21 December 2023: Clinical Research
Evaluation of Safety and Efficacy of Preoperative Coronal MRI-Guided Minimally Invasive Surgery for Cervical Spondylotic Radiculopathy
Yuan Liu1EF, Tianlong Wu1B, Jinghong Yuan1C, Jianye Tan1E, Chongzhi Pan1B, Xinxin Miao1C, Dingwen He1B, Xigao Cheng1234AG*DOI: 10.12659/MSM.942137
Med Sci Monit 2023; 29:e942137
Abstract
BACKGROUND: Key-hole surgery is a minimally invasive technique that has shown promise in various surgical procedures. This study aimed to assess the clinical effectiveness of preoperative coronal MRI-assisted key-hole surgery for the treatment of patients with cervical spondylotic radiculopathy (CSR).
MATERIAL AND METHODS: A total of 30 patients diagnosed with CSR and undergoing key-hole surgery with CMRI assistance were included in the study. Various parameters, including surgical segments, incision length, disease duration, operative time, intraoperative fluoroscopy times, intraoperative blood loss, complications, and length of hospitalization, were recorded. Precise measurements of Cobb angles and intervertebral space height were taken before and after the surgical procedure. Surgical outcomes were evaluated using modified Macnab criteria, visual analogue scale (VAS), Japanese Orthopaedic Association Scores (JOA), and neck disability index (NDI).
RESULTS: The average duration of disease was 6.47±3.29 months, with an average incision length of 1.94±0.15 cm and operative time of 57.83±4.34 minutes. The average intraoperative blood loss was 33.70±9.28 ml, with an average of 3.50±0.73 intraoperative fluoroscopies. The average duration of hospitalization was 4.10±1.27 days. Preoperative and postoperative measurements showed no statistically significant difference in C2-C7 Cobb angles and intervertebral space height. However, there were significant improvements in postoperative VAS, NDI, and JOA scores compared to preoperative scores. The surgical effectiveness rate was 100%, with a high rate of good and excellent outcomes.
CONCLUSIONS: The findings of this study suggest that preoperative CMRI-assisted key-hole surgery for single-segment CSR is a safe and effective treatment option with low complication rates. The clinical benefits include high security and good outcomes. Further research and larger studies are warranted to validate these findings.
Keywords: Multiparametric Magnetic Resonance Imaging, radiculopathy, Treatment Outcome
Background
Cervical spondylotic radiculopathy (CSR), a globally pervasive ailment associated with the aging process, imposes a significant burden on healthcare and imaging resources [1,2]. Commonly observed symptoms among patients include neck pain, numbness in the upper limbs, and muscle weakness [3]. This condition often arises from nerve root compression caused by cervical stenosis and degenerative cervical discs [4]. The classical surgical approach for treating CSR is anterior cervical discectomy and fusion [5]. However, cervical fusion can impact the mobility of the treated cervical segment and can potentially lead to adjacent segment disease [6]. As the field of minimally invasive spine surgery continues to advance at a rapid pace, the cervical key-hole technique has been widely used in the treatment of CSR, owing to minimal trauma, faster recovery, and maintenance of the stability of the cervical spine in the operative segment [7]. The key steps in key-hole surgery involve the exploration of the protruding intervertebral disc and the relief of compression on the responsible segmental nerve root. In cases in which the location of the compressed nerve is uncertain preoperatively, excessive traction on the nerve root and dura mater can be required during endoscopy, potentially exacerbating nerve root damage and compromising surgical outcomes.
Conventional magnetic resonance imaging (MRI) is the preferred diagnostic modality for confirming CSR [8]. However, the diagnostic accuracy of MRI for CSR is limited. It is unable to display the continuous direction of nerve roots, and it also has a false-negative rate in assessing cervical nerve root compression at the intervertebral foramina [9–11]. In addition to using MRI for diagnosis, computerized tomography (CT) myelography with contrast enhancement is used to confirm CSR [12,13]. Guidelines recommend its application to assess patients who present clinical symptoms or signs inconsistent with MRI findings [14]. Selective diagnostic nerve root blocks can also be used to confirm the compression of nerve roots [15]. Both CT myelography and selective diagnostic nerve root blocks are effective in localizing compressed nerve roots but are invasive procedures and not the preferred diagnostic option.
There is a growing body of research on coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI), which is regarded as a non-invasive and reliable new technology [16]. One advantage of CMRI over traditional MRI is its ability to maintain high sensitivity in visualizing nerve roots by leveraging the long T2 characteristics of water, even when the magnetization vectors of other tissues have fully decayed [16]. Furthermore, after reconstructing coronal images, CMRI clearly ascertains the spatial relationship between the disc herniation and nerve roots [17]. Because of its noninvasive nature and reliable characteristics, CMRI is actually superior to CT myelography and MRI in terms of diagnostic efficacy [18]. In our previous research, CMRI was used for the diagnosis of extraforaminal disc herniation and multi-segmental lumbar disc herniation, as well as for differentiating between mimicking tumor discs and nerve sheath tumors [18–20]. To the best of our knowledge, there have been no reports on the use of CMRI for localizing compressed nerve roots in CSR. Therefore, this research aimed to investigate the impact of preoperative CMRI on the intraoperative conditions of cervical key-hole surgery, as well as its influence on postoperative pain intensity and cervical stability.
Material and Methods
PATIENT SELECTION:
This was a retrospective single-arm cohort study that analyzed a total of 30 patients who underwent key-hole surgery with precise localization of the compressed cervical nerve root using CMRI for CSR at the Second Affiliated Hospital of Nanchang University, from 2018 to 2023. It received approval from our institution’s Medical Ethics Committee and Internal Review Board, and we obtained written informed consent from all participants. Figure 1 shows the patient flowchart.
The inclusion criteria were as follows: (1) cervical spine MRI and CMRI revealed a unilateral single-segment disc protrusion compressing the nerve root, which was consistent with the patient’s symptoms and signs; (2) patients who underwent key-hole surgery for CSR; (3) conservative treatment for CSR was strictly followed for a duration of more than 6 months without significant improvement; (4) lateral posterior soft disc herniation; and (5) patient’s relevant examinations did not indicate any surgical contraindications or underlying conditions that would affect postoperative recovery.
The exclusion criteria were as follows: (1) history of previous cervical spine surgery or multi-segmental cervical disc herniation; (2) patients presenting with cervical spondylotic myelopathy, spinal tumors, spinal tuberculosis, or ossification of the posterior longitudinal ligament; and (3) follow-up period of less than 1 year after the surgery.
SURGICAL TECHNIQUE:
Following the administration of general anesthesia, the patient was positioned in a prone orientation, and the Macintosh headframe was used to secure the head. Perspective positioning was used to identify and mark the responsible segment. An approximately 8-mm longitudinal incision was made 2 cm laterally to the midline of the responsible segment. A working channel was inserted based on preoperative localization. Under endoscopic visualization, a minimal amount of soft tissue on the surface of the surgical segment’s lamina was cleared, and the upper and lower lamina as well as the medial intersection of the articular process joint (V-point) were identified. The V-point served as a marker point to grind the window opening. The upper edge of the lower lamina, lower edge of the upper lamina, and a portion of the medial aspect of the articular process joint were ground down to access the spinal canal. The ligamentum flavum was detached, the herniated cervical disc was removed according to preoperative CMRI positioning, and the cervical nerve was explored. The incision was rinsed and closed sequentially.
INSPECTION TECHNIQUES:
The scanning parameters for CMRI are referenced in our previous study [21].
OUTCOME MEASURES:
General data, including sex, age, surgical segment, surgical duration, intraoperative blood loss, number of intraoperative fluoroscopic procedures, duration of hospitalization, and intraoperative complications, were collected for statistical analysis.
Patients were followed up for at least 1 year after the surgery. Prior to the surgery and at postoperative months 1, 3, 6, and 12, the visual analogue scale (VAS) was used to assess the level of pain in the neck and upper extremities. Functional capacity was evaluated using the neck disability index (NDI). Neurological function was assessed using the Japanese Orthopaedic Association (JOA) score. At the final follow-up, the modified MacNab criteria were used to assess the surgical outcomes.
The preoperative, 6-month postoperative, and final follow-up assessments involved evaluating and analyzing radiographic examinations to record the C2–C7 Cobb angle and the height of the intervertebral space at the surgical segment. These measurements were used to assess the impact of CMRI-assisted key-hole surgery on the local stability of the cervical spine.
STATISTICAL ANALYSIS:
Statistical analyses were conducted in the present study using SPSS20.0 statistical software. Measurement data were presented as mean ± standard deviation, and the
Results
PATIENT CHARACTERISTICS:
A total of 30 patients (21 men and 9 women) were included in this study. The patients had an average age of 49.83±11.61 years and an average disease duration of 6.47±3.29 months. All surgical procedures were exclusively conducted on the lower cervical spine, specifically involving C4/5 in 5 cases, C5/6 in 10 cases, and C6/7 in 13 cases. The key-hole surgery technique was used for all patients, with preoperative use of CMRI for nerve root localization and decompression performed through a unilateral approach. All patients underwent successful surgeries. The mean incision length was 1.94±0.15 cm, intraoperative fluoroscopy was performed 3.50±0.73 times, surgical duration was 57.83±4.34 min, intraoperative blood loss was 33.70±9.28 mL, and the duration of hospitalization averaged 4.10±1.27 days (Table 1).
IMAGING RESULTS:
Throughout the 6-month and 1-year postoperative follow-up period, none of the included patients experienced segmental instability. The C2–C7 Cobb angles in patients followed up at 6 months and 1 year postoperatively showed no significant improvement compared with preoperative values, with no statistically significant differences. There were no significant changes in intervertebral space height, with no statistically significant differences observed (Table 2).
FUNCTIONAL RESULTS:
The preoperative, postoperative 1-week, postoperative 3-month, postoperative 6-month, and postoperative 1-year VAS scores for neck pain were 4.33±1.03, 2.93±1.01, 2.53±1.28, 2.07±0.94, and 1.47±0.68, respectively. The preoperative, postoperative 1-week, postoperative 3-month, postoperative 6-month, and postoperative 1-year VAS scores for upper limb pain were 8.50±1.01, 3.53±1.01, 2.00±0.59, 1.40±0.50, and 1.07±0.25, respectively. The preoperative, postoperative 1-week, postoperative 3-month, postoperative 6-month, and postoperative 1-year NDI scores were 35.23±5.69, 10.43±1.83, 6.17±1.74, 4.77±1.77, and 3.43±1.74, respectively. The preoperative, postoperative 1-week, postoperative 3-month, postoperative 6-month, and postoperative 1-year JOA scores were 11.73±1.14, 13.60±1.07, 14.60±1.13, 14.47±1.04, and 14.90±0.80, respectively.
Significant reductions in VAS scores (neck and upper limb) and NDI scores were observed at each postoperative follow-up time point compared with preoperative values, with statistically significant differences. Moreover, significant improvements in JOA scores were observed at each postoperative follow-up time point compared with preoperative values, with statistically significant differences. One year after follow-up, based on the modified MacNab criteria for evaluating treatment effectiveness, 21 cases were rated as excellent, 9 cases as good, 0 cases as fair, and 0 cases as poor, resulting in a combined excellent and good rating of 100% (Table 3).
CASE 1: A male patient, aged 55 years, was admitted to our department with a concern of left-sided neck and shoulder pain for approximately 2 months. MRI revealed no significant spinal canal stenosis in the multi-level cervical spine, but CMRI showed disc herniation at the left C7/T1 level, with nerve root filling defects (Figure 2A). Key-hole surgery was performed to remove the protruding intervertebral disc (Figure 2B), and the patient’s pain was immediately relieved, leading to discharge on the third day after surgery. Throughout the follow-up period, the patient’s functional improvement was satisfactory (Figure 2C, 2D).
CASE 2: A male patient, 43 years of age, presented with a chief concern of radiating pain and weakness in the left upper limb persisting for a duration of 6 months. MRI showed disc herniation at the right C4/C5 and C5/C6 levels, with no significant narrowing at the C6/C7 level. The clinical symptoms of the patient did not match the MRI findings. Subsequently, based on the CMRI, the left-side protruding disc at the C6/C7 level was compressing the nerve (Figure 3A). Key-hole surgery was successfully performed (Figure 3B), and his pain symptoms were immediately relieved, leading to discharge on the third day after surgery. Throughout the follow-up period, the patient’s functional improvement was satisfactory (Figure 3C, 3D).
CASE 3: A male patient, aged 37 years, was admitted to the hospital for cervical pain and left upper arm pain. CMRI revealed left foraminal stenosis at the C6/C7 segment (Figure 4A). The protruding intervertebral disc was excised during a cervical key-hole surgery (Figure 4B). The patient experienced immediate relief of pain and was discharged within 3 days. Three months after key-hole surgery, the patient exhibited significant improvement in functionality (Figure 4C, 4D).
Comprehensive details regarding the 3 cases are shown in Figures 5–7.
Discussion
CSR typically manifests as radicular pain and is usually managed conservatively [22]. In cases in which conservative management fails, surgical intervention can be required to alleviate symptoms by relieving compression [23]. MRI scans with a slice thickness of only 3 to 5 mm often fail to clearly demonstrate the morphology of nerve roots, leading to ambiguous or missed diagnoses of CSR [24]. CMRI is a T2-weighted imaging technique that suppresses the fat component between the intra-neural fascicles and peripheral. It utilizes a combination of fat suppression techniques and T2-weighting to specifically visualize the fluid within the endoneurial space of the nerve bundles [25]. Unlike MRI, CMRI imaging provides enhanced visualization of the complete extent of the spinal nerve root [17]. Furthermore, Byun et al noted that CMRI allows for the 3-dimensional visualization of the relationship between the nerve root and intervertebral disc [16]. The present study is the first to advocate a combination of the cervical key-hole technique with preoperative CMRI. In this study, all patients underwent preoperative CMRI to accurately identify the responsible segment causing symptoms. Precise decompression of the responsible segment was performed, resulting in effective relief of postoperative symptoms. This further validates the accuracy of CMRI in identifying the responsible segment. The fastest relief occurred for neck pain and radicular pain, with most patients experiencing significant relief of symptoms within 1 week after surgery, followed by continued gradual improvement. In 1 case we found that the radicular pain symptoms persisted for 1 week after surgery, possibly due to prolonged nerve root edema. However, symptoms were ultimately relieved after treatment with mannitol dehydration and analgesia. There were no cases of collapse of the intervertebral space at the responsible segment in any of the patients. At the last follow-up, there was no statistically significant difference in cervical curvature compared to preoperative measurements, indicating that this technique effectively protected the stability of the facet joints and surgical segments.
In the present study, the advantages of preoperative CMRI for accurate localization of the compressed cervical nerve in treating CSR with key-hole surgery were shown by its smaller surgical trauma, shorter incision length of 1.94±0.15 cm, shorter operating time of 57.83±4.34 min, fewer intraoperative fluoroscopy times of 3.50±0.73, less bleeding of 33.70±9.28 mL, and lower incidence of postoperative complications in patients. There can be 2 possible reasons for this. First, preoperative CMRI can accurately determine the location of the cervical intervertebral disc herniation, reducing the need for excessive traction on the cervical nerve root and dura mater during key-hole surgery to locate the disc, thereby reducing interference with nerve tissue and lowering the incidence of cervical nerve injury. Second, after grinding and opening the window during key-hole surgery, the cervical nerve can be directly decompressed without the need for further probing to locate the intervertebral space, avoiding excessive stripping of the surrounding soft tissue in the neck.
Minimally invasive spine surgery has gained increasing attention [26]. Ruetten et al first reported the use of key-hole surgery to treat patients with cervical radiculopathy [27]. In contrast to the previous approach of using air as the imaging medium in microendoscopic posterior cervical foraminotomy, the use of uninterrupted flow of physiological saline as the medium in key-hole surgery offers enhanced surgical visualization, thereby yielding superior surgical outcomes [28]. Researchers have employed diverse methodologies to augment the therapeutic efficacy of key-hole surgery. In a report by Zhang et al [29], of 42 patients with CSR who underwent key-hole surgery with O-arm navigation assistance, 38 achieved favorable treatment outcomes. Liu et al used ultrasonic bone osteotome as a supplementary approach in conjunction with key-hole surgery [7]. Previous reports have described the use of the novel Delta system for key-hole surgery [30]. Nevertheless, the larger and wider threaded channel implies a longer incision length. The aforementioned studies have demonstrated favorable clinical outcomes in terms of improving accuracy, relieving nerve compression, and reducing surgical time. However, they also have challenges such as complex utilization procedures, steep learning curves [7], high probability of postoperative complications [30], intraoperative radiation exposure [29], and inability to enhance the preoperative diagnostic confirmation rate of nerve root-type cervical spondylosis [7,29,30]. As a result, widespread adoption and application have not been achieved. On the basis of key-hole surgical treatment, the preoperative use of CMRI in the present study exhibited improved therapeutic outcomes and a reduced occurrence of unimproved symptoms compared with previous reports. In addition, this study also focused on the changes in postoperative cervical spine stability following key-hole surgery.
This study had several limitations, including a retrospective observational design and a relatively small sample size, which can result in clinical heterogeneity. To further establish the clinical effectiveness of this treatment approach, it is imperative to conduct larger, long-term, prospective randomized controlled studies in the future.
Conclusions
The results of this study showed that using preoperative CMRI to accurately identify the responsible segment combined with performing key-hole surgery is an effective treatment method for single-segment CSR patients, with advantages such as smaller surgical trauma, fewer complications, and faster recovery.
Figures
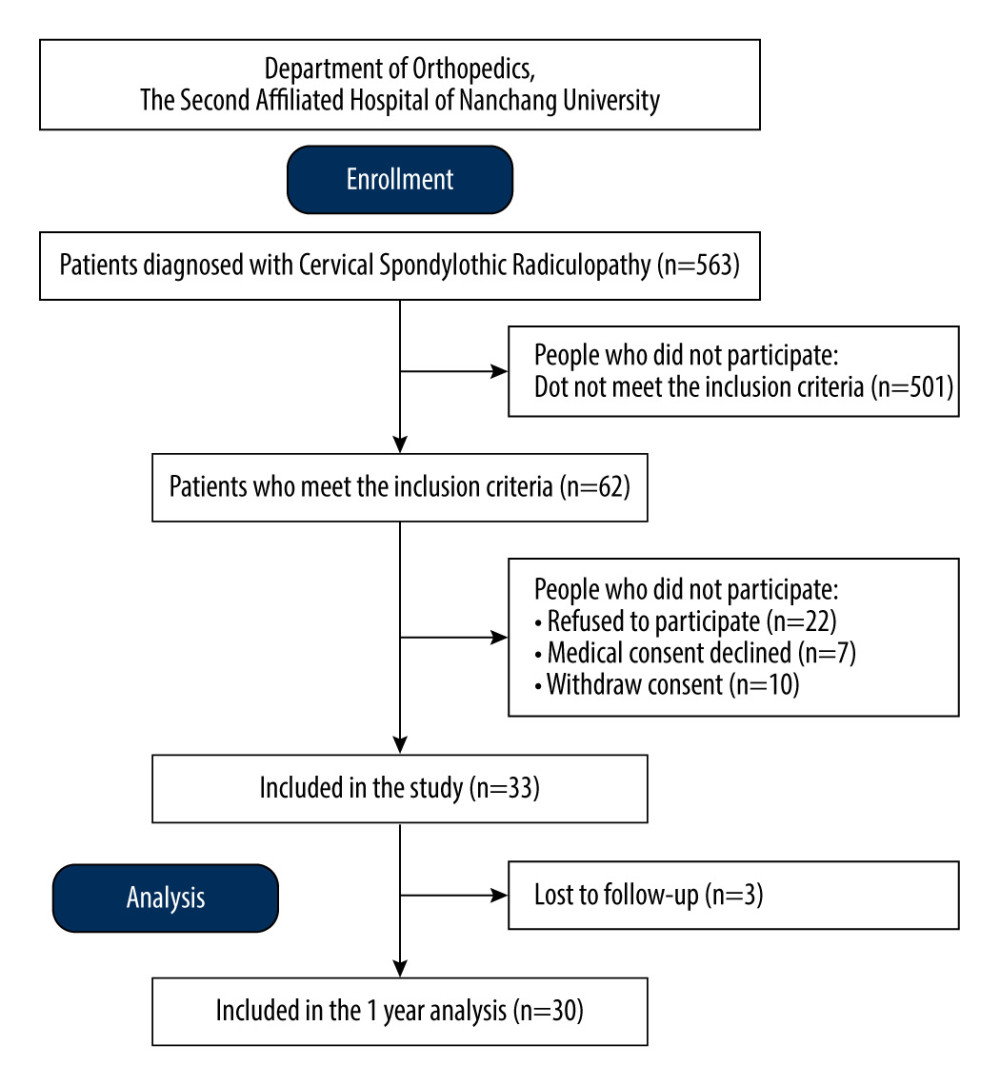 Figure 1. Flow diagram of enrollment, follow-up, and analysis.
Figure 1. Flow diagram of enrollment, follow-up, and analysis. 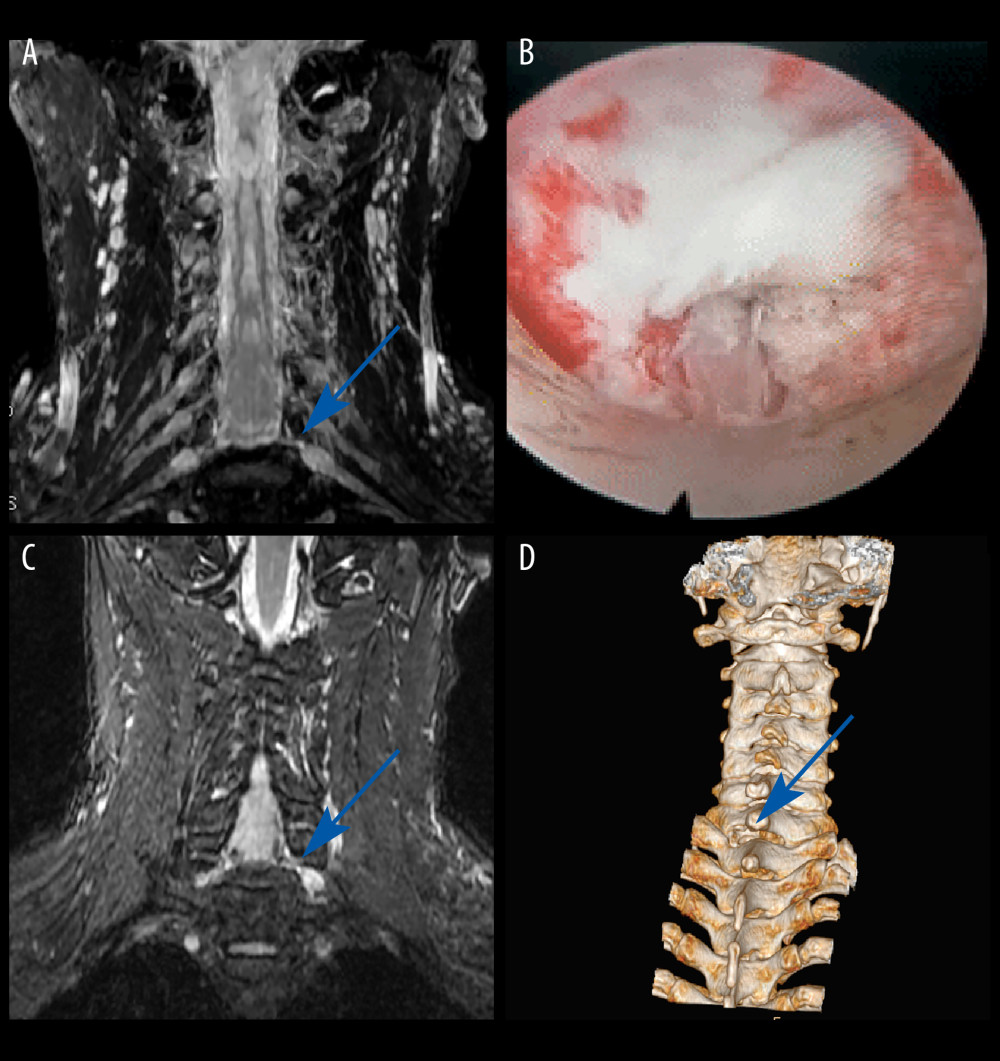 Figure 2. Case 1: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (B) Never root decompression performed, with good dural pulsation. (C) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 2. Case 1: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (B) Never root decompression performed, with good dural pulsation. (C) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. 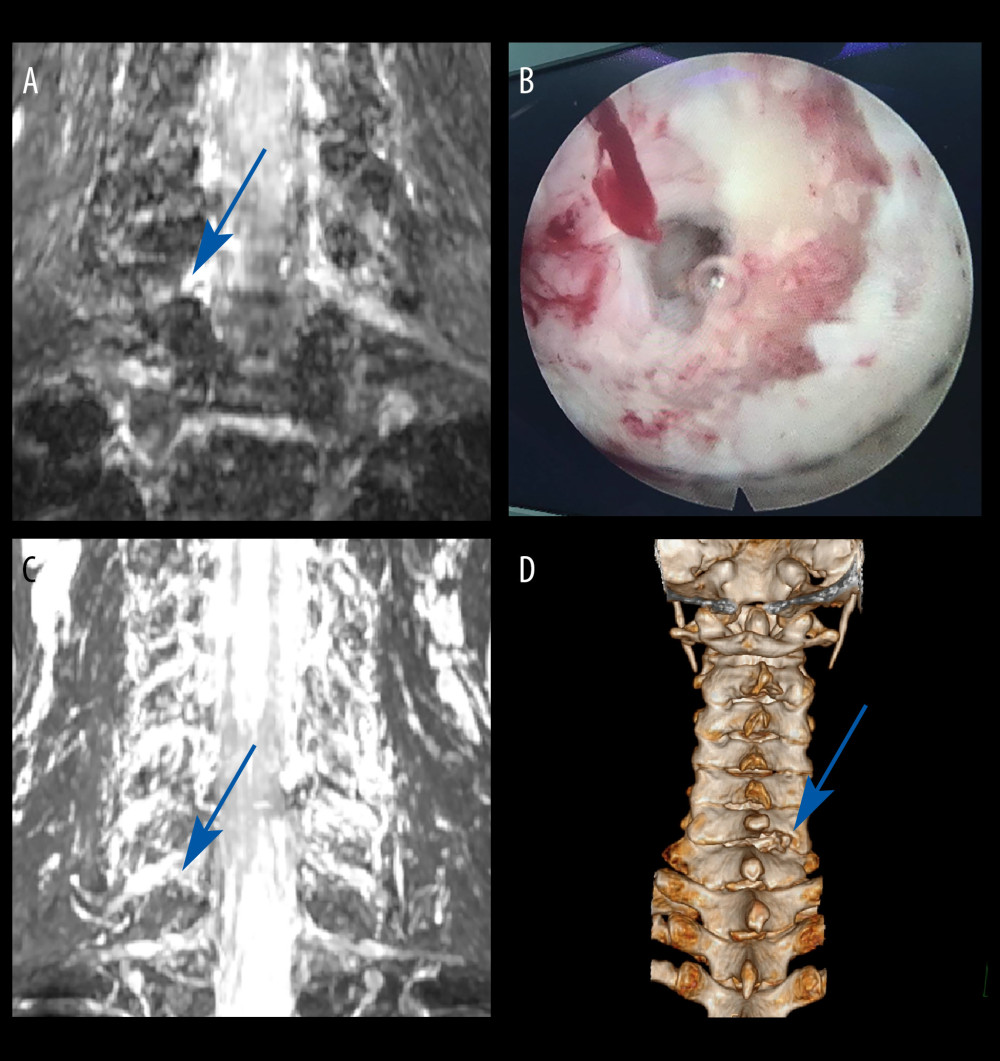 Figure 3. Case 2: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (blue arrow). (B) Surgical specimen of the removed intervertebral disc. (C) Postoperative CMRI showed good decompression of the right C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 3. Case 2: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (blue arrow). (B) Surgical specimen of the removed intervertebral disc. (C) Postoperative CMRI showed good decompression of the right C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. 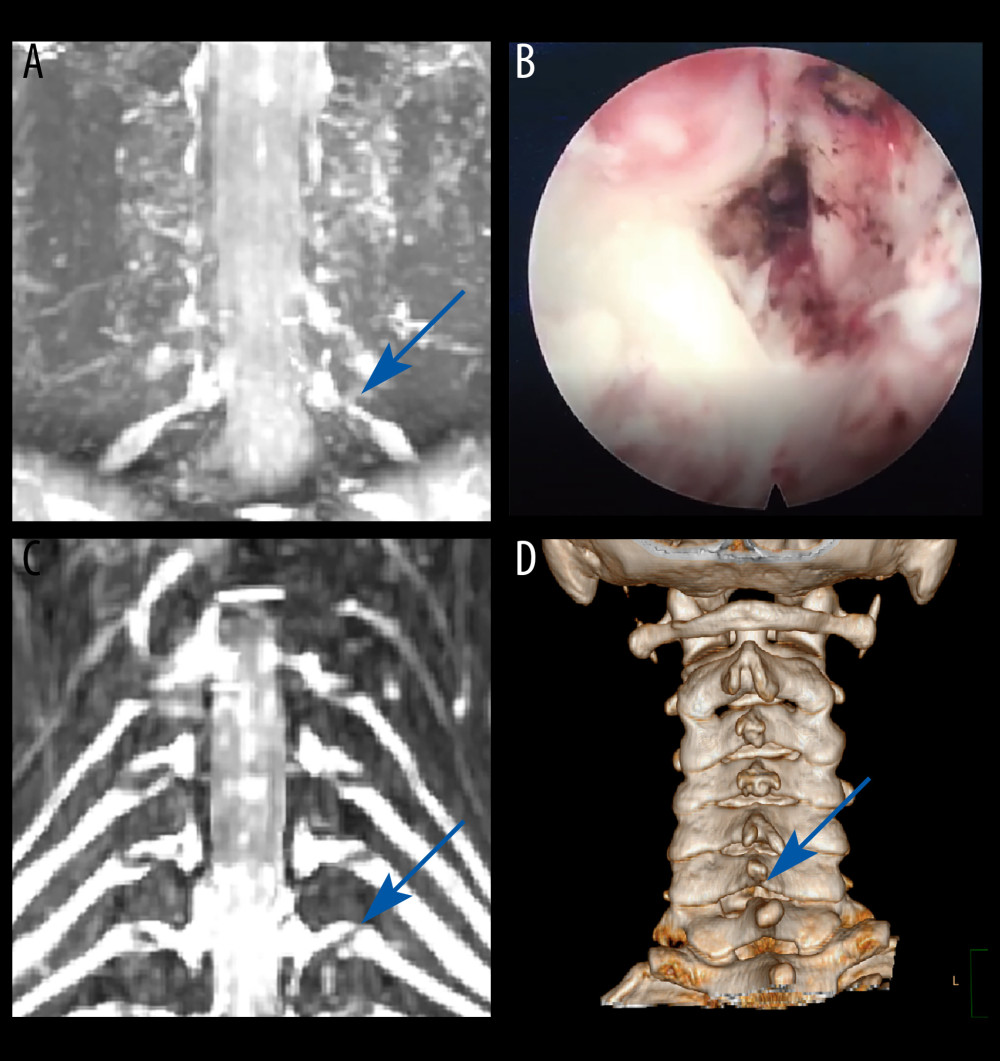 Figure 4. Case 3: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (B) Never root decompression performed. (C) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 4. Case 3: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (B) Never root decompression performed. (C) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. 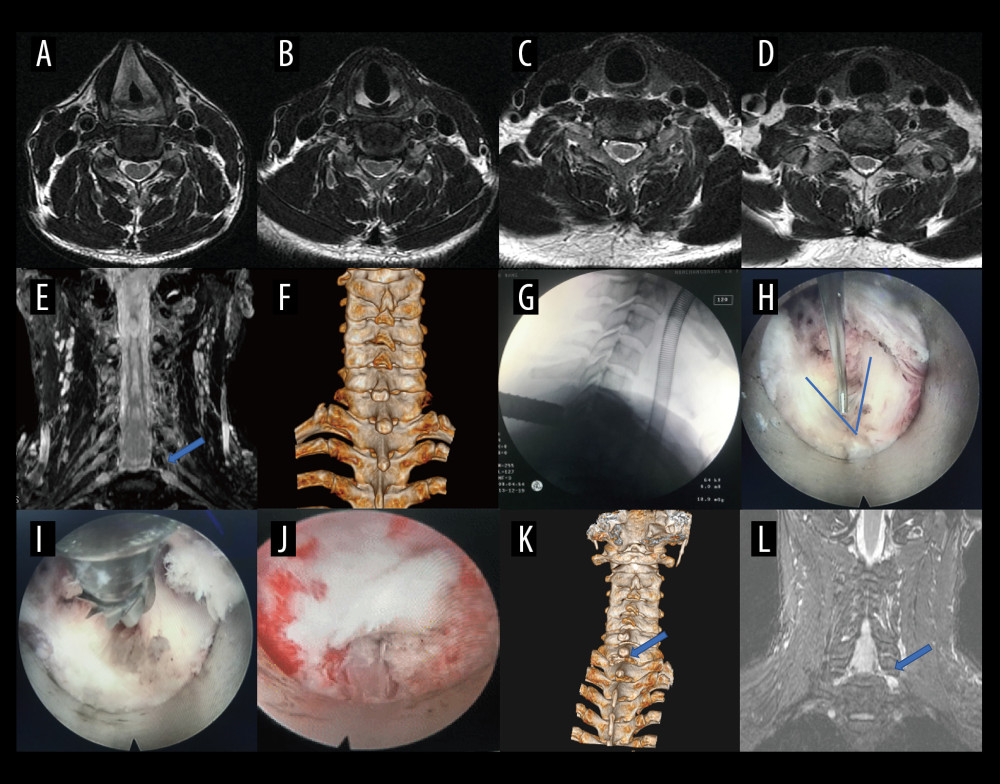 Figure 5. A typical case: case 1. (A-D) Cervical spine magnetic resonance imaging (MRI) showed no significant cervical spinal canal stenosis in segments C4/C5, C5/C6, C6/C7, and C7/T1. (E) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (F) Preoperative CT imaging. (G) After pre-operative localization by CMRI imaging, insertion of a working cannula. (H) Exposure of the V-point (arrow). (I) Using a high-speed drilling to remove the vertebral plate and articular process around the V-point. (J) Never root decompression performed, with good dural pulsation. (K) Postoperative computed tomography image with appropriate windowing (blue arrow). (L) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 5. A typical case: case 1. (A-D) Cervical spine magnetic resonance imaging (MRI) showed no significant cervical spinal canal stenosis in segments C4/C5, C5/C6, C6/C7, and C7/T1. (E) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (F) Preoperative CT imaging. (G) After pre-operative localization by CMRI imaging, insertion of a working cannula. (H) Exposure of the V-point (arrow). (I) Using a high-speed drilling to remove the vertebral plate and articular process around the V-point. (J) Never root decompression performed, with good dural pulsation. (K) Postoperative computed tomography image with appropriate windowing (blue arrow). (L) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). Vue PACS, v12.2.6, Philips. 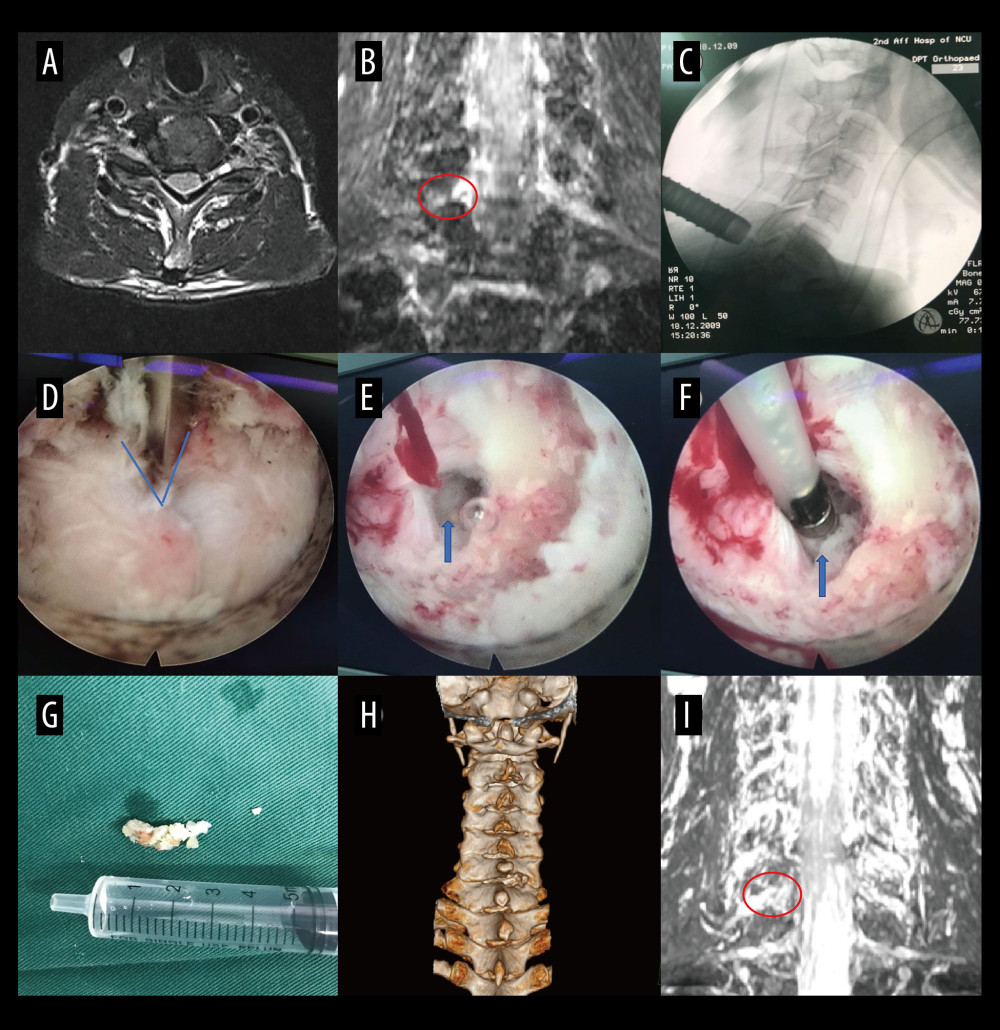 Figure 6. A typical case: case 2. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent cervical spinal canal stenosis in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (red circle). (C) After pre-operative localization by CMRI imaging, insertion of a working cannula. (D) Exposure of the V-point (blue arrow). (E) Exposure of the protruding intervertebral disc (blue arrow). (F) Surgical specimen of the removed intervertebral disc. (G) Degenerated disc tissue for comparison. (H) Postoperative computed tomography image with appropriate windowing. (I) Postoperative CMRI showed good decompression of the right C7 nerve root (red circle). Vue PACS, v12.2.6, Philips.
Figure 6. A typical case: case 2. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent cervical spinal canal stenosis in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (red circle). (C) After pre-operative localization by CMRI imaging, insertion of a working cannula. (D) Exposure of the V-point (blue arrow). (E) Exposure of the protruding intervertebral disc (blue arrow). (F) Surgical specimen of the removed intervertebral disc. (G) Degenerated disc tissue for comparison. (H) Postoperative computed tomography image with appropriate windowing. (I) Postoperative CMRI showed good decompression of the right C7 nerve root (red circle). Vue PACS, v12.2.6, Philips. 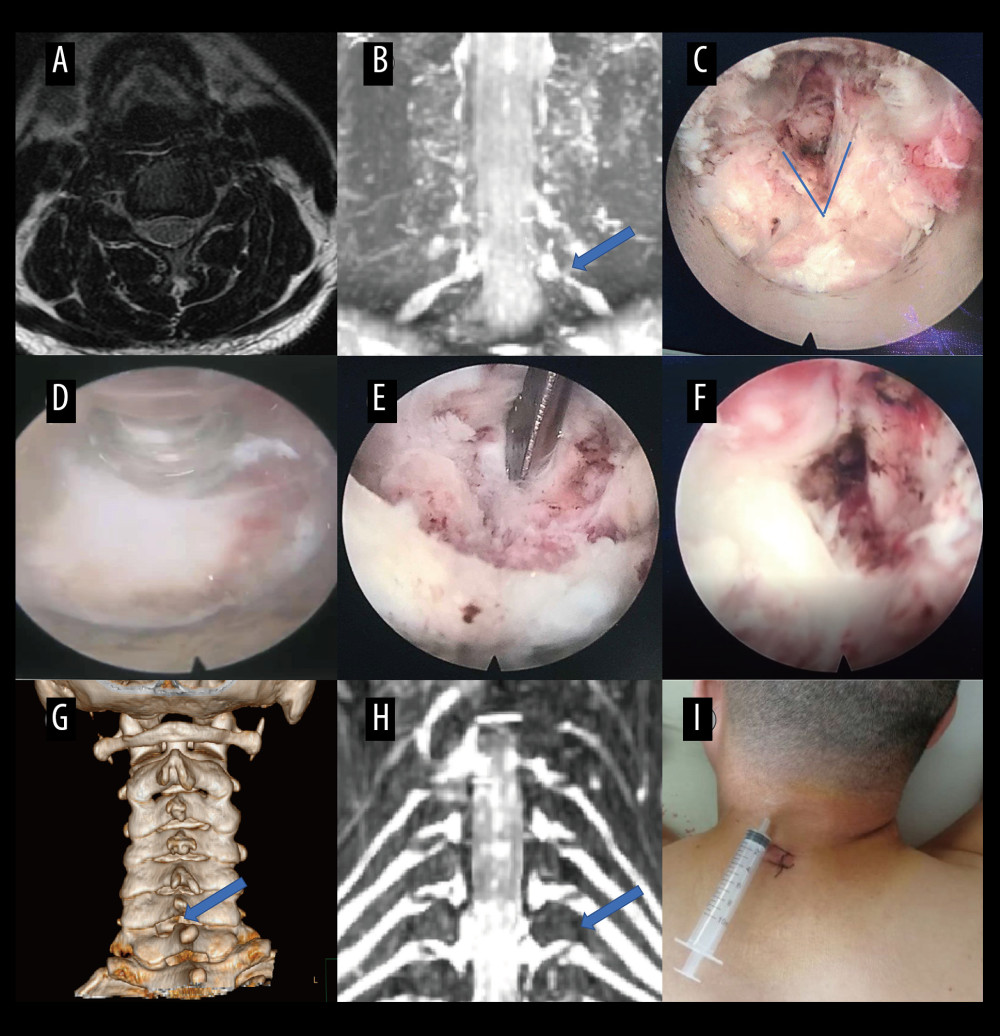 Figure 7. A typical case: case 3. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent disc herniation in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (C) Exposure of the V-point. (D, E) Pictures during endoscopic surgery. (F) Never root decompression performed. (G) Postoperative computed tomography image with appropriate windowing (blue arrow). (H) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (I) Postoperative small incision. Vue PACS, v12.2.6, Philips.
Figure 7. A typical case: case 3. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent disc herniation in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (C) Exposure of the V-point. (D, E) Pictures during endoscopic surgery. (F) Never root decompression performed. (G) Postoperative computed tomography image with appropriate windowing (blue arrow). (H) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (I) Postoperative small incision. Vue PACS, v12.2.6, Philips. References
1. Hu J, Chen F, Qiu G, Jingshu Keli for treating cervical spondylotic radiculopathy: The first multicenter, randomized, controlled clinical trial: J Orthop Translat, 2021; 27; 44-56
2. Luyao H, Xiaoxiao Y, Tianxiao F, Management of cervical spondylotic radiculopathy: A systematic review: Global Spine J, 2022; 12(8); 1912-24
3. Persson LC, Carlsson JY, Anderberg L, Headache in patients with cervical radiculopathy: A prospective study with selective nerve root blocks in 275 patients: Eur Spine J, 2007; 16(7); 953-59
4. Benditz A, Brunner M, Zeman F, Effectiveness of a multimodal pain management concept for patients with cervical radiculopathy with focus on cervical epidural injections: Sci Rep, 2017; 7(1); 7866
5. Mazzucchi E, La Rocca G, Perna A, Single-level anterior cervical discectomy and interbody fusion: A comparison between porous tantalum and polyetheretherketone cages: J Pers Med, 2022; 12(6); 986
6. Kwok WCH, Wong CYY, Law JHW, Risk factors for adjacent segment disease following anterior cervical discectomy and fusion with plate fixation: A systematic review and meta-analysis: J Bone Joint Surg Am, 2022; 104(21); 1915-45
7. Liu J, Kong Q, Feng P, Clinical effect of channel assisted cervical key hole technology combined with ultrasonic bone osteotome in the treatment of single segment cervical spondylotic radiculopathy: Front Surg, 2022; 9; 1029028
8. Oshina M, Oshima Y, Tanaka S, Utility of oblique sagittal reformatted and three-dimensional surface reconstruction computed tomography in foraminal stenosis decompression: Sci Rep, 2018; 8(1); 16011
9. McKay G, Torrie PA, Bertram W, Myelography in the assessment of degenerative lumbar scoliosis and its influence on surgical management: Korean J Spine, 2017; 14(4); 133-38
10. Schwarz D, Kele H, Kronlage M, Diagnostic value of magnetic resonance neurography in cervical radiculopathy: Plexus patterns and peripheral nerve lesions: Invest Radiol, 2018; 53(3); 158-66
11. Birchall D, Connelly D, Walker L, Hall K, Evaluation of magnetic resonance myelography in the investigation of cervical spondylotic radiculopathy: Br J Radiol, 2003; 76(908); 525-31
12. Bartlett RJ, Hill CR, Gardiner E, A comparison of T2 and gadolinium enhanced MRI with CT myelography in cervical radiculopathy: Br J Radiol, 1998; 71(841); 11-19
13. Modic MT, Masaryk TJ, Mulopulos GP, Cervical radiculopathy: prospective evaluation with surface coil MR imaging, CT with metrizamide, and metrizamide myelography: Radiology, 1986; 161(3); 753-59
14. Bono CM, Ghiselli G, Gilbert TJ, An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders: Spine J, 2011; 11(1); 64-72
15. Anderberg L, Annertz M, Rydholm U, Selective diagnostic nerve root block for the evaluation of radicular pain in the multilevel degenerated cervical spine: Eur Spine J, 2006; 15(6); 794-801
16. Byun WM, Jang HW, Kim SW, Three-dimensional magnetic resonance rendering imaging of lumbosacral radiculography in the diagnosis of symptomatic extraforaminal disc herniation with or without foraminal extension: Spine (Phila Pa 1976), 2012; 37(10); 840-44
17. Shen J, Wang HY, Chen JY, Liang BL, Morphologic analysis of normal human lumbar dorsal root ganglion by 3D MR imaging: Am J Neuroradiol, 2006; 27(10); 2098-103
18. Yuan J, Du Z, Wu Z, Differential diagnosis of mimicking tumor discs using coronal magnetic resonance imaging of three-dimensional fast-field echo with water-selective excitation: A single center retrospective study: Orthop Surg, 2022; 14(12); 3330-39
19. Jia J, Ding R, Liu X, Coronal magnetic resonance imaging of three-dimensional fast-field echo with water-selective excitation improves the sensitivity and reliability of identification of extraforaminal lumbar disc herniation: J Int Med Res, 2019; 47(12); 6053-60
20. Li J, Jia J, Wu T, Accuracy of coronal magnetic resonance imaging diagnosis of multi-segmental lumbar disc herniation: A single-center retrospective analysis: Med Sci Monit, 2023; 29; e938577
21. Tang T, Yuan J, Yin J, Case report: The coronal magnetic resonance imaging of three-dimensional fast-field echo with water-selective excitation can identify the wrapping of spinal nerve fibers into subdural tumors prior to operation: Front Neurol, 2022; 13; 945299
22. Wang C, Gu Z, Yu J, Clinical observation of Long chiropractic treatment on patients with neurogenic cervical spondylosis: Study protocol for a randomized controlled trial: Medicine (Baltimore), 2022; 101(9); e28861
23. Zhang P, Jin Y, Zhu B, Unilateral biportal endoscopic foraminotomy and diskectomy combined with piezosurgery for treating cervical spondylotic radiculopathy with neuropathic radicular pain: Front Neurol, 2023; 14; 1100641
24. Zarrabian MM, Diehn FE, Kotsenas AL, Dorsal lumbar disc migrations with lateral and ventral epidural extension on axial MRI: A case series and review of the literature: Am J Neuroradiol, 2016; 37(11); 2171-77
25. Byun WM, Kim JW, Lee JK, Differentiation between symptomatic and asymptomatic extraforaminal stenosis in lumbosacral transitional vertebra: Role of three-dimensional magnetic resonance lumbosacral radiculography: Korean J Radiol, 2012; 13(4); 403-11
26. Wu B, Wei T, Yao Z, A real-time 3D electromagnetic navigation system for percutaneous transforaminal endoscopic discectomy in patients with lumbar disc herniation: A retrospective study: BMC Musculoskelet Disord, 2022; 23(1); 57
27. Ruetten S, Komp M, Merk H, Godolias G, A new full-endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9-mm endoscopes: Prospective 2-year results of 87 patients: Minim Invasive Neurosurg, 2007; 50(4); 219-26
28. Wu PF, Li YW, Wang B, Posterior cervical foraminotomy via full-endoscopic versus microendoscopic approach for radiculopathy: A systematic review and meta-analysis: Pain Physician, 2019; 22(1); 41-52
29. Zhang C, Wu J, Xu C, Minimally invasive full-endoscopic posterior cervical foraminotomy assisted by O-arm-based navigation: Pain Physician, 2018; 21(3); E215-E23
30. Haijun M, Xiaobing Z, Bin G, Trans-interlamina percutaneous endoscopic cervical discectomy for symptomatic cervical spondylotic radiculopathy using the new Delta system: Sci Rep, 2020; 10(1); 10290
Figures
 Figure 1. Flow diagram of enrollment, follow-up, and analysis.
Figure 1. Flow diagram of enrollment, follow-up, and analysis. Figure 2. Case 1: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (B) Never root decompression performed, with good dural pulsation. (C) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 2. Case 1: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (B) Never root decompression performed, with good dural pulsation. (C) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. Figure 3. Case 2: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (blue arrow). (B) Surgical specimen of the removed intervertebral disc. (C) Postoperative CMRI showed good decompression of the right C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 3. Case 2: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (blue arrow). (B) Surgical specimen of the removed intervertebral disc. (C) Postoperative CMRI showed good decompression of the right C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. Figure 4. Case 3: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (B) Never root decompression performed. (C) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 4. Case 3: example of a typical case. (A) Coronal magnetic resonance image of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (B) Never root decompression performed. (C) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (D) Postoperative computed tomography image with appropriate windowing (blue arrow). Vue PACS, v12.2.6, Philips. Figure 5. A typical case: case 1. (A-D) Cervical spine magnetic resonance imaging (MRI) showed no significant cervical spinal canal stenosis in segments C4/C5, C5/C6, C6/C7, and C7/T1. (E) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (F) Preoperative CT imaging. (G) After pre-operative localization by CMRI imaging, insertion of a working cannula. (H) Exposure of the V-point (arrow). (I) Using a high-speed drilling to remove the vertebral plate and articular process around the V-point. (J) Never root decompression performed, with good dural pulsation. (K) Postoperative computed tomography image with appropriate windowing (blue arrow). (L) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). Vue PACS, v12.2.6, Philips.
Figure 5. A typical case: case 1. (A-D) Cervical spine magnetic resonance imaging (MRI) showed no significant cervical spinal canal stenosis in segments C4/C5, C5/C6, C6/C7, and C7/T1. (E) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C7/T1 level, with nerve root filling defects (blue arrow). (F) Preoperative CT imaging. (G) After pre-operative localization by CMRI imaging, insertion of a working cannula. (H) Exposure of the V-point (arrow). (I) Using a high-speed drilling to remove the vertebral plate and articular process around the V-point. (J) Never root decompression performed, with good dural pulsation. (K) Postoperative computed tomography image with appropriate windowing (blue arrow). (L) Postoperative CMRI showed good decompression of the left C8 nerve root (blue arrow). Vue PACS, v12.2.6, Philips. Figure 6. A typical case: case 2. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent cervical spinal canal stenosis in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (red circle). (C) After pre-operative localization by CMRI imaging, insertion of a working cannula. (D) Exposure of the V-point (blue arrow). (E) Exposure of the protruding intervertebral disc (blue arrow). (F) Surgical specimen of the removed intervertebral disc. (G) Degenerated disc tissue for comparison. (H) Postoperative computed tomography image with appropriate windowing. (I) Postoperative CMRI showed good decompression of the right C7 nerve root (red circle). Vue PACS, v12.2.6, Philips.
Figure 6. A typical case: case 2. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent cervical spinal canal stenosis in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed disc herniation at the left C6/C7 level, with nerve root filling defects (red circle). (C) After pre-operative localization by CMRI imaging, insertion of a working cannula. (D) Exposure of the V-point (blue arrow). (E) Exposure of the protruding intervertebral disc (blue arrow). (F) Surgical specimen of the removed intervertebral disc. (G) Degenerated disc tissue for comparison. (H) Postoperative computed tomography image with appropriate windowing. (I) Postoperative CMRI showed good decompression of the right C7 nerve root (red circle). Vue PACS, v12.2.6, Philips. Figure 7. A typical case: case 3. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent disc herniation in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (C) Exposure of the V-point. (D, E) Pictures during endoscopic surgery. (F) Never root decompression performed. (G) Postoperative computed tomography image with appropriate windowing (blue arrow). (H) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (I) Postoperative small incision. Vue PACS, v12.2.6, Philips.
Figure 7. A typical case: case 3. (A) Cervical spine magnetic resonance imaging (MRI) showed no apparent disc herniation in segments C6/C7. (B) Coronal MRI of 3-dimensional fast-field echo with water-selective excitation (CMRI) showed foraminal stenosis at the left C6/C7 level (blue arrow). (C) Exposure of the V-point. (D, E) Pictures during endoscopic surgery. (F) Never root decompression performed. (G) Postoperative computed tomography image with appropriate windowing (blue arrow). (H) Postoperative CMRI showed good decompression of the left C7 nerve root (blue arrow). (I) Postoperative small incision. Vue PACS, v12.2.6, Philips. Tables
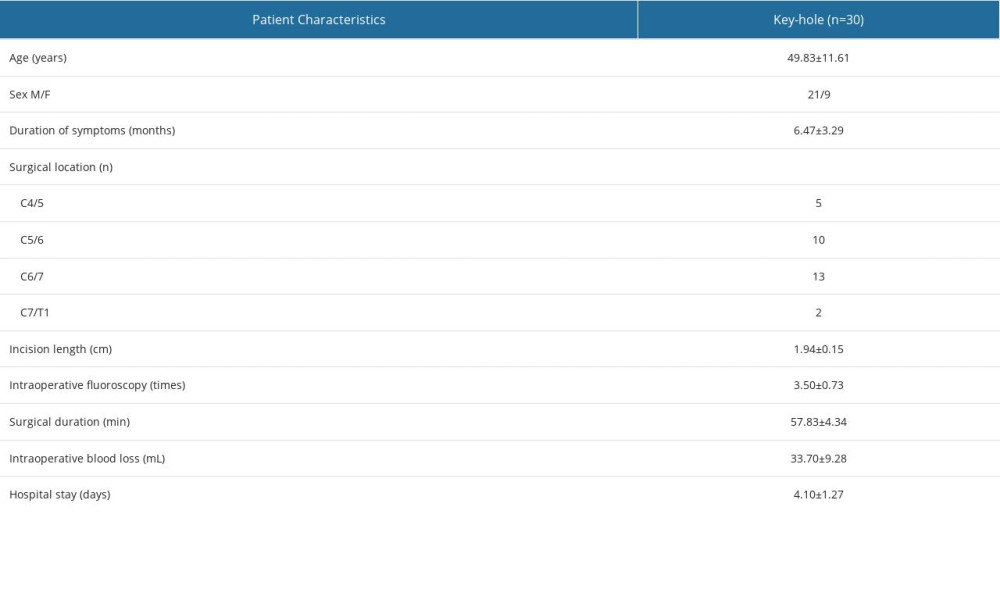 Table 1. Summary of baseline characteristics of patients.
Table 1. Summary of baseline characteristics of patients.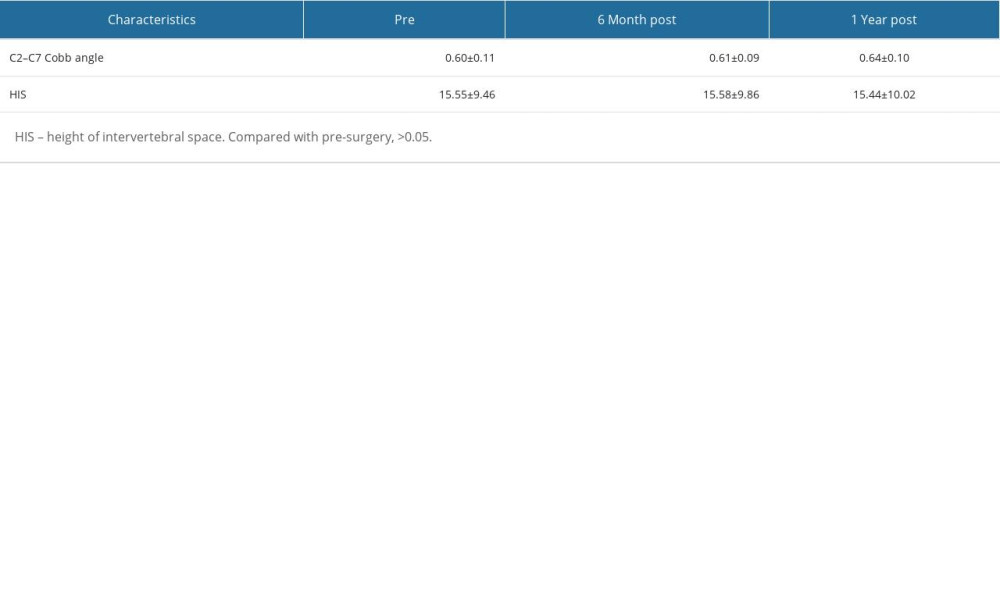 Table 2. Comparison of preoperative and postoperative C2–C7 Cobb angle and height of intervertebral space.
Table 2. Comparison of preoperative and postoperative C2–C7 Cobb angle and height of intervertebral space.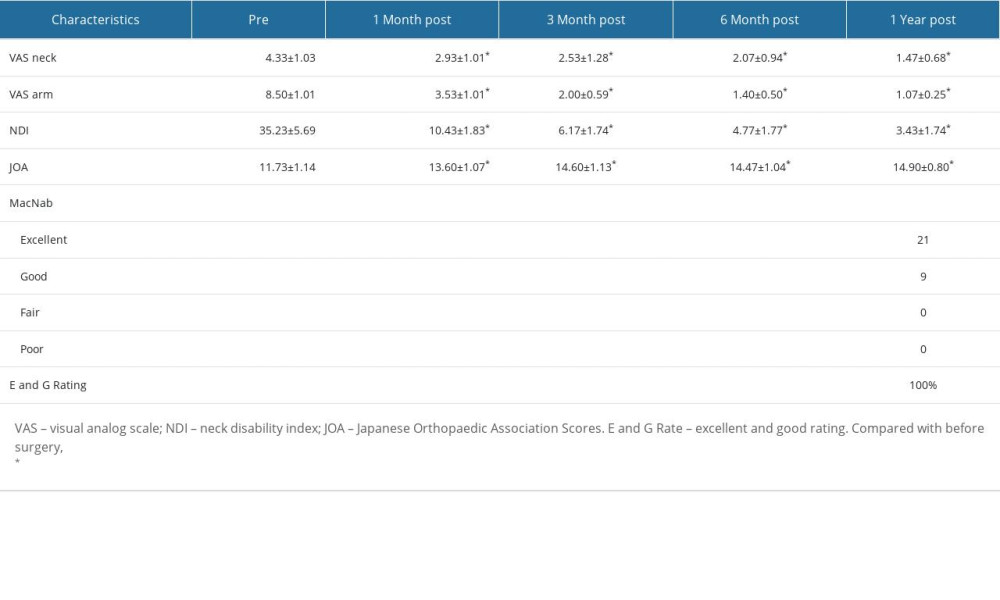 Table 3. Comparison of the preoperative and postoperative functional scores.
Table 3. Comparison of the preoperative and postoperative functional scores. Table 1. Summary of baseline characteristics of patients.
Table 1. Summary of baseline characteristics of patients. Table 2. Comparison of preoperative and postoperative C2–C7 Cobb angle and height of intervertebral space.
Table 2. Comparison of preoperative and postoperative C2–C7 Cobb angle and height of intervertebral space. Table 3. Comparison of the preoperative and postoperative functional scores.
Table 3. Comparison of the preoperative and postoperative functional scores. In Press
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








