15 December 2023: Clinical Research
Lamina-Lifting Suspension Modification in Bridge Crane Technique in Treatment of Severe Thoracic Ossification of the Ligamentum Flavum (TOLF)
Ye Zhang12ABCDEFG, Yunsheng Ou12ADG, Wei Luo12BC, Wanyuan Qin12DE, Tuotuo Xiong12EF, Yong Zhu12AEF*DOI: 10.12659/MSM.941803
Med Sci Monit 2023; 29:e941803
Abstract
BACKGROUND: The aim of this study was to investigate the effectiveness and potential complications of combining a lamina-lifting suspension system with the bridge crane technique in treating thoracic ossification of the ligamentum flavum (TOLF) with thoracic myelopathy.
MATERIAL AND METHODS: A patient with severe TOLF and myelopathy was treated using a lamina-lifting suspension system combined with bridge crane technique. The brief surgical procedure involved implantation of internal fixation, separation of laminae, installation of cross-bridges, reverse lifting, and fixation of cross-bridges. The modified Japanese Orthopaedic Association (mJOA) scale, Hirabayashi recovery rate, and ASIA grade of the patient were recorded. The canal occupation ratio (COR) and spinal cord status were evaluated by imaging data.
RESULTS: The surgical intervention significantly enhances the patient’s lower limb function, as evidenced by an increase in mJOA score from 5 preoperatively to 11 at terminal follow-up. The Hirabayashi recovery rate after surgery ranges between 25% and 50%. Additionally, ASIA classification improved to grade E. Imaging data showed that the ossification of the thoracic vertebrae had subsided, while the volume of the local spinal canal had recovered and the spinal cord injury had been completely relieved. No adverse effects or complications were observed.
CONCLUSIONS: The lamina-lifting suspension system preserves the benefits of bridge crane technique while also augmenting the traction of a post laminae-OLF complex (LOC) suspension, rendering it more secure and manageable. Nevertheless, further sample analysis and research are required in the future.
Keywords: Spinal Diseases, Ossification, Heterotopic, Spinal Cord Injuries, Surgical Procedures, Operative
Background
Ossification of the ligamentum flavum (OLF) is a form of heterotopic ossification disease that affects spinal ligaments, characterized by the transformation of fibrous tissue within the ligament into bone tissue [1,2]. OLF can occur in the cervical, thoracic, and lumbar vertebrae, with the lower thoracic vertebrae (T9–T12) being the most commonly affected. While OLF predominantly affects east Asians (particularly in Japan, South Korea, and China), there is also a growing number of reports from other regions [3,4]. The prevalence of thoracic ossification of the ligamentum flavum (TOLF) has been reported to vary in recent epidemiological investigations, ranging from 3.8% to 63.9% [3,5,6]. Increased use of CT and MRI allows more TOLF to be detected early. TOLF is the primary etiology of thoracic spinal canal stenosis. The insidious onset of this pathological condition poses challenges in its early detection. When ossification compresses the nerves in the spinal cord, patients may experience sensory and motor dysfunction, as well as difficulty with bowel movements. Surgery remains the only effective treatment for this condition.
Currently, conventional methods for treating TOLF typically involve posterior decompression [7] such as laminectomy, lamina fenestration, or expansive laminoplasty to preserve the spinal nerve function by expanding the volume of the spinal canal. Unfortunately, direct surgical intervention within the spinal canal often brings high surgical risks and postoperative complications [8,9]. Hence, the development of safe and efficient decompression methods remains a persistent technical challenge. Sun [10,11] first proposed the “bridge crane technique” in 2018, which enables direct decompression while preserving the integrity of the posterior column. Based on the aforementioned concepts, we have developed a lamina-lifting suspension system that optimizes utilization of the bridge crane technique in TOLF. Mechanically, this system achieves a gradual, stable, and controllable decompression process while being securely fastened instantly, which markedly reduces the risk of surgical complications and is also conducive to patient rehabilitation.
Material and Methods
PATIENT:
This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and the patient provided consent for the surgical procedure and consent form. The study recruited a patient with severe thoracic myelopathy who was admitted to the Orthopedics Department of the First Affiliated Hospital of Chongqing Medical University. The patient presented with numbness and weakness in both lower limbs, along with sensory disturbance and motor dysfunction that have persisted for 1 year. The ASIA classification was assigned as grade D. Preoperatively, a CT scan revealed a high-density mass protruding into the spinal canal at levels T8–T10 and an enlarged and fused ossification projecting into the ventral aspect of the spinal canal, with a canal occupation ratio (COR) ranging from 26.69% to 38.36%. The spinal canal at the corresponding level exhibited a clear stenosis (approximately 3 mm at its narrowest point), along with osteophyte formation in facet joints. An MRI scan revealed stenosis of the spinal canal at the T8–T10 levels, resulting in compression of the adjacent thoracic cord with observed edema signal (Figure 1).
ASSESSMENT METHODS:
The modified Japanese Orthopaedic Association (mJOA) [11,12] scale and ASIA classification before surgery, after surgery, and at 1-year follow-up were compared. Hirabayashi recovery rate [mJOA(post)-mJOA(pre)]/[17-mJOA(pre)] after surgery (at 3-month follow-up) and at 1-year follow-up were also compared. The canal occupation ratio (COR): spinal stenosis rate was based on axial CT scan=axial ossification mass area/axial spinal canal area)×100% (Figure 2).
STRUCTURE OF THE LAMINA-LIFTING SUSPENSION SYSTEM:
The lamina-lifting suspension system consists of 2 components: a cross-bridge and a lift rod (Figure 3). The cross-bridge is a curved plate made of titanium alloy (tantalum, or niobium zirconium alloy is also permitted) that is used to securely fasten the free lamina. The plate body has extension parts at both ends for fixation to the pedicle, while the middle part features a connecting orifice for fixing the screw and laminae. The cross-bridge employs the bowstring effect to continuously pull and fixate the lamina. Together with the fixed screws of the lamina, it provides stable support by connecting multiple segments of the lamina and the vertebral body. Additionally, fixed orifices are present on both sides of the curved plate body. The lift rod is used to raise the free lamina by passing it through the connecting orifice of the cross-bridge. The lift rod consists of a first threaded section at its lower end for connecting to the lamina and a second threaded section (either double-threaded or rectangular-threaded) at its upper end. Notably, the second thread section consists of dual threads, enabling more efficient power transmission and achieving twice as much lifting distance with each revolution. The lifting nut has a cylindrical body positioned on the second thread section, accompanied by a pair of driving lugs located on both sides of the main body. This configuration allows for easy rotation of the lifting screw by engaging with the driving lugs, thereby facilitating upward movement of the lifting rod. The lower part of the lifting nut is equipped with a conical contact section to enhance stability during the lifting process, ensuring contact with the inner surface of the connecting orifice in the lamina. In summary, this system has a clear and consistent capacity for controlled lifting.
MECHANISM TRANSMISSION METHODS AND FORMULA DERIVATION:
The lead screw is a commonly used mechanical transmission element in various mechanical equipment. The lead pitch of the lead screw refers to the linear distance traveled by the thread per revolution, which is an important performance indicator. When designing and selecting a lead screw, it is essential to calculate its lead based on specific requirements. The lead calculation formula is a crucial tool in the design and selection of lead screws, enabling us to swiftly determine the appropriate lead for optimal screw specifications. In practical applications, specific transmission requirements necessitate the careful selection of suitable screw types and specifications. The formula for calculating the lead of a lead screw is: P=πd/p, where P represents the lead pitch of the lead screw, d denotes the diameter of the thread, and p represents the screw pitch. The diameter of a lead screw refers to the size of its threaded portion and is typically measured in millimeters (mm). Screw pitch is how far the thread advances with each complete turn and also uses millimeters (mm) as units; π has an approximate value of 3.14. Therefore, in this study, the lead pitch of the lifting rod was 3.768 mm (
SURGICAL TECHNIQUE:
After general endotracheal anesthesia, the skin was disinfected and sterile towels were placed. A posterior median longitudinal incision was made at T7–11 levels, with successive cutting of the skin, subcutaneous tissue, and fascia, removal of paravertebral muscle, and exposure of the facet joint. The positioning needles were inserted at T7–T11 levels once the pedicle was found. Pedicle screws were implanted into T7–11 vertebrae after confirmation by a C-arm X-ray machine. The machine underwent a secondary inspection and screws were subsequently extracted. The spinous processes of T8–T10, as well as a portion of T7 and T11, were excised. The laminae from the lower part of the T7 to the upper part of the T11 were drilled and dissociated using a speed burr. Subsequently, pedicle screws were re-implanted into the vertebrae spanning from T7 to T11. Two titanium rods of appropriate length were selected and implanted into the “U” slot of the pedicle nail, followed by fastening and locking nuts. The 3 cross-bridges extensions were then horizontally attached to the titanium rod. After that, the lift rod was inserted through the connecting orifice of the cross-bridge to raise the free lamina to the desired position (the first threaded section of the lift rod was used to connect the free lamina via the pedicle connecting orifice, while the second threaded section was connected with a lifting nut that can be pulled upwards by rotating it on top of the cross-bridge). After using the temporary fixation screw to provisionally secure laminae through the fixed orifice, we detached the lifting rod from the connecting orifice and then used the laminar fixation screw to secure the unattached laminae. Finally, we removed the temporary fixation device from its position within the fixed orifice. Depending on actual circumstances, it is possible that the fixed orifice may also serve as an auxiliary screw aperture where an auxiliary screw can be implanted without requiring removal. The screw position was confirmed to be optimal using a C-arm X-ray machine. Bipolar electrocoagulation and gelatin were employed for hemostasis, while 2 drainage tubes were inserted into the incision. The fascia, subcutaneous tissue, and skin were closed with antibacterial VICRYL Plus. The surgical procedure is shown in brief in Figures 4–7 and Supplementary Video 1.
Results
SURGICAL OUTCOMES:
The surgical procedure lasted 3 h 25 min, with an estimated blood loss of 200 milliliters, and no adverse incidents were encountered during the operation.
CLINICAL OUTCOMES:
The successful implementation of the surgery markedly improved the patient’s lower limb function, as evidenced by an increase in the mJOA score from 5 points preoperatively to 9 points postoperatively, ultimately reaching 11 points during follow-up in the terminal stage (Table 1). The postoperative Hirabayashi recovery rate was 25–50%, resulting in complete resolution of the lower limb disfunction. Additionally, the ASIA classification improved to grade E following surgery.
RADIOLOGICAL OUTCOMES:
The compression of the spinal cord caused by the ossification was successfully alleviated. X-ray imaging revealed an optimal position for the internal fixation without any deviation or displacement. The preoperative kyphosis (Cobb angle of T7–T11) was 3.8°, which remained stable after surgery and at the 1-year follow-up, indicating local stability. The CT scan shown regression of the ossification in the thoracic vertebrae, along with recovery of local spinal canal volume. The COR improved significantly from 26.69–38.36% preoperatively to 0% postoperatively. The MRI examination exhibited no apparent compression or deformation of the thoracic spinal cord, and normal spinal signals were observed (Figure 6).
Discussion
Thoracic ossification of the ligamentum flavum (TOLF) is a form of heterotopic ossification of the spinal ligaments [13], a process that converts fibrous tissue inside the ligaments into bone. TOLF is the most prevalent cause of thoracic myelopathy, which can result in thoracic stenosis, spinal cord compression, and neurological function impairment in severe instances [9,13]. The principles of TOLF surgery share many similarities with those utilized in the treatment of myelopathy [14,15]: decompress the spinal cord and, if necessary, stabilize or articulate the spine to prevent recurrence or late abnormalities. As a result, open decompression has become a common method for spinal cord decompression and TOLF excision. Virous methods has been reported, including laminectomy, lamina fenestration, hemilaminectomy, isolated laminectomy, and foraminotomy.
Generally, the medial half of the laminae and the articular process, which form the posterior wall of the spinal canal, are often included in posterior decompression procedures, which have been widely adopted [7]. This approach has a positive impact and a generally safe operating area. However, direct intraspinal surgery often yields unfavorable outcomes [1,2,4], such as cerebrospinal fluid leakage, dural tear, epidural hematoma, and spinal cord nerve injury. In a systematic review of 137 thoracic laminectomy patients [16], preoperative mJOA scores improved from 5.08 to 8.29, and the incidences of dural tear, infection, and early neurological damage were 18.4%, 5.8%, and 5.7% respectively. As a result, research efforts are focused on adequate decompression while minimizing adverse surgical consequences.
A prevalent surgical concept is to use minimally invasive treatments to prevent soft tissue and ligament injury, such as laminae fenestration, which retain the facet joints and posterior column, eliminating the need for fusion. Although there is little evidence on laminae fenestration, a study [17] reported that patients’ mJOA scores improved from 4.88 before surgery to 7 after using this approach. Similarly, isolated laminectomy has been well described in myelopathy due to thoracic stenosis [18] and has also been accepted as a viable treatment for TOLF (12% dural tears due to adhesion). Sun et al [11] first proposed the “bridge crane” technique for the treatment of TOLF in 2018. This method separated and lifted the laminae-OLF complex (LOC) backward as a whole without inserting any instruments into the spinal canal, thus achieving direct decompression of the spinal cord. After 19-month follow-up, satisfactory clinical outcomes were demonstrated. Sun et al [10] also compared the treatment of TOLF patients with “bridge crane” technique and traditional laminectomy, and found that the former had a higher recovery rate and mJOA score, as well as a lower complication rate. Based on this foundation, we have designed and developed a novel lamina-lifting suspension system that achieves a gradual, stable, and controllable decompression process through mechanical principles while remaining firmly fixed. In essence, this innovation enhances the “LOC suspension” procedure within the “bridge crane” technique. We have employed this system to substitute the procedure of lifting LOC with Prolene suture. Instead, a stable and regulated process of pulling LOC is implemented. In this study, the mJOA score of the patient obviously recovered after surgery and basically returned to normal life. Postoperative imaging examination showed that the ratio of ossification invasion was reduced, the spinal cord compression was markedly relieved. The patient reported feeling well, and the sensory and motor functions of both lower limbs were basically recovered after surgery. At the final follow-up, the patient had no functional disorders in both lower limbs.
Cerebrospinal fluid (CSF) leakage, commonly caused by dural adhesions or ossification [19,20], can result in various complications such as pseudocysts, poor wound healing, respiratory obstruction, and even meningitis. Repeated intraspinal manipulation of instruments and the process of separating dural adhesions in traditional surgery may enhance the risk of dural rupture and CSF leakage [21]. Eun et al [17] reported an incidence of 11.7% of dural adhesions in 17 TOLF patients who underwent spinal fenestration decompression, and 1 patient had an intraoperative dural tear. Similarly, Osman et al [16] reported an 18.4% incidence for dural tears in a systematic review involving 137 patients undergoing thoracic laminectomy. Endoscopic decompression has a relatively low incidence of dural tears (6.7%), but it may not be suitable for nodular and fused TOLF due to the challenges in accessing central ossification and achieving adequate decompression via catheterization. The bridge crane technique is a direct decompression procedure that involves the complete separation and suspension of the ossifying ligamentum flavum through use of an internal fixation bar (Figure 4). With this technique, decompression can be achieved without removing the ligamentum flavum, considerably reducing the danger of CSF leakage. A study comparing laminectomy with the bridge crane technique for TOLF [10] found that while both procedures significantly reduced spinal cord compression, the latter had a distinct advantage in terms of JOA improvement (3.8 bridge crane vs 3.0 laminectomy) and recovery rate (71.0% bridge crane vs 54.4% laminectomy). Additionally, compared to the laminectomy group, which experienced a 13.6% leakage rate of cerebral fluid, the bridge crane group experienced 5.3% leakage. In the bridge crane technique, the LOC is pulled backward by tightening the sutures that are secured to it. However, this procedure is based on the surgeon’s experience, judgment, and manipulation perception, and a certain risk of instability still remains. Our new technique achieves stable and controlled lifting, which theoretically could reduce surgical complications caused by excessive dissociation. Our technique can also enlarge the volume of the spinal canal directly without addressing the abnormal dura mater, which remains attached to the ossified ligament complex after ascension. In theory, separation of the dura and ossifying ligaments could be avoided and the incidence of CSF leakage could be reduced.
Acute neurological deterioration is a common complication associated with traditional laminectomy procedures. Complete removal of the thoracic lamina often entails a heightened risk of acute neurological decline. In a study conducted by William et al [22], the incidence of acute neurological deterioration following thoracic laminectomy was reported to be 14.5% (14/96), which significantly exceeds the rates observed in cases of myelopathic cervical spondylosis treatment. Several theories have been postulated to elucidate the occurrence of postoperative neurological decline subsequent to laminectomy and decompression [12,22,23], including vascular injury, hypotension or localized ischemia, direct trauma, and traction exerted on the spinal cord nerves. Similarly, some scholars propose that prolonged symptom duration, multiple compression sites [24], or the presence of thoracic stenosis evident in preoperative CT scans [25] are unfavorable prognostic indicators. Anatomically [22], the volume of the thoracic spinal canal is comparatively smaller than that of the cervical or lumbar regions, thus providing limited space for the spinal cord. Furthermore, thoracic laminectomy disrupts the tension band located posterior to the spine, potentially resulting in instability and neurological deterioration. Additionally, intraoperative manipulation of the spinal canal or the formation of hematomas can damage the spinal cord. Takai et al [26] reported that the external space narrowing caused by TOLF can trigger collateral venous drainage within ossification lesions, while surgical resection can damage compensatory venous drainage pathways and worsen nerve status. Additionally, manipulation of the narrowest segments also poses a potential risk to the spinal cord [26,27]. Here, our technique involves a gradual and cautious retraction of the laminae without extensive resection. This approach preserves the anatomical integrity of the thoracic laminae, ensuring the stability of the spinal posterior column. Furthermore, it safeguards the original collateral circulation vessels located within the laminae and maintains the direct blood supply to the local spinal nerves.
Conclusions
Our technique preserves the technical advantages of the bridge crane technique, while also enhancing the pulling of a post-LOC suspension to make it safer and more manageable. However, this study has some limitations: (1) This study only reported 1 case, so additional sample analysis and research are required; (2) This technique is only applicable to the thoracic vertebrae from neutral to the lordotic segment, and the effect on the kyphotic spine may be limited due to the risk of insufficient spinal cord floating; and (3) During the procedure, incomplete removal of the posterior lamina hindered the surgeon’s ability to observe the effects of spinal decompression and intraspinal hemostasis.
Supplementary Material
Figures
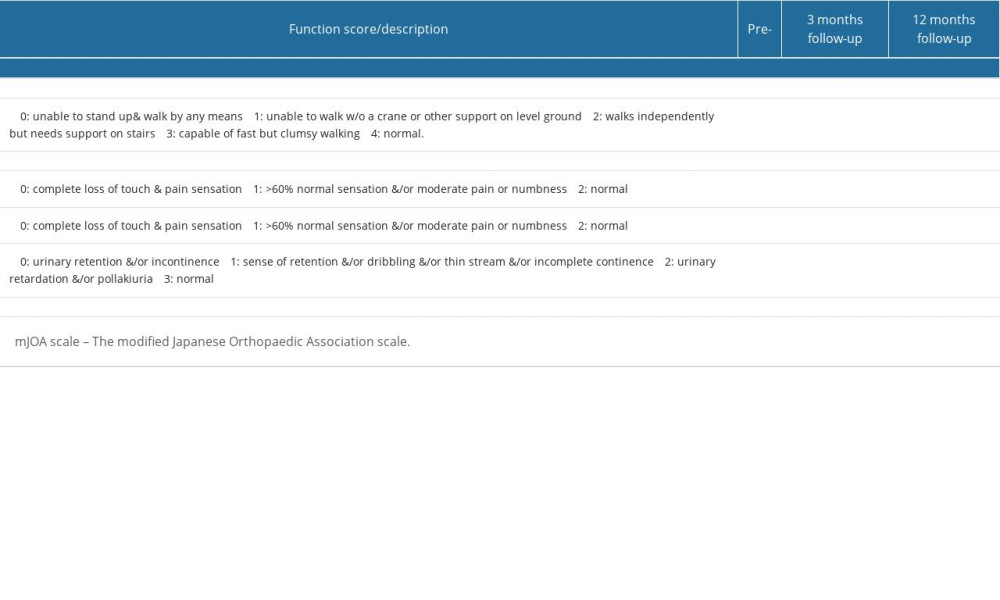
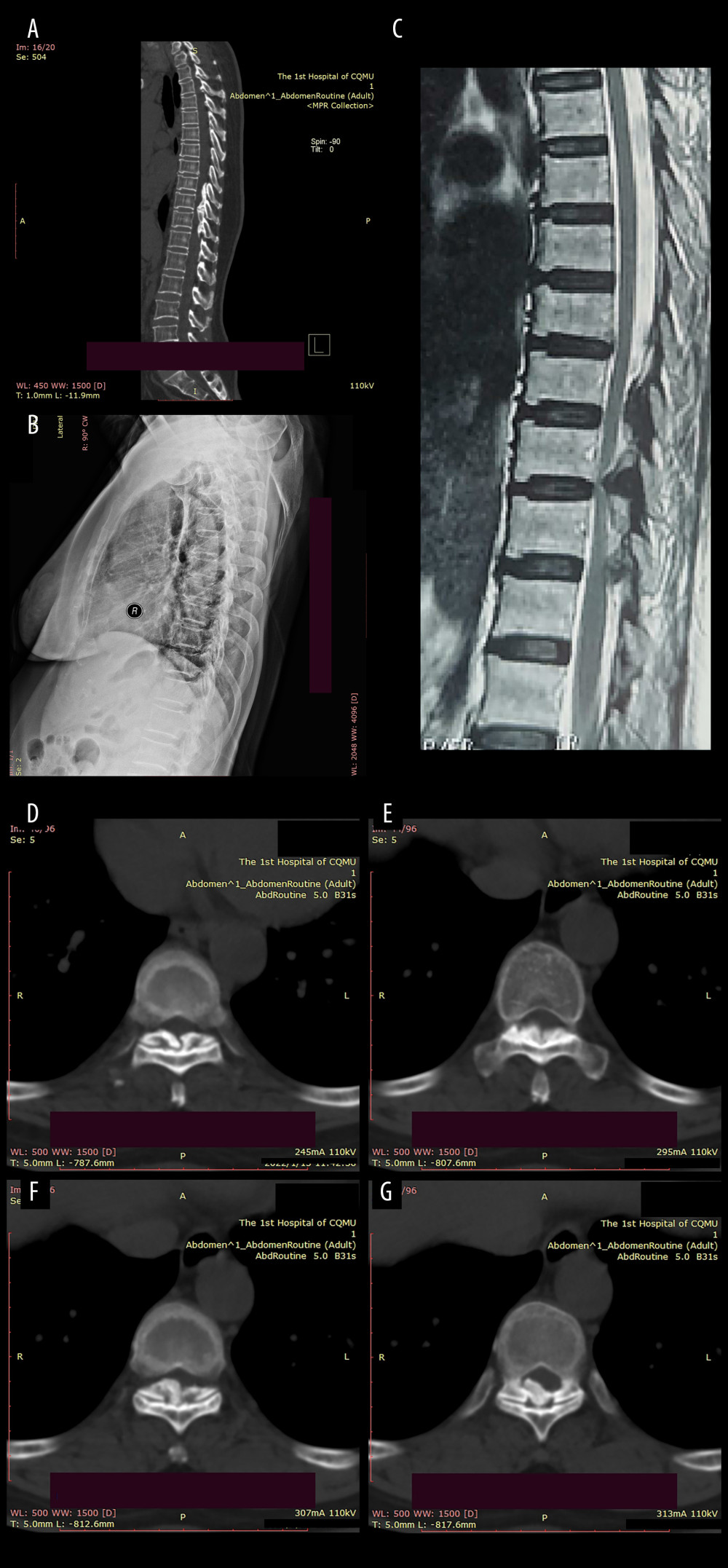 Figure 1. Preoperative imaging data of the patient:(A) Sagittal CT revealed a high-density mass protruding into the spinal canal at the levels of T8–T10, indicating potential spinal cord compression; (B) Preoperative sagittal X-ray of the patient; (C) The MRI plain scan revealed significant stenosis of the spinal canal at the corresponding levels of T8–T10, accompanied by marked compression and narrowing of the adjacent thoracic cord; (D–G) The coronal CT plain scan at T8–10 level revealed high-density ossification that had ruptured into the spinal canal, resulting in significant narrowing of the corresponding canal.
Figure 1. Preoperative imaging data of the patient:(A) Sagittal CT revealed a high-density mass protruding into the spinal canal at the levels of T8–T10, indicating potential spinal cord compression; (B) Preoperative sagittal X-ray of the patient; (C) The MRI plain scan revealed significant stenosis of the spinal canal at the corresponding levels of T8–T10, accompanied by marked compression and narrowing of the adjacent thoracic cord; (D–G) The coronal CT plain scan at T8–10 level revealed high-density ossification that had ruptured into the spinal canal, resulting in significant narrowing of the corresponding canal. 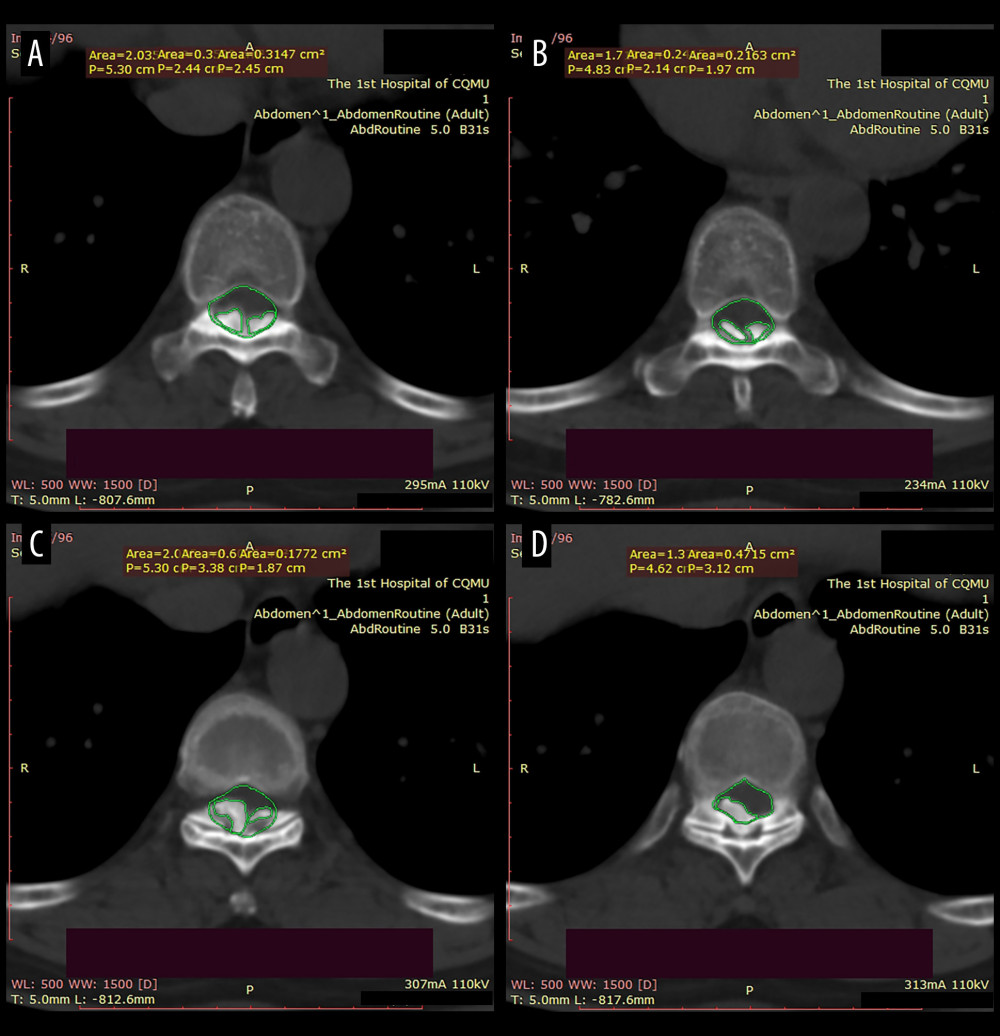 Figure 2. The canal occupancy ratio (COR) of T8–10.(A, B) The COR for T8/T9 is 26.69–32.67%; (C) The COR for T9/T10 is 38.36%; (D) The COR for T10 is 34.19%; The green area corresponds to the level of the spinal canal, whereas the red areas denote ossifications that have infiltrated into it.
Figure 2. The canal occupancy ratio (COR) of T8–10.(A, B) The COR for T8/T9 is 26.69–32.67%; (C) The COR for T9/T10 is 38.36%; (D) The COR for T10 is 34.19%; The green area corresponds to the level of the spinal canal, whereas the red areas denote ossifications that have infiltrated into it.  Figure 3. Structure diagram of the cross-bridge and lifting rod.(A) 1. Curved plate body (11. Laminar connecting orifice; 12. Fixing orifice); 2. Extend the fixed part; 3. Laminae fixation screws; (B) 1. Curved plate body; 2. Extension part; 4. Pull rod (41. First thread section; 42. Second thread section; 43. Standard quick interface); 5. Pull nut (51. Cylindrical body; 52. Drive lugs; 511. Tapered contact part). (C) Structure diagram of the lifting rod.
Figure 3. Structure diagram of the cross-bridge and lifting rod.(A) 1. Curved plate body (11. Laminar connecting orifice; 12. Fixing orifice); 2. Extend the fixed part; 3. Laminae fixation screws; (B) 1. Curved plate body; 2. Extension part; 4. Pull rod (41. First thread section; 42. Second thread section; 43. Standard quick interface); 5. Pull nut (51. Cylindrical body; 52. Drive lugs; 511. Tapered contact part). (C) Structure diagram of the lifting rod. 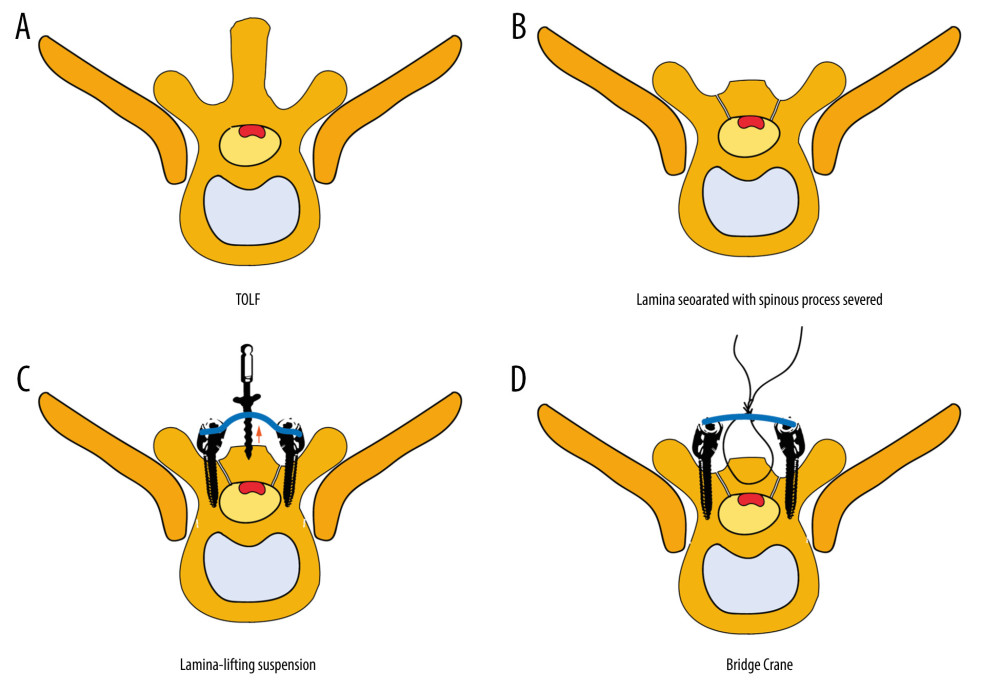 Figure 4. Brief surgical diagram.(A) Part of the spinal section of thoracic ossification of the ligamentum flavum (TOLF). The red area represents ossification. (B) The procedure of excising the spinous process and grooving the corresponding segment of the lamina. (C) Surgical diagram of lamina-lifting suspension system combined with bridge crane technique. The blue part represents the cross-bridge. (D) Surgical diagram of the traditional bridge crane technique, using sutures to achieve laminae-OLF complex (LOC) pulling.
Figure 4. Brief surgical diagram.(A) Part of the spinal section of thoracic ossification of the ligamentum flavum (TOLF). The red area represents ossification. (B) The procedure of excising the spinous process and grooving the corresponding segment of the lamina. (C) Surgical diagram of lamina-lifting suspension system combined with bridge crane technique. The blue part represents the cross-bridge. (D) Surgical diagram of the traditional bridge crane technique, using sutures to achieve laminae-OLF complex (LOC) pulling. 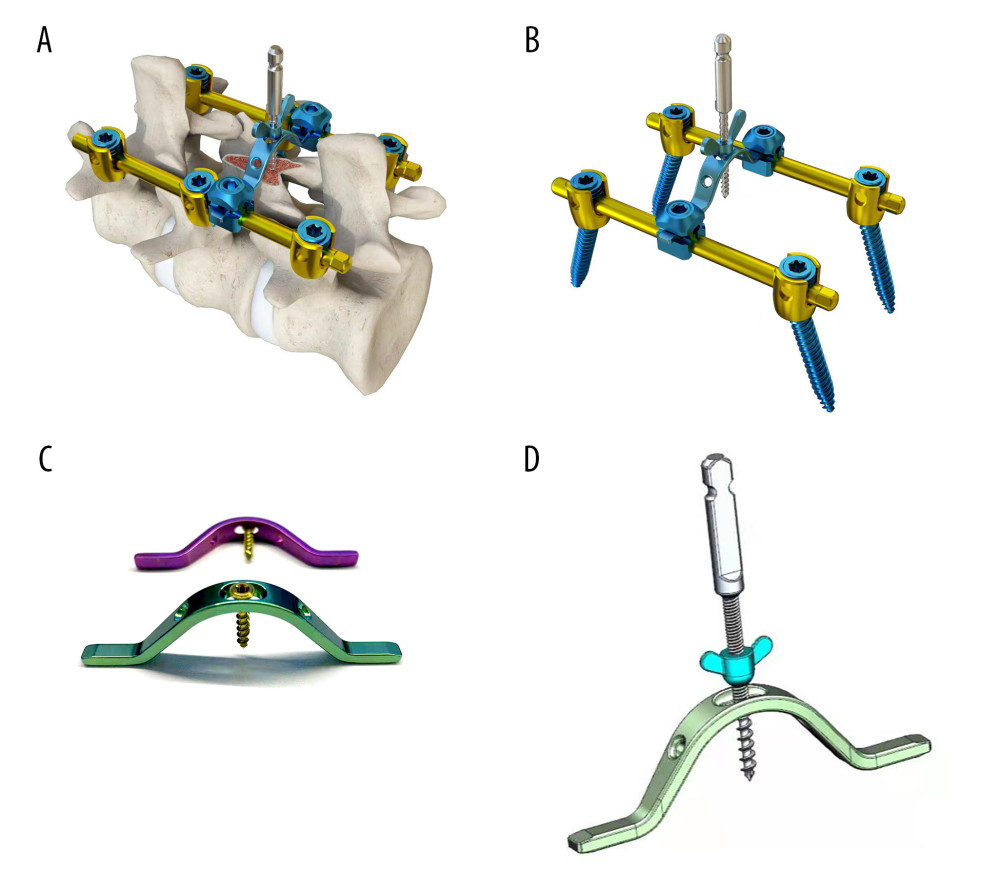 Figure 5. Local structure of the laminae-lifting suspension system.(A) Surgical 3D diagram of the laminae-lifting suspension system. (B) Local structure diagram of the laminae-lifting suspension system. (C, D) Local construction of the cross-bridge.
Figure 5. Local structure of the laminae-lifting suspension system.(A) Surgical 3D diagram of the laminae-lifting suspension system. (B) Local structure diagram of the laminae-lifting suspension system. (C, D) Local construction of the cross-bridge. 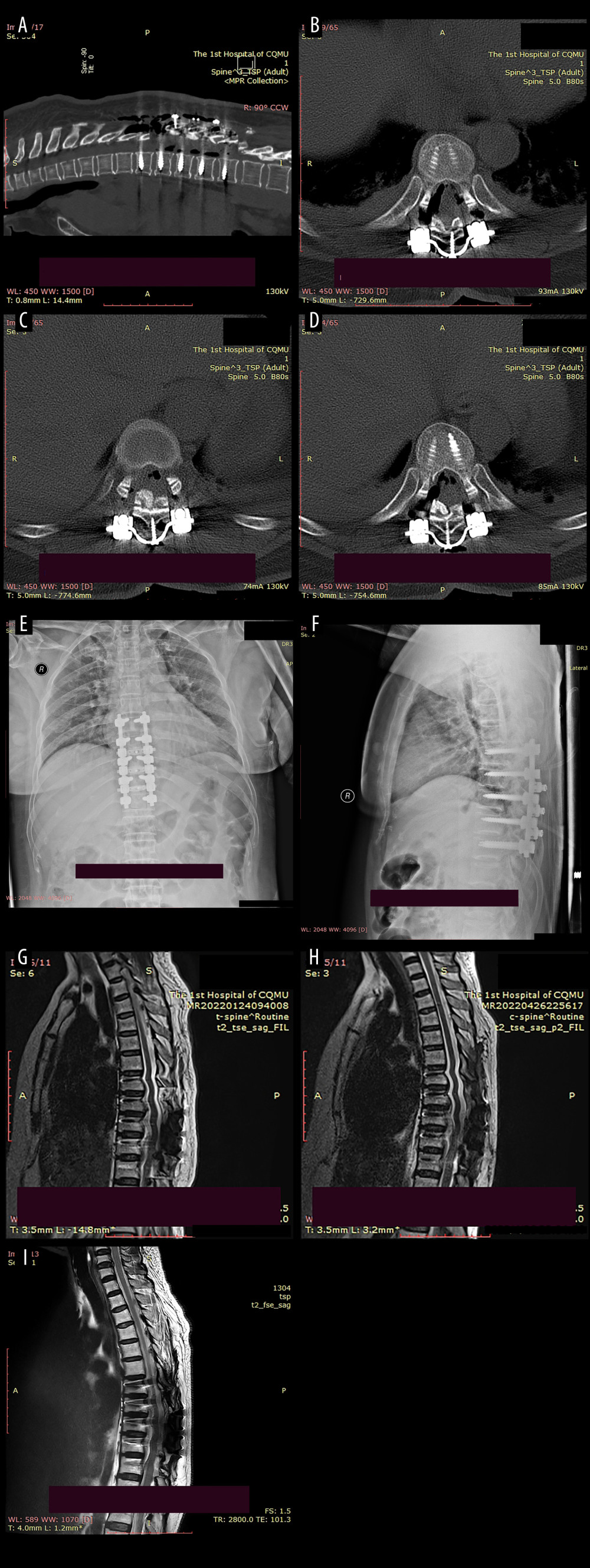 Figure 6. Postoperative imaging data of the patient.(A) The postoperative CT scan revealed a posterior shift of the ossifications and restoration of canal volume. The red area indicates the laminae-OLF complex (LOC). (B) Cross-bridge and screws in T8/T9. (C) Cross-bridge and screws in T9/T10. (D) Cross-bridge and screws in T10/T11. (E, F) Postoperative radiographs of the patient indicated local internal fixation and cross-bridges are in place without deviation. (G–I) MRI scans at 1 week, 3 months, and 1-year after surgery revealed a posterior migration of ossifications, with no abnormal signals detected in the spinal cord.
Figure 6. Postoperative imaging data of the patient.(A) The postoperative CT scan revealed a posterior shift of the ossifications and restoration of canal volume. The red area indicates the laminae-OLF complex (LOC). (B) Cross-bridge and screws in T8/T9. (C) Cross-bridge and screws in T9/T10. (D) Cross-bridge and screws in T10/T11. (E, F) Postoperative radiographs of the patient indicated local internal fixation and cross-bridges are in place without deviation. (G–I) MRI scans at 1 week, 3 months, and 1-year after surgery revealed a posterior migration of ossifications, with no abnormal signals detected in the spinal cord. 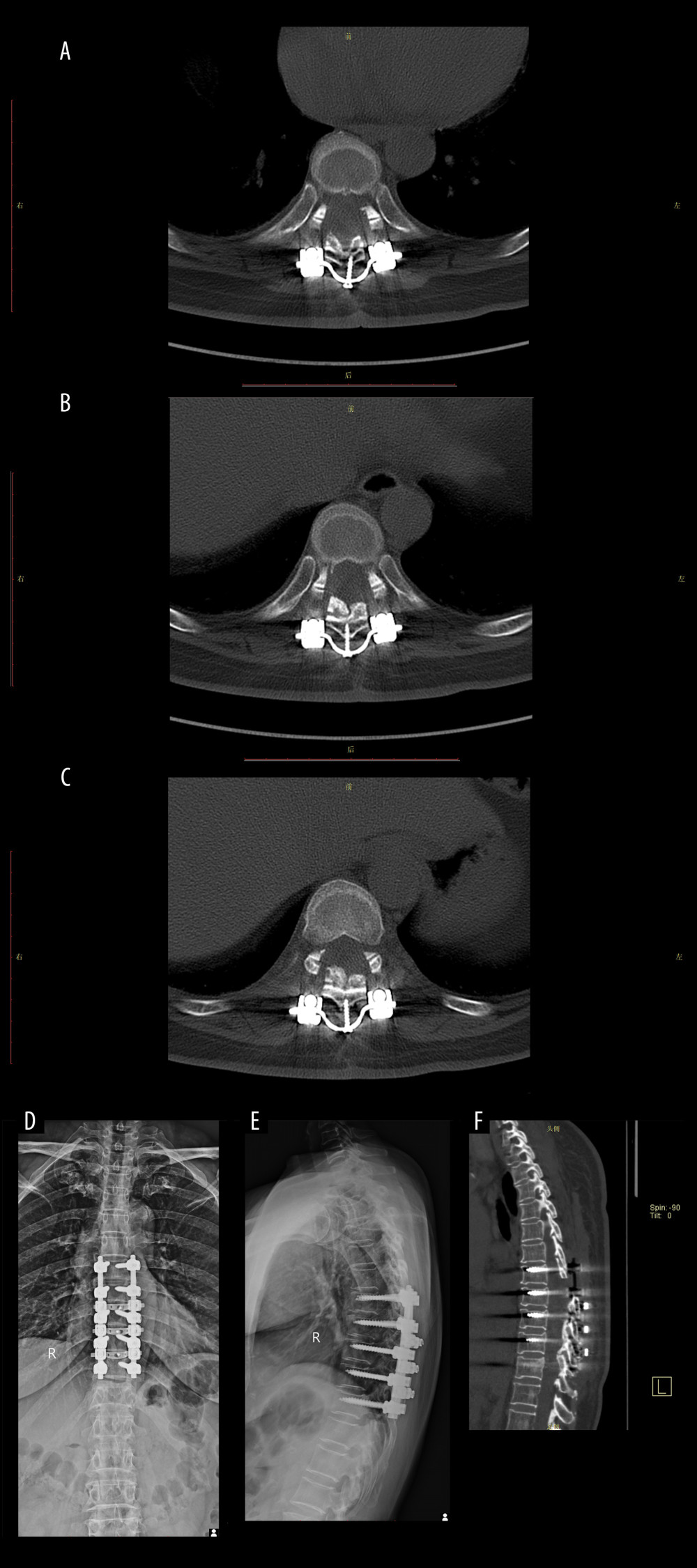 Figure 7. Imaging data of the patient after 6 months of follow-up.(A) Cross-bridge and screws in T8/T9. (B) Cross-bridge and screws in T9/T10. (C) Cross-bridge and screws in T10/T11. (D, E) Plain radiography of the spine showed screws, rods, and cross-bridge were still all in good position during the follow-up. (F) The sagittal sections of a CT scan.
Figure 7. Imaging data of the patient after 6 months of follow-up.(A) Cross-bridge and screws in T8/T9. (B) Cross-bridge and screws in T9/T10. (C) Cross-bridge and screws in T10/T11. (D, E) Plain radiography of the spine showed screws, rods, and cross-bridge were still all in good position during the follow-up. (F) The sagittal sections of a CT scan. References
1. Kägi S, Ciurea A, Micheroli R, Ossification of the ligamentum flavum: Rheumatology (Oxford), 2020; 59(7); 1616
2. Wang CH, Cui WL, Xue JL, Liao Z, Transforaminal en bloc resection for the treatment of thoracic ossification of the ligamentum flavum: Retrospective cohort study: Int J Surg, 2018; 54(Pt A); 278-84
3. Guo JJ, Luk KD, Karppinen J, Prevalence, distribution, and morphology of ossification of the ligamentum flavum: A population study of one thousand seven hundred thirty-six magnetic resonance imaging scans: Spine (Phila Pa 1976), 2010; 35(1); 51-56
4. Christiano LD, Assina R, Goldstein IM, Ossification of the ligamentum flavum: A unique report of a Hispanic woman: Neurosurg Focus, 2011; 30(3); E15
5. Williams DM, Gabrielsen TO, Latack JT, Ossification in the cephalic attachment of the ligamentum flavum. An anatomical and CT study: Radiology, 1984; 150(2); 423-26
6. Kim SI, Ha KY, Lee JW, Kim YH, Prevalence and related clinical factors of thoracic ossification of the ligamentum flavum – a computed tomography-based cross-sectional study: Spine J, 2018; 18(4); 551-57
7. Daniels AH, McDonald CL, Basques BA, Kuris EO, Ossified ligamentum flavum: Epidemiology, treatment, and outcomes: J Am Acad Orthop Surg, 2022; 30(12); e842-e51
8. Yu L, Li B, Yu Y, The relationship between dural ossification and spinal stenosis in thoracic ossification of the ligamentum flavum: J Bone Joint Surg Am, 2019; 101(7); 606-12
9. Zhao Y, Xiang Q, Jiang S, Incidence and risk factors of dural ossification in patients with thoracic ossification of the ligamentum flavum: J Neurosurg Spine, 2023; 38(1); 131-38
10. Sun K, Sun X, Zhu J, Comparison of surgical results of the bridge crane technique versus laminectomy for the treatment of thoracic myelopathy caused by ossification of the ligamentum flavum: Global Spine J, 2023; 13(2); 384-92
11. Sun J, Sun K, Shi J, The bridge crane technique for the treatment of the severe thoracic ossification of the ligamentum flavum with myelopathy: Eur Spine J, 2018; 27(8); 1846-55
12. Li KK, Chung OM, Chang YP, So YC, Myelopathy caused by ossification of ligamentum flavum: Spine (Phila Pa 1976), 2002; 27(12); E308-12
13. Machino M, Sakai K, Yoshii T, Treatment for the thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum: J Clin Med, 2022; 11(16); 4690
14. Chen Y, Lu XH, Yang LL, Chen DY, Ossification of ligamentum flavum related to thoracic kyphosis after tuberculosis: Case report and review of the literature: Spine (Phila Pa 1976), 2009; 34(1); E41-44
15. Ohara Y, Ossification of the ligaments in the cervical spine, including ossification of the anterior longitudinal ligament, ossification of the posterior longitudinal ligament, and ossification of the ligamentum flavum: Neurosurg Clin N Am, 2018; 29(1); 63-68
16. Osman NS, Cheung ZB, Hussain AK, Outcomes and complications following laminectomy alone for thoracic myelopathy due to ossified ligamentum flavum: A systematic review and meta-analysis: Spine (Phila Pa 1976), 2018; 43(14); E842-E48
17. Eun SS, Kumar R, Choi WG, Lamina fenestration technique for treatment of thoracic ossified ligamentum flavum: 2-year follow-up result: J Neurol Surg A Cent Eur Neurosurg, 2017; 78(3); 286-90
18. Zhong ZM, Wu Q, Meng TT, Clinical outcomes after decompressive laminectomy for symptomatic ossification of ligamentum flavum at the thoracic spine: J Clin Neurosci, 2016; 28; 77-81
19. Zhai J, Guo S, Zhao Y, The role of cerebrospinal fluid cross-section area ratio in the prediction of dural ossification and clinical outcomes in patients with thoracic ossification of ligamentum flavum: BMC Musculoskelet Disord, 2021; 22(1); 701
20. Muthukumar N, Dural ossification in ossification of the ligamentum flavum: A preliminary report: Spine (Phila Pa 1976), 2009; 34(24); 2654-61
21. Ju JH, Kim SJ, Kim KH, Clinical relation among dural adhesion, dural ossification, and dural laceration in the removal of ossification of the ligamentum flavum: Spine J, 2018; 18(5); 747-54
22. Young WF, Baron E, Acute neurologic deterioration after surgical treatment for thoracic spinal stenosis: J Clin Neurosci, 2001; 8(2); 129-32
23. Trojan DA, Pouchot J, Pokrupa R, Diagnosis and treatment of ossification of the posterior longitudinal ligament of the spine: Report of eight cases and literature review: Am J Med, 1992; 92(3); 296-306
24. Ahorukomeye P, Saniei S, Pennacchio CA, Outcomes in surgical treatment for tandem spinal stenosis: Systematic literature review: Spine J, 2022; 22(11); 1788-800
25. Kang KC, Lee CS, Shin SK, Ossification of the ligamentum flavum of the thoracic spine in the Korean population: J Neurosurg Spine, 2011; 14(4); 513-19
26. Takai K, Matsumoto T, Yabusaki H, Surgical complications associated with spinal decompression surgery in a Japanese cohort: J Clin Neurosci, 2016; 26; 110-15
27. Ito Z, Matsuyama Y, Ando M, Postoperative paralysis from thoracic ossification of posterior longitudinal ligament surgery risk factor of neurologic injury: nationwide multiinstitution survey: Spine (Phila Pa 1976), 2016; 41(19); E1159-E63
Figures
 Figure 1. Preoperative imaging data of the patient:(A) Sagittal CT revealed a high-density mass protruding into the spinal canal at the levels of T8–T10, indicating potential spinal cord compression; (B) Preoperative sagittal X-ray of the patient; (C) The MRI plain scan revealed significant stenosis of the spinal canal at the corresponding levels of T8–T10, accompanied by marked compression and narrowing of the adjacent thoracic cord; (D–G) The coronal CT plain scan at T8–10 level revealed high-density ossification that had ruptured into the spinal canal, resulting in significant narrowing of the corresponding canal.
Figure 1. Preoperative imaging data of the patient:(A) Sagittal CT revealed a high-density mass protruding into the spinal canal at the levels of T8–T10, indicating potential spinal cord compression; (B) Preoperative sagittal X-ray of the patient; (C) The MRI plain scan revealed significant stenosis of the spinal canal at the corresponding levels of T8–T10, accompanied by marked compression and narrowing of the adjacent thoracic cord; (D–G) The coronal CT plain scan at T8–10 level revealed high-density ossification that had ruptured into the spinal canal, resulting in significant narrowing of the corresponding canal. Figure 2. The canal occupancy ratio (COR) of T8–10.(A, B) The COR for T8/T9 is 26.69–32.67%; (C) The COR for T9/T10 is 38.36%; (D) The COR for T10 is 34.19%; The green area corresponds to the level of the spinal canal, whereas the red areas denote ossifications that have infiltrated into it.
Figure 2. The canal occupancy ratio (COR) of T8–10.(A, B) The COR for T8/T9 is 26.69–32.67%; (C) The COR for T9/T10 is 38.36%; (D) The COR for T10 is 34.19%; The green area corresponds to the level of the spinal canal, whereas the red areas denote ossifications that have infiltrated into it. Figure 3. Structure diagram of the cross-bridge and lifting rod.(A) 1. Curved plate body (11. Laminar connecting orifice; 12. Fixing orifice); 2. Extend the fixed part; 3. Laminae fixation screws; (B) 1. Curved plate body; 2. Extension part; 4. Pull rod (41. First thread section; 42. Second thread section; 43. Standard quick interface); 5. Pull nut (51. Cylindrical body; 52. Drive lugs; 511. Tapered contact part). (C) Structure diagram of the lifting rod.
Figure 3. Structure diagram of the cross-bridge and lifting rod.(A) 1. Curved plate body (11. Laminar connecting orifice; 12. Fixing orifice); 2. Extend the fixed part; 3. Laminae fixation screws; (B) 1. Curved plate body; 2. Extension part; 4. Pull rod (41. First thread section; 42. Second thread section; 43. Standard quick interface); 5. Pull nut (51. Cylindrical body; 52. Drive lugs; 511. Tapered contact part). (C) Structure diagram of the lifting rod. Figure 4. Brief surgical diagram.(A) Part of the spinal section of thoracic ossification of the ligamentum flavum (TOLF). The red area represents ossification. (B) The procedure of excising the spinous process and grooving the corresponding segment of the lamina. (C) Surgical diagram of lamina-lifting suspension system combined with bridge crane technique. The blue part represents the cross-bridge. (D) Surgical diagram of the traditional bridge crane technique, using sutures to achieve laminae-OLF complex (LOC) pulling.
Figure 4. Brief surgical diagram.(A) Part of the spinal section of thoracic ossification of the ligamentum flavum (TOLF). The red area represents ossification. (B) The procedure of excising the spinous process and grooving the corresponding segment of the lamina. (C) Surgical diagram of lamina-lifting suspension system combined with bridge crane technique. The blue part represents the cross-bridge. (D) Surgical diagram of the traditional bridge crane technique, using sutures to achieve laminae-OLF complex (LOC) pulling. Figure 5. Local structure of the laminae-lifting suspension system.(A) Surgical 3D diagram of the laminae-lifting suspension system. (B) Local structure diagram of the laminae-lifting suspension system. (C, D) Local construction of the cross-bridge.
Figure 5. Local structure of the laminae-lifting suspension system.(A) Surgical 3D diagram of the laminae-lifting suspension system. (B) Local structure diagram of the laminae-lifting suspension system. (C, D) Local construction of the cross-bridge. Figure 6. Postoperative imaging data of the patient.(A) The postoperative CT scan revealed a posterior shift of the ossifications and restoration of canal volume. The red area indicates the laminae-OLF complex (LOC). (B) Cross-bridge and screws in T8/T9. (C) Cross-bridge and screws in T9/T10. (D) Cross-bridge and screws in T10/T11. (E, F) Postoperative radiographs of the patient indicated local internal fixation and cross-bridges are in place without deviation. (G–I) MRI scans at 1 week, 3 months, and 1-year after surgery revealed a posterior migration of ossifications, with no abnormal signals detected in the spinal cord.
Figure 6. Postoperative imaging data of the patient.(A) The postoperative CT scan revealed a posterior shift of the ossifications and restoration of canal volume. The red area indicates the laminae-OLF complex (LOC). (B) Cross-bridge and screws in T8/T9. (C) Cross-bridge and screws in T9/T10. (D) Cross-bridge and screws in T10/T11. (E, F) Postoperative radiographs of the patient indicated local internal fixation and cross-bridges are in place without deviation. (G–I) MRI scans at 1 week, 3 months, and 1-year after surgery revealed a posterior migration of ossifications, with no abnormal signals detected in the spinal cord. Figure 7. Imaging data of the patient after 6 months of follow-up.(A) Cross-bridge and screws in T8/T9. (B) Cross-bridge and screws in T9/T10. (C) Cross-bridge and screws in T10/T11. (D, E) Plain radiography of the spine showed screws, rods, and cross-bridge were still all in good position during the follow-up. (F) The sagittal sections of a CT scan.
Figure 7. Imaging data of the patient after 6 months of follow-up.(A) Cross-bridge and screws in T8/T9. (B) Cross-bridge and screws in T9/T10. (C) Cross-bridge and screws in T10/T11. (D, E) Plain radiography of the spine showed screws, rods, and cross-bridge were still all in good position during the follow-up. (F) The sagittal sections of a CT scan. Tables
In Press
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952