16 December 2022: Review Articles
A Review of the Roles of Apelin and ELABELA Peptide Ligands in Cardiovascular Disease, Including Heart Failure and Hypertension
Jan RozwadowskiDOI: 10.12659/MSM.938112
Med Sci Monit 2022; 28:e938112
Abstract
ABSTRACT: Apelin and ELABELA (ELA), which are peptides belonging to the adipokines group, are endogenous peptide ligands of their receptor, APJ, which together constitute the apelinergic system. The apelinergic system is expressed in numerous human tissues and organs, including the heart, blood vessels, adipose tissue, central nervous system, lungs, kidneys, and liver. Apelin, being the most widely studied member of the apelinergic system, plays a key role in the cardiovascular system and exerts a pleiotropic effect in tissues. Under physiological conditions, the peripheral actions of apelin include augmented cardiac contractility, increased left ventricular stroke volume, vasodilation, increased diuresis, and lowered systemic blood pressure. Multiple studies suggest that activation of the apelinergic system exerts beneficial effects on the treatment of cardiovascular diseases (CVD), including hypertension and heart failure, whereas the silencing of the apelin/APJ axis results in attenuation of inflammatory processes and prevents formation of atherosclerotic plaques. As numerous effects of apelin are not entirely explained, further studies of the cardiovascular actions of apelin and ELA are necessary to help establish effective pharmacological treatments of CVDs. This article aims to review the roles of apelin and elabela peptide ligands in cardiovascular diseases, including heart failure and hypertension.
Keywords: APLNR Protein, Human, apelin, APELA Protein, Human, Cardiovascular Diseases, Humans, Apelin Receptors, Heart Failure, Hypertension, Ligands
Background
The apelinergic system is composed of a group of endogenous peptides: apelin, ELABELA (ELA), and a G-protein-coupled receptor called APJ [1,2]. Apelin is an endogenous neuropeptide produced from a 77-amino acid precursor, preproapelin, which can be cleaved by endopeptidases to form C-terminal biologically active peptides, including apelin-13, -16, -17, -19, and -36 [3,4]. Apelin-13 is the most potent activator of cell lines expressing the APJ receptor and is susceptible to additional posttranslational modifications, which result in the production of its more stable, pyroglutaminated form, called [Pyr1]-apelin-13, which is the most abundant form in cardiac tissue [5–8]. Shin et al have shown that the bioactivity of Apelin-55 isoform greatly increases the number of potential therapeutic targets for the apelinergic system [9]. Apelin-13, -16, -17, -19, and -36 are widely expressed in various types of human tissue, predominantly in adipose tissue, as well as in the central nervous system, heart, lungs, kidneys, and liver [8]. Apelinergic signaling is crucial for the functioning of the cardiovascular system, energy metabolism, fluid homeostasis, angiogenesis, human immunodeficiency virus-1 (HIV-1) infection, and the neuroendocrine stress response [10–12]. ELA is a recently discovered novel endogenous peptidic ligand of the APJ receptor [13]. Human ELA consists of 3 exons on chromosome 4 [13]. ELA mRNA contains a conserved open reading frame that encodes a polypeptide of 54 amino acids (aa), which is a highly conserved protein with a predicted N-terminal signal sequence of 22 residues [13]. 22 N-terminal aa are susceptible to form a 32-aa mature peptide, which is a novel peptide hormone [13]. Peverelli et al demonstrated that ELA can inhibit cyclic AMP (cAMP) production, activate the extracellular signal-regulated kinases (ERK1/2), and mobilize intracellular calcium through APJ internalization [14]. It has been found to play a key function in early embryonic development, which is critical to cell motility during gastrulation and essential for mesendoderm differentiation during embryogenesis [13,15]. This peptide is also important for the functionality of adult organs, mainly the blood vessels, heart, and kidneys [16]. ELA has been found to have a similar cardiovascular profile to apelin, as they both act through the APJ and both were identified as the endogenous ligands of the human orphan receptor APJ [4,17]. The best known and most widely studied pathway of signal transduction within the apelinergic system is the binding of apelin to the APJ. APJ is a G-protein-coupled transmembrane receptor (GCPR) for apelin, which closely resembles the angiotensin receptor 1 (AT-1), since they share over 50% of the amino acids in the transmembrane regions [18]. The APJ receptor contains consensus sites for palmitoylation, glycosylation, and phosphorylation by (cAMP)-dependent protein kinase [18].
Immunohistochemistry studies have revealed that APJ is located in the endothelium of blood vessels, in smooth muscle cells, and in cardiomyocytes, as well as other places [19]. Langelaan et al (2013) presented the three-dimensional structure of the APJ receptor, having observed a high-resolution nuclear magnetic resonance (NMR) structure of the N-terminus and the first transmembrane segment of APJ, which comprises residues essential for apelin binding in dodecylphosphocholine micelles [20].
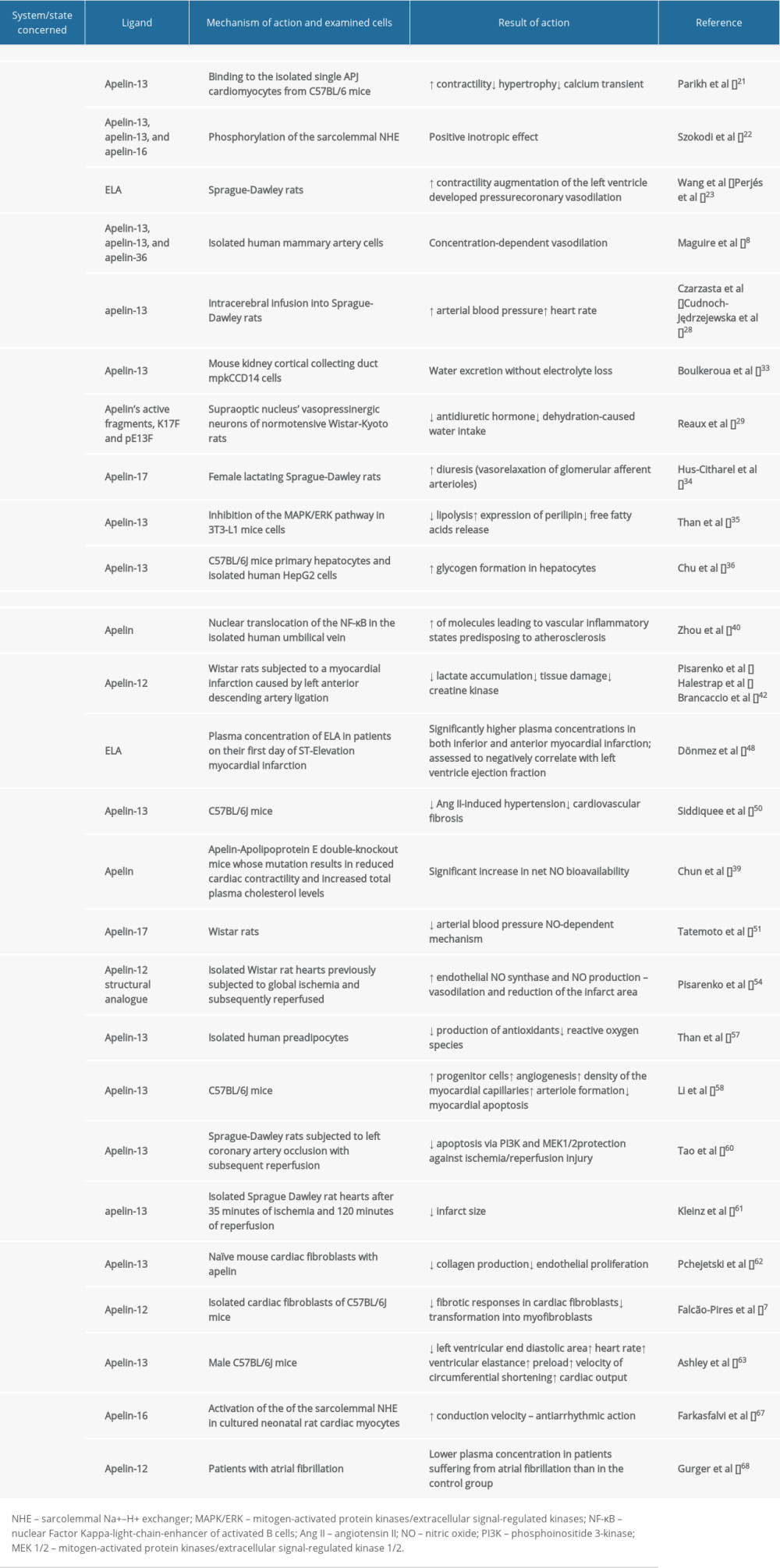
Recent studies have focused on the pleiotropic actions of the apelin/APJ axis in human health and disease. Since apelin and their receptor APJ exert a cardioprotective function, their role in the pathogenesis of cardiovascular diseases (CVD) has been widely studied. This article aims to review the roles of apelin and elabela peptide ligands in cardiovascular diseases, including heart failure and hypertension. The role of the apelinergic system in physiological conditions and cardiovascular diseases is shown in Table 1.
Physiology of the Apelin/APJ Axis
CARDIAC MUSCLE:
Parikh et al (2018) found a decreased contractile response to stretch in APJ−/− cardiomyocytes compared with APJ+/+ cardiomyocytes in isolated single cardiomyocytes from C57BL/6 mice [21]. The calcium transient did not change with stretch in either APJ−/− or APJ+/+ cardiomyocytes, while administration of apelin to APJ+/+ cardiomyocytes resulted in decreased calcium transient [21]. These authors suggested that APJ stretch transduction is mediated specifically by myocardial APJ; therefore, APJ is necessary for stretch-induced increases in contractility, and apelin opposes APJ’s stretch-mediated hypertrophy [21]. A study conducted in neonatal rat ventricular myocytes isolated from Sprague-Dawley rats has proven that [Pyr1]apelin-13, apelin-13, and apelin-16 phosphorylate the sarcolemmal Na+–H+ exchanger (NHE). NHE phosphorylation results in cardiac myofilament sensitization to intracellular calcium ions, which exerts a positive inotropic effect. The same study also demonstrated the significant role of the phospholipase C-phosphokinase C (PLC-PKC) cascade in cardiac contractility. When apelin was infused with staurosporine, which is a PLC-PKC cascade inhibitor, the inotropic response was significantly attenuated. This phenomenon suggests that the abovementioned kinase cascade is the main regulatory pathway of the myocardial contractile force and the positive inotropic effect of apelin [22]. Moreover, ELA has been shown to play a role in the physiology of cardiomyocytes [16]. ELA strengthens cardiac contractility, augments left ventricle (LV) developed pressure [23], and promotes coronary vasodilation [24].
VASCULAR FUNCTION AND BLOOD PRESSURE:
Apelin has been found to have a vasodilatory effect on peripheral vessels. In vitro studies have proven that incubation of vascular smooth muscle cells (VSMC) isolated from the aorta of C57BL/6J mice with apelin-13 enhances angiogenesis and VSMC proliferation via activation of the Jagged-1/Notch 3 pathway [25]. [Pyr1]apelin-13, apelin-13, and apelin-36 have been found to promote concentration-dependent vasodilation in isolated human mammary artery cells by up to 50% of its diameter due to the release of cyclooxygenase products [8]. These authors have also proven that mammary artery vasodilation induced by [Pyr1]apelin-13 was abolished after endothelium removal [8]. Apelin-recruited Aplnr+ cells were shown to improve myocardial neovascularization by the paracrine function [26].
When administered into the central nervous system, apelin has been found to increase both arterial blood pressure (BP) and heart rate (HR). Seyedabadi et al (2002) have demonstrated that intracerebral microinjections of apelin-13 into the nucleus tractus solitarius and rostral ventrolateral medulla of Sprague-Dawley rats resulted in transient elevation of arterial BP by 10 to 20 mmHg [27]. Czarzasta et al (2016) have confirmed these results, as they have also shown that the intracerebral infusion of apelin-13 into Sprague-Dawley rats significantly increased arterial BP [28]. A study by Reaux et al (2001), in which apelin fragments pE13F and R10F were administered intracerebroventricularly (i.c.v.) into Wistar-Kyoto rats, found they did not affect mean arterial BP or HR in the 30 min following injection [29]. The aforementioned apelin fragments injected into the femoral vein of Wistar-Kyoto rats elicited an immediate, small but significant decrease in mean arterial BP and a robust increase in HR [29]. However, a study by Kagiyama et al (2004) has shown that the i.c.v. injection of [Pyr1]apelin-13 into Wistar rats dose-dependently increases their mean arterial BP and HR. An i.v. injection of [Pyr1]apelin-13 has also dose-dependently increased mean arterial pressure (MAP) and HR; however, the peripheral effects of apelin appeared relatively weak compared with its central effects [30]. A study by Cudnoch-Jędrzejewska et al (2015) has shown that the pressor effect of i.c.v. admission of apelin-13 depends on diet and exposure to stress. In Sprague-Dawley rats fed a normal diet and not exposed to stress, apelin-13 elicited an increase in MAP and HR. On the contrary, in rats fed with a high-fat diet and not exposed to stress, in rats fed with a high-fat diet and exposed to stress, and in rats fed with a normal-fat diet and exposed to stress, the pressor effect of i.c.v. apelin-13 admission was abolished [31]. Also, a recent study by Wojno et al (2020) demonstrated that apelin-13 i.c.v. infusion into Sprague-Dawley rats significantly increases MAP and that the apelin-13 pressor effect is diminished by the i.c.v. admission of V1aRANT, which is a V1a receptor antagonist [32].
FLUID HOMEOSTASIS:
It has been proven that the apelin/APJ axis is importantly involved in the regulation of fluid homeostasis. Boulkeroua et al (2019) found that apelin-13 antagonizes antidiuretic hormone (ADH)-induced aquaporin-2 (AQP2) expression in the mouse kidney cortical collecting duct mpkCCD14 cells, demonstrating that the apelin/APJ system exerts an aquaretic role in fluid homeostasis at the kidney level by excreting water without electrolyte loss [33]. Therefore, it may be assumed that the APJ/apelin system acts in an opposite way to ADH antidiuretic activity [33]. Reaux et al (2001) were the first to detect in male normotensive Wistar-Kyoto rats the presence of apelinergic neurons in the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus, which are the cerebral structures releasing ADH. These authors found that direct administration of apelin’s active fragments, K17F and pE13F, into the SON vasopressinergic neurons inhibits the release of ADH, which induces a decrease in dehydration-caused water intake [29]. A study by Hus-Citharel (2008) has revealed that i.v. injection of apelin-17 into female lactating rats progressively increased diuresis by the vasorelaxation of glomerular afferent arterioles [34].
CARBOHYDRATE AND LIPID METABOLISM:
The apelin/APJ system also regulates glucose and lipid metabolism. It has been proven that apelin suppresses lipolysis in the 3T3-L1 cells of mice by inhibiting the mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) pathway, which inactivates the adipogenic transcriptional factor, peroxisome proliferator-activated receptor gamma (PPAR-γ) [35]. The apelin/APJ mediated activation of MAPK/ERK stimulates the expression of perilipin, which is a lipid-droplet-associated protein that stabilizes the lipid droplets from fragmentation and remodeling, protecting them from degradation by lipases and thus reducing free fatty acid release [35]. Chu et al (2013) demonstrated that administration of tumor necrosis factor α (TNF-α) in C57BL/6J mice primary hepatocytes and isolated human HepG2 cells reduced glycogen synthesis via improvement of JNK/insulin receptor substrate 1/glycogen synthase kinase functioning [36]. These authors concluded that apelin administration into the abovementioned cells increases glycogen formation in hepatocytes, which suggests that apelin has anti-insulin resistance properties, since TNF-α is frequently elevated in insulin resistance states [36].
The Role of the Apelin/APJ System in Cardiovascular Diseases
ATHEROSCLEROSIS:
Atherosclerosis is a chronic inflammatory disease of the arterial endothelium in which the balance of pro-inflammatory and inflammation-resolving mechanisms is crucial [37]. Apelin can alleviate processes involved in the pathogenesis of atherosclerosis [38]. Activation of the apelin-APJ pathway, which regulates Angiotensin II (Ang II) signaling in the human vascular wall, has also been found to abrogate Ang II-induced vascular effects in Apelin-Apolipoprotein E (ApoE) double-knockout mice [39]. As apelin stimulates inflammatory-related atherosclerosis, it is very likely that the pharmacological silencing of the APJ pathway and lowering the plasma apelin levels would be beneficial in prevention of atherosclerosis [38]. Activation of the apelin-APJ system via the nuclear translocation of the Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) in the isolated human umbilical vein stimulates the expression of adhesion molecules such as Intracellular Adhesion Molecule 1 (ICAM-1), Vascular Adhesion Molecule 1 (VCAM-1), and chemokines, including Monocyte Chemoattractant Protein-1 (MCP-1), which together are involved in monocyte recruitment and the development of vascular inflammatory states predisposing to atherosclerosis [40]. Chun et al (2008) have shown in ApoE-KO mice, an Apolipoprotein E deficient atherosclerotic model, that Ang II induces atherosclerosis and abdominal aortic aneurysm formation in their vessels [39]. Kadoglou et al (2012) have shown that serum apelin concentration in patients with carotid atherosclerosis are lower than in healthy controls [41].
CORONARY ARTERY DISEASE:
Multiple studies suggest that apelin’s activity might be beneficial in coronary artery disease (CAD) thanks to its vasodilatory effects on coronary arteries and its cardioprotective potential. Pisarenko et al (2013) have proved that i.v. injection of apelin-12 into Wistar rats after a myocardial infarction (MI) caused by left anterior descending (LAD) artery ligation reduces lactate accumulation in the area at risk [42]. Lactate reduction results in better cellular oxygenation, thus diminishing tissue damage, enabling glycolysis and proper contraction to take place [43]. It also lowers creatine kinase, which is a marker of sarcomeric damage, suggesting that apelin activity may be beneficial in acute coronary syndrome treatment [42,44]. Tempel et al (2012) found that apelin also enhances the recruitment of cKit+/Flk1+/Aplnr+ cells into the hearts of C57BL/6J mice after MI [26]. A meta-analysis by Chen et al (2017) revealed that the mutant allele of the APJ rs9943582 gene increases the risk of CAD [45]. Moreover, these authors have shown that the circulating apelin concentration was significantly lower in CAD patients than in controls, and that the plasma concentration of apelin rises on the fifth day after percutaneous coronary intervention [45]. Li et al (2008) revealed that the mean apelin plasma concentrations in patients with stable angina were significantly lower than in the control group without angina [46]. Moreover, Akboga et al (2013) reported that the plasma apelin concentration was higher in patients with partial or complete filling of the epicardial artery by the collateral vessels (2/3 grade in the Rentrop-Cohen classification) than in patients with no filling of any collateral vessels or in patients with filling of side branches of the artery by collateral vessels without visualization of the epicardial segment (0/1 grade in the Rentrop-Cohen classification) [47]. These authors suggest that higher plasma apelin concentration resulting in wider coronary collateral development is a promising target strategy for anti-ischemic treatment [47]. Dönmez et al (2019) measured ELA plasma concentration in patients on their first day of ST-elevation MI and compared the results with the healthy population [48]; ELA plasma concentrations were significantly higher in both inferior and anterior MI, and there was a positive correlation between N-terminal brain natriuretic peptide, troponin I, and apelin levels [48]. ELA has also been found to be negatively correlated with LV ejection fraction [48].
HYPERTENSION:
Apelin can neutralize the activity of Ang II; therefore, it may exert beneficial effects in lowering blood pressure and may be a potential target in the pharmacotherapy of hypertension. L-NAME, which is an apelin inhibitor, has been found to block apelin-mediated amelioration of Ang II-exacerbated vascular disease [39]. In APJ knockout mice, the pressor effect of Ang II is enhanced; therefore, it has been suggested that apelin has a counter-regulatory role in relation to the renin–angiotensin system [49]. Siddiquee et al (2011) discovered that subcutaneous apelin administration into C57BL/6J mice protects them against Ang II-induced hypertension and cardiovascular fibrosis [50]. Apelin administered into Apelin-ApoE double-knockout mice, whose mutation results in reduced cardiac contractility and increased total plasma cholesterol levels, has been found to significantly increase net nitric oxide (NO) bioavailability, measured using electron paramagnetic resonance spectroscopy [39]. Its perivascular and cardiac antifibrotic effects are mediated through antagonism of Ang II signaling and increased nitric oxide synthase (NOS) expression, resulting in decreased profibrotic gene expression, including plasminogen activator inhibitor-1 (PAI-1) [50]. Moreover, apelin-17 is a very potent vasodilating adipokine, which has been proven to reduce arterial BP in Wistar rats via a nitric oxide (NO)-dependent mechanism [51].
Sarzani et al (2007) have also discovered that, among patients with coronary artery disease (CAD), carriers of at least 1 copy of the G212A allele of APJ are more protected against the development of hypertension and they have a 10% lower risk of death from heart failure (HF) caused by progressive hemodynamic deterioration than patients with the G212 homozygous variant [52].
It has been suggested that ELA may also be related to the pathophysiology of hypertension, but this needs confirmation in further research. A recent study by Li et al (2019) is the first to demonstrate that ELA plasma concentration in hypertensive patients is remarkably lower when compared with controls, and its levels have been negatively correlated with systolic and diastolic BP [53].
ISCHEMIA/REPERFUSION INJURY:
Several experimental studies suggest the cardioprotective role of apelin in ischemia/reperfusion injury (IRI). Pisarenko et al (2015) have proven that the structural analog of apelin-12, infused directly into isolated Wistar rat hearts previously subjected to global ischemia and subsequently reperfused, increases endothelial NO synthase (eNOS) and NO production through signal transduction via phosphoinositide 3-kinase (PI3K) and MAPK/ERK kinase 1/2 (MEK1/2), which promotes vasodilation and reduces the infarct area [54]. In IRI, the role of the mitochondrial pores is crucial – as they open, the organelle releases its calcium content into the cytosol, increasing cellular edema, affecting cellular homeostasis and inflammation, and leading to cellular death [55]regarding the pathophysiology of IRI and its management with hyperbaric oxygen (HBO. These authors used isolated Wistar rat hearts, subjected to 35 min of global ischemia and 30 min of reperfusion, to prove that the infusion of the analogs of apelin, 5 min prior to ischemia, augments the production of NO and consequently activates the mitochondrial ATP-dependent potassium channel (KATP channel) [56]. Activation of the KATP channel inhibits mitochondrial pore opening, which is a known trigger for cardiomyocyte apoptosis and necrosis [56]. In isolated human preadipocytes, administration of [Pyr1]apelin-13 increased the production of antioxidants, including superoxide dismutase, glutathione peroxidase and catalase, which reduced the cellular levels of reactive oxygen species (ROS), which are known factors involved in cellular damage during IRI [57].
Li et al (2012) demonstrated that intraperitoneal (i.p.) apelin-13 administration in C57BL/6J mice increased the number of progenitor cells and promoted angiogenesis via the SDF-1/CXCR4 signaling pathway after transient ligation of the left descending artery with subsequent reperfusion [58]. Upregulation of SDF-1/CXCR4 expression leads to an increase in the density of the myocardial capillaries and enhances arteriole formation, which results in improvement of cardiac function by suppressing myocardial apoptosis [58]. Moreover, research conducted by Liu et al (2015) has shown that in Apln-CreER; Rosa26RFP/+ mice, which are a line able to indicate if apelin is expressed in the vasculature, higher expression of apelin in the coronary endothelial cells leads to an active sprouting of angiogenic endothelial cells after MI [59]. Additionally, in vivo apelin-13 intra-arterial administration into Sprague-Dawley rats subjected to left coronary artery occlusion with subsequent reperfusion has been found to inhibit endoplasmic reticulum-dependent apoptosis via PI3K and MEK1/2, protecting the cardiomyocytes against IRI [60]. [Pyr1]apelin-13 administered intra-arterially after 35 min of ischemia and 120 min of reperfusion significantly decreased infarct size in isolated Sprague-Dawley rat hearts [61].
HEART FAILURE:
Apelin exerts a beneficial effect on the development of HF through inhibition of adverse cardiac remodeling and a reduction of the extent of myocardial fibrosis. In vitro experiments performed in mouse cardiac fibroblasts obtained from normal and pressure-overloaded hearts have revealed that pretreatment of naive cardiac fibroblasts with apelin inhibited the production of collagen and decreased the spontaneous production of collagen in cardiac fibroblasts isolated from the hearts after aortic banding [62]. Moreover, these authors have revealed that prevention of collagen accumulation by apelin was mediated by a reduction in sphingosine kinase 1 (SphK1) activity [62], which is responsible for endothelial proliferation in physiological conditions [53]. The administration of apelin in isolated cardiac fibroblasts of C57BL/6J mice inhibits the transforming growth factor β (TGF-β)-induced fibrotic responses in cardiac fibroblasts, and their transformation into myofibroblasts, but the anti-fibrotic effect is reached even in the absence of TGF-β, as apelin still affects AMP kinase, which acts as a master regulator of cellular energy metabolism in cardiomyocytes [7].
A study on male C57BL/6J mice has revealed a decrease in the left ventricular end-diastolic area (LVEDA) in echocardiographic examination, an increase in HR, an increase in ventricular elastance, and an increase in preload recruitable stroke work, without significant changes in diastolic measures after acute i.p. administration of apelin [63]. Moreover, long-term i.p. infusion of apelin for 2 weeks has been found to increase the velocity of circumferential shortening and cardiac output (CO), while a histopathological investigation found no features of cardiomyocyte hypertrophy. These authors therefore suggest that apelin reduces the preload and the afterload of LV and increases contractile reserve without concomitant hypertrophy [63]. In vivo studies using fibroblasts from the C57BL6/J mice aortic banding model indicated that i.p. pretreatment with apelin attenuated the development of myocardial fibrotic remodeling and inhibited cardiac SphK1 activity [62]. Moreover, administration of apelin 2 weeks after aortic banding prevented cardiac remodeling by inhibiting myocyte hypertrophy, cardiac fibrosis, and ventricular dysfunction [62]. Parikh et al (2018) bred mice with the conditional elimination of APJ in the endothelium (APJendo−/−) and myocardium (APJmyo−/−) [21]. They demonstrated that APJendo−/− mice subjected to transaortic constriction (TAC) displayed decreased LV systolic function and increased wall thickness, which was not observed in APJmyo−/− mice subjected to TAC [21]. Czarzasta et al (2019), in a study on Sprague-Dawley rats, demonstrated that the crucial factor influencing APJ expression in the hypothalamus is HF [13]. The observed changes of receptor expression have systemic effects – apelin synthesized in the central nervous system caused an increase in the hemodynamic parameters in rats on a normal-fat diet [64].
ATRIAL FIBRILLATION:
Apelin exerts direct effects on cardiomyocyte contractility and electrophysiological properties and thus can play an important role in atrial fibrillation (AF) pathogenesis [65,66]. Farkasfalvi et al (2007) conducted a study which proved the antiarrhythmic action of apelin by the activation of NHE, which increases the conduction velocity in cultured neonatal rat cardiac myocytes [67]. Moreover, it has been proven by Gurger et al (2014) that serum apelin-12 levels were lower in patients with AF than in the control group [68]. An increase in plasma apelin concentration has been observed in patients with AF on its own lasting more than 3 months and with no other underlying conditions, after electrical cardioversion and restoration of sinus rhythm [69]. Falcone et al (2010) revealed that patients with AF on its own with a plasma apelin concentration below the median of 640 pg/mL are 3 times more susceptible to AF recurrence after a cardioversion than those with apelin levels higher than the abovementioned median level [70]. Moreover, these authors have shown that patients with supraventricular tachycardias with both a low apelin and a high brain natriuretic peptide (BNP) plasma concentration possess a higher risk of ischemic incidents than people with low apelin or enhanced BNP alone [70,71].
Therapeutic Potential of Apelin/APJ Axis
As discussed above, experimental and clinical studies suggest the pivotal cardioprotective role of the apelin/APJ axis and suggest its therapeutic potential in numerous CVDs. It has been shown that i.v. administration of [Pyr1]apelin-13 to patients with HF at New York Heart Association Functional Classification II and III level increases CO and reduces BP and peripheral vascular resistance (PVR) [72,73]. These findings indicate the promising therapeutic potential of apelin in HF, as it reduces both the afterload and preload and also acts as a vasodilator in the peripheral vessels [73]. Japp et al (2008) also concluded that [Pyr1]apelin-13 and apelin-36 injected i.v. into 24 healthy male volunteers increased forearm blood flow, most likely due to the apelin NO-induced vasodilatory effects on the peripheral resistance vessels [72]. These authors suggest that augmented blood flow by exogenous apelin might be beneficial as an alternative treatment for HF patients and possibly for people with vascular diseases [72]. A study by Barnes et al (2013) supports previous findings by documenting that i.v. administration of apelin-13 in patients with chronic HF induced vasodilation and lowered mean arterial pressure, which was measured by cardiac bioimpedance of peripheral brachial vessels [74]. In another study, Gao et al (2009) assessed patients with postinfarction HF who underwent intracoronary implantation of bone marrow mononuclear cells (BMMC), and revealed increased urine output, relief of dyspnea, and increased LV ejection fraction 7 days after the procedure [75]. Therefore, the apelin-APJ axis might be crucial for cardiac function improvement with BMMC transplantation in the course of HF and presumably also hypertension, as it enhances dieresis [75]. Moreover, 2-h i.v. infusion of [Pyr1]apelin-13 in high doses in overweight patients with DM2 caused a significant increase in the Δ glucose infusion rate (ΔGIR), which is a reliable marker of insulin sensitivity [76]. Considering the cardiovascular effects of apelin, it was necessary to discontinue infusion immediately in the case of systolic BP above 160 mmHg or below 90 mmHg, diastolic BP above 100 mmHg or below 50 mmHg, or HR below 50 bpm or above 110 bpm [76]. These effects suggest a significant influence of the APJ/apelin system on glucose tolerance, especially in diabetic patients; therefore, exogenously administered apelin may serve as a novel therapeutic strategy in insulin resistance states [77].
Accordingly, the apelin/APJ axis may be a promising new target for the pharmacological treatment of CVDs. However, the potential use of the apelin/APJ axis elements (including ELA) is restricted by the fact that the mechanisms of its action in the pathophysiology of CVDs, and its potential adverse effects and safety in humans, are not yet fully understood.
Conclusions
The apelin/APJ axis is involved in modulation of the cardiovascular physiology and pathophysiology; therefore, it has been suggested that the modification of apelin levels might be therapeutic in CVDs. Clinical and experimental studies imply that targeting the apelin/APJ axis in patients with CVDs such as atherosclerosis, CAD, hypertension, HF, and AF can delay disease progression. For many years, a molecule that could be used as a potential therapeutic in CVD in the apelin signaling pathway has been sought. It seems that ELA might be such a peptide, but comprehensive experimental studies of ELA are still needed. Therefore, further studies focusing on the apelin/APJ axis may help to establish an effective pharmacological treatment of CVDs.
References
1. Shin K, Kenward C, Rainey JK, Apelinergic system structure and function: Comprehensive physiology [Internet], 2017; 407-50, Wiley Available from: [cited 2022 Jan 12]https://onlinelibrary.wiley.com/doi/10.1002/cphy.c170028
2. Murza A, Sainsily X, Coquerel D, Discovery and structure – activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions: J Med Chem, 2016; 59(7); 2962-72
3. Chaves-Almagro C, Castan-Laurell I, Dray C, Apelin receptors: From signaling to antidiabetic strategy: Eur J Pharmacol, 2015; 763; 149-59
4. Tatemoto K, Hosoya M, Habata Y, Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor: Biochem Biophys Res Commun, 1998; 251(2); 471-76
5. Habata Y, Fujii R, Hosoya M, Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum: Biochim Biophys Acta, 1999; 1452(1); 25-35
6. Kawamata Y, Habata Y, Fukusumi S, Molecular properties of apelin: Tissue distribution and receptor binding: Biochim Biophys Acta, 2001; 1538(2–3); 162-71
7. Falcão-Pires I, Ladeiras-Lopes R, Leite-Moreira AF, The apelinergic system: A promising therapeutic target: Expert Opin Ther Targets, 2010; 14(6); 633-45
8. Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP, [Pyr 1]Apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease: Hypertension, 2009; 54(3); 598-604
9. Shin K, Chapman NA, Sarker M, Bioactivity of the putative apelin proprotein expands the repertoire of apelin receptor ligands: Biochim Biophys Acta, 2017; 1861(8); 1901-12
10. Masri B, Knibiehler B, Audigier Y, Apelin signalling: A promising pathway from cloning to pharmacology: Cell Signal, 2005; 17(4); 415-26
11. O’Carroll AM, Lolait SJ, Harris LE, Pope GR, The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis: J Endocrinol, 2013; 219(1); R13-35
12. Pitkin SL, Maguire JJ, Bonner TI, Davenport APInternational Union of Basic and Clinical Pharmacology, LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function: Pharmacol Rev, 2010; 62(3); 331-42
13. Chng SC, Ho L, Tian J, Reversade B, ELABELA: A hormone essential for heart development signals via the apelin receptor: Dev Cell, 2013; 27(6); 672-80
14. Peverelli E, Mantovani G, Lania AG, Spada A, cAMP in the pituitary: An old messenger for multiple signals: J Mol Endocrinol, 2014; 52(1); R67-77
15. Pauli A, Norris ML, Valen E, Toddler: An embryonic signal that promotes cell movement via apelin receptors: Science, 2014; 343(6172); 1248636
16. Xu J, Chen L, Jiang Z, Li L, Biological functions of Elabela, a novel endogenous ligand of APJ receptor: J Cell Physiol, 2018; 233(9); 6472-82
17. Liu W, Yan J, Pan W, Tang M, Apelin/Elabela-APJ: A novel therapeutic target in the cardiovascular system: Ann Transl Med, 2020; 8(5); 243
18. O’Dowd BF, Heiber M, Chan A, A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11: Gene, 1993; 136(1–2); 355-60
19. Kleinz MJ, Skepper JN, Davenport AP, Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells: Regul Pept, 2005; 126(3); 233-40
20. Langelaan DN, Reddy T, Banks AW, Structural features of the apelin receptor N-terminal tail and first transmembrane segment implicated in ligand binding and receptor trafficking: Biochim Biophys Acta, 2013; 1828(6); 1471-83
21. Parikh VN, Liu J, Shang C, Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition: Am J Physiol Heart Circ Physiol, 2018; 315(2); H348-56
22. Szokodi I, Tavi P, Földes G, Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility: Circ Res, 2002; 91(5); 434-40
23. Wang Z, Yu D, Wang M, Elabela-Apelin receptor signaling pathway is functional in mammalian systems: Sci Rep, 2015; 5(1); 8170
24. Perjés Á, Kilpiö T, Ulvila J, Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart: Basic Res Cardiol, 2016; 111(1); 2
25. Li L, Li L, Xie F, Jagged-1/Notch3 signaling transduction pathway is involved in apelin-13-induced vascular smooth muscle cells proliferation: Acta Biochim Biophys Sin (Shanghai), 2013; 45(10); 875-81
26. Tempel D, de Boer M, van Deel ED, Apelin enhances cardiac neovascularization after myocardial infarction by recruiting Aplnr+ circulating cells: Circ Res, 2012; 111(5); 585-98
27. Seyedabadi M, Goodchild AK, Pilowsky PM, Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration: Autonomic Neuroscience, 2002; 101(1–2); 32-38
28. Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, The role of apelin in central cardiovascular regulation in rats with post-infarct heart failure maintained on a normal fat or high fat diet: Clin Exp Pharmacol Physiol, 2016; 43(10); 983-94
29. Reaux A, De Mota N, Skultetyova I, Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain: Physiological role of apelin: J Neurochem, 2001; 77(4); 1085-96
30. Kagiyama S, Fukuhara M, Kiyoshi M, Central and peripheral cardiovascular actions of apelin in conscious rats: Regul Pept, 2005; 125(1–3); 55-59
31. Cudnoch-Jedrzejewska A, Gomolka R, Szczepanska-Sadowska E, High-fat diet and chronic stress reduce central pressor and tachycardic effects of apelin in Sprague-Dawley rats: Clin Exp Pharmacol Physiol, 2015; 42(1); 52-62
32. Wojno O, Czarzasta K, Puchalska L, Central interaction between the apelinergic and vasopressinergic systems in the regulation of the haemodynamic parameters in rats maintained on a high-fat diet: Clin Exp Pharmacol Physiol, 2020; 47(12); 1902-11
33. Boulkeroua C, Ayari H, Khalfaoui T, Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in kidney collecting duct cells: Cell Physiol Biochem, 2019; 53(4); 687-700
34. Hus-Citharel A, Bouby N, Frugière A, Effect of apelin on glomerular hemodynamic function in the rat kidney: Kidney Int, 2008; 74(4); 486-94
35. Than A, Cheng Y, Foh LC, Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways: Mol Cell Endocrinol, 2012; 362(1–2); 227-41
36. Chu J, Zhang H, Huang X, Apelin ameliorates TNF-α-induced reduction of glycogen synthesis in the hepatocytes through G protein-coupled receptor APJ: PLoS One, 2013; 8(2); e57231
37. Bäck M, Yurdagul A, Tabas I, Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities: Nat Rev Cardiol, 2019; 16(7); 389-406
38. Lu Y, Zhu X, Liang GX, Apelin–APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-κB/JNK signal pathway in human umbilical vein endothelial cells: Amino Acids, 2012; 43(5); 2125-36
39. Chun HJ, Ali ZA, Kojima Y, Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis: J Clin Invest, 2008; 118(10); 3343-54
40. Zhou Q, Cao J, Chen L, Apelin/APJ system: A novel therapeutic target for oxidative stress-related inflammatory diseases (review): Int J Mol Med, 2016; 37(5); 1159-69
41. Kadoglou NPE, Sailer N, Moumtzouoglou A, Adipokines: A novel link between adiposity and carotid plaque vulnerability: Apelin and Visfatin in Carotid Plaque Vulnerability: Eur J Clin Invest, 2012; 42(12); 1278-86
42. Pisarenko O, Serebryakova L, Studneva I, Effects of structural analogues of apelin-12 in acute myocardial infarction in rats: J Pharmacol Pharmacother, 2013; 4(3); 198
43. Halestrap AP, Wang X, Poole RC, Lactate transport in heart in relation to myocardial ischemia: Am J Cardiol, 1997; 80(3); 17A-25A
44. Brancaccio P, Maffulli N, Limongelli FM, Creatine kinase monitoring in sport medicine: Br Med Bull, 2007; 81-82(1); 209-30
45. Chen T, Wu B, Lin R, Association of apelin and apelin receptor with the risk of coronary artery disease: A meta-analysis of observational studies: Oncotarget, 2017; 8(34); 57345-55
46. Li Z, Bai Y, Hu J, Reduced apelin levels in stable angina: Intern Med, 2008; 47(22); 1951-55
47. Akboga MK, Akyel A, Sahinarslan A, Relationship between plasma apelin level and coronary collateral circulation: Atherosclerosis, 2014; 235(2); 289-94
48. Dönmez Y, Acele A, Increased Elabela levels in the acute ST segment elevation myocardial infarction patients: Medicine (Baltimore), 2019; 98(43); e17645
49. Ishida J, Hashimoto T, Hashimoto Y, Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo: J Biol Chem, 2004; 279(25); 26274-79
50. Siddiquee K, Hampton J, Khan S, Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production: J Hypertens, 2011; 29(4); 724-31
51. Tatemoto K, Takayama K, Zou MX, The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism: Regul Pept, 2001; 99(2–3); 87-92
52. Sarzani R, Forleo C, Pietrucci F, The 212A variant of the APJ receptor gene for the endogenous inotrope apelin is associated with slower heart failure progression in idiopathic dilated cardiomyopathy: J Card Fail, 2007; 13(7); 521-29
53. Li Y, Yang X, Ouyang S, Declined circulating Elabela levels in patients with essential hypertension and its association with impaired vascular function: A preliminary study: Clin Exp Hypertens, 2020; 42(3); 239-43
54. Pisarenko OI, Shulzhenko VS, Studneva IM, Signaling pathways of a structural analogue of apelin-12 involved in myocardial protection against ischemia/reperfusion injury: Peptides, 2015; 73; 67-76
55. Sánchez EC, Pathophysiology of ischemia-reperfusion injury and its management with hyperbaric oxygen (HBO): A review: J Emerg Crit Care Med, 2019; 3; 22
56. Pisarenko O, Shulzhenko V, Studneva I, Structural apelin analogues: Mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury: Cardiometabolic protection by apelin peptides: Br J Pharmacol, 2015; 172(12); 2933-45
57. Than A, Zhang X, Leow MKS, Apelin attenuates oxidative stress in human adipocytes: J Biol Chem, 2014; 289(6); 3763-74
58. Li L, Zeng H, Chen JX, Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction: Am J Physiol Heart Circ Physiol, 2012; 303(5); H605-18
59. Liu Q, Hu T, He L, Genetic targeting of sprouting angiogenesis using Apln-CreER: Nat Commun, 2015; 6(1); 6020
60. Tao J, Zhu W, Li Y, Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion: Am J Physiol Heart Circ Physiol, 2011; 301(4); H1471-86
61. Kleinz MJ, Baxter GF, Apelin reduces myocardial reperfusion injury independently of PI3K/Akt and P70S6 kinase: Regul Pept, 2008; 146(1–3); 271-77
62. Pchejetski D, Foussal C, Alfarano C, Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1: Eur Heart J, 2012; 33(18); 2360-69
63. Ashley E, Powers J, Chen M, The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo: Cardiovas Res, 2005; 65(1); 73-82
64. Czarzasta K, Wojno O, Zera T, The influence of post-infarct heart failure and high fat diet on the expression of apelin APJ and vasopressin V1a and V1b receptors: Neuropeptides, 2019; 78; 101975
65. Salska A, Dziuba M, Salski W, Apelin and atrial fibrillation: The role in the arrhythmia recurrence prognosis: Dis Markers, 2018; 2018; 5285392
66. Yu XH, Tang ZB, Liu LJ, Apelin and its receptor APJ in cardiovascular diseases: Clin Chim Acta, 2014; 428; 1-8
67. Farkasfalvi K, Stagg MA, Coppen SR, Direct effects of apelin on cardiomyocyte contractility and electrophysiology: Biochem Biophys Res Commun, 2007; 357(4); 889-95
68. Gurger M, Celik A, Balin M, The association between apelin-12 levels and paroxysmal supraventricular tachycardia: J Cardiovasc Med (Hagerstown), 2014; 15(8); 642-46
69. Kallergis EM, Manios EG, Kanoupakis EM, Effect of sinus rhythm restoration after electrical cardioversion on apelin and brain natriuretic peptide prohormone levels in patients with persistent atrial fibrillation: Am J Cardiol, 2010; 105(1); 90-94
70. Falcone C, Buzzi MP, D’Angelo A, Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation: Int J Immunopathol Pharmacol, 2010; 23(3); 917-25
71. Chang KW, Hsu JC, Toomu A, Clinical applications of biomarkers in atrial fibrillation: Am J Med, 2017; 130(12); 1351-57
72. Japp AG, Cruden NL, Amer DAB, Vascular effects of apelin in vivo in man: J Am Coll Cardiol, 2008; 52(11); 908-13
73. Japp AG, Cruden NL, Barnes G, Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure: Circulation, 2010; 121(16); 1818-27
74. Barnes GD, Alam S, Carter G, Sustained cardiovascular asctions of APJ agonism during renin–angiotensin system activation and in patients with heart failure: Circ Heart Fail, 2013; 6(3); 482-91
75. Gao LR, Xu RY, Zhang NK, Increased apelin following bone marrow mononuclear cell transplantation contributes to the improvement of cardiac function in patients with severe heart failure: Cell Transplant, 2009; 18(12); 1311-18
76. Gourdy P, Cazals L, Thalamas C, Apelin administration improves insulin sensitivity in overweight men during hyperinsulinaemic-euglycaemic clamp: Diabetes Obes Metab, 2018; 20(1); 157-64
77. Antushevich H, Wójcik M, Review: Apelin in disease: Clin Chim Acta, 2018; 483; 241-48
In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952