (trabeculectomy, express valve implantation). Table 2 provides the inclusion and exclusion criteria for the comparison group. The PPV was performed for the following reasons: 1) retinal detachment (4 patients received silicone oil as an endotamponade); 2) state after uveitis or PDR (3 patients received BSS as an endotamponade); and 3) hemorrhage into the vitreous chamber (5 patients received silicone oil as an endotamponade).
TOPICAL TREATMENT AFTER CW-TSCP:
Topical treatment after CW-TSCP was selected individually for each patient, and during observation we did note any complications. We assumed that the CW-TSCP procedure was effective when 1) the IOP value in the postoperative period was between 11 and 21 mmHg, 2) it was not necessary to repeat the operation or implement laser therapy, 3) there were no postoperative complications, and 4) there was no need to increase the dose of local anti-glaucoma drugs or to include systemic treatment.
INTRAOCULAR PRESSURE MEASUREMENT:
A Goldmann tonometer was used to assess IOP, with a value of 11–21 mmHg regarded as normal. During the ongoing follow-up, all patients in the study group and the control group were under the care of the hospital eye clinic according to their individual treatment and follow-up plan. If an increase in IOP was noted during follow-up after CW-TSCP, treatment was implemented, with the goals of “IOP reduction with initial monotherapy should be at least 20% from baseline values” and “an IOP reduction of less than 10% should be considered as no response to the drug,” following the current standards of the Polish Ophthalmological Society [48].
COMPUTER-BASED OPTICAL COHERENCE TOMOGRAPHY (OCT):
OCT (RTVue; Optovue, Inc., Fremont, California) was performed on each patient 4 times according to the standard protocol.
CW-TSCPC PROCEDURE:
CW-TSCPC surgeries were performed at 180 degrees of the lower hemisphere (excluding 3 and 9 o’clock) using an A.R.C. diode laser FOX at power settings between 2300 mW and 2500 mW or the Cyclo G6® laser system and G-Probe® delivery device (IRIDEX Corp., Mountain View, CA) at settings of 1250 mW and a duration of 4000 ms for eyes with dark irises and 1500 mW and a duration of 3500 ms for eyes with light irises.
The G-Probe was held perpendicular to the sclera so that the laser beam was directed 1.2 mm back toward the ciliary processes. Typically, 6 or 7 applications were made in each quadrant, sparing the meridians at 3 and 9 o’clock to avoid damage to the long ciliary blood vessels and nerves. The postoperative regimen included 0.1% dexamethasone for 4 weeks and 1% atropinum for 7–14 days.
STATISTICAL ANALYSIS:
Statistical analysis was atropine made via using Statplus software (AnalystSoft Inc, Brandon, FL 33511, USA) with the threshold of statistical significance
Results
CHANGES IN IOP VALUES DEPENDING ON THE ENDOTAMPONADE USED DURING PPV:
The study and comparison group patients showed a decrease in IOP between the start of follow-up (0 days) and 180 days after surgery (P<0.05; Table 3). Statistical analysis showed that in the study group, when silicone oil was used as an endotamponade, we observed IOP decreasing between the start of follow-up (0 days) and 180 days after surgery (Table 3; P<0.00001). Further, the IOP values recorded at 180 days of follow-up in the study group when silicone oil was used as the endotamponade were significantly lower than in the comparison group (Table 3; P=0.04593).
In the study group patients, when SF6 was used as an endotamponade, IOP was significantly lower on the 180th day of follow-up than at the beginning of the follow-up (Table 3; P=0.01446).
When BSS was used as an endotamponade, statistically significant differences were found in the study group between the beginning (0 days) and the 180th day of observation (Table 3; P=0.00008), as well as in the comparison group (Table 3; P=0.00017). Moreover, the variances in the IOP values between the study and comparison groups at baseline (0 days) and at 180 days of follow-up were statistically significant (Table 3; P=0.10097 and P=0.01391, respectively). The median number of topical hypotensive drugs was reduced, and there was no need for systemic treatment (Table 3).
EVALUATION OF THE EFFECTIVENESS OF CW-TSCP TREATMENT IN THE STUDY AND COMPARISON GROUPS:
In the next stage of the analysis, we evaluated the effectiveness of CW-TSCP treatment in the study and comparison groups based on the type of endotamponade used. In the study group, when silicone oil was used as an endotamponade, there was 1 case in which the applied treatment did not produce the expected results, which was presented in the material and methods section (Table 4).
In the study group, when both SF36 and BSS were used as an endotamponade, the treatment had the expected results in all patients, and thus the number of patients for whom the treatment did not have the expected results was 0, which made it impossible to perform a chi-square analysis (Table 4).
Meanwhile, in the comparison group, we found that achieving the expected outcome did not depend on the type of endotamponade used (Table 4; χ2=0.80; P=0.371). In the comparison group, 1 patient for whom silicone was used as an endotamponade did not have the expected outcome; this was also true for 1 patient for whom BSS was used (Table 4).
CHANGES IN IOP VALUES AFTER THE CW-TSCPC PROCEDURE, DEPENDING ON THE ETIOLOGY FOR WHICH PPV WAS PREVIOUSLY PERFORMED AND THE KIND OF ENDOTAMPONADE USED:
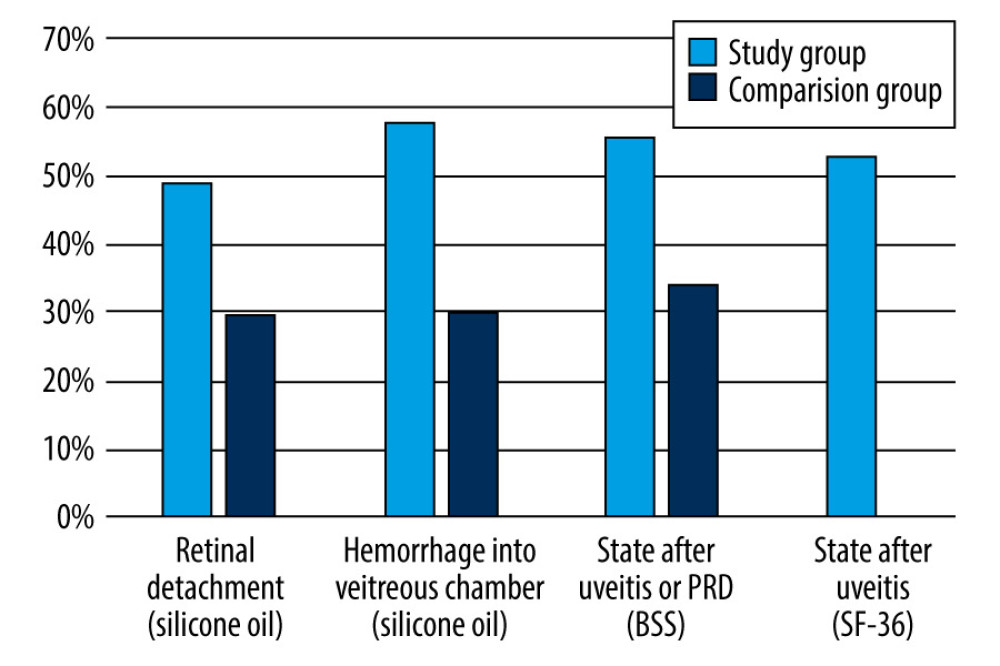
When PPV was performed due to retinal detachment or due to hemorrhage into the vitreous chamber, a statistically significant decrease in IOP was only found in the study group between the start and the 180th day of follow-up (Table 5; P<0.05).
In contrast, for patients whose indication for PPV was the state after uveitis or proliferative diabetic retinopathy (PRD), when BSS was used as an endotamponade, statistically significant reductions in IOP were observed in the study group between 0 and 6 months of follow-up (Table 5; P=0.00010), in the comparison group between 0 and 6 months of follow-up (Table 5; P=0.00027), and for comparisons of IOP values at baseline (0 days) between the study and comparison groups (Table 5; P=0.000604). When the indication for IOP was the state after uveitis or PRD and sulfur hexafluoride (SF-36) was used, there was a significant decrease in IOP between the start (0 days) and 180 days of follow-up in the study group (Table 5; P=0.00908).
Figure 1 shows the percentage by which IOP decreased between days 0 and 180 of follow-up in the study and comparison groups, taking into account the reason for PPV and the endotamponade used.
Discussion
In our study, we evaluated the validity and efficacy of using the CW-TSCPC procedure as a first-line invasive treatment in patients after PPV who experienced an increase in IOP and for whom drug treatment did not result in the intended improvement and IOP reduction.
We demonstrated that use of the CW-TSCPC procedure as the first-choice invasive treatment for refractory glaucoma contributed to a significant reduction in IOP during follow-up, regardless of the endotamponade used during the IOP procedure (
Currently, however, there is a changing view on the use and safety of CW-TSCPC in glaucoma therapy [18,49–51]. Bernardi et al showed that 3 months after the first CW-TSCPC procedure, IOP decreased by 24.80%, while 3 months after the second procedure, the decrease in IOP was 45.60% [52]. Similar to our study, Bernardi et al found no complications after the CW-TSCPC procedure [52]. We did not observe any complications during follow-up of 180 days. Long-term complications, such as chronic hypotonia, atrophy of the eyeball, and loss of vision leading to blindness, were not assessed. However, as suggested by Khodeiry et al [53], the lack of complications or a significant reduction in their incidence could be due to the fact that the CW-TSCPC procedure was performed using a lower laser power with a concomitant increase in exposure time and avoidance of pop sound generation [53]. Currently, however, there is a changing view on the use and safety of CW-TSCPC in glaucoma therapy [39].
Based on the available literature, the results of this study are the first of their kind, as IOP changes after CW-TSCP were evaluated in patients after previous PPV surgery, considering the different indications for the PPV procedure and the type of endotamponade used [53].
Nevertheless, it is important to take note of the study of Khodeiry et al, who evaluated the efficacy and safety of CW-TSCPC in 18 patients (18 eyes) with glaucoma who had a previous PPV procedure and used silicone oil as an endotamponade [53]. The authors reported a decrease in IOP from 29.7±9.60 mmHg at baseline to 14.40±5.50 mmHg at 6 months (180 days) of follow-up, which is a 51.52% decrease [53]. In contrast, in our study, when silicone oil was used, the decrease in IOP in the study group ranged from 46% to 58%, and in the control group it was 70% [53]. Khodeiry et al reported no decrease in IOP during the follow-up period in 5 patients (27.80%), while 1 patient had hypotonia (5.60%) [53]. It is possible that the lack of the expected treatment effect in 27.80% of patients could be due to the fact that Khodeiry et al included ethnically diverse participants (white, Hispanic, African American, Asian) in the study [47,54].
Although the removal of silicone oil is considered an alternative to filtering surgery, such as CW-TSCPC, for patients with glaucoma after PPV, it is important to note that a study by Jonas et al showed that long-term reduction in IOP after silicone oil removal was only achieved in 91% of cases, despite an initial decrease in IOP of approximately 93% [55]. Therefore, the decision to adopt this therapeutic strategy should be made after carefully evaluating the risk of retinal detachment recurrence and engaging in comprehensive discussions with the patient [55].
The safety of CW-TSCPC has also been evaluated by Khodeiry et al, who used this procedure in patients diagnosed with pseudophakic glaucoma. They reported a 42% decrease in IOP from 27.50±9.80 mmHg before the procedure to 16.10±6.30 mmHg after surgery [56].
It should be noted that the CW-TSCP procedure has a high success rate, as confirmed by our observations. However, the groups in the study were not very large, which may have led to absence of the expected result. Nevertheless, our findings are comparable to observations made by other researchers. Ghazi-Nouri et al reported success (IOP <21 mmHg) in 73.70% of patients after CW-TSCPC during a 12-month follow-up period [57]. Bloom et al reported successful outcomes for 72% of patients after 1-year of follow-up (IOP <22 mmHg) [58]. In addition, we observed a decrease in the number of topical IOP-lowering drugs during the 180-day follow-up period and no need for systemic treatment, which is consistent with the observations of other investigators [46,53,57].
There are some limitations of our study that should be noted. The number of participants in the study and control groups was relatively small, which may be due to the single-center, retrospective nature of our study. In addition, the observation period was only 180 days, and monitoring patients’ IOP for a longer period of time would have allowed us to determine the durability and long-term effectiveness of the CW-TSCPC procedure. Further, it would have been interesting to include patients who did not adhere to the post-treatment recommendations. Despite these limitations, our results have clinical value and may change the perception of the CW-TSCPC procedure, supporting its use as the first-choice procedure for invasive treatment of glaucoma, including after PPV.
Conclusions
The analysis demonstrated that the CW-TSCPC procedure, performed for elevated IOP following PPV when conservative treatment is ineffective, is both safe and effective. Notably, we did not observe any adverse effects, such as vision loss, hyphema, macular edema, hypotony, persistent inflammation, phthisis bulbi, or sympathetic ophthalmia. These findings suggest that the CW-TSCPC procedure can be recommended as the primary invasive treatment option for patients with increased IOP after PPV. However, further studies with larger sample sizes are needed.
Tables
Table 1. Inclusion and exclusion criteria for the study group.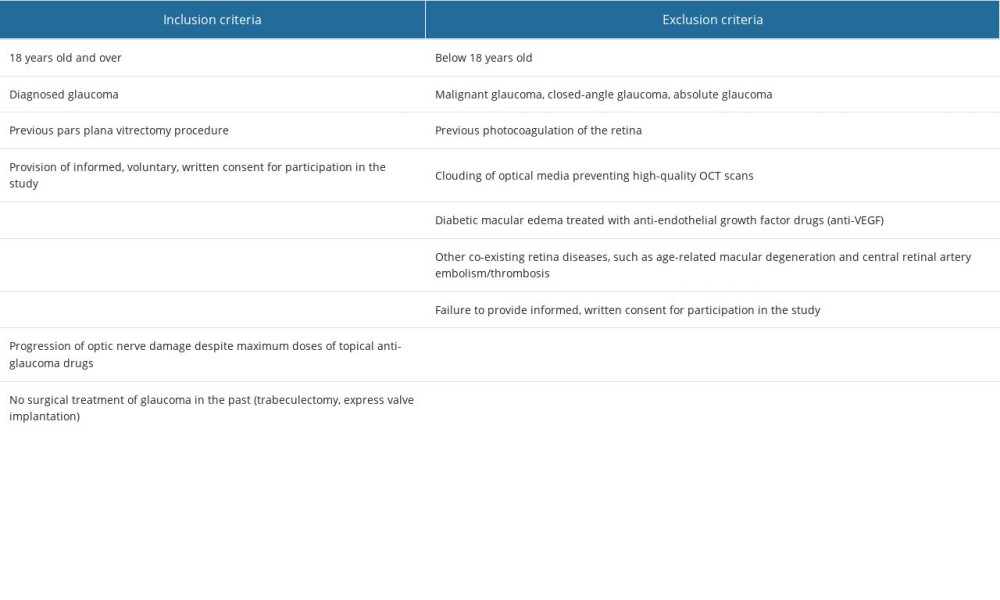 Table 2. Inclusion and exclusion criteria for the control group.
Table 2. Inclusion and exclusion criteria for the control group. Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period.
Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period.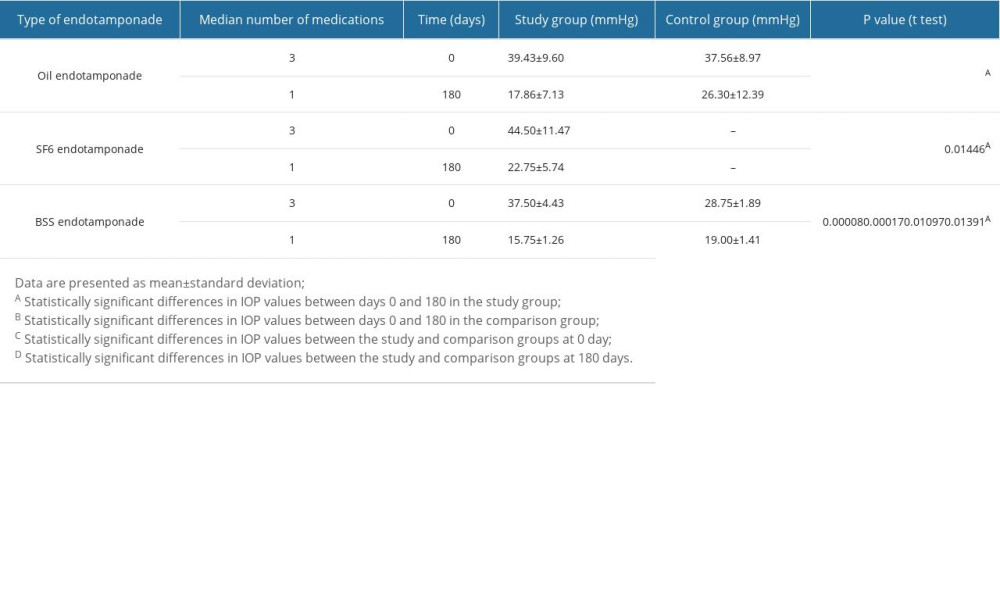 Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure.
Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure.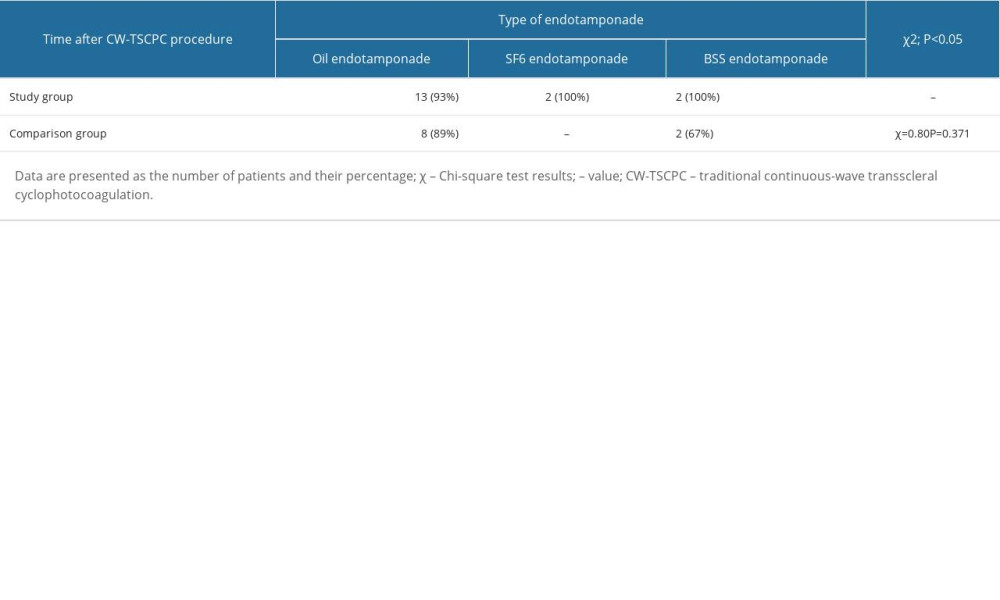 Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed.
Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed.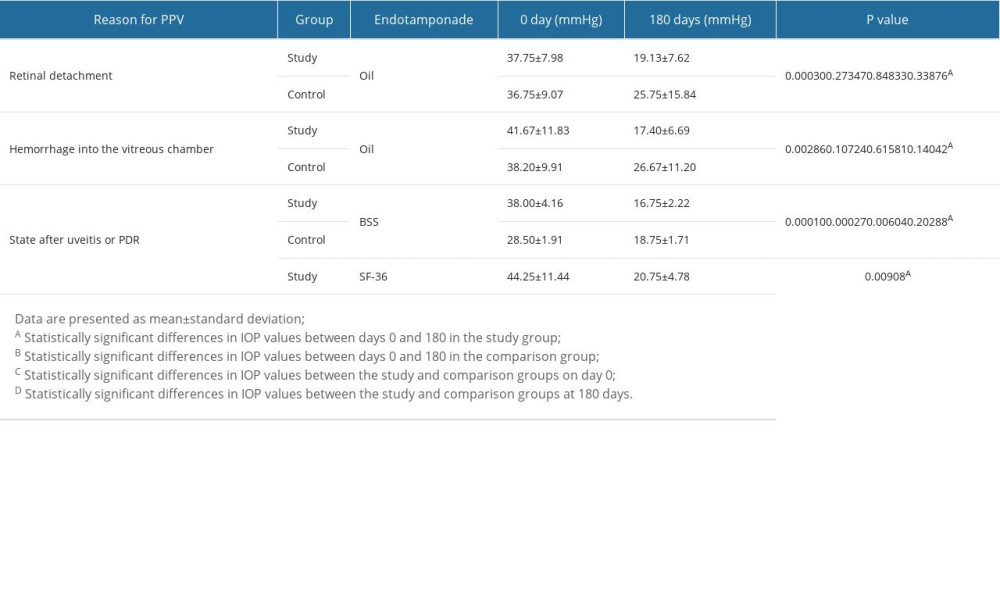
References
1. Allison K, Patel D, Alabi O, Epidemiology of glaucoma: The past, present, and predictions for the future: Cureus, 2020; 12(11); e11686
2. Schuster AK, Erb C, Hoffmann EM, The diagnosis and treatment of glaucoma: Dtsch Ärztebl Int, 2020; 117(13); 225-34
3. Stein JD, Khawaja AP, Weizer JS, Glaucoma in adults – screening, diagnosis, and management: A review: JAMA, 2021; 325(2); 164-74
4. Zhang N, Wang J, Li Y, Jiang B, Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review: Sci Rep, 2021; 11(1); 13762
5. Grzybowski A, Och M, Kanclerz P, Primary open angle glaucoma and vascular risk factors: a review of population-based studies from 1990 to 2019: J Clin Med, 2020; 9(3); 761
6. Fiedorowicz M, Choragiewicz T, Turski WA, Tryptophan pathway abnormalities in a murine model of hereditary glaucoma: Int J Mol Sci, 2021; 22(3); 1039
7. Quigley HA, Stone EM, Fingert JH, Familial glaucoma – a pedigree revisited with genetic testing after 70 years: JAMA Ophthalmol, 2022; 140(5); 543-44
8. Matlach J, Bender S, König J, Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression: Clin Ophthalmol Auckl NZ, 2019; 13; 9
9. Douglass A, Dattilo M, Feola AJ, Evidence for menopause as a sex-specific risk factor for glaucoma: Cell Mol Neurobiol, 2023; 43(1); 79-97
10. Al-Beishri AS, Malik R, Freidi A, Ahmad S, Risk factors for glaucoma drainage device exposure in a middle-eastern population: J Glaucoma, 2019; 28(6); 529-34
11. Ahmad A, Ahmad SZ, Khalique N, Prevalence and associated risk factors of glaucoma in Aligarh, India – a population-based study: Delhi Journal of Ophthalmology, 2020; 31(1); 36-40
12. Aquino MCD, Barton K, Tan AMWT, Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study: Clin Exp Ophthalmol, 2015; 43(1); 40-46
13. Ramli N, Htoon HM, Ho CL, Risk factors for hypotony after transscleral diode cyclophotocoagulation: J Glaucoma, 2012; 21(3); 169-73
14. Tan AM, Chockalingam M, Aquino MC, Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma: Clin Exp Ophthalmol, 2010; 38(3); 266-72
15. Cvenkel B, Kolko M, Current medical therapy and future trends in the management of glaucoma treatment: J Ophthalmol, 2020; 2020; 6138132
16. Jayanetti V, Sandhu S, Lusthaus JA, The latest drugs in development that reduce intraocular pressure in ocular hypertension and glaucoma: J Exp Pharmacol, 2020; 12; 539-48
17. Yadav KS, Rajpurohit R, Sharma S, Glaucoma: Current treatment and impact of advanced drug delivery systems: Life Sci, 2019; 221; 362-76
18. Anand N, Klug E, Nirappel A, Solá-Del Valle D, A review of cyclodestructive procedures for the treatment of glaucoma: Semin Ophthalmol, 2020; 35(5–6); 261-25
19. Miglani T, Ullah S, A review of the surgical management of neovascular glaucoma: Curr Surg Rep, 2023; 1-6
20. Kuchar S, Moster MR, Reamer CB, Waisbourd M, Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma: Lasers Med Sci, 2016; 31(2); 393-96
21. Maslin JS, Chen PP, Sinard J, Histopathologic changes in cadaver eyes after MicroPulse and continuous wave transscleral cyclophotocoagulation: Can J Ophthalmol, 2020; 55(4); 330-35
22. Pastor SA, Singh K, Lee DA, Cyclophotocoagulation: A report by the american academy of ophthalmology: Ophthalmology, 2001; 108(11); 2130-38
23. Osman EA, Al-Muammar A, Mousa A, Controlled Cyclophotocoagulation with diode laser in refractory glaucoma and long term follow up at King Abdulaziz University Hospital, Riyadh: Saudi J Ophthalmol, 2010; 24(1); 9-13
24. Fujikawa M, Sawada O, Kakinoki M, Long-term intraocular pressure changes after vitrectomy for epiretinal membrane and macular hole: Graefes Arch Clin Exp Ophthalmol, 2014; 252(3); 389-93
25. Mansukhani SA, Barkmeier AJ, Bakri SJ, The risk of primary open-angle glaucoma following vitreoretinal surgery – a population-based study: Am J Ophthalmol, 2018; 193; 143-55
26. Rahman R, Marler J, Stephenson J, Gillibrand W, Risk factors for elevated intraocular pressure on first day postoperative review following pars plana vitrectomy: J Vitreoretin Dis, 2017; 1(6); 397-400
27. Wu L, Berrocal MH, Rodriguez FJ, Intraocular pressure elevation after uncomplicated pars plana vitrectomy: Results of the Pan American Collaborative Retina Study Group: Retina, 2014; 34(10); 1985-89
28. Pillai GS, Varkey R, Unnikrishnan UG, Radhakrishnan N, Incidence and risk factors for intraocular pressure rise after transconjunctival vitrectomy: Indian J Ophthalmol, 2020; 68(5); 812-17
29. Toklu E, Altinisik M, Elbay A, Koytak A, Comparison of postoperative anterior segment changes associated with pars plana vitrectomy with and without vitreous base shaving: Int J Ophthalmol, 2020; 13(11); 1745-52
30. Yuan S, Fan W, Zhang C: Risk factors for intraocular pressure rise after pars plana vitrectomy with silicone oil tamponade, 2023
31. Rossi T, Ripandelli G, Pars plana vitrectomy and the risk of ocular hypertension and glaucoma: Where are we?: J Clin Med, 2020; 9(12); 3994
32. Rush RB, Penella ADV, Reinauer RM, Silicone oil versus perfluoropropane gas tamponade during vitrectomy for tractional retinal detachment or fibrous proliferation: A randomized clinical trial: Retina, 2021; 41(7); 1407-15
33. Zhang Y, Li X, Pan G, Efficacy of PPV combined with air tamponade for treatment of inferior retinal breaks: J Ophthalmol, 2021; 2021; 9597854
34. Deaner JD, Aderman CM, Bonafede L, Regillo CD, PPV, Retinectomy, and silicone oil without scleral buckle for recurrent RRD from proliferative vitreoretinopathy: Ophthalmic Surg Lasers Imaging Retina, 2019; 50(11); e278-e87
35. Cheng YH, Wang H, Li B, Vitrectomy with air tamponade for surgical repair of rhegmatogenous retinal detachment by eye position guided fluid-air exchange: Int J Ophthalmol, 2020; 13(9); 1417-22
36. Quan AV, Yannuzzi N, Chen J, Gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with secondary open angle glaucoma following vitreoretinal surgery: J Glaucoma, 2020; 29(4); e23
37. Seol BR, Lee SY, Kim YJ, Comparison of the efficacy and safety of trabeculectomy with mitomycin C according to concentration: A prospective randomized clinical trial: J Clin Med, 2020; 10(1); 59
38. Christakis PG, Zhang D, Budenz DL, Five-year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed versus Baerveldt study: Am J Ophthalmol, 2017; 176; 118-26
39. Pandav SS, Seth NG, Thattaruthody F, Long-term outcome of low-cost glaucoma drainage device (Aurolab aqueous drainage implant) compared with Ahmed glaucoma valve: Br J Ophthalmol, 2020; 104(4); 557-62
40. Vernon SA, Koppens JM, Menon GJ, Negi AK, Diode laser cycloablation in adult glaucoma: Long-term results of a standard protocol and review of current literature: Clin Experiment Ophthalmol, 2006; 34(5); 411-20
41. Ndulue JK, Rahmatnejad K, Sanvicente C, Evolution of cyclophotocoagulation: J Ophthalmic Vis Res, 2018; 13(1); 55
42. Sanchez FG, Lerner F, Sampaolesi J: Arch Soc Esp Oftalmol Engl Ed, 2018; 93(12); 573-79
43. Frezzotti P, Mittica V, Martone G, Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma: Acta Ophthalmol (Copenh), 2010; 88(1); 150-55
44. Murphy CC, Burnett CAM, Spry PGD, A two-centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma: Br J Ophthalmol, 2003; 87(10); 1252-57
45. Yildirim N, Yalvac IS, Sahin A, A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: A long-term follow-up: J Glaucoma, 2009; 18(3); 192-96
46. Albahlal A, Dhibi HA, Shahwan SA, Sympathetic ophthalmia following diode laser cyclophotocoagulation: Br J Ophthalmol, 2014; 98(8); 1101-6
47. Williams AL, Moster MR, Rahmatnejad K, Clinical efficacy and safety profile of micropulse transscleral cyclophotocoagulation in refractory glaucoma: J Glaucoma, 2018; 27(5); 445-49
48. Szaflik J, Dobrowolski D, Grabska-Liberek I: Wytyczne diagnostyki i leczenia jaskry (aktualizacja 2022) [in Polish]https://www.pto.com.pl/wytyczne
49. Ezzouhairi SM, Naciri L, Mba T, Jomaa R, A new approach for CW-TSCPC to improve its safety and efficacy in glaucoma treatment: J Fr Ophtalmol, 2022; 45(1); 93-103
50. Ling Q, Cai Z, Zhang X, Duan X, The efficacy and safety of micropulse transscleral laser treatment in glaucoma: A systematic review and meta-analysis: BMC Ophthalmol, 2023; 23(1); 263
51. Souissi S, Le Mer Y, Metge F, An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma: Acta Ophthalmol (Copenh), 2021; 99(5); e621-e53
52. Bernardi E, Töteberg-Harms M, First and second transscleral cyclophotocoagulation treatments provide similar intraocular pressure-lowering efficacy in patients with refractory glaucoma: Int Ophthalmol, 2022; 42(8); 2363-69
53. Khodeiry MM, Liu X, Sheheitli H, Slow coagulation transscleral cyclophotocoagulation for postvitrectomy patients with silicone oil-induced glaucoma: J Glaucoma, 2021; 30(9); 789-94
54. Emanuel ME, Grover DS, Fellman RL, Micropulse cyclophotocoagulation: Initial results in refractory glaucoma: J Glaucoma, 2017; 26(8); 726-29
55. Jonas JB, Knorr HL, Rank RM, Budde WM, Intraocular pressure and silicone oil endotamponade: J Glaucoma, 2001; 10(2); 102-8
56. Khodeiry MM, Sheheitli H, Sayed MS, Treatment outcomes of slow coagulation transscleral cyclophotocoagulation in pseudophakic patients with medically uncontrolled glaucoma: Am J Ophthalmol, 2021; 229; 90-99
57. Ghazi-Nouri SMS, Vakalis AN, Long-term results of the management of silicone oil-induced raised intraocular pressure by diode laser cycloablation: Eye Lond Engl, 2005; 19(7); 765-69
58. Bloom PA, Tsai JC, Sharma K, “Cyclodiode”. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma: Ophthalmology, 1997; 104(9); 1508-19 discussion 1519–20
Tables
 Table 1. Inclusion and exclusion criteria for the study group.
Table 1. Inclusion and exclusion criteria for the study group. Table 2. Inclusion and exclusion criteria for the control group.
Table 2. Inclusion and exclusion criteria for the control group. Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period.
Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period. Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure.
Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure. Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed.
Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed. Table 1. Inclusion and exclusion criteria for the study group.
Table 1. Inclusion and exclusion criteria for the study group. Table 2. Inclusion and exclusion criteria for the control group.
Table 2. Inclusion and exclusion criteria for the control group. Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period.
Table 3. Changes in IOP values depending on the endotamponade used during PPV over the 180-day observation period. Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure.
Table 4. The effectiveness of the CW-TSCPC procedure in patients after PPV depends on the endotamponade used during the procedure. Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed.
Table 5. Changes in IOP values after the CW-TSCPC procedure depending on the etiology of the disease for which PPV was previously performed. In Press
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952