13 December 2023: Clinical Research
Comparative Evaluation of Contrast-Enhanced Spectral Mammography and Digital Breast Tomosynthesis for Diagnosing and Treating Breast Cancer
Di Yang1ABCDEF, Fei Gong2AG*DOI: 10.12659/MSM.941880
Med Sci Monit 2023; 29:e941880
Abstract
BACKGROUND: The aim of this study was to evaluate the efficacy of contrast-enhanced spectral mammography (CESM) and digital breast tomosynthesis (DBT) in the diagnosis and chemotherapy of breast cancer.
MATERIAL AND METHODS: We retrospectively analyzed data on 125 lesions of 115 patients with breast diseases in Lanzhou First People’s Hospital from January 2020 to June 2022. Patients were examined by digital breast tomographic fusion and contrast-enhanced spectral mammography after chemotherapy. We compared the diagnostic accuracy of the 2 imaging techniques, and the diagnostic efficacy was evaluated with ROC curves.
RESULTS: There were significant differences in the type and degree of CESM enhancement between benign and malignant lesions. Malignant lesions mostly showed moderate to severe enhancement, while benign lesions mostly showed mild to moderate enhancement. There was no significant difference in DBT manifestations between benign and malignant lesions. After neoadjuvant chemotherapy, 88 patients had pathological remission, and the remission rate was 70.40%. Thirty-seven patients did not respond (nonresponse rate: 29.60%). The accuracy of CESM lesion size assessment was 84.00% (105/125), with high consistency. The accuracy of DBT lesion size assessment was 68.00% (85/125), and the consistency was poor. BI-RADS 4B was the truncation point. CESM had significantly higher sensitivity, specificity, accuracy, positive predictive value, and negative predictive value than DBT. In premenopausal patients and patients aged less than 50 years, the diagnostic efficacy of DBT and CESM was significantly different.
CONCLUSIONS: The diagnostic efficacy of CESM was significantly better than DBT in premenopausal women and patients under age 50. Diagnosis and treatment of breast diseases may be enhanced by the use of CESM.
Keywords: Breast Cancer 3, Mammography, Tomography
Background
According to survey data of the World Health Organization in 2020, the incidence of breast cancer has jumped to first place among malignant tumors, and it is also the most common cancer among women worldwide [1]. Early detection, early diagnosis, and individualized treatment are key to improving the survival rate. Mammography is currently recognized as the preferred screening method for breast cancer. However, mammography also has limitations (eg, the display of non-calcifying lesions is greatly affected by breast density). Mammography should be performed after compression of the breast, and it is difficult to avoid masking of lesions by overlapping of fibroglandular tissue and lesions, and the dense fibroglandular tissue can also locally overlap to affect the diagnosis [2].
Digital breast tomosynthesis (DBT) is the latest development trend of full-field digital mammography (FFDM). DBT technology obtains 3D image information of the mammary gland by back projection tomography reconstruction of 2D projection data collected from different angles, which overcomes many shortcomings of traditional digital breast projection imaging and can more clearly display the lesions covered by glands in the breast, thus improving the diagnostic efficiency and the ability to accurately assess the size of the tumor before surgery, especially for dense breasts [3]. In addition to diagnostic functions, DBT can also be used to guide biopsies and preoperative localization. For small untouchable lesions (eg, high-risk lesions such as suspicious microscopic calcification distributed in clusters and structural distortion shown only by DBT) it is feasible to guide needle biopsy, or to implant a positioning guide wire before surgery to achieve surgical-precision excision biopsy [4]. However, DBT also has limitations: (1) Simultaneous DBT and mammography examination will increase the radiation dose; (2) The reconstructed tomography image shows clearly in the middle part, but in the edge part near the skin the accuracy needs to be improved; and (3) Although breast cancer detection and recall rates were improved in patients of various age groups and breast density, the advantages were significantly reduced in women with extremely dense breast tissue [5].
Contrast-enhanced spectral mammography (CESM) is a new technique based on traditional radiography combined with intravenous iodine contrast agent, which can assess the blood supply characteristics of the lesion, remove the overlap of surrounding normal glandular tissue, and show the lesion more clearly. Previous studies have shown that imaging features of CESM are correlated with some clinicopathological features of breast cancer [6,7]. At present, CEM is mainly used in the following aspects: (1) Evaluation of lesions found in breast DM, especially dense breast lesions; (2) Preoperative staging of newly diagnosed breast cancer; and (3) Evaluation of pathological complete response to neoadjuvant therapy [8]. It has been reported that CEM has high specificity and sensitivity, and may have broader prospects in evaluating the response to neoadjuvant therapy [9]. However, CEM also has limitations, such as anaphylaxis and extravasation of contrast agents, increased radiation dose during multiple evaluations of response to neoadjuvant breast cancer, and inability to fully evaluate axillary lymph nodes and display lesions close to the chest wall or the medial breast [10].
Neoadjuvant chemotherapy (NACT) refers to systemic and systematic chemotherapeutic therapy for patients before local surgical treatment [11]. The correct evaluation and monitoring of the response of locally advanced breast cancer after NACT is helpful for clinicians to select appropriate sensitive drugs according to the response to chemotherapy, formulate appropriate surgical treatment plans, reduce postoperative recurrence and metastasis, and improve the survival rate of patients. In addition, imaging evaluation can determine whether chemotherapy is effective from multiple angles due to the advantages of noninvasive examination, determining the scope of surgery, measuring tumor volume, observing the metabolism of lesions and changes in peripheral lymph nodes, and understanding the blood supply of lesions [12], which has received increasing attention from scholars in China and abroad and has been widely used in recent years. At present, there are few reports on the application of DBT and CESM in neoadjuvant chemotherapy. This study explored the application of CESM and DBT in the diagnosis and evaluation of chemotherapy effect in breast cancer.
Material and Methods
GENERAL DATA:
A total of 115 patients with breast diseases who met the following criteria in Lanzhou First People’s Hospital from January 2020 to June 2022 were retrospectively analyzed. All patients were female, ranging in age from 30 to 69 years, with a median age of 50 years. Inclusion criteria were: (1) Accepting DBT and CESM inspection at the same time; (2) Confirmed by biopsy or surgical pathology; and (3) According to the classification criteria of breast imaging reporting and data system (BI-RADS), the breast was classified as dense breast (including class c and d) based on DBT assessment. Exclusion criteria were: (1) The glands were of fatty type or scattered fibroadandular type; (2) No pathological results; and (3) The image quality was poor and could not be used for analysis.
IMAGING EXAMINATION METHODS:
DBT and CESM were performed using the Senographe Essential digital mammography device (GE, USA). For DBT examination, the healthy cranio-caudal (CC) side of the breast was taken first, and then the affected side of breast CC, followed by the healthy side of medial lateral oblique (MLO) and the affected side of MLO. During DBT imaging, the X-ray tube takes 9 low-dose exposures around the breast at a 25° scanning angle to obtain a series of low-dose two-dimensional (2D) images, and then reassembles a series of three-dimensional (3D) tomography and 2D (V-preview) images with different layers through computer post-processing software.
For CESM examination, a Medrad Mark 7 Arterion high-pressure syringe was used for intravenous injection of ioparol (370 mgI/mL) from Shanghai Bolaike Xinyi Pharmaceutical Company through the elbow at a dose of 1.22 mL/kg and a flow rate of 3 mL/s. After 2 min of contrast injection, the healthy side of breast CC was taken first, and then the affected side of breast CC, followed by the healthy side of MLO and the affected side of MLO. Four position images were taken within 7 min, and those with unqualified images were reshot soon thereafter. For each view, the CESM technique made it possible to obtain 2 images: a low-energy acquisition at 26–30 kVp and a high-energy acquisition at 45–49 kVp, with these values depending on breast density and thickness. Motion blur could be sometimes observed on subtraction images due to the acquisition of motion between low- and high-energy images. After the examination, the patient was observed for 30 min, told to drink more water, and the indwelling needle was removed after confirming there was no adverse reaction.
IMAGE ANALYSIS:
All DBT and CESM examinations were carried out 2 times: just before the beginning of NACT and as a follow-up examination 2 weeks before the end of chemotherapy to evaluate its effect (and to inform decisions about possible changes in therapeutic strategies). Two senior radiologists experienced in breast imaging diagnosis read the films of DBT and CESM without knowing the pathology results and wrote down the results. The diagnostic results of each imaging technique were compared with pathology findings. If the 2 disagreed, an associate chief physician was invited to reach an agreement. The analysis included breast density, lesion type, lesion enhancement type, enhancement degree, and diagnosis classification. According to the author’s experience and relevant literature reports, the degree of lesion enhancement in this study was divided into no enhancement, mild enhancement (the intensity of lesion enhancement was within 2050), moderate enhancement (the intensity of lesion enhancement was between 2050 and 2100), and severe enhancement (the intensity of lesion enhancement was above 2100). For the diagnosis of breast CESM images, the BI-RADS diagnostic criteria of mammography and MRI were referenced. The reader first read the DBT image and recorded the results and then read the CESM image to make the diagnosis and record the results again. To avoid the influence of DBT on the outcome of CESM diagnosis, the interval between DBT and CESM diagnosis of the same patient was more than 15 days. In this study, the BI-RADS 1–4A category was considered benign, and the 4B-5 category was considered malignant.
PATHOLOGICAL DIAGNOSIS:
The specimens were fixed, embedded, and sliced, and examined by immunohistochemical staining and hematoxylin and eosin (HE) staining. The sections were diagnosed by an experienced senior pathologist and the corresponding histopathological type was obtained. Pathological diagnosis defines malignant histology as positive and benign histology as negative.
OBSERVATION INDICATORS:
1) The coincidence rate, misdiagnosis rate, missed diagnosis rate, and diagnostic accuracy of CESM and DBT in different types of breast cancer were compared. 2) Evaluation of chemotherapy efficacy: Based on the response evaluation criteria of solid tumors, the effect of chemotherapy was evaluated by the smallest CESM reduction of the lesion after chemotherapy. Complete response: the lesion disappeared; Partial response: maximum diameter and reduction of target lesion ≥30%; Stable disease: the maximum diameter of target lesions was decreased by <30% or increased by <20%, and no new lesions; Disease progression: Maximum diameter and increase of target lesion ≥20%. Response rate=complete response rate+partial response rate, nonresponse rate=stable disease rate+progression rate.
STATISTICAL ANALYSIS:
The pathological findings were taken as the criterion standard for diagnosis. The consistency of image diagnosis and pathological results was determined by Kappa index, Kappa ≥0.75, indicating good consistent; 0.4< Kappa <0.75, indicating the consistency is medium; Kappa <0.4, indicating poor consistency. The results of this study were processed using SPSS 21.0 statistical software, in which the count data were expressed as%, the comparison between groups was expressed as χ2 test result, which was used to measure the degree of fit between the actual observed values and the theoretical inferred values of 2 or more samples. The normally distributed measurement data were expressed as (x±s), the comparison between groups was analyzed by
Results
PATHOLOGICAL DIAGNOSIS:
A total of 125 lesions were detected in 122 mammary glands of 115 patients, including 70 cases of breast cancer lesions, including 50 cases of invasive ductal carcinoma, 14 cases of ductal carcinoma in situ (DCIS) or with microinvasion, 2 cases of invasive lobular carcinoma, 1 case of invasive micropapillary carcinoma, 1 case of apocrine carcinoma, 1 case of solid papillary carcinoma in situ, and 1 case of intraductal papillary carcinoma with multifocal microinvasion. There were 55 benign lesions, including 23 fibroadenomas, 17 adenopathies, 8 intraductal papillomas, 4 chronic inflammation, 1 granulomatous lobular mastitis, 1 myofibroblastomatous hyperplasia, and 1 cyst secondary infection.
MANIFESTATIONS AND DIAGNOSIS OF DBT AND CESM LESIONS:
The DBT and CESM imaging findings and pathological results of 125 breast lesions are shown in Figure 1. There were significant differences in CESM enhancement type and enhancement degree between benign and malignant lesions (ES=0.89, P<0.05; ES=0.75, P<0.05). The malignant lesions showed moderate to severe enhancement, while the benign lesions showed mild to moderate enhancement (Figure 1A). There was no significant difference in DBT manifestations between benign and malignant lesions (P>0.05) (Figure 1B).
COMPARISON OF CESM AND DBT TO EVALUATE THE EFFICACY OF BREAST CANCER CHEMOTHERAPY:
After neoadjuvant chemotherapy, 88 patients had pathological remission, and the remission rate was 70.40%. Thirty-seven patients had no response, and the nonresponse rate was 29.60%. In the evaluation of the chemotherapy effect, the accuracy of CESM lesion size assessment was 84.00% (105/125), with good consistency. The accuracy of DBT lesion size assessment was 68.00% (85/125), with poor consistency, as shown in Figure 2 (ES=0.73, P<0.05).
BI-RADS CLASSIFICATION OF ALL BREAST LESIONS BY DBT AND CESM AND THE CORRESPONDING PATHOLOGICAL RESULTS:
Four false negatives were diagnosed by CESM (all were classified as BI-RADS 4A, including 1 DCIS, 1 invasive ductal carcinoma, 1 intraductal papillary carcinoma with multifocal microinvasion, and 1 invasive micropapillary carcinoma). DCIS showed cluster pleomorphic microcalcification in DBT and was diagnosed as BI-RADS 4B class. No enhancement was found in the corresponding area on the subtraction map, so it was downgraded to 4A. Invasive micropapillary carcinoma showed focal asymmetry with microcalcification on DBT and was diagnosed as BI-RADS class 4B. Due to obvious background enhancement, the lesions in the corresponding areas were not obvious on the subtraction map, so they were downgraded to 4A. Invasive ductal carcinoma showed only scattered coarse calcification in DBT, without obvious mass or suspected malignant microcalcification, and was diagnosed as BI-RADS class 2. The subtraction image showed multiple nodular enhancements and was diagnosed as BI-RADS class 4A. Intraductal papillary carcinoma with multifocal microinfiltration showed focal asymmetry on DBT and was diagnosed as BI-RADS class 3. The subtraction image showed mild nonlumpy enhancement and was diagnosed as BI-RADS class 4A. In addition, 15 false positives were diagnosed by CESM (6 cases of BI-RADS class 4B, 2 cases of BI-RADS class 4C, 7 cases of BI-RADS class 5, including 6 intraductal papilloma, 4 fibroadenoma, 3 adenopathy, 1 granulomatous lobular mastitis, and 1 cyst secondary infection). Granulomatous lobular mastitis showed focal asymmetry in DBT and was diagnosed as BI-RADS class 4B. The subtraction image showed severe enhancement, and the enhancement degree of granulomatous lobular mastitis was significantly reduced at 7 min, so it was diagnosed as BI-RADS class 5. The secondary infection of the cyst showed an irregular isodense mass in DBT with burrs at the edge, which was diagnosed as BI-RADS class 5. The subtraction image showed a marginally enhanced mass, maintaining the original diagnosis. One case of adenosis was diagnosed as BI-RADS class 4C with irregular and slightly high-density masses with rough edges on DBT and was downgraded to 4B without enhancement in the subtraction image. Other adenopathies, intraductal papilloma, and fibrovascular adenoma were misdiagnosed as malignant due to moderate to severe enhancement on subtraction, as shown in Table 1.
DIAGNOSTIC EFFICACY OF DBT AND CESM FOR BREAST LESIONS:
The sensitivity, specificity, accuracy, and positive and negative predictive values of DBT were 81.4% (57/70), 54.5% (30/55), 69.6% (87/125), 69.5% (57/82), and 69.8% (30/43), respectively. The sensitivity, specificity, accuracy, and positive and negative predictive values of CESM were 94.3% (66/70), 72.7% (40/55), 84.8% (106/125), 81.5% (66/81), and 90.9% (40/44), respectively. Compared with DBT, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of CESM were significantly different (P<0.001), as shown in Table 2.
ROC CURVE ANALYSIS RESULTS OF EACH GROUP:
The diagnostic results of DBT and CESM of 125 breast lesions were compared with their pathology results, and ROC curves are shown in Figure 3. The areas under the curve were 0.716 (95% CI: 0.626~0.806, P<0.001) and 0.877 (95% CI: 0.811–0.943, P<0.001), and the difference between them was statistically significant (Z value=7.922, P<0.001), as shown in Figure 3A. In the premenopausal group, the areas under the ROC curves of DBT and CESM were 0.724 (95% CI: 0.619–0.828, P<0.001) and 0.882 (95% CI: 0.809–0.955, P<0.001), respectively, and the area under the ROC curve of CESM was greater than that of DBT (Z=5.975, P<0.001) (Figure 3B). In the postmenopausal group, the areas under the ROC curve of DBT and CESM were 0.873 (95% CI: 0.747–0.999, P=0.001) and 0.857 (95% CI: 0.681–1.000, P=0.001), respectively, and the difference was not statistically significant (Z=0.021, P=0.983) (Figure 3C). In the age group ≤50 years old, the areas under the ROC curves of DBT and CESM were 0.716 (95% CI: 0.611–0.820, P<0.001) and 0.886 (95% CI: 0.815–0.956, P<0.001), respectively. The area under the ROC curve of CESM was larger than that of DBT, and the difference was statistically significant (Z=7.040, P<0.001), as shown in Figure 3D. In the age group >50 years old, the areas under the ROC curves of DBT and CESM were 0.709 (95% CI: 0.523–0.895, P=0.074) and 0.839 (95% CI: 0.644–1.000, P=0.004), respectively, and the difference was not statistically significant (Z=0.898, P=0.369) (Figure 3E).
Discussion
Breast cancer ranks first in the incidence of female malignant tumors in China, and the trend of occurrence at a younger age is obvious [13]. Early detection, diagnosis, and treatment of breast cancer are the key to improving the cure rate, prolonging the survival time, and reducing the mortality rate [14]. Early detection and active treatment can lead to a better prognosis, especially when lesions cannot be detected by clinical physical examination [15]. X-ray is one of the most effective imaging examination methods in clinical practice. Due to the overlapping effect caused by compression of glands during examination, some false negative and false positive cases inevitably occur. The dense mammary glands of Asian women are most prevalent [16]. Due to the obscuration and overlap of lesions and glandular tissues, many lesions are not easy to find, or normal breast tissue may be mistaken as lesions, limiting the clinical application of X-ray imaging. Therefore, in recent years, many new medical imaging technologies have emerged, among which DBT is a new 3D mammography technology that can reduce or eliminate the superposition effect of traditional mammography, find small lesions covered by glands, and effectively improve the detection rate of breast masses. CESM is a novel structural and functional imaging method with a short inspection time and low cost. CESM has 2 kinds of images – low-energy images and high-energy images – low-energy images are equivalent to molybdenum targets, high-energy images are equivalent to enhancement examinations, and the 2 images are digitally subtracted to allow the lesion to appear [17].
DBT technology can reduce the overlap of fibroglandular tissue and lesions and show the lesions more clearly to improve the detection rate, sensitivity, specificity, and accuracy of the diagnosis of dense breast lesions. In the 2013 edition of the mammography BI-RADS classification, 4 categories were subdivided into 4A, 4B, and 4C, and the malignant possibility of 4A lesions was only 2–10%, suggesting that benign lesions accounted for the vast majority in this classification. Therefore, in this study, the classification of BI-RADS equal to or greater than 4B was diagnosed as malignant to improve the pathological correlation. The results of this study showed that the sensitivity, specificity, accuracy, and area under the curve of DBT in the diagnosis of dense breast lesions were 81.4%, 54.5%, 69.6%, and 0.716, respectively. However, it has been reported that the sensitivity, specificity, accuracy, and area under the curve of DBT for the diagnosis of dense breast masses were 83.3%, 80.9%, 80.9% and 0.821, respectively [18]. The specificity, accuracy, and area under the curve of this study were lower than those of the above report [18]. This difference could be explained by the fact that all types of lesions in dense breasts were used in this study as research objects, as well as the fact that most of the enrolled cases had difficulties distinguishing benign from malignant by DBT, which was a result of selection bias. The radiologists’ experience in DBT reading may also be one of the factors that made the difference.
The low-energy images of CESM are similar to those of conventional DM, showing significant advantages in microcalcification, while the subtraction images can eliminate the masking effect of fibroglandular tissue. Studies have shown that CESM is significantly more sensitive to breast cancer than DM, especially for patients with dense breasts [19]. The results of this study showed that the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of CESM for the diagnosis of breast lesions were 94.3%, 72.7%, 84.8%, 81.5%, and 90.9%, respectively, which were significantly improved compared with DBT and similar to the results of Li et al [20]. However, CESM images had significantly higher sensitivity but lower specificity, which demonstrated that imaging techniques may not be able to distinguish between residual invasive lesions and co-existing inflammatory/reactive lesions, even after intravenous administration of contrast agents. In this study, the area under the ROC curve of CESM diagnosis was 0.877, which was basically consistent with the results of Wang et al [21] and significantly lower than the results of previous studies [22]. The reason for this discrepancy was considered to be related to the different selection of research objects, which included all glandular types of mammary glands, while this study was limited to dense mammary glands. In addition, this study discussed the diagnostic efficacy of DBT and CESM according to menopausal status and age of patients as grouping factors, providing a theoretical basis for the selection of appropriate examination methods for clinical diagnosis and treatment. The results showed that CESM was more effective than DBT in patients ≤50 years of age and premenopausal patients, while no significant difference was found in patients >50 years of age and postmenopausal patients. There is a certain correlation between breast density, age, and hormone level. Younger age and higher hormone levels will lead to higher breast density, and the advantages of CESM will be more obvious, but there will also be cases of missed diagnosis. When the mammary gland is dense, background parenchymal enhancement (BPE) is obvious, which obscures the enhancement of the lesion. Small enhancement lesions are not easily detected in BPE. In this study, 2 malignant lesions showed no abnormal enhancement in CESM subtraction images: one was invasive micropapillary carcinoma, and the other was medium-nuclear grade DCIS. The invasive micropapillary carcinomatous lesions were small, and the BPE was obvious, resulting in no lesions in the subtraction image. In previous studies on CESM [23,24], no enhancement of DCIS was found, which may be related to the insignificant increase in the blood supply of DCIS. In view of the above, subtraction of CESM should be combined with a low-energy diagram for diagnosis in clinical work. In addition, most benign lesions in this study showed varying degrees of enhancement on subtraction images, suggesting that the diagnosis of benign and malignant lesions should not solely rely on whether there is enhancement. The results of this study showed that malignant lesions were mainly characterized by moderate and severe enhancement, while benign lesions were mainly characterized by mild and moderate enhancement, and there was a significant difference in the degree of enhancement between benign and malignant lesions (P<0.001), which was consistent with the findings of Patel et al [25].
Our study has several limitations. First, it had a retrospective design. Second, the number of female patients in this single-institution study was relatively small. In addition, all DBT and CESM exams were conducted on a single-vendor system. Therefore, future multi-institutional studies, including larger patient populations, are warranted to validate our findings. At present, there are few CESM applications in China, doctors have little clinical experience, and there is no fixed standard for the interpretation of image signs. Therefore, it is recommended to strengthen standardized training for imaging doctors to improve their skills, so that CESM can become one of the routine clinical imaging methods for breast cancer.
Conclusions
CESM is significantly better than DBT in the overall diagnosis of breast lesions in patients after NACT, especially in patients who are premenopausal and ≤50 years old. Therefore, our findings might be adopted as reference data for future prospective studies designed to evaluate the impact of CESM on surgical decision-making for patients with breast cancer.
Figures
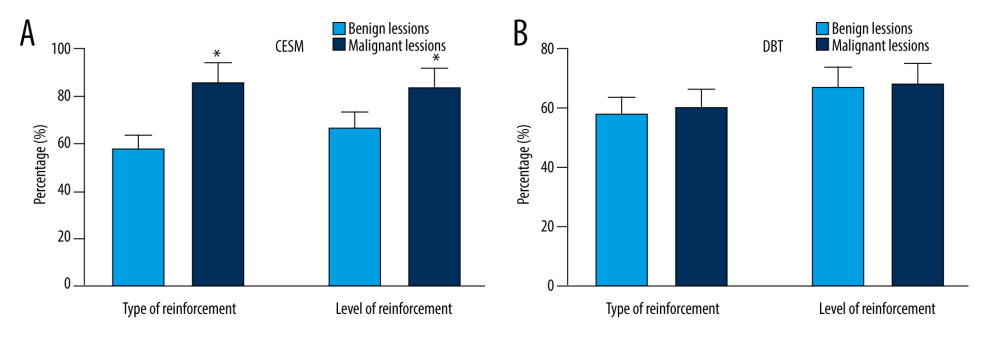 Figure 1. Manifestations and diagnostic results of (A) CESM lesions and (B) DBT. * P<0.05, compared with the CESM group. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 1. Manifestations and diagnostic results of (A) CESM lesions and (B) DBT. * P<0.05, compared with the CESM group. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). 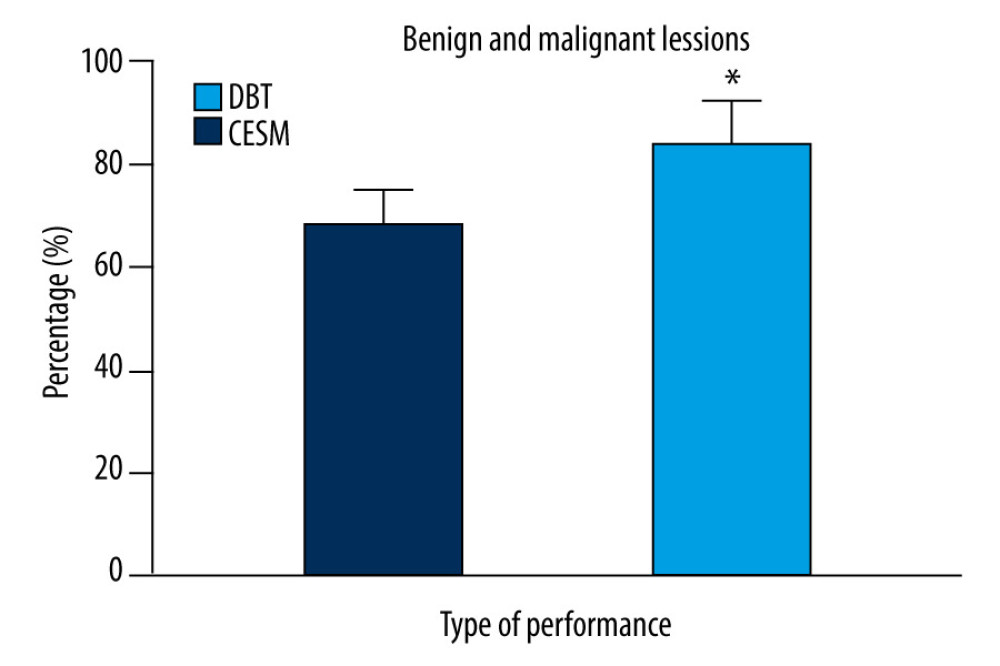 Figure 2. Efficacy of breast cancer chemotherapy evaluated by CESM and DBT. * P<0.05. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 2. Efficacy of breast cancer chemotherapy evaluated by CESM and DBT. * P<0.05. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). 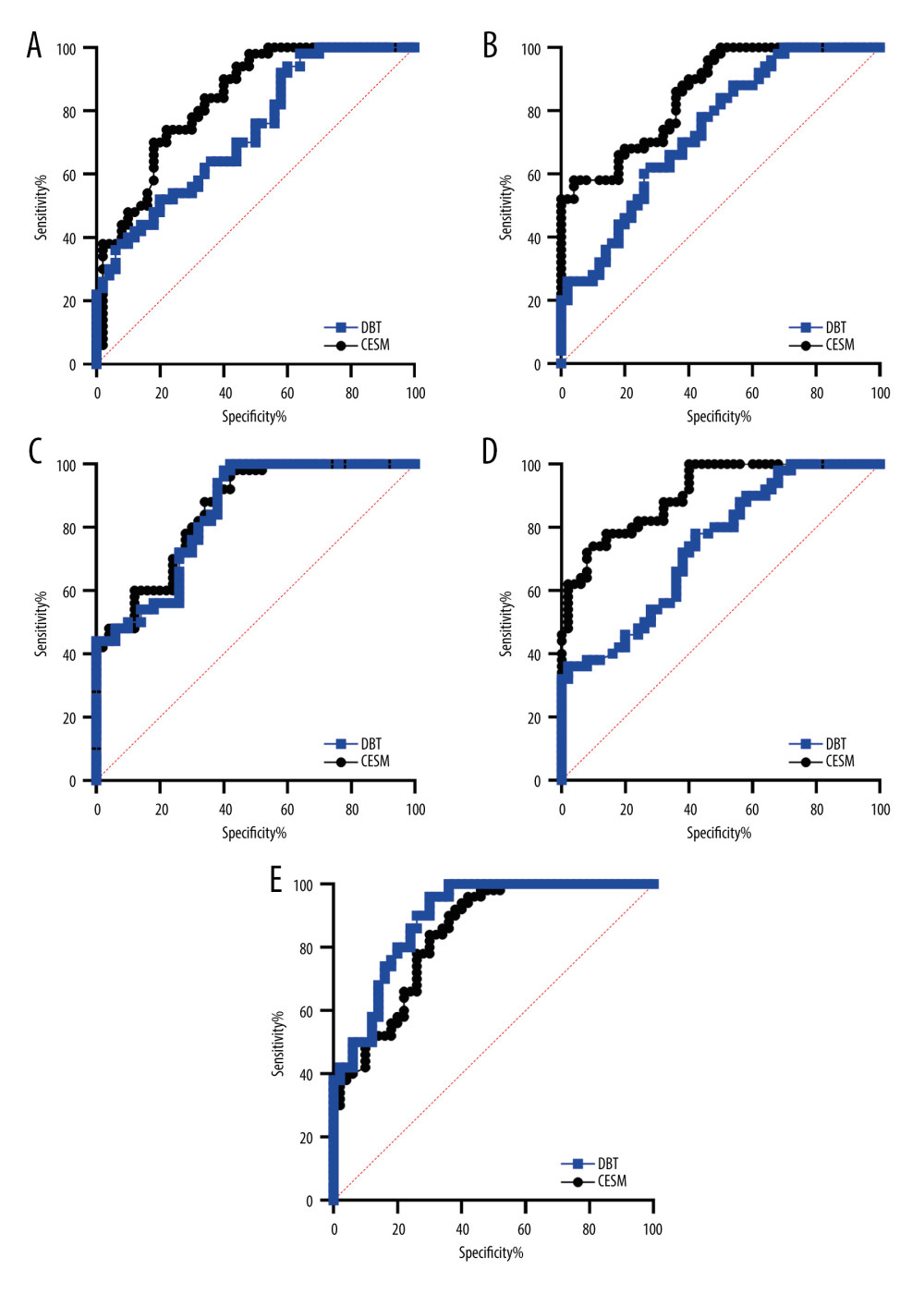 Figure 3. ROC curve analysis results of patients in each group. (A) diagnostic results of DBT and CESM. (B) premenopausal group. (C) postmenopausal group. (D) age group ≤50 years old. (E) age group >50 years old. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 3. ROC curve analysis results of patients in each group. (A) diagnostic results of DBT and CESM. (B) premenopausal group. (C) postmenopausal group. (D) age group ≤50 years old. (E) age group >50 years old. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). References
1. Barzaman K, Karami J, Zarei Z, Breast cancer: Biology, biomarkers, and treatments: Int Immunopharmacol, 2020; 84; 106535
2. Siviengphanom S, Gandomkar Z, Lewis SJ, Brennan PC, Mammography-based radiomics in breast cancer: A scoping review of current knowledge and future needs: Acad Radiol, 2022; 29(8); 1228-47
3. Chong A, Weinstein SP, McDonald ES, Conant EF, Digital breast tomosynthesis: Concepts and clinical practice: Radiology, 2019; 292(1); 1-14
4. Lotter W, Diab AR, Haslam B, Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach: Nat Med, 2021; 27(2); 244-49
5. Heindel W, Weigel S, Gerß J, Digital breast tomosynthesis plus synthesised mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): A multicentre, open-label, randomised, controlled, superiority trial: Lancet Oncol, 2022; 23(5); 601-11
6. Sogani J, Mango VL, Keating D, Contrast-enhanced mammography: Past, present, and future: Clin Imaging, 2021; 69; 269-79
7. Mao N, Shi Y, Lian C, Intratumoral and peritumoral radiomics for preoperative prediction of neoadjuvant chemotherapy effect in breast cancer based on contrast-enhanced spectral mammography: Eur Radiol, 2022; 32(5); 3207-19
8. Covington MF, Contrast-enhanced mammography implementation, performance, and use for supplemental breast cancer screening: Radiol Clin North Am, 2021; 59(1); 113-28
9. Zhang K, Lin J, Lin F, Radiomics of contrast-enhanced spectral mammography for prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer: J Xray Sci Technol, 2023; 31(4); 669-83
10. Savaridas SL, Whelehan P, Warwick VR, Contrast-enhanced digital breast tomosythesis and breast MRI to monitor response to neoadjuvant chemotherapy: patient tolerance and preference: Br J Radiol, 2022; 95(1134); 20210779
11. Wang H, Mao X, Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer: Drug Des Devel Ther, 2020; 14; 2423-33
12. Montemurro F, Nuzzolese I, Ponzone R, Neoadjuvant or adjuvant chemotherapy in early breast cancer?: Expert Opin Pharmacother, 2020; 21(9); 1071-82
13. Lei S, Zheng R, Zhang S, Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020: Cancer Commun (Lond), 2021; 41(11); 1183-94
14. Criscitiello C, Corti C, Breast cancer genetics: Diagnostics and treatment: Genes (Basel), 2022; 13(9); 1593
15. Travieso-Aja MDM, Maldonado-Saluzzi D, Naranjo-Santana P, Diagnostic performance of contrast-enhanced dual-energy spectral mammography (CESM): A retrospective study involving 644 breast lesions: Radiol Med, 2019; 124(10); 1006-17
16. Zhu X, Huang JM, Zhang K, Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: Systematic review and meta-analysis: Clin Breast Cancer, 2018; 18(5); e985-e95
17. Suter MB, Pesapane F, Agazzi GM, Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions: A systematic review and meta-analysis: Breast, 2020; 53; 8-17
18. Rhodes KM, Turner RM, Savović J, Between-trial heterogeneity in meta-analyses may be partially explained by reported design characteristics: J Clin Epidemiol, 2018; 95; 45-54
19. Hoyer A, Hirt S, Kuss O, Meta-analysis of full ROC curves using bivariate time-to-event models for interval-censored data: Res Synth Methods, 2018; 9(1); 62-72
20. Li L, Roth R, Germaine P, Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions: Diagn Interv Imaging, 2017; 98(2); 113-23
21. Wang Q, Li K, Wang L, Preclinical study of diagnostic performances of contrast-enhanced spectral mammography versus MRI for breast diseases in China: Springerplus, 2016; 5(1); 763
22. Qin Y, Liu Y, Zhang X, Contrast-enhanced spectral mammography: A potential exclusion diagnosis modality in dense breast patients: Cancer Med, 2020; 9(8); 2653-59
23. Sorin V, Sklair-Levy M, Dual-energy contrast-enhanced spectral mammography (CESM) for breast cancer screening: Quant Imaging Med Surg, 2019; 9(11); 1914-17
24. La Forgia D, Catino A, Dentamaro R, Role of the contrast-enhanced spectral mammography for the diagnosis of breast metastases from extramammary neoplasms: J BUON, 2019; 24(4); 1360-66
25. Patel BK, Hilal T, Covington M, Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy: Ann Surg Oncol, 2018; 25(5); 1350-56
Figures
 Figure 1. Manifestations and diagnostic results of (A) CESM lesions and (B) DBT. * P<0.05, compared with the CESM group. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 1. Manifestations and diagnostic results of (A) CESM lesions and (B) DBT. * P<0.05, compared with the CESM group. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). Figure 2. Efficacy of breast cancer chemotherapy evaluated by CESM and DBT. * P<0.05. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 2. Efficacy of breast cancer chemotherapy evaluated by CESM and DBT. * P<0.05. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). Figure 3. ROC curve analysis results of patients in each group. (A) diagnostic results of DBT and CESM. (B) premenopausal group. (C) postmenopausal group. (D) age group ≤50 years old. (E) age group >50 years old. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA).
Figure 3. ROC curve analysis results of patients in each group. (A) diagnostic results of DBT and CESM. (B) premenopausal group. (C) postmenopausal group. (D) age group ≤50 years old. (E) age group >50 years old. The figure was created using GraphPad Prism 9.5.0 for Windows. GraphPad software (San Diego, California USA). Tables
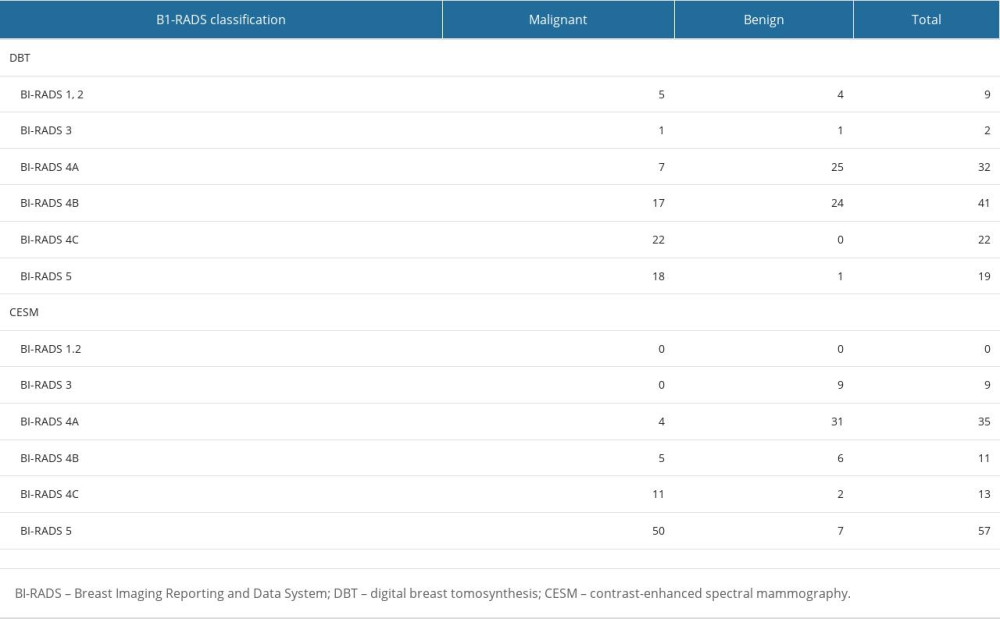 Table 1. BI-RADS classification and pathological results of DBT and CESM.
Table 1. BI-RADS classification and pathological results of DBT and CESM.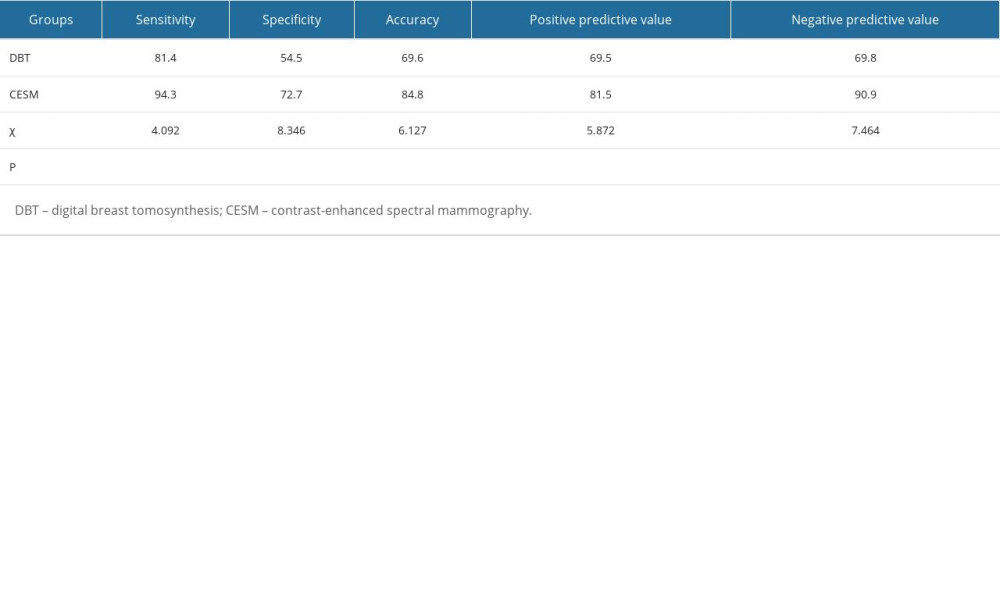 Table 2. Comparison of the diagnostic efficacy of DBT and CESM for breast lesions.
Table 2. Comparison of the diagnostic efficacy of DBT and CESM for breast lesions. Table 1. BI-RADS classification and pathological results of DBT and CESM.
Table 1. BI-RADS classification and pathological results of DBT and CESM. Table 2. Comparison of the diagnostic efficacy of DBT and CESM for breast lesions.
Table 2. Comparison of the diagnostic efficacy of DBT and CESM for breast lesions. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








