07 February 2024: Clinical Research
Predicting Severity in Hypertriglyceridemia-Induced Acute Pancreatitis: The Role of Neutrophils, Calcium, and Apolipoproteins
Ying-qiu Hu1DE, Xia Tao1B, Hai-bo Wu1E, Wu-gen Li2D, Ding-yi Chen1F, Yuan-fei Liu1G, Shao-yu Tao1F, Kai-yang Wang1A*DOI: 10.12659/MSM.942832
Med Sci Monit 2024; 30:e942832
Abstract
BACKGROUND: Hypertriglyceridemia-induced acute pancreatitis (HTG-AP), representing 10% of all acute pancreatitis cases, is characterized by younger onset age and more severe progression, often leading to higher ICU admission rates. This condition poses a significant challenge due to its rapid progression and the potential for severe complications, including multiple organ failure. HTG-AP is distinct from other forms of pancreatitis, such as those caused by cholelithiasis or alcohol, in terms of clinical presentation and outcomes. It's essential to identify early markers that can predict the severity of HTG-AP to improve patient management and outcomes.
MATERIAL AND METHODS: This study divided 127 HTG-AP patients into mild acute pancreatitis (MAP, n=71) and moderate-to-severe acute pancreatitis (MSAP/SAP, n=56) groups. Blood biological indicators within the first 24 hours of admission were analyzed. Risk factors for HTG-AP progression were determined using binary logistic regression and ROC curves.
RESULTS: Elevated levels of HCT, NLR, TBI, DBI, AST, Cre, and AMS were noted in the MSAP/SAP group, with lower levels of LYM, Na⁺, Ca²⁺, ApoA, and ApoB compared to the MAP group (p<0.05). NEUT%, Ca²⁺, ApoA, and ApoB were significantly linked with HTG-AP severity. Their combined ROC analysis yielded an area of 0.81, with a sensitivity of 61.8% and specificity of 90%.
CONCLUSIONS: NEUT%, Ca²⁺, ApoA, and ApoB are significant risk factors for progressing to MSAP/SAP in HTG-AP. Their combined assessment provides a reliable predictive measure for early intervention in patients at risk of severe progression.
Keywords: Apolipoprotein A-I, Apolipoprotein B (3304-3317), Calcium, Hypertriglyceridemia, Neutrophils, Pancreatitis
Background
The most common cause of acute pancreatitis (AP) is cholelithiasis. In recent years, the number of patients with pancreatitis caused by hypertriglyceridemia (HTG) has increased year by year. It is reported that acute pancreatitis caused by HTG has risen to 10% of all cases of pancreatitis, and has become the third-leading pathogenic factor after cholelithiasis and alcohol in countries outside China [1,2]. Compared with other causes of AP, HTG-AP has a younger onset age, faster progression, and a higher proportion of patients who need to be admitted to the intensive care unit (ICU) [3,4]. In our clinical work, we found that patients with HTG-AP may have mild symptoms at the onset of the disease, but they can quickly progress to severe disease, with multiple organ failure. Wenhua He [5] found higher proportions of pancreatic necrosis, infection, and organ failure in patients with HTG-AP than in the non-HTG-AP group. The severity in patients with HTG-AP was significantly higher than in those with biliary AP or alcoholic AP, and systemic complications were significantly higher in patients with HTG-AP than in those with biliary AP [6]. Early prediction of whether a patient will develop severe disease could help provide intervention as soon as possible and reduce the possibility of the patient developing severe disease.
Therefore, in this retrospective study, we reviewed and collected the data of 127 patients with HTG-AP in our hospital, analyzed the blood biological indicators within 24 h of admission, and analyzed the possible risk factors for progressing to severe of HTG-AP.
Material and Methods
MATERIAL AND CRITERIA:
This was a retrospective study. In our hospital electronic medical record database, we accessed information on 225 patients with HTG-AP diagnosed in the Second Affiliated Hospital of Nanchang University from October 2018 to July 2023. The inclusion criteria were:
GROUPING:
The patients were divided into 2 groups, MAP and MSAP/SAP, based on the final outcome of the progression. The classification of MAP, MSAP, and SAP were according to the Atlanta classification and definition revised in 2012 [7], in which: MAP is defined as no local or systemic complications and organ failure; MSAP is defined as local or systemic complications or transient organ failure (<48 h), or both; and SAP is defined as local or systemic complications and persistent single or multiple organ failure (>48 h). According to the final outcome of pancreatitis, patients were divided into the MAP group (n=71) and the MSAP/SAP group (n=56).
OBSERVATION INDICATORS:
We recorded data on general patient characteristics, including: age, sex, time from abdominal pain to hospital visit, history of pancreatitis, hyperlipidemia, diabetes, and hypertension. Hematological indicators within 24 h of admission included: WBC, PLT, HCT, percentage of neutrophils (NEUT%), absolute value of neutrophils (NEUT), absolute value of lymphocytes (LYM), and ratio of neutrophils to lymphocytes (NLR). Biochemical indicators were: total protein (TP), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea nitrogen (UREA), creatinine (Cre), blood sodium (Na+), blood calcium (Ca2+), blood glucose (GLU), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), non-high-density lipoprotein (NHDL), apolipoprotein A (ApoA), apolipoprotein B (ApoB), apolipoprotein E (ApoE), lipoprotein a (LPa), amylase (AMS), and lipase (LIP).
STATISTICAL METHODS:
SPSS 25 statistical software was used for data analysis and graphing, and the K-S method was used for all normally distributed data. The measurement data conforming to normal distribution was expressed in (X±s), and independent sample
Results
GENERAL CLINICAL CHARACTERISTICS:
The average age of the 127 patients was 38.7 years, with a minimum age of 17 years and a maximum age of 68 years. Male patients accounted for 80%. Among all, 36.2% had a history of pancreatitis, 36.2% had a history of hyperlipidemia, 25.3% had a history of diabetes, and 11.1% had a history of hypertension. The indicator of WBC, NEUT%, TC, TG, ApoE, GLU, AMS, and LIP in HTG-AP patients were higher than the normal reference range, while LYM, Na+, HDL, ApoA, and ApoB were lower than the normal reference range.
There was no significant difference between the MSAP/SAP group and MAP group in age, sex, time from abdominal pain to hospital visit, history of pancreatitis, history of hyperlipidemia, history of diabetes, history of hypertension, WBC, PLT, NEUT, TP, AP, IBIL, ALT, ALP, UREA, TC, TG, HDL, LDL, NHDL, ApoE, LPa, GLU, LIP (P>0.05). HCT, NLR, TBI, DBI, AST, Cre, and AMS in the MSAP/SAP group were higher than those in the MAP group, while LYM, Na+, Ca2+, ApoA, and ApoB were lower than in the MAP group (P<0.05) (Table 1).
BINARY LOGISTIC REGRESSION ANALYSIS:
Binary logistic univariate regression analysis showed that HCT (OR=1.11, 95% CI: 1.027, 1.199, P=0.009), NEUT% (OR=1.09, 95% CI: 1.035, 1.148, P=0.001), and Cre (OR=1.017, 95% CI: 1.005, 1.030, P=0.005) were independent risk factors for developing severe HTG-AP, while LYM (OR=0.666, 95% CI: 0.447, 0.991, P=0.045), Ca2+ (OR=0.025, 95% CI: 0.004, 0.142, P<0.001), ApoA (OR=0.271, 95% CI: 0.088, 0.834, P=0.023), and ApoB (OR=0.189, 95% CI: 0.065, 0.55, P=0.002) were independent protective factors against developing severe HTG-AP (Table 2). We selected HCT, NEUT%, Cre, LYM, Ca2+, ApoA, ApoB, and other parameters with P<0.05 in the binary logistic univariate regression analysis to carry out the binary logistic multivariate regression analysis. The results showed that NEUT% (OR=1.084, P=0.04, 95% CI: 1.004, 1.171), Ca2+ (OR=0.059, P=0.005, 95% CI: 0.008, 0.417), ApoA (OR=0.259, P=0.037, 95% CI: 0.073, 0.92), and ApoB (OR=0.291, P=0.049, 95% CI: 0.085, 0.994) are related to developing severe HTG-AP. The higher the NEUT%, the greater the possibility of developing severe pancreatitis, and a lower NEUT% is associated with less severity. Lower Ca2+, ApoA, and ApoB were associated with higher risk of developing severe pancreatitis, whereas higher Ca2+, ApoA, and ApoB were associated with less risk of developing severity disease (Table 3).
ROC CURVE:
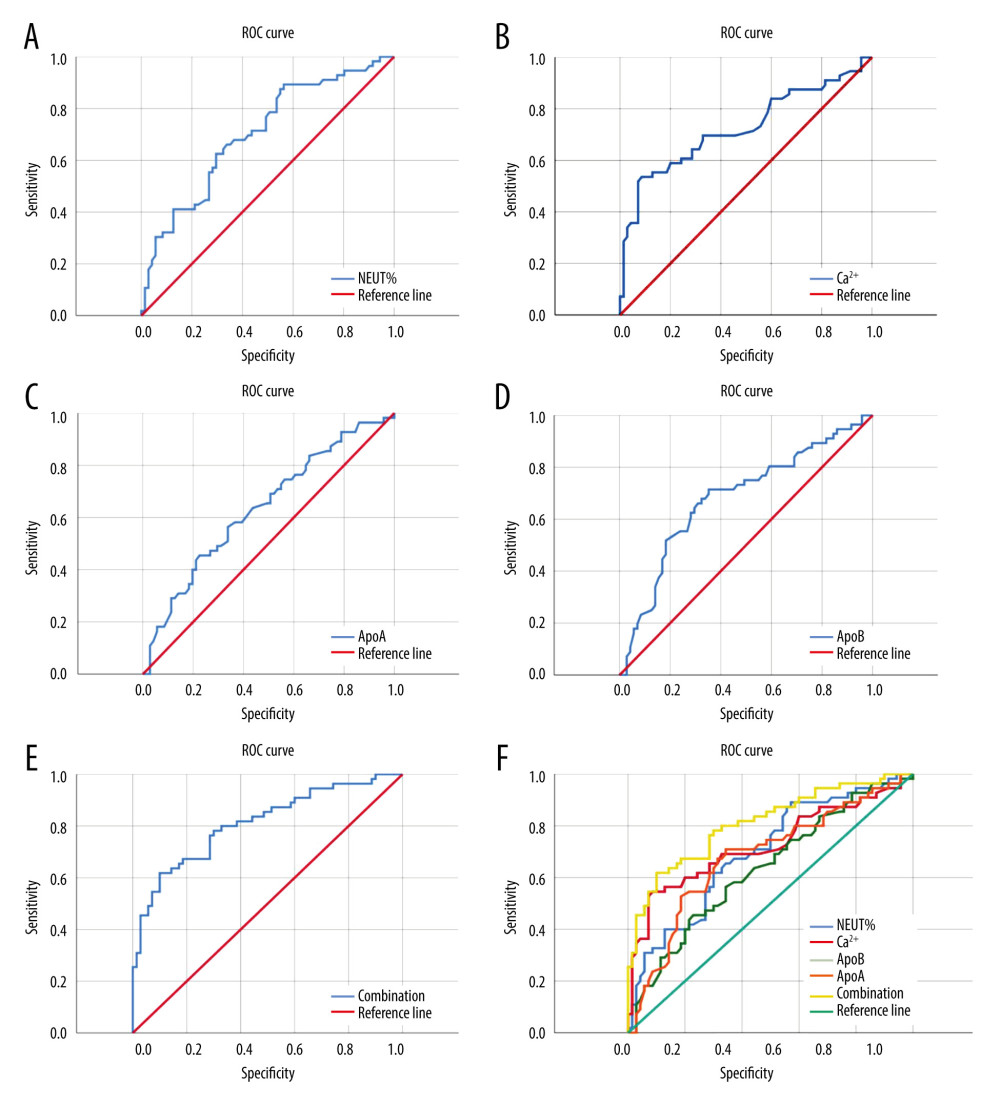
The ROC curve of predictive efficacy of NEUT%, Ca2+, ApoA, and ApoB for the development of MSAP/SAP in patients with HTG-AP showed that the areas under the curve of NEUT%, Ca2+, ApoA, and ApoB predicting progression to MSAP/SAP were 0.70, 0.73, 0, 63, and 0.68, respectively, with their cutoff values of 78.55%, 2.175 mmol/L, 0.705 umol/L, and 0.495 umom/L, respectively, with sensitivity of 89.3%, 53.6%, 45.5%, and 71.4%, and specificity of 43.7%, 91.4%, 77.5%, and 64.8%, respectively. The combined prediction of NEUT%, Ca2+, ApoA, and ApoB for developing severe pancreatitis has an area under the ROC curve of 0.81, a sensitivity of 61.8%, and a specificity of 90% (Figure 1, Table 4).
Discussion
To predict progression of AP, clinical studies have used multiple scoring systems, imaging scores, and biomarkers, such as Ranson score, APACHEII score, CTSI and MCTSI imaging scores, IL-6, and serum Ca2+solubility [10–14]. However, most scoring systems need dynamic observation, and there are too many complex parameters to be included, and the clinical practicability is poor. The prediction accuracy of single biomarkers varies greatly, and the impact of pancreatitis of different etiologies on biomarker is also different [15]. The chief characteristic of patients with HTG-AP is the presence of abnormal lipid metabolism. Currently, it is generally believed that the pathophysiological mechanism of HTG-AP is related to excessive TG and the increase in free fatty acids produced after its metabolism [16]. Therefore, the level of TG is considered to be the main risk factor for whether HTG-AP will develop into severe illness. The incidence of severe illness, organ injury, and mortality in patients with high TG are higher than those in patients with low TG [17,18]. However, some studies have found that there is no significant correlation between TG and the severity of HTG-AP. For example, Hutchison et al [19] reported that there is no significant correlation between Ranson’s score and the TG level of HTG-AP patients. Wang et al [20] used 2648 mg/dL as the TG threshold, and divided 144 HTG-AP patients into high TG and low TG groups, finding no statistically significant difference in the length of ICU hospitalization or mortality in the 2 groups. They also confirmed that TG, TC, and progression to severe HTG-AP were not correlated, but they found that ApoA and ApoB were correlated with progressing to severe HTG-AP, and the higher ApoA and ApoB are, the less likely patients are to develop severe disease. Perhaps because HTG levels deplete apolipoproteins and lead to lower levels of apolipoproteins, the results showed that ApoA and ApoB were associated with the progression of a patient’s disease severity, but not with TG.
ApoA is considered to be the main protein component of HDL [21]. It has a vital role in reverse cholesterol transport and maintaining cellular cholesterol homeostasis. It is also deemed a protective lipid particle in preventing atherosclerosis, which can potentially reduce the risk of cardiovascular diseases [22]. With the study of ApoA, we have discovered that it has functions that are not limited to cardiovascular protection alone, but has multiple roles in immune and inflammatory responses. ApoA can reduce inflammation by modifying the accumulation of cholesterol in lymphocytes, as well as their activation and proliferation, resulting in improved effectiveness of the T-regulatory response in the lymph nodes [23,24]. Additionally, ApoA can act as an anti-inflammatory agent by impeding the interaction between monocytes and T cells, and disrupting the signaling process between them, thus reducing the production of TNF-α and IL-1β. ApoA can suppress the inflammatory function of monocytes present in peripheral blood mononuclear cells that have been activated through antigen or lectin stimulation [25]. The development of severe HTG-AP may be related to the loss of anti-inflammatory function caused by low ApoA, and timely correction of low ApoA may slow progression of the disease.
ApoB is the major structure of low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), and very low-density lipoprotein (VLDL) [21]. ApoB is thought to promote inflammation in the body. ApoB can enhance the ingress of lipoproteins into the wall of blood vessels and encourage macrophage phagocytosis, thus triggering inflammation [26]. In the context of rheumatoid arthritis, ApoB can aggravate the inflammatory response [27]. Our study found a negative correlation between ApoB and progression to severe disease in patients, and ApoB appears to have anti-inflammatory properties similar to ApoA in HTG-AP. However, further investigation is needed to elucidate the function of ApoB.
The ROC curve showed that in patients with HTG-AP, the specificity of NEUT% for the development of MSAP/SAP in patients with HTG-AP was lower, and the sensitivity of Ca2+ and ApoA were lower also. However, ApoB seems to be a reliable predictor of the likelihood of developing MSAP/SAP in patients with HTG-AP, both in sensitivity (61.8%) and specificity (90%). The combined prediction value of NEUT%, Ca2+, ApoA, and ApoB is the best. Therefore, in clinical work, when the initial blood test indicators of patients show NEUT%>78.55%, Ca2+ <2.175 mmol/L, ApoA <0.705 umol/L, and ApoB <0.495 umol/L, they may be developing severe illness. ApoB seems to be a reliable predictor for the likelihood of developing MSAP/SAP in patients.
Conclusions
Most HTG-AP patients are male, with a younger age of onset, and they often have a history of diabetes and hyperlipidemia. The indicator of WBC, NEUT%, TC, TG, ApoE, GLU, AMS, and LIP in HTG-AP patients are higher than the normal reference range, while LYM, Na+, HDL, ApoA, and ApoB are lower than the normal reference range.TG may be only an initiating factor for HTG-AP, and its level has no correlation with whether HTG-AP becomes severe. Univariate analysis showed that HCT, NEUT%, LYM, Cre, Ca2+, ApoA, and ApoB were associated with progressing to MSAP/SAP of HTG-AP, but in multivariate analysis, only NEUT%, Ca2+, ApoA, and ApoB were found to be associated with progressing to MSAP/SAP of HTG-AP. Compared with NEUT%, Ca2+, and ApoA, ApoB seems to be a reliable predictor of the likelihood of developing MSAP/SAP in patients with HTG-AP. The combination of NEUT%, Ca2+, ApoA, and ApoB can predict whether a patient will develop severe HTG-AP.
Tables
Table 1. General clinical characteristics of MSAP/SAP and MAP groups.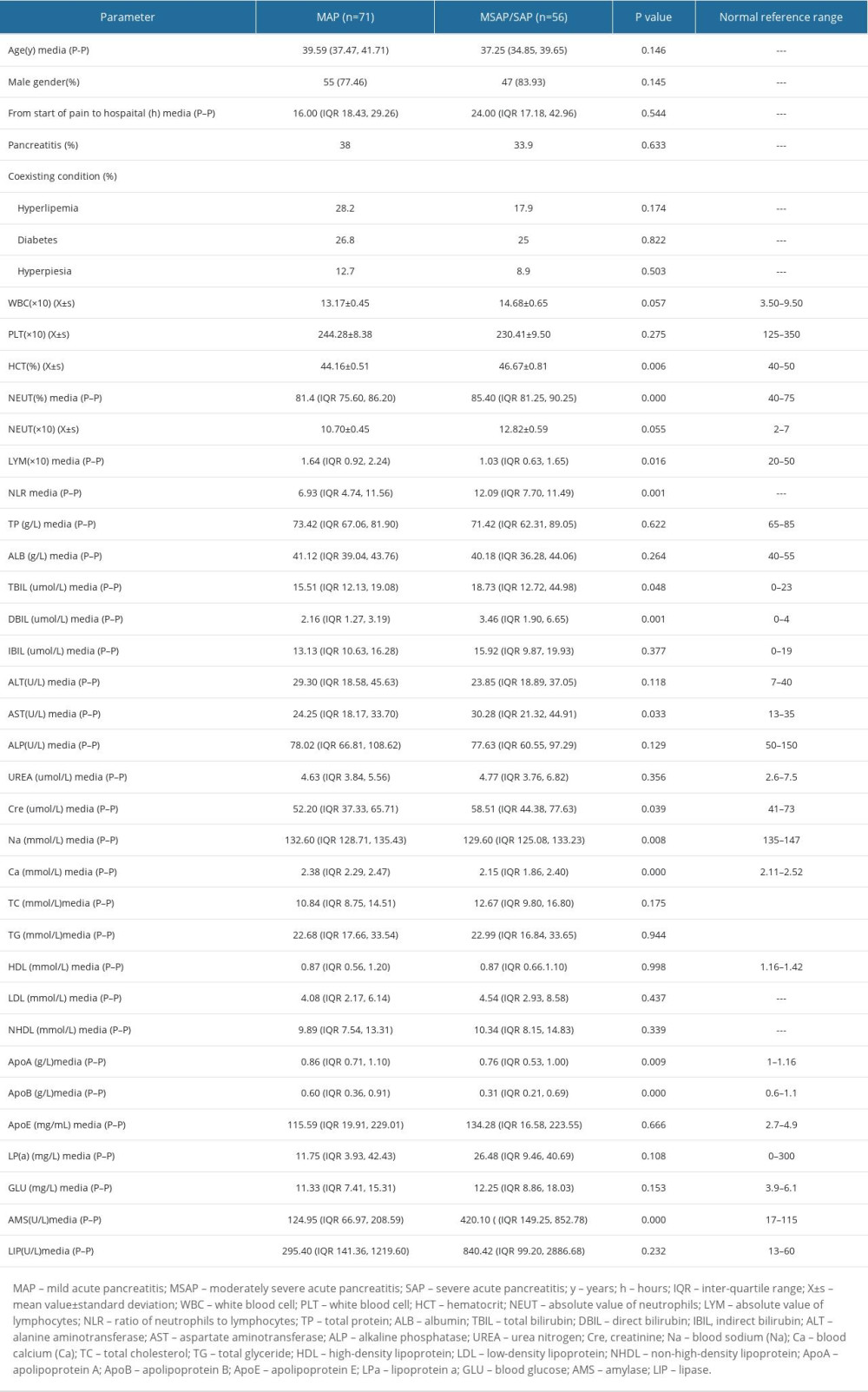 Table 2. Binary logistic single factor regression analysis.
Table 2. Binary logistic single factor regression analysis.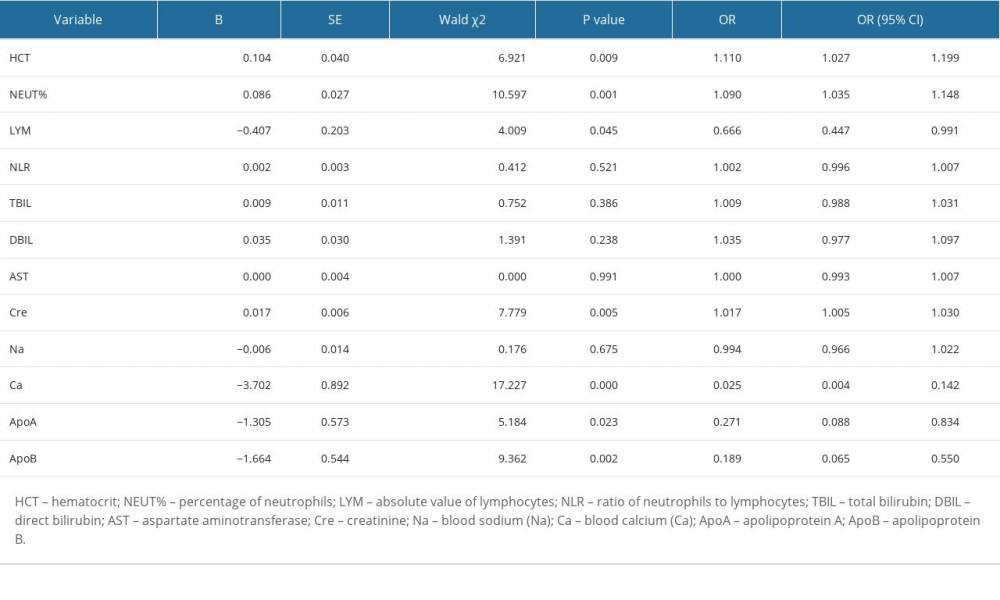 Table 3. Binary logistic multifactor regression analysis.
Table 3. Binary logistic multifactor regression analysis.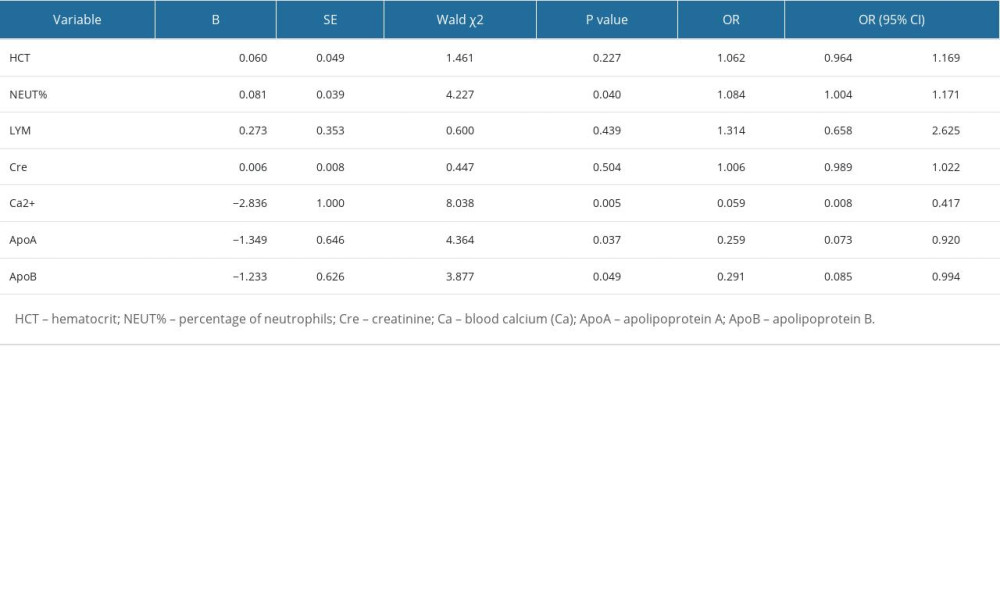 Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP.
Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP.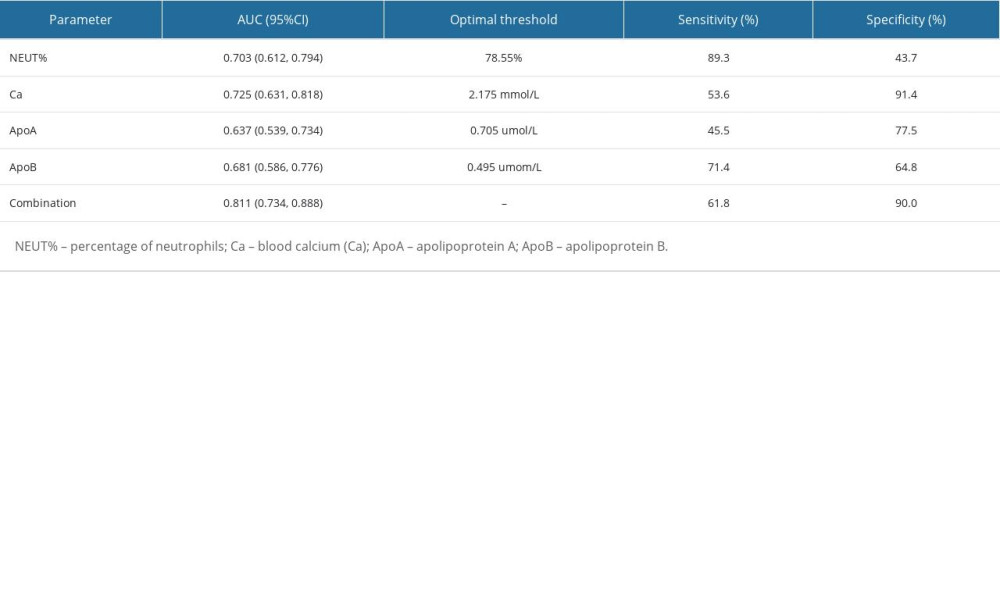
References
1. Winslet M, Hall C, London NJ, Neoptolemos JP, Relation of diagnostic serum amylase evels to aetiology and severity of acute pancreatitis: Gut, 1992; 33; 982-86
2. Nathyros VG, Giouleme OI, Nikolaidis NL, Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis: J Clin Gastroenterol, 2002; 34(4); 472-75
3. Deng LH, Xue P, Xia Q, Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis: World J Gastroenterol, 2008; 14(28); 4558-61
4. Bosques-Padilla FJ, Vazquez-Elizondo G, Gonzalez-Santiago O, Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome: Am J Med Sci, 2015; 349(3); 206-11
5. He W, Zhu Y, Zhu Y, Comparison of severity and clinical outcoms between hypertriglyceridemia pancreatitis and acute pancreatitis due to other causes: Natl Med J China, 2016; 96(32); 2569-72
6. Zhang X-L, Li F, Zhen Y-M, Clinical study of 224 patients with hypertriglyceridemia pancreatitis: Chinese Medical Journal, 2015; 128(15); 2044-49
7. Banks PA, Bollen TL, Dervenis C, Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus: Gut, 2013; 62(1); 102-11
8. Pancreatic Surgery Group, Branch of Surgery, Chinese Medical Association guidelines for the diagnosis and treatment of acute pancreatitis (2014): Chinese Journal of Practical Surgery, 2015; 35(1); 4-7
9. , Expert consensus on the diagnosis and treatment of hypertriglyceridemic acute pancreatitis: Chinese General Practice Medicine, 2021; 24(30); 3781-93
10. Ranson JH, Rifkind KM, Roses DF, Prognostic signs and the role of operative management in acute pancreatitis: Surg Gynecol Obstet, 1974; 139(1); 69-81
11. Knaus WA, Draper EA, Wagner DP, APACHE II: A severity of disease classification system: Crit Care Med, 1985; 13(10); 818-29
12. Balthazar EJ, Robinson DL, Megibow AJ, Acute pancreatitis: Value of CT in establishing prognosis: Radiol, 1990; 174(2); 331-36
13. Mortele KJ, Mergo PJ, Taylor HM, Peripancreatic vascular abnormalities complicating acute pancreatitis: Contrast-enhanced helical CT findings: Eur J Radiol, 2004; 52(1); 67-72
14. van den Berg FF, de Bruijn AC, van Santvoort HC, Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis: Pancreatol, 2020; 20(7); 1302-11
15. Kun G, Cheng Q, Zhizhi T, Weiqin L, Evaluation of severity and prediction of severity of acute pancreatitis: Chinese Journal of Practical Internal Medicine, 2021; 41(1); 3-9
16. de Pretis N, Amodio A, Frulloni L, Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management: United European Gastroenterol J, 2018; 6(5); 649-55
17. Anderson F, Thomson SR, Clarke DL, Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes: Pancreatology, 2009; 9; 252-57
18. Chang YT, Chang MC, Su TC, Association of cystic fibrosis transmembrane conductance regulator (CFTR) mutation/variant/haplotype and tumor necrosis factor (TNF) promoter polymorphism in hyperlipidemic pancreatitis: Clin Chem, 2008; 54; 131-38
19. Hutchison B, Collins J, Makar RS, Retrospective analysis of outcomes in patients with acute hypertriglyceridemic pancreatitis treated without therapeutic plasma exchange: Transfusion, 2021; 61; 537-45
20. Wang S-H, Chou Y-C, Shangkuan W-C, Relationship between plasma triglyceride level and severity of hypertriglyceridemic pancreatitis: PLoS One, 2016; 11(10); e0163984
21. Lamarche B, Couture P, Dietary fatty acids, dietary patterns, and lipoprotein metabolism: Curr Opin Lipidol, 2015; 26(1); 42-47
22. Mangaraj M, Nanda R, Panda S, Apolipoprotein A-I: A molecule of diverse function: Indian J Clin Biochem, 2016; 31(3); 253-59
23. Wilhelm AJ, Zabalawi M, Owen JS, Apo lipoprotein A1 modulates regulatory T cells in auto immune LDL r−/−, Apo A 1−/− mice: J Biol Chem, 2010; 285(46); 16158
24. Catapano AL, Pirillo A, Bonacina F, Norata GD, HDL in innate and adaptive immunity: Cardiovasc Res, 2014; 103(3); 372-83
25. Hyka N, Dayer JM, Modoux C, Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes: Blood, 2001; 97; 2381-89
26. Feng M, Rached F, Kontush A, Chapman MJ, Impact of lipoproteins on atherobiology: emerging insights: Cardiol Clin, 2018; 36; 193-201
27. Lee JY, Apolipoprotein B binds to enolase-1 and aggravates infammation in rheumatoid arthritis: Ann Rheum Dis, 2018; 77; 1480-89
Tables
 Table 1. General clinical characteristics of MSAP/SAP and MAP groups.
Table 1. General clinical characteristics of MSAP/SAP and MAP groups. Table 2. Binary logistic single factor regression analysis.
Table 2. Binary logistic single factor regression analysis. Table 3. Binary logistic multifactor regression analysis.
Table 3. Binary logistic multifactor regression analysis. Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP.
Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP. Table 1. General clinical characteristics of MSAP/SAP and MAP groups.
Table 1. General clinical characteristics of MSAP/SAP and MAP groups. Table 2. Binary logistic single factor regression analysis.
Table 2. Binary logistic single factor regression analysis. Table 3. Binary logistic multifactor regression analysis.
Table 3. Binary logistic multifactor regression analysis. Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP.
Table 4. The predictive value of NEUT%, Ca2+, ApoA, ApoB, and their combination for the severity of HTGP. In Press
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952