29 February 2024: Review Articles
Review of the Evaluation of Pulmonary Hypoplasia as an Important Determinant of Clinical Outcomes in Infants with Congenital Diaphragmatic Hernia
Daria KuchnowskaDOI: 10.12659/MSM.943259
Med Sci Monit 2024; 30:e943259
Abstract
ABSTRACT: Pulmonary hypoplasia is one of main causes of neonatal mortality and morbidity in patients with congenital diaphragmatic hernia. With most cases diagnosed prenatally, the emphasis is put on prediction of the severity of this defect. Several attempts are made to reduce the mortality and provide optimal prenatal and postnatal care. Appropriate estimation of risk of pulmonary hypoplasia also provides an important inclusion criterion for prenatal intervention. The main tool used for the detection and prediction of pulmonary hypoplasia is ultrasound, with an increasing number of available formulas to estimate the risk of occurrence of this phenomenon and complication associated with it. For most of the formulas used in this measurement method, the main limitations are either gestational-age dependency or limited research. Other imaging methods used to assess the risk of pulmonary hypoplasia involve magnetic resonance imaging and vascular assessment of affected lungs. The limitation in these remains the limited accessibility. Currently, the most widely used indexes are observed-to-expected lungs-to-head ratio and presence of liver herniation. These are the 2 most commonly used measurement methods, as they are the basis for patient qualification for fetoscopic endoluminal tracheal occlusion. This article aims to review the evaluation of pulmonary hypoplasia or hypoplastic lung disease as an important determinant of clinical outcomes in infants with congenital diaphragmatic hernia. In this review, we emphasize the importance of early prenatal diagnosis of congenital diaphragmatic hernia and present a summary of different methods of prenatal risk assessment of lung hypoplasia in congenital diaphragmatic hernia.
Keywords: Ultrasonography, Hernias, Diaphragmatic, Congenital, Perinatology, Fetoscopy, Fetal Therapies
Background
Congenital diaphragmatic hernia (CDH) is a rare congenital defect that occurs in around 1 in every 3000 to 4000 pregnancies [1]. In this defect, a migration of organs from the abdomen into the thorax takes place because of diaphragm maldevelopment. This phenomenon reduces the volume in the thorax available for the lungs to develop. It results in a lung hypoplasia, a dysfunction where incomplete development of lungs and a low number of alveoli cause a series of postnatal complications [2–4]. The most serious include pulmonary artery hypertension and insufficient ventilation, which both can lead to the death of neonates.
On average, the diagnosis of CDH is made at 24 weeks of gestation; at this point, the assessment of the coexisting malformations is needed as well as a qualification for prenatal intervention and stratification of the risk of postnatal complications [5,6].
There are several imaging methods aimed at stratifying the risk of pulmonary hypoplasia antenatally to improve decision-making processes and planning of patient care postnatally [7]. Over the years, ultrasound has become the most common method to manage cases of CDH. Due to its variety of markers, it allows clinicians to efficiently qualify patients for the fetoscopic endoluminal tracheal occlusion (FETO) procedure as well as to predict postnatal outcomes [8]. Other imaging methods, such as magnetic resonance imaging (MRI), despite its less common use, aim to help with precise diagnosis and aid management [9]. In spite of many methods and markers being available, all of them have substantial limitations.
This article aims to review the evaluation of pulmonary hypoplasia, or hypoplastic lung disease, as an important determinant of clinical outcomes in infants with CDH.
Epidemiology
CDH is diagnosed in approximately 1 in 3000 live births, with the occurrence ranging between 2 to 4 in 10 000 among studies [1,10–13]. The prenatal detection rate reported from 1986 to 2003 was between 52% and 54% [13–15]. More current research suggests that in the recent years this rate has increased to 68% [16]. The same study reported a higher survival rate in a group of CDH cases diagnosed postnatally, which might correlate with a smaller diaphragm defect [16]. One-year survival is reported to be 45%, with isolated CDH having better prognosis than that associated with other defects [11–14,16]. Right-sided hernia, which makes up 10% to 15% of cases of CDH, is most often missed during the scan and diagnosed postnatally [5]. This type of hernia, as well as bilateral (about 1% of cases), is generally associated with worse prognosis when compared with left-sided CDH, at 44% vs 53%, respectively [1,17].
Etiology
With 70% of cases being isolated CDH, the exact cause of this defect remains unknown. Several causes are being investigated, although it seems to be affected by several factors at once [1,18]. With an increasing number of cases being diagnosed in the first trimester, chorionic villus sampling is recommended as a part of diagnosis [17]. The result of genetic testing is further needed when qualifying a patient for FETO in cases of isolated CDH. In the other 10% to 30% of cases, CDH is a part of multi-organ defects that are often accompanied by aneuploidies, such as trisomy 21, 18, or 13, cytogenic rearrangements, or single gene mutations [17,19]. The aforementioned single gene mutations involving GATA4, ZFPM2, NR2F2, and WT1, as well as the retinoid acid pathway, affect not only the development of the diaphragm but also show effect in the development of the heart and lung, independently of the herniation [1].
Pathophysiology
Fetal development of the lungs starts at 4 weeks of gestation, when the primal trachea is formed from the laryngotracheal groove, further forming lobar buds. Till the end of the embryonic stage, which lasts up till week 6 of gestation, the lobar buds are subdividing to form bronchopulmonary segments. Between weeks 6 and 16, the pseudoglandular stage takes place, when the formation of the tracheobronchal tree is occurring. This process is unique to this stage, as in later phases more alveoli can be created; however, past week 16, further division of the tracheobronchal tree is impossible. In the canalicular phase, lasting between weeks 16 and 28 of gestation, the basic unit of the lung is formed: the acinus. The acinus is built of bronchioles, alveolar ducts, sacs, and alveoli and is responsible for gas exchange. Later, it differentiates into pneumocytes type I and type II. In the saccular stage, ending in week 36 of gestation, the airways end with crests, which turn into saccules and then subsaccules, which mature into alveoli. This maturation process promotes a rapid increase in the gas exchange area. Throughout the end of the saccular stage, maturation of pneumocytes type II takes place, providing efficient cells for the production of surfactant [2].
In cases of CDH, during fetal development, malformation of the diaphragm is observed. The most common locations where the loss of continuity is observed are the posterolateral side, Bochdalek hernia, and the anterior part, Morganini hernia. Bochdalek hernia is associated with the respiratory issues at birth, while Morganini hernia is claimed to be most often diagnosed accidentally during childhood [19–21]. With the loss of integrity of the diaphragm, the migration of abdominal organs into the chest cavity occurs. In cases of left-sided hernia, it is the stomach, intestinal loops, and spleen, and in severe cases, the liver, while in right-sided hernia, the liver and intestines are primarily observed in the chest cavity [1].
There are several theories explaining the mechanism of impairment of lung development in fetuses affected by CDH. The primary reason, and the best documented, is the restriction of intrathoracic space in this population [2,3]. With cases being diagnosed as early as the first trimester, in these cases of CDH the development of the tracheobronchal tree is impaired, which further translates into changes observed in the airways of the neonates delivered [2,3,19]. Moreover, the minimization of space for the development of alveoli disrupts the expansion of the gas exchange area by preventing the widening and lengthening of alveoli in the saccular stage [3,4]. The occlusion of trachea and increase of intrapulmonary pressure stimulates the development and maturation of the lungs [2–4].
Ultrasound Assessment
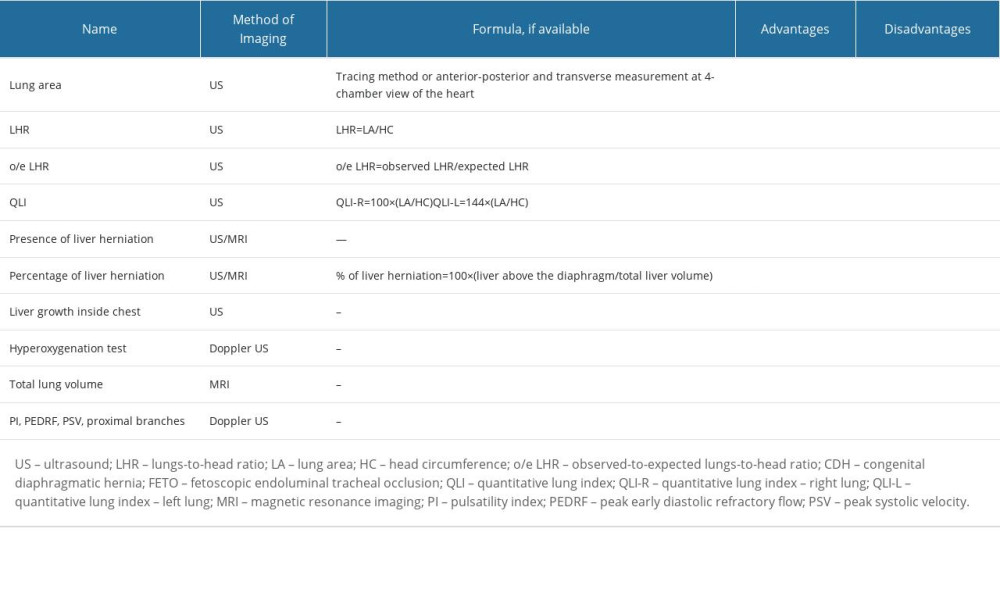
The wide availability, safety, and reproducibility of ultrasound allowed for inventing measurement methods in fetuses with CDH to predict the severity of disease prenatally, with lung area measurements and lungs-to-head ratio (LHR) becoming the most frequently used method of stratification. The lung area calculation includes 3 methods of measurement: anterior-posterior, transverse length, and tracing. They require the view of lungs at the 4 chambers of the heart level. The calculation methods are easily reproducible; however, the tracing method has been proven to provide more accurate results, as the anterior-posterior and transverse measurements often overestimate the area [22]. Although the accuracy of the measurement is not affected by gestational age, there is limited ability for interpretation, as the results are gestational-age dependent [9,22]. Similar to lung area measurement, LHR is also an index that is gestational age-related: both lung area and head circumference grow at a different rate. Moreover, lung area can be affected by a number of factors throughout a CDH pregnancy. Similar to lung area measurement, the tracing method proved to be more accurate rather than diagonal measurements [22–24]. It was shown that LHR <1.0 and liver herniation in this population are strong indicators of postnatal need for extracorporeal membrane oxygenation use, classifying a given case as severe [25]. Although the initial attempt of this method was to avoid gestational age dependency, it has been shown that the most accurate results were obtained in the third trimester [9,22,26].
The observed-to-expected (o/e) LHR aims to overcome this limitation and provide measurement that is independent of gestational age. Here, the observed LHR is the measurement of affected fetuses, while the expected LHR is a mean measurement of healthy fetuses of certain gestational age [24]. It has been observed that in the population of CDH fetuses, the o/e LHR was 39% on average, and in 19% of cases this measurement fell below the 5th percentile. Subsequently, Alfaraj et al observed that in fetuses with an o/e LHR greater than 45%, the survival rate was 100%, therefore providing a strong indicator for prediction of pulmonary hypoplasia for these fetuses [27]. This prediction was better if the ultrasound check was performed at 32 to 33 weeks rather than 22 to 23 weeks [22]. Despite its limitations, the o/e LHR has become the measurement that is used to assess patient eligibility for the FETO procedure and is most frequently used to assess the severity of the condition [8].
Another parameter that is used as an alternative to the o/e LHR is the lung-to-thorax transverse area ratio [28]. Measured as an area of a contralateral lung (tracing method) to the area of the thorax (limited by the ribs, sternum, and vertebrae), this parameter shows the linear correlation of the o/e LHR, with a cut-off value of high mortality at <0.08 [29,30]. Meta-analysis of several prognostic factors of pulmonary hypoplasia in fetuses with CDH shows that the lung-to-thorax transverse area ratio measurement is as precise as the o/e-LHR measurement in prediction of poor postnatal outcomes in this group of patients [31].
The quantitative lung index (QLI-R/L) was introduced as a gestational age-independent index describing lung hypoplasia. Its formula requires measurements of head circumference and lung area either in millimeters or centimeters, with 2 different formulas for each lung [32]. Hislop et al found that the level of hypoplasia in both lungs of neonates were different at birth, and this difference was observed until the discharge of the patients [33]. Research comparing the o/e LHR, QLI, and o/e lung area of the contralateral lung was conducted, showing that despite the advantages of the QLI, the o/e LHR was a sufficient method of predicting lung hypoplasia for fetuses with CDH [34]. In recent studies, lung growth was assessed, and a cut-off value for >1st percentile was proposed as a tool to accurately determine the increased risk for pulmonary hypoplasia [32,35]. Its reproducibility in calculating lung size shows promise as an alternative tool of monitoring lung growth among fetuses after tracheal occlusion. It has been noted that the increase of this index could suggest neonatal survival; however, more studies are needed to confirm this observation [32]. There is an urgent need for new suitable and accurate ultrasound markers for precise patient selection for intrauterine minimally invasive intervention (FETO). So far, most clinicians use the o/e LHR for distinguishing severe congenital diaphragmatic hernia cases with a high risk of pulmonary hypoplasia suitable for the FETO procedure. However, it seems that the new parameters QLI-R and QLI-L may add a valuable quality and more precision in the ultrasound assessment of babies with CDH. Kontopoulos et al showed that within a group of left-sided CDH, a cut-off value of the QLI-R of 0.45 or lower is predicative of neonatal death from pulmonary hypoplasia. This marker also was proposed as a useful alternative to monitor fetal lung growth after tracheal occlusion [32].
Liver herniation, or its absence, has also become a widely used indicator of survival of patients with CDH, with researchers aiming at quantifying the risk by the percentage of liver herniation that is independent of gestational age. Olutoye et al found that the percentage of liver herniation affected the outcomes of fetuses with CDH, mostly those related to pulmonary hypertension [36]. Their research has shown that each 1% increase in liver herniation increases the odds of sildenafil use at discharge by a factor of 1.12 [36]. Nonetheless, this remains a less commonly used method, as the percentage of herniation is subject to measurement inconsistencies. Similarly, Ott et al examined the relations between herniated liver volume and lung development dependent on the side of herniation in their research [37]. They showed that liver growth inside the thoracic cavity adversely affects lung development. This means that monitoring liver growth inside the thoracic cavity throughout gestation might be a useful addition in the decision-making process.
Selected methods of prenatal risk assessment of lung hypoplasia in congenital diaphragmatic hernia are presented in Table 1.
3-D Ultrasonography
To assess the volume of lungs, 3-dimensional ultrasonography was engaged in 2 methods: stacked contour and virtual organ computer-aided analysis measurements. It seems that both methods improve the prediction of lung hypoplasia, especially when compared with estimated fetal weight. Stacked contour proved to be the more effective method; however, both face the same limitations of imaging as 2-dimensional ultrasound, namely oligohydramnios, maternal obesity, and artifacts from bony structures and movements [9].
Doppler Ultrasound
With pulmonary artery hypertension being one of the symptoms of lung hypoplasia, several attempts to identify these patients prenatally have arisen. Despite the fact that the mortality of patients with CDH can be estimated or even predicted by ultrasound markers, the markers seem to have little value in the prediction of pulmonary artery hypoplasia [38]. The vascular assessment of fetal lungs proposed by Sokol et al is to measure the main pulmonary vessel diameter of the ipsilateral branch; the authors showed that there is a correlation with postnatal outcome of the fetus [39].
To identify poorly developing lungs, there are several attempts to assess their vasculature by measuring the pulsatility index, peak early diastolic refractory flow, and peak systolic value in the proximal branches of the contralateral lung. All of these measurements have shown strong correlation of loss in blood perfusion in lungs to o/e LHR; however, there is little data on the validation of these indices [23].
Another attempt to assess lung vasculature and function to predict risk of pulmonary artery hypertension is a hyperoxygenation test. Patients were given a 60% oxygen flow mask, and pulsatility index value in the proximal branches was measured before and after. A decrease of ≥20% in the pulsatility index value was considered a positive result. Non-reactive results indicated a high risk of pulmonary artery hypertension after delivery and poor response to inhaled, with 100% of patients in the trial developing this complication (n=9) of which 77% (n=7) died [40]. A hyperoxygenation test could prove a positive predictive value of neonatal pulmonary artery hypertension, with several further studies supporting the aforementioned thesis and showing that those changes are not observed in cases of healthy lung tissue [40,41]. Small study groups also suggest an urgent need for further research in this field.
Magnetic Resonance Imaging
Despite its not being a primary choice of imaging during pregnancy, MRI allows for accurate measurement of lung volume. With more time needed to perform the examination, its T2 sequence provides multiple slices of lungs, which obtain precise total lung volumetry [42]. In fetuses with a high risk of pulmonary hypoplasia, the ratio of total lung volume to estimated fetal weight is significantly lower than that of healthy fetuses, yet the cut-off value for increased risk of mortality is still known [43]. In the population of fetuses affected by CDH, it has been noticed that higher o/e total lung volume helps to predict respiratory morbidity in neonates [44]. However, this seems unique for this population, as the prediction of lung hypoplasia in other congenital defects has similar results to those provided by an ultrasound. Moreover, a meta-analysis provided by Oluyomi-Obi et al shows that o/e total lung volume has similar prognostic value in predicting the risk of pulmonary hypoplasia to o/e-LHR, with <25% value correlating to significant neonatal mortality (
Due to the high water content in the lungs, the T2-weighted sequence allows for more precise assessment of hypoplastic lungs than does ultrasound [23]. This not only helps in detecting coexisting pulmonary defects, such as sequestration, but also shows promise in the functional assessment of fetal lungs. In a recent study, Sakuma et al suggest that a low o/e lung-to-liver signal intensity ratio might represent the histological characteristics of hypoplastic lungs [46].
While ultrasound is the method of choice for CDH diagnosis with preliminary assessment of the contents of the hernia sac, it can be limited by a number of factors. MRI plays a supplementary role in that diagnosis, as its T1-weighted sequence allows for a more precise outline of the liver, stomach, and intestines. It could further affect diagnosis and management, as more research is focused on quantifying the liver herniation as an independent factor for prediction of poor neonatal outcome [9].
Clinical Implications
With the recent development of prenatal interventions and tracheal occlusion in the population of fetuses with CDH, the need for adequate risk stratification and assessment of eligibility for the procedures has risen. This is particularly important in the era of the
On the other hand, a recent study showed that the decision to continue a pregnancy was based on the severity of congenital diaphragmatic hernia and the risk of postnatal complications. Among 222 patients with CDH diagnosed before 24 weeks of gestation, 28% (n=66) decided to terminate the pregnancy [7]. The factors affecting the decision-making process were not only the presence of the defect, but also the postnatal risk of pulmonary artery hypertension, as well as the presence of additional malformations. Russo et al emphasize that the majority of parents need to obtain immediate and reliable information about the condition at the moment of diagnosis [48]. This further shows the need for the future development and introduction of reproducible and reliable markers for lung hypoplasia.
Intrauterine Interventions
In the era of the TOTAL trial, the adequate qualification for the FETO procedure became a primary step in the management of fetuses with CDH. The severity estimation through the calculation of o/e LHR and the assessment of liver herniation are considered while counseling patients and planning treatment. The procedures FETO-IN and FETO-OUT are performed between 26 and 28 weeks and 33 and 34 weeks, respectively. During FETO-IN, a balloon is inserted percutaneously into the trachea of the fetus causing build-up of lung fluid, which induces airway proliferation. Afterward, between 33 and 34 weeks, the FETO-OUT procedure is performed to reverse the occlusion, which promotes lung maturation [8]. All of these processes combined provide for 7-fold increased odds for survival in cases of severe CDH, when compared with expectant management [1]. The same trend was not observed for moderate cases of CDH, in which the odds were similar between the 2 groups [8].
Future Directions
When discussing the prediction of pulmonary hypoplasia, it is important to focus on the need for a simple, repeatable form of measurement that would detect the risk for pulmonary hypoplasia in fetuses regardless of etiology of that defect. Such promise is shown by the QLI-R/L formula, which could provide fetal medicine specialists with a simple and easily accessible form of predicting the risk of a neonate being affected by lung hypoplasia. However, further research is needed to apply the QLI-R/L formula, not only to qualify for FETO or general management of CDH cases, but also to compare against lung hypoplasia of another origin. Hopefully, some new therapies will be introduced to prevent severe pulmonary hypoplasia in fetuses with CDH. This might be the most promising and clinically relevant future direction of research in CDH.
Conclusions
This review has highlighted the importance of early diagnosis of CDH in fetuses and infants and has emphasized the importance of recognizing that pulmonary hypoplasia, or hypoplastic lung disease, is an important determinant of clinical outcomes, which also requires accurate clinical evaluation.
References
1. Kosinski P, Wielgos M, Congenital diaphragmatic hernia: Pathogenesis, prenatal diagnosis and management – literature review: Ginekol Pol, 2017; 88(1); 24-30
2. Laudy JA, Wladimiroff JW, The fetal lung. 1: Developmental aspects: Ultrasound Obstet Gynecol, 2000; 16(3); 284-90
3. Kinane TB, Lung development and implications for hypoplasia found in congenital diaphragmatic hernia: Am J Med Genet C Semin Med Genet, 2007; 145C(2); 117-24
4. Schittny JC, Development of the lung: Cell Tissue Res, 2017; 367(3); 427-44
5. Deprest JA, Nicolaides K, Gratacos E, Fetal surgery for congenital diaphragmatic hernia is back from never gone: Fetal Diagn Ther, 2011; 29(1); 6-17
6. Garne E, Haeusler M, Barisic I, Congenital diaphragmatic hernia: Evaluation of prenatal diagnosis in 20 European regions: Ultrasound Obstet Gynecol, 2002; 19(4); 329-33
7. Horn-Oudshoorn EJJ, Peters NCJ, Franx A, Termination of pregnancy after a prenatal diagnosis of congenital diaphragmatic hernia: Factors influencing the parental decision process: Prenat Diagn, 2023; 43(1); 95-101
8. Deprest JA, Nicolaides KH, Benachi A, Randomized trial of fetal surgery for severe left diaphragmatic hernia: N Engl J Med, 2021; 385(2); 107-18
9. Triebwasser JE, Treadwell MC, Prenatal prediction of pulmonary hypoplasia: Semin Fetal Neonatal Med, 2017; 22(4); 245-49
10. Wynn J, Yu L, Chung WK, Genetic causes of congenital diaphragmatic hernia: Semin Fetal Neonatal Med, 2014; 19(6); 324-30
11. McGivern MR, Best KE, Rankin J, Epidemiology of congenital diaphragmatic hernia in Europe: A register-based study: Arch Dis Child Fetal Neonatal Ed, 2015; 100(2); F137-44
12. Dott MM, Wong LY, Rasmussen SA, Population-based study of congenital diaphragmatic hernia: risk factors and survival in Metropolitan Atlanta, 1968–1999: Birth Defects Res A Clin Mol Teratol, 2003; 67(4); 261-67
13. Gallot D, Boda C, Ughetto S, Prenatal detection and outcome of congenital diaphragmatic hernia: A French registry-based study: Ultrasound Obstet Gynecol, 2007; 29(3); 276-83
14. Balayla J, Abenhaim HA, Incidence, predictors and outcomes of congenital diaphragmatic hernia: A population-based study of 32 million births in the United States: J Matern Fetal Neonatal Med, 2014; 27(14); 1438-44
15. Graham G, Devine PC, Antenatal diagnosis of congenital diaphragmatic hernia: Semin Perinatol, 2005; 29(2); 69-76
16. Burgos CM, Frenckner B, Luco M, Prenatally versus postnatally diagnosed congenital diaphragmatic hernia – side, stage, and outcome: J Pediatr Surg, 2019; 54(4); 651-55
17. Cordier AG, Russo FM, Deprest J, Benachi A, Prenatal diagnosis, imaging, and prognosis in Congenital Diaphragmatic Hernia: Semin Perinatol, 2020; 44(1); 51163
18. Dumpa V, Chandrasekharan P, Congenital diaphragmatic hernia: StatPearls, 2023, Treasure Island (FL)
19. Kardon G, Ackerman KG, McCulley DJ, Congenital diaphragmatic hernias: From genes to mechanisms to therapies: Dis Model Mech, 2017; 10(8); 955-70
20. Al-Salem AH, Congenital hernia of Morgagni in infants and children: J Pediatr Surg, 2007; 42(9); 1539-43
21. Zaleska-Dorobisz U, Baglaj M, Sokolowska B, Late presenting diaphragmatic hernia: clinical and diagnostic aspects: Med Sci Monit, 2007; 13(Suppl 1); 137-46
22. Peralta CF, Cavoretto P, Csapo B, Assessment of lung area in normal fetuses at 12–32 weeks: Ultrasound Obstet Gynecol, 2005; 26(7); 718-24
23. Claus F, Sandaite I, DeKoninck P, Prenatal anatomical imaging in fetuses with congenital diaphragmatic hernia: Fetal Diagn Ther, 2011; 29(1); 88-100
24. Jani J, Nicolaides KH, Keller RL, Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia: Ultrasound Obstet Gynecol, 2007; 30(1); 67-71
25. Deprest J, Jani J, Gratacos E, Fetal intervention for congenital diaphragmatic hernia: The European experience: Semin Perinatol, 2005; 29(2); 94-103
26. Britto IS, Araujo E, Sangi-Haghpeykar H, Reference ranges for 2-dimensional sonographic lung measurements in healthy fetuses: A longitudinal study: J Ultrasound Med, 2014; 33(11); 1917-23
27. Alfaraj MA, Shah PS, Bohn D, Congenital diaphragmatic hernia: Lung-to-head ratio and lung volume for prediction of outcome: Am J Obstet Gynecol, 2011; 205(1); 43.e1-8
28. Hasegawa T, Kamata S, Imura K, Use of lung-thorax transverse area ratio in the antenatal evaluation of lung hypoplasia in congenital diaphragmatic hernia: J Clin Ultrasound, 1990; 18(9); 705-9
29. Usui N, Okuyama H, Kanamori Y, The lung to thorax transverse area ratio has a linear correlation with the observed to expected lung area to head circumference ratio in fetuses with congenital diaphragmatic hernias: J Pediatr Surg, 2014; 49(8); 1191-96
30. Hidaka N, Murata M, Sasahara J, Correlation between lung to thorax transverse area ratio and observed/expected lung area to head circumference ratio in fetuses with left-sided diaphragmatic hernia: Congenit Anom (Kyoto), 2015; 55(2); 81-84
31. Masahata K, Yamoto M, Umeda S, Prenatal predictors of mortality in fetuses with congenital diaphragmatic hernia: A systematic review and meta-analysis: Pediatr Surg Int, 2022; 38(12); 1745-57
32. Kontopoulos E, Bulman M, Gordienko I, Clinical assessment of the fetal right Quantitative Lung Index: J Matern Fetal Neonatal Med, 2023; 36(2); 2242555
33. Hislop A, Reid L, Persistent hypoplasia of the lung after repair of congenital diaphragmatic hernia: Thorax, 1976; 31(4); 450-55
34. Ruano R, Takashi E, da Silva MM, Haeri S, Quantitative lung index, contralateral lung area, or lung-to-head ratio to predict the neonatal outcome in isolated congenital diaphragmatic hernia?: J Ultrasound Med, 2013; 32(3); 413-17
35. Kontopoulos EV, Quintero LF, Chmait R, Quintero RA, The quantitative lung index: The left lung: J Matern Fetal Neonatal Med, 2022; 35(21); 4142-48
36. Olutoye OO, Mehl SC, Moturu A, Risk stratification by percent liver herniation in congenital diaphragmatic hernia: J Surg Res, 2023; 282; 168-73
37. Ott KC, Bi M, Scorletti F, The interplay between prenatal liver growth and lung development in congenital diaphragmatic hernia: Front Pediatr, 2022; 10; 983492
38. Basurto D, Russo FM, Papastefanou I, Pulmonary hypertension in congenital diaphragmatic hernia: Antenatal prediction and impact on neonatal mortality: Prenat Diagn, 2022; 42(10); 1303-11
39. Sokol J, Shimizu N, Bohn D, Fetal pulmonary artery diameter measurements as a predictor of morbidity in antenatally diagnosed congenital diaphragmatic hernia: A prospective study: Am J Obstet Gynecol, 2006; 195(2); 470-77
40. Done E, Allegaert K, Lewi P, Maternal hyperoxygenation test in fetuses undergoing FETO for severe isolated congenital diaphragmatic hernia: Ultrasound Obstet Gynecol, 2011; 37(3); 264-71
41. DeKoninck P, Lewi P, Done E, Sonographic evaluation of vascular pulmonary reactivity following oxygen administration in fetuses with normal lung development: Prenat Diagn, 2012; 32(13); 1300-4
42. Williams G, Coakley FV, Qayyum A, Fetal relative lung volume: Quantification by using prenatal MR imaging lung volumetry: Radiology, 2004; 233(2); 457-62
43. Tanigaki S, Miyakoshi K, Tanaka M, Pulmonary hypoplasia: Prediction with use of ratio of MR imaging-measured fetal lung volume to US-estimated fetal body weight: Radiology, 2004; 232(3); 767-72
44. Jani J, Cannie M, Sonigo P, Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia: Ultrasound Obstet Gynecol, 2008; 32(6); 793-99
45. Oluyomi-Obi T, Kuret V, Puligandla P, Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH): J Pediatr Surg, 2017; 52(5); 881-88
46. Sakuma J, Nakata M, Takano M, Prenatal evaluation of functional pulmonary hypoplasia via fetal magnetic resonance imaging: J Obstet Gynaecol Res, 2021; 47(9); 3100-6
47. Tisekar OR, Ak AK, Hypoplastic lung disease: StatPearls, 2023, Treasure Island (FL)
48. Russo FM, Debeer A, De Coppi P, What should we tell parents? Congenital diaphragmatic hernia: Prenat Diagn, 2022; 42(3); 398-407
In Press
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
12 Mar 2024 : Clinical Research
Comparing Neuromuscular Blockade Measurement Between Upper Arm (TOF Cuff®) and Eyelid (TOF Scan®) Using Miv...Med Sci Monit In Press; DOI: 10.12659/MSM.943630
11 Mar 2024 : Clinical Research
Enhancement of Frozen-Thawed Human Sperm Quality with Zinc as a Cryoprotective AdditiveMed Sci Monit In Press; DOI: 10.12659/MSM.942946
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952