15 January 2024: Clinical Research
Enhancement of Levator Ani Muscle Strength in Postpartum Women: The Impact of Pelvic Floor Muscle Training
R.M. Sonny Sasotya1ABCDEFG*, Arnova ReswariDOI: 10.12659/MSM.942758
Med Sci Monit 2024; 30:e942758
Abstract
BACKGROUND: Levator ani muscle injuries during vaginal childbirth can lead to pelvic organ prolapse (POP). Pelvic floor muscle training (PFMT) is an effective conservative approach to alleviate these symptoms. This study aimed to compare outcomes with and without 3 months of PFMT in 34 women with levator ani muscle injury following vaginal delivery.
MATERIAL AND METHODS: In a quasi-experimental study, 34 postpartum women were divided into 2 groups: one received PFMT along with home-based materials and regular follow-ups, while the other served as the control. We measured basal tone and maximal levator ani muscle contraction using the Peritron perineometer and assessed changes after 3 months.
RESULTS: The basal tone and maximal contraction of the levator ani muscle significantly increased following a 3-month intervention period both in PFMT and control group (P=0.0001). The maximal contraction of the levator ani muscle after a 3-month intervention period was significantly higher in PFMT group compared with control group (36.59±1.45 vs 27.76±13.35, P=0.0001), respectively. A significant positive correlation was found between basal tone and maximal contraction (r=0.806, P=0.0001).
CONCLUSIONS: A 3-month PFMT program effectively increased levator ani muscle strength in postpartum women compared to those who did not undergo PFMT.
Keywords: Delivery, Obstetric, Exercise Therapy, Pelvic Floor
Background
Pelvic floor dysfunction (PFD) arises due to the impairment, malfunction, and breakdown of the supportive structures within the pelvic region [1]. This encompasses conditions such as pelvic organs prolapse (POP), discomfort in the pelvic region, difficulties in sexual function, loss of urinary control, and challenges with anal continence [1–3]. The prevalence of PFD in the United States reaches 23.7%, while in Indonesia the prevalence is unclear [4]. The exact cause of PFD remains unclear, with no specific inciting event identified [5]. Multiple factors, including poor evacuation techniques, lifestyle habits such as avoiding urination or bowel movements, and potential trauma from surgery or obstetrics, are thought to contribute to this condition [5]. The diagnosis of PFD relies on a combination of clinical assessment and supplementary tests [5]. Management of PFD are lifestyle modification, including diet, weight loss, Kegel exercises, and core exercises. Another management is medication, invasive procedure, or even surgery [5]. The incidence of PFD subsequent to natural childbirth is estimated to be 13–36% [6]. During the initial year following childbirth, roughly 40–91% of primiparas experience sexual difficulties, pelvic discomfort, and indications of POP [7]. Resulting from PFD and the postpartum sequelae of pelvic floor organ trauma, these issues contribute to health challenges and a diminished sense of well-being among women [7].
Multiparity is the predominant risk factor linked to the occurrence of POP. In women with multiple childbirths, the initial vaginal delivery is connected with an escalated risk of POP and urinary incontinence [7–9]. Assisted vaginal delivery methods, like forceps or vacuum delivery, substantially elevate the likelihood of PFD, particularly POP, in contrast to unassisted spontaneous vaginal delivery [4,9]. Vaginal childbirth influences the dynamics of the levator ani muscle, even in the absence of any damage to the muscle itself [10]. Additionally, vaginal delivery impacts the width of the genital hiatus, thereby raising the likelihood of developing prolapse of the anterior vaginal wall in the future [11].
Pelvic floor muscle training (PFMT) is a helpful way to manage mild to moderate POP symptoms [12]. PFMT is a primary component of behavioral treatments, often recommended as the initial non-surgical approach for addressing urinary incontinence and overactive bladder symptoms [13,14]. PFMT uses exercises targeting enhancement of pelvic floor muscle strength, power, endurance, and relaxation, or a combination of these factors [13]. A Cochrane review concluded that engaging in PFMT during and after pregnancy is effective in averting symptoms related to PFD (eg, stress urinary and fecal incontinence) [14]. Notably, PFMT offers superior outcomes compared to no intervention or placebos for women dealing with stress, urge, and mixed urinary incontinence [14]. Furthermore, multiple prior investigations have indicated increased pelvic floor muscle strength following PFMT [15–17].
PFMT can enhance pelvic floor muscle function through 2 primary mechanisms. First, rigorous training of these muscles can increase muscle mass, lifting the levator plate by promoting muscle hypertrophy and enhancing the rigidity of the surrounding connective tissue. Second, deliberate contractions before and during moments of heightened intra-abdominal pressure prevent urinary incontinence [9]. Consistent PFMT can decrease the occurrence of urinary incontinence and related PFD, while also increasing the strength and stamina of these muscles within the first year after childbirth [7].
A similar study investigated whether the implementation of PFMT immediately after vaginal delivery could contribute to improved tissue healing. However, the results revealed that a structured program of supervised PFMT, in combination with at-home exercises during the early postpartum period, did not result in a greater reduction in the occurrence of complete levator ani avulsion or a decrease in the levator hiatus area when compared to the natural remission process [18]. The study aimed to compare outcomes with and without 3 months of PFMT in 34 women with levator ani muscle injury following vaginal delivery.
Material and Methods
ETHICS APPROVAL:
This study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of Hasan Sadikin Hospital, Bandung. Every participant provided written informed consent, and all procedures were conducted according to pertinent guidelines and regulations.
STUDY DESIGN AND PARTICIPANTS:
We conducted a study using a quasi-experimental design, involving 34 women who had undergone spontaneous postpartum deliveries from November 2022 to April 2023 at Hasan Sadikin Hospital. Inclusion criteria consisted of a history of spontaneous vaginal delivery within the past 3 months, age under 40 years, a body mass index below 25 kg/m2, a baby birth weight below 4000 grams, willingness to participate for a 3-month duration, and the absence of comorbidities like diabetes, hypertension, stroke, and obesity. We excluded women diagnosed with complications resulting from vaginal delivery (eg, grade III and IV perineal tears, rectovaginal fistula, and urinary incontinence), as well as those who withdrew from the study.
SAMPLE SIZE AND DATA COLLECTION:
Sample size calculations indicated a requirement of 17 participants per group. Consecutive sampling was utilized, resulting in the enrolment of 34 patients who met the inclusion criteria. To ensure the homogeneity of the study population, we collected information from medical records, including patients’ age, parity, last baby’s birth weight, and history of perineal tears, while BMI was determined during physical examinations [2,19]. The patients were then divided into 2 groups: the experimental group (17 subjects) performed PFMT, and the control group (17 subjects) did not. For both groups, basal tone and maximal levator ani muscle contraction were measured using the Peritron perineometer. After 3 months, the subjects were evaluated for changes in basal tone and maximal levator ani contraction.
EVALUATION OF PFD:
A digital rectal examination was performed to capture data for the Modified Oxford Scale, which was employed to determine the presence and extent of pelvic floor dysfunction [20,21]. The examination was conducted as a preparatory step before the measurement of levator ani muscle strength, ensuring comprehensive evaluation of the pelvic floor’s condition and function.
EVALUATION OF LEVATOR ANI MUSCLE:
We used Peritron perineometer gain basal to and maximal contraction of levator ani to evaluate the levator ani muscle strength. The procedure for evaluating levator ani muscle was adapted from a previous study [20]. Participants were evaluated with empty urinary bladders and bowels. During the examination, they were positioned in the lithotomy posture on gynecological examination chairs. The examination was conducted by a qualified examiner following standardized procedures. The subjects were instructed to contract their pelvic muscles with the guidance “imagine trying to hold in gas or feces.” For assessing basal tone, the subjects were asked to relax their pelvic floor muscles, after which the Peritron probe was gently inserted into the vagina. To measure maximal contraction, the subjects were instructed to contract the pelvic floor muscles for as long as possible.
METHODS OF PFMT:
The procedure of PFMT was adapted from a previous study [13,18]. The experimental group was instructed to independently perform PFMT at home. The initial step in PFMT involved instructing the subjects on how to identify and effectively contract and relax their pelvic floor muscles. This instruction was facilitated using verbal feedback based on digital assessment. Following enrolment, the subjects were instructed to independently perform PFMT at home for a duration of 3 months (3 sets of 8–12 contractions per day) [18]. Although supervised PFMT is considered ideal, we aimed to mitigate this limitation by providing study participants with verbal education, informative brochures, and instructional videos on conducting PFMT independently at home. To ensure compliance, we also distributed logbooks to track and document PFMT activities every 2 weeks.
EVALUATION OF TREATMENT:
The decision to use a 3-month period aligns with the study design from previous research [9]. After this 3-month experimental period, we assessed changes in basal tone and maximal levator ani contraction following the interventions.
STATISTICAL ANALYSIS:
We used SPSS version 25.0 (IBM, USA) for Windows to analyze the data. For patients’ age, IMT, last baby birth weight, basal tone, and maximal levator ani contraction were treated as continuous variables, presented as mean±SD and median (min–max). Parity and history of perineal tears wee categorical variables, presented as frequency and percent. Bivariate analyses were performed to compare the basal tone before and after the experimental period using a
Results
CHARACTERISTICS OF THE STUDY PARTICIPANTS:
The study subjects’ characteristics encompassed factors such as age, parity, body mass index (BMI), baby birth weight, and perineal tears, as illustrated in Table 1. Among the 34 women, the average age was 25.29±5.37 years. Regarding parity, there were 15 primiparous women (44.1%) and 8 women (23.5%) with parity greater than 3. The average BMI was 22.97±1.98 kg/m2, while the mean baby birth weight was 2876.18±414.14 grams. Most subjects (67.6%) had no history of perineal tears.
BASAL TONE:
The comparison of basal tone before and after 3 months of treatment PFMT group and in the control group are presented in Table 2. In the PFMT group, there was a significant increase in basal tone from the initial measurement (30.72±1.96) to the measurement taken 3 months after treatment (32.86±2.20) (P=0.003). Similarly, the control group also demonstrated a significant increase in basal tone from the initial measurement (30.98±1.81) to the measurement obtained 3 months after the treatment period (25.65±12.33) (P=0.003).
MAXIMAL LEVATOR ANI CONTRACTION:
Table 2 illustrates the comparisons of maximal levator ani muscle contractions before and after 3 months of treatment in both the PFMT and control groups. In the PFMT group, a significant enhancement in maximal levator ani contraction was observed, increasing from the initial measurement (33.76±2.23) to the measurement taken 3 months after treatment (36.59±1.45) (P=0.001). Similarly, in the control group, a significant increase in maximal levator ani contraction was noted, with measurements rising from the initial value (34.20±2.20) to the value obtained 3 months after treatment (27.76±13.35) (P=0.001).
EXPERIMENTAL GROUP VS CONTROL GROUP:
We assessed and compared maximum levator ani muscle contraction after 3 months in both groups. The results revealed a significantly higher maximal levator ani muscle contraction in the PFMT group compared to the control group (36.59±1.45 vs 27.76±13.35, respectively, P<0.05), as presented in Table 3. The Pearson correlation test demonstrated a positive correlation between basal tone and maximal levator ani contraction (r=0.806, P=0.0001), signifying that a stronger basal tone corresponded to a more robust maximal contraction.
Discussion
We studied 34 postpartum women divided into the PFMT group and the control group. To ensure the homogeneity of the study population, we confirmed that there were no differences in baseline characteristics such as age, parity, body mass index (BMI), baby birth weight, and perineal tears. We found that both the PFMT and control groups exhibited increased maximal contraction of the levator ani muscle after a 3-month intervention. Importantly, the PFMT group exhibited a significantly higher maximal contraction of the levator ani muscle at 3 months after treatment when compared to the control group. In contrast to our findings, a different outcome was reported in a similar study conducted by Hilde et al, which found that, even though there was a reduction in levator ani muscle changes in both the PFMT group and the control group, the difference between the 2 groups was not statistically significant [18].
Following implementation of the 3-month experimental period, we observed substantial improvements in the basal tone and maximal contraction of the levator ani muscle in the PFMT group. Notably, the basal tone increased from 30.72±1.96 to 32.86±2.20 cmH2O (
A key finding was that the PFMT group had significantly higher maximal levator ani muscle contraction compared to the control group after the 3-month experimental period (36.59±1.45 vs 27.76±13.35, respectively,
Moreover, a positive correlation was observed between basal tone and maximal levator ani muscle contraction (r=0.806,
Our study has several limitations: 1) PFMT sessions were not supervised by experienced trainers; 2) Lack of information related to the participants’ delivery, including duration of the second stage of labor and the baby’s head circumference; 3) Absence of imaging techniques, such as ultrasound and MRI, to assess pelvic floor defects; 4) Relatively short intervention duration. These limitations provide valuable insights for designing future research to address these aspects more comprehensively.
Conclusions
The 3-month PFMT program effectively increased levator ani muscle strength in postpartum women compared to those who did not undergo PFMT.
Tables
Table 1. Comparison of study participants’ characteristics between postpartum women in the PFMT and control groups.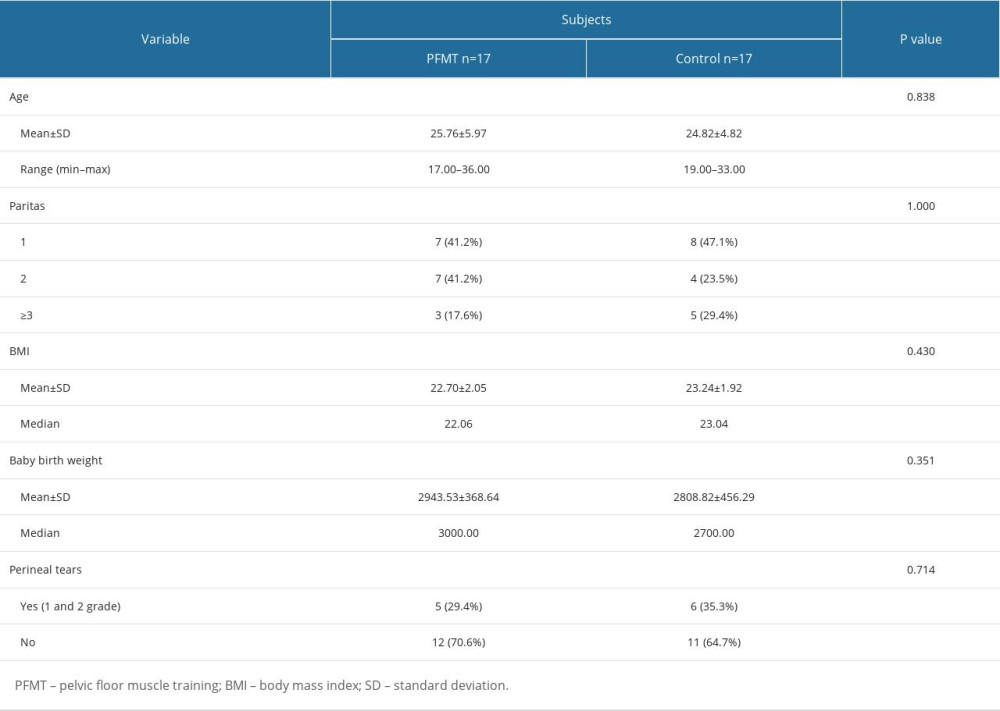 Table 2. Comparison of levator ani muscle strength changes before and after a 3-month intervention in PFMT vs control group.
Table 2. Comparison of levator ani muscle strength changes before and after a 3-month intervention in PFMT vs control group.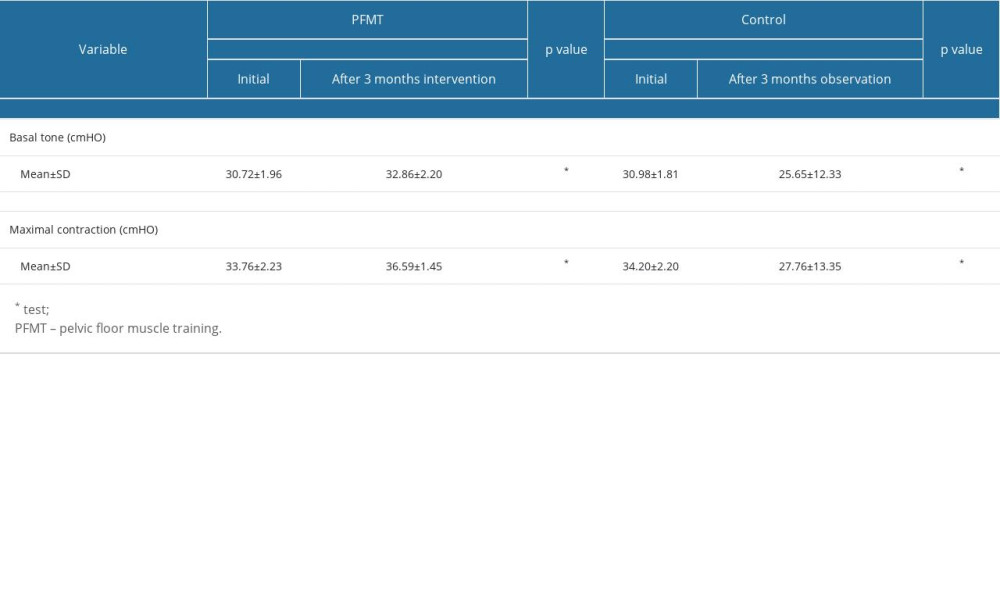 Table 3. Maximal levator ani muscle contraction after 3 months intervention in PFMT vs Control groups.
Table 3. Maximal levator ani muscle contraction after 3 months intervention in PFMT vs Control groups.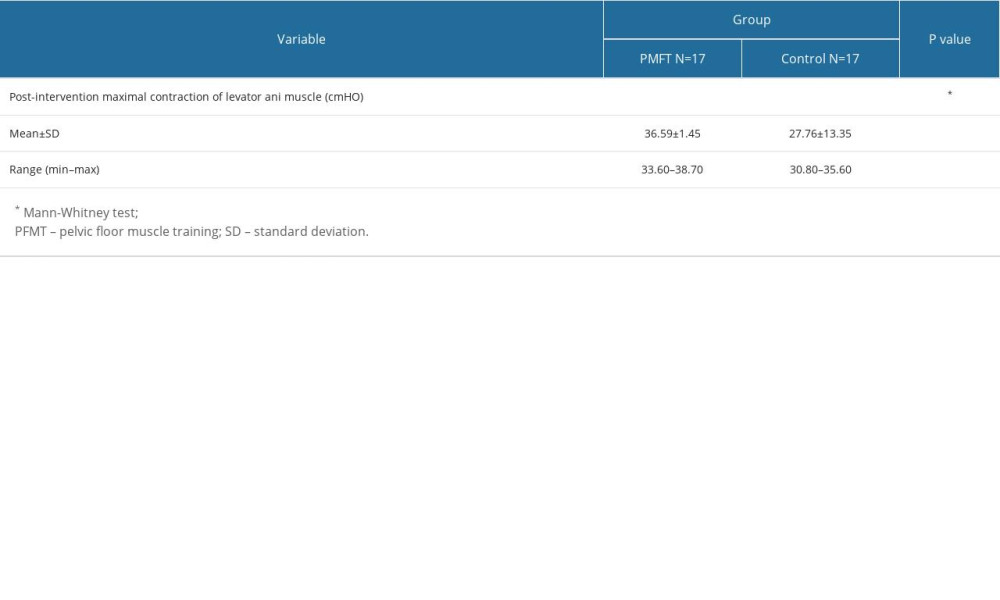
References
1. Pan H, Bao Y, Cao H, The effectiveness of magnetic stimulation for patients with pelvic floor dysfunction: A systematic review and meta-analysis: Heortology and Urodynamics, 2018; 37(8); 2368-81
2. Blomquist JL, Carroll M, Muñoz A, Handa VL, Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery: Am J Obstet Gynecol, 2020; 222(1); 62e1-e8
3. Jacomo RH, Nascimento TR, Lucena Da Siva M, Exercise regimens other than pelvic floor muscle training cannot increase pelvic muscle strength – a systematic review: J Bodywork Mov Ther, 2020; 24(4); 568-74
4. Hallock JL, Handa VL, The epidemiology of pelvic floor disorders and childbirth: Obstet Gynecol Clin North Am, 2016; 43(1); 1-13
5. Grimes WR, Stratton M, Pelvic floor dysfunction: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing [cited 2023 Nov 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK559246/
6. Van Delft K, Sultan A, Thakar R, Schwertner-Tiepelmann N, Kluivers K, The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction: BJOG, 2014; 121(9); 1164-72
7. Sigurdardottir T, Steingrimsdottir T, Geirsson RT, Can postpartum pelvic floor muscle training reduce urinary and anal incontinence?: Am J Obstet Gynecol, 2020; 222(3); 247e1-e8
8. Dietz HP, Shek KL, Chantarasorn V, Langer SEM, Do women notice the effect of childbirth-related pelvic floor trauma?: Aust NZJ Obstet Gynaecol, 2012; 52(3); 277-81
9. Van Delft K, Thakar R, Sultan A, The natural history of levator avulsion one year following childbirth: A prospective study: BJOG, 2015; 122(9); 1266-73
10. Kearney R, Miller JM, Ashton-Miller JA, DeLancey JOL, Obstetric factors associated with levator ani muscle injury after vaginal birth: Obstet Gynecol, 2006; 107(1); 144-49
11. Kamisan Atan I, Gerges B, Shek K, Dietz H, The association between vaginal parity and hiatal dimensions: A retrospective observational study in a tertiary urogynaecological centre: BJOG, 2015; 122(6); 867-72
12. Wang T, Wen Z, Li M, The effect of pelvic floor muscle training for women with pelvic organ prolapse: A meta-analysis: Int Urogynecol J, 2022; 33(7); 1789-801
13. Cho ST, Kim KH, Pelvic floor muscle exercise and training for coping with urinary incontinence: J Exerc Rehabil, 2021; 17(6); 379-87
14. Woodley SJ, Lawrenson P, Boyle R, Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women: Cochrane Database Syst Rev, 2020; 5(5); CD007471
15. Alves FK, Riccetto C, Adami DBV, A pelvic floor muscle training program in postmenopausal women: A randomized controlled trial: Maturitas, 2015; 81(2); 300-5
16. Ouchi M, Kato K, Gotoh M, Suzuki S, Physical activity and pelvic floor muscle training in patients with pelvic organ prolapse: A pilot study: Int Urogynecol J, 2017; 28(12); 1807-15
17. Liu YJ, Ting SWH, Hsiao SM, Efficacy of bio-assisted pelvic floor muscle training in women with pelvic floor dysfunction: Eur J Obstet Gynecol Reprod Biol, 2020; 251; 206-11
Tables
 Table 1. Comparison of study participants’ characteristics between postpartum women in the PFMT and control groups.
Table 1. Comparison of study participants’ characteristics between postpartum women in the PFMT and control groups. Table 2. Comparison of levator ani muscle strength changes before and after a 3-month intervention in PFMT vs control group.
Table 2. Comparison of levator ani muscle strength changes before and after a 3-month intervention in PFMT vs control group. Table 3. Maximal levator ani muscle contraction after 3 months intervention in PFMT vs Control groups.
Table 3. Maximal levator ani muscle contraction after 3 months intervention in PFMT vs Control groups. In Press
15 Mar 2024 : Clinical Research
Impact of One-Lung Ventilation on Oxygenation and Ventilation Time in Thoracoscopic Heart Surgery: A Compar...Med Sci Monit In Press; DOI: 10.12659/MSM.943089
14 Mar 2024 : Clinical Research
Differential DHA and EPA Levels in Women with Preterm and Term Births: A Tertiary Hospital Study in IndonesiaMed Sci Monit In Press; DOI: 10.12659/MSM.943895
15 Mar 2024 : Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial FibrillationMed Sci Monit In Press; DOI: 10.12659/MSM.943526
09 Apr 2024 : Clinical Research
Correlation between Thalamocortical Tract and Default Mode Network with Consciousness Levels in Hypoxic-Isc...Med Sci Monit In Press; DOI: 10.12659/MSM.943802
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








