26 June 2023: Clinical Research
Glasgow Coma Scale as an Indicator of Patient Prognosis: A Retrospective Study of 257 Patients with Heatstroke from 3 Medical Centers in Guangdong, China
HuaiSheng Chen1CE, Ronglin Chen2B, Xinghui WuDOI: 10.12659/MSM.939118
Med Sci Monit 2023; 29:e939118
Abstract
BACKGROUND: Coma has been considered as a valuable symptom of heatstroke. This study aimed to evaluate the role of the Glasgow Coma Scale (GCS) as an indicator of prognosis of patients with heatstroke.
MATERIAL AND METHODS: From Jan 1st, 2013 to Dec 31st, 2020, the clinical courses of 257 heatstroke patients from 3 medical centers in Guangdong, China, were observed. Diagnosis of heatstroke was made according to Expert Consensus in China. GCSs were calculated on the 1st, 3rd, and 5th days after admission to intensive care units (ICUs). GCS £8, as a coma criterion, was employed to predict the outcomes.
RESULTS: Seventy-five patients (29.18%) were comatose at admission. Twenty-seven (10.50%) patients, including 24 (24/75, 32.00%) coma patients and 3 (3/182,1.65%) non-coma patients died during ICU stay (P<0.0001). Patients with GCS ≤8 had a 2-fold higher risk of death as compared with those with GCS >8. The area under curves (AUCs) of GCSs on the 1st, 3rd, and 5th days to predict mortality were 0.81 (0.70-0.91), 0.91 (0.84-0.98), and 0.91 (0.82-0.99), respectively. Each additional 1 year of age, 1/min of respiratory rate (RR), and 1% of hematocrit (HCT) increased the risk of death of coma patients by 3%, 6%, and 4%, respectively (all P≤0.05). Patients with improving GCSs had lower mortality rates than non-improving patients (5.71% vs 55.00%, P<0.0001) within 5 days after admission.
CONCLUSIONS: GCS ≤8 at admission predicted worse outcomes in heatstroke patients, which possibly enhanced the risks of death for other factors, including age, RR, and HCT.
Keywords: Glasgow Coma Scale, Heat Stroke, Mortality, Prognosis
Background
Heatstroke has attracted the attention of clinicians for about 100 years. In 1886, 48 heatstroke patients with a 52% mortality rate were reported in the Assouan area of Egypt due to a heatwave [1]. Since clinicians found that brain injury was a common phenomenon in heatstroke in 1888 [2], coma has been considered as a valuable symptom in subsequent studies [3]. Although still controversial, the definition of heatstroke proposed by Dr. Bouchamais, which defined it as a core body temperature over 40°C and which induced multiple organs dysfunction dominated by central nervous system abnormalities such as delirium, convulsion, or coma, is used clinically [4,5]. The impact of an extremely hot environment on health has become a growing concern and a burden on public health [6]. According to the data provided by the largest emergency department (ED) data system from 2009 to 2010 in the USA, the number of emergency visits because of heatstroke was estimated to be 8251, 54.6% of whom needed hospitalization, and 3.5% of whom died in EDs or hospitals [7]. In a recent study, 1152 cases of severe heatstroke with 10% mortality were reported from 2013 to 2017 in Shanghai, China [8]. It is particularly important to explore the high-risk factors for poor outcomes in heatstroke. Central nervous system abnormalities, a common phenomenon, may be a potential risk factor for poor outcomes in heatstroke [9]. A cohort study assessed the prognostic factors of heatstroke, which shows Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment Score (SOFA), and Japanese Association for Acute Medicine (JAAM) Disseminated intravascular coagulation (DIC) score, but not Glasgow Coma Scale (GCS), can predict in-hospital mortality in heatstroke [10]. However, the severity of the patients was mild or moderate, as their mean APACHE II score was below 15, which possibly masked the potential efficacy of the predictors.
Coma, a state of unarousable unconsciousness due to dysfunction of the brain’s ascending reticular activating system (ARAS), which is responsible for arousal and maintaining wakefulness [11], is a common phenomenon in patients with heatstroke. In the early stage of HS, brain CT usually has no positive results. Diffuse edema of the brain parenchyma may appear after 2–5 days. Most brain edema in HS patients is reversible. When the condition is stable, it can gradually disappear after 7–10 days [4]. In some extreme cases, punctate hemorrhages can be found by susceptibility-weighted imaging [12]. Clinically, although many patients had encephalopathy early, they experienced different trajectories depending on whether they had transient or persistent coma; therefore, the clinical picture and kinetics of encephalopathy need to be elucidated in heatstroke. The Glasgow Coma Scale (GCS), introduced in 1974 to standardize assessment of the level of consciousness in brain-injured patients, has been widely used in monitoring neurological status and predicting patient outcome [13]. The GCS consists of 3 parameters: best eye response (E), best verbal response (V), and best motor response (M). The total Coma Score has values of 3–15, with 3 being the worst and 15 being the best [14]. GCS ≤8, a criterion of coma, is associated with severe brain injury and poor prognosis. Neurological injury markers can predict poor prognosis of exertional heat stroke (EHS) [15]. The GCS was selected as one of the predictors of the mortality rate of EHS patients [16]. The EHSS score involves 12 parameters – T, GCS, potential hydrogen value (pH), lactate (Lac), platelet (PLT), prothrombin time (PT), fibrinogen (Fib), troponin I (TnI), aspartate aminotransferase (AST), total bilirubin (TBIL), serum creatinine (SCr), and acute gastrointestinal injury (AGI) classification – with a predictive value with AUC higher than 0.96 [17]. Another study with 763 patients found that GCS and platelets were independent predictors of poor outcomes [18]. The present retrospective study from 3 medical centers in Guangdong, China focused on the kinetics of GCS and its prognostic value in heatstroke patients, aiming to evaluate the role of the GCS as an indicator of prognosis in 257 patients with heatstroke.
Material and Methods
ETHICAL STATEMENT:
The protocol of the study was approved by the Medical Ethics Review Committee of General Hospital of Southern Theatre Command of PLA (No. NZLLKZ2022047). As a retrospective study, the requirement for informed consent was waived.
STUDY POPULATION:
From Jan 1st, 2013 to Dec 31st, 2020, patients with heatstroke admitted to the 3 intensive care units (ICUs) in Guangdong province of China, were retrospectively enrolled. Heatstroke was diagnosed according to the Expert Consensus on Diagnosis and Treatment of Heatstroke in China published by the Expert Group on Prevention and Treatment of Heatstroke and Critical Care Committee of the General Hospital of Southern Theatre Command of PLA [4]. We excluded patients with chronic liver and kidney diseases, chronic cardiac insufficiency, chronic pulmonary insufficiency, underlying central nervous system (CNS) diseases, metabolic disorders, or those using sedation and analgesia medications. Researchers from the 3 centers were trained by doctors from the General Hospital of Southern Theatre Command of PLA. All patients were treated by cooling, fluid resuscitation, and organ support according to their conditions. Antibiotics were also administered if necessary.
DATA COLLECTION:
GCSs were calculated on the 1st, 3rd, and 5th days after admission. The patients were divided into survival and non-survival groups. GCS ≤8 was considered to be an indicator of coma. The max body temperature (Tmax) out-of-hospital was recorded. The body temperature at admission (Tadmit), heart rate (HR), respiratory rate (RR), mean artery pressure (MAP), and laboratory indexes at 1st, 3rd, and 5th days were also recorded. APACHE II and SOFA scores were determined to evaluate the disease severity. Baseline demographic characteristics were recorded at admission (day 1). Representative brain computed tomography (CT) from surviving and non-surviving patients was employed to evaluate the severity of brain injury.
STATISTICAL ANALYSIS:
Means (M)±standard deviations (SD) of outcome measurements were calculated and compared using R language with EmpowerStats 2.0 software, and forest plots were drawn using Python 3.8. Repeated measurements were analyzed using IBM SPSS Statistics v 20.0 software. Kaplan-Meier (KM) curve and receiver operating characteristic (ROC) curves were employed to assess the prognosing values of GCS. Odds ratio (OR) and 95% confidence interval (CI) were also calculated.
Results
CHARACTERISTICS OF THE PATIENTS:
A total of 260 heatstroke patients were admitted to the 3 ICUs during the period covered. Three patients were excluded due to lack of GCS values at admission. The remaining 257 patients (33±15 years old, 90% males) were included. Twenty-seven (10.50%) patients died during ICU stay. GCSs in non-surviving patients (5.67±3.55) were significantly lower than those in surviving patients (12.35±4.05, P<0.0001). Seventy-five patients (29.18%) were comatose (GCS ≤8), of whom 24 (88.89%) died and 51 (22.17%) survived. The rates of mortality in coma and non-coma patients were 32.00% (24/75) and 1.65% (3/182), respectively (P<0.0001). Baseline demographic characteristics of non-surviving and surviving patients are listed in Table 1. HR, MAP, Tmax, and Tadmit at admission in non-surviving patients were all significantly higher than those in surviving patients. Hematocrit (HCT), platelets (PLT), total bilirubin (TBIL), albumin (ALB), serum creatinine (SCr), and creatine kinase isoenzyme (CK-MB) were also significantly worse in non-surviving patients.
UNIVARIATE ANALYSIS OF DEATH-ASSOCIATED RISK FACTORS AT ADMISSION:
Tachycardia, hyperthermia, Tmax, coma, and higher TBIL, SCr, and CK-MB at admission were risk factors for death (Table 2).
SUB-GROUP ANALYSIS OF CO-EFFECTS OF COMA ON OTHER DEATH-ASSOCIATED RISK FACTORS AT ADMISSION:
Among coma patients (GCS ≤8), an additional 1 year of age, 1/min of RR, 1% of HCT, and 1×109/L of PLT increased the risks of death by 3%, 6%, 4%, and 0.3%, respectively (all P≤0.05) (Figure 1).
MULTIPLE REGRESSION ANALYSIS OF DEATH-ASSOCIATED RISK FACTORS AT ADMISSION:
If adjusted by coma, age and HR became more important factors than Tmax, and SCr to predict heatstroke mortality (Table 3). Coma was a mortality risk factor for patients with heatstroke [OR=3.57 (2.55, 4.99), P<0.0001]. Importantly, if adjusted by age, HR, RR, MAP, Tmax, Tadmit, leukocyte count, HCT, PLT, TBIL, ALB, CR, and CK-MB, coma still had significant effect on mortality [OR=2.01 (1.09, 3.69), P=0.02].
:
Kaplan-Meier curve analysis indicated significantly different length of ICU stay between patients with GCS ≤8 and those with GCS >8 at admission [log rank (Mantel-Cox) (P=0.002), Breslow (generalized Wilcoxon) (P=0.002), and Tarone-Ware (P=0.001)] (Figure 2A). GCSs were analyzed kinetically, which showed GCSs on the 1st, 3rd, and 5th in non-surviving patients were significantly lower than those in surviving patients (P<0.0001) (Figure 2B). Receiver operating characteristic (ROC) curves indicated GCSs on the 1st, 3rd, and 5th day could predict mortality, with areas under the curve (AUCs) of 0.81 [95% confidence interval (CI) 0.70–0.91], 0.91 (95% CI 0.84–0.98), and 0.91 (95% CI 0.82–0.99) (Figure 2C) and the corresponding cut-off values of 8.5, 9, and 9, respectively. As the AUCs of GCSs on the 3rd and 5th days were greater than that of GCSs on the 1st day, we hypothesized that persistent coma indicates a worsen outcome in heatstroke. Dynamic analysis of the impact of GCS on predicting mortality may have greater clinical significance.
Among the 75 patients with GCS ≤8 at admission, 35 (46.67%) presented GCSs increasing to more than 8 on the 3rd (25) or 5th day (10). The rate of mortality in patients with GCS that did not non-improve (55.00%, 22/40) was significantly higher than that in patients with GCS that improved (5.71%, 2/35) (
Kaplan-Meier (KM) curve analysis showed improving GCS predicted better outcomes, which was verified by log rank test (Mantel-Cox) (P=0.01), Breslow test (generalized Wilcoxon) (P=0.01), and Tarone-Ware test (P=0.01) (Figure 2D).
REPRESENTATIVE IMAGES FEATURES:
A 22-year-old male heatstroke patient with APACHE II score of 31 and GCS of 3 at admission was persistently comatose and died 5 days after admission. A brain computed tomography (CT) scan showed diffuse encephalic swelling, compressed or occluded ventricles, sulci, and cisterna, and extensive subarachnoid hemorrhage on the 3rd day after admission (Figure 3A). His GCS remained less than 5 during ICU stay. Another 27-year-old male heatstroke patient admitted with APACHE II score of 20 and GCS of 5 survived. Although his brain CT scan did not show swelling or edema at admission (Figure 3B1), a light encephalic swelling was shown by a CT scan 6 days later (Figure 3B2). The patient’s brain CT scan images returned to normal on the 17th day. Accordingly, his GCS improved to 10 and 15 on the 5th and 9th day after admission, respectively.
Discussion
LIMITATIONS:
The present study has certain limitations. Firstly, although it was demonstrated that persistent coma from admission onwards (GCS ≤8) was valuable in predicting outcomes in heatstroke, due to lack of relative indexes, we could not explain the mechanism of coma. Secondly, indexes used to assess brain injury are limited. Attention should be focused on multiple brain injury-related indicators, including clinical signs, radiography, electroencephalogram, and biomarkers, all of which together can comprehensively evaluate the changes of brain after injury. Thirdly, even though there were no sex differences between the non-survival and survival groups, males accounted for 92% of the total. This added bias to the study and needs to be improved in future research. Finally, this was a retrospective observational study, and we can only analyze existing data to find risk factors.
Conclusions
GCS ≤8 at admission could be an ideal predictor of clinical outcomes in heatstroke patients with GCS ≤8 at admission, which may have enhanced the risks of mortality due to other factors, including age, RR, and HCT.
Figures
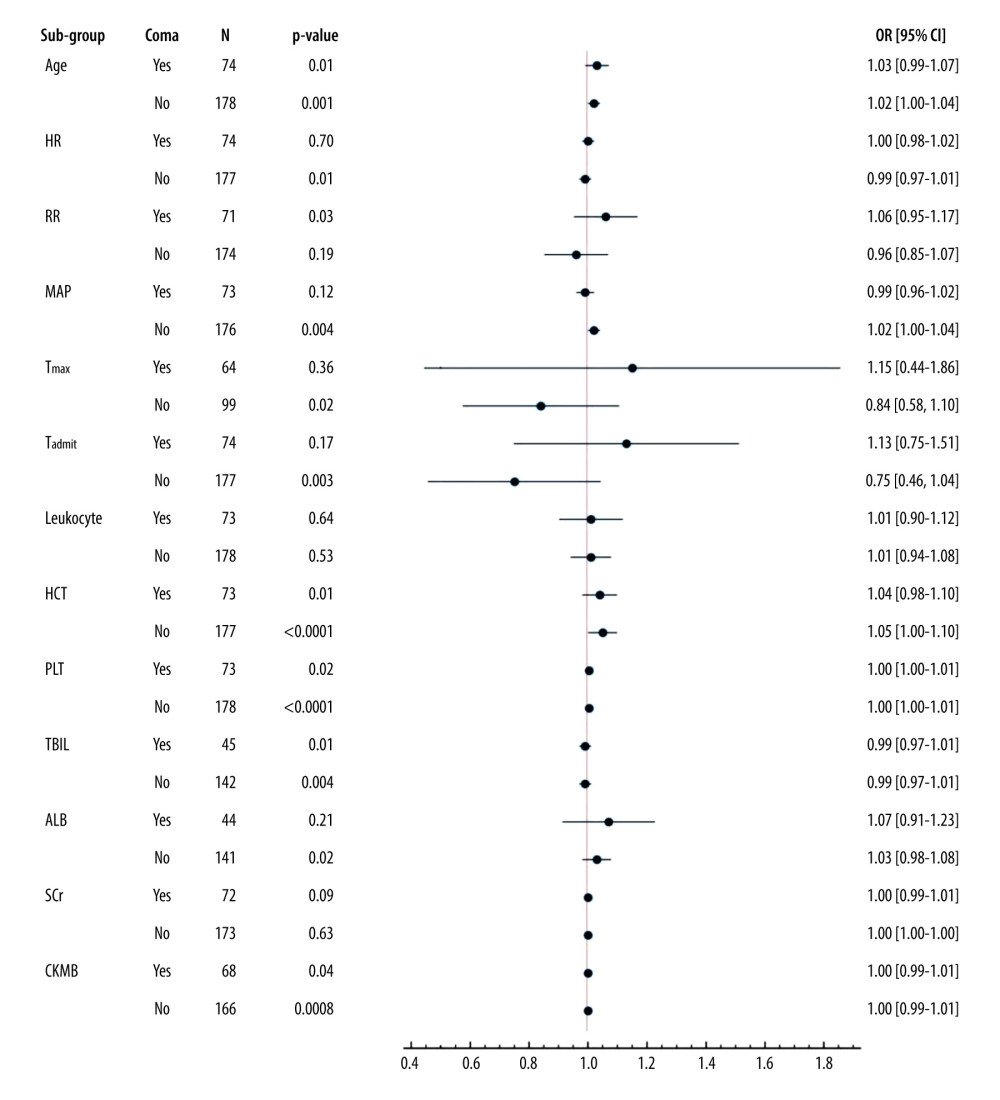 Figure 1. Sub-group analysis of co-effects of coma on other mortality-associated risk factorsAll patients were grouped according to whether they were comatose or not. Coma (GCS ≤8) was applied as a stratification factor. Among the coma patients, older age and higher RR, HCT, PLT, and CKMB were all harmful factors (OR≥1, and P value less than 0.05) (all P≤0.05). These results indicated that an additional 1 year of age, 1/min of RR, 1% of HCT, and 1×109/L of PLT increased the risks of death by 3%, 6%, 4%, and 0.3%, respectively (all P≤0.05). GCS – Glasgow Coma Scale; APACHE II score – Acute Physiology and Chronic Health Evaluation II score; SOFA score – Sequential Organ Failure Assessment Score; HR – heart rate; RR – respiratory rate; MAP – mean arterial pressure; Tmax – the max body temperature; Tadmit – the body temperature at admission; HCT – hematocrit; PLT – platelets; TBIL – total bilirubin; ALB – albumin; SCr – serum creatinine; CK-MB – creatine kinase isoenzyme. The data of the figure were calculated and compared using R language with EmpowerStats 2.0 software, and the forest plot was drawn using Python 3.8.
Figure 1. Sub-group analysis of co-effects of coma on other mortality-associated risk factorsAll patients were grouped according to whether they were comatose or not. Coma (GCS ≤8) was applied as a stratification factor. Among the coma patients, older age and higher RR, HCT, PLT, and CKMB were all harmful factors (OR≥1, and P value less than 0.05) (all P≤0.05). These results indicated that an additional 1 year of age, 1/min of RR, 1% of HCT, and 1×109/L of PLT increased the risks of death by 3%, 6%, 4%, and 0.3%, respectively (all P≤0.05). GCS – Glasgow Coma Scale; APACHE II score – Acute Physiology and Chronic Health Evaluation II score; SOFA score – Sequential Organ Failure Assessment Score; HR – heart rate; RR – respiratory rate; MAP – mean arterial pressure; Tmax – the max body temperature; Tadmit – the body temperature at admission; HCT – hematocrit; PLT – platelets; TBIL – total bilirubin; ALB – albumin; SCr – serum creatinine; CK-MB – creatine kinase isoenzyme. The data of the figure were calculated and compared using R language with EmpowerStats 2.0 software, and the forest plot was drawn using Python 3.8. 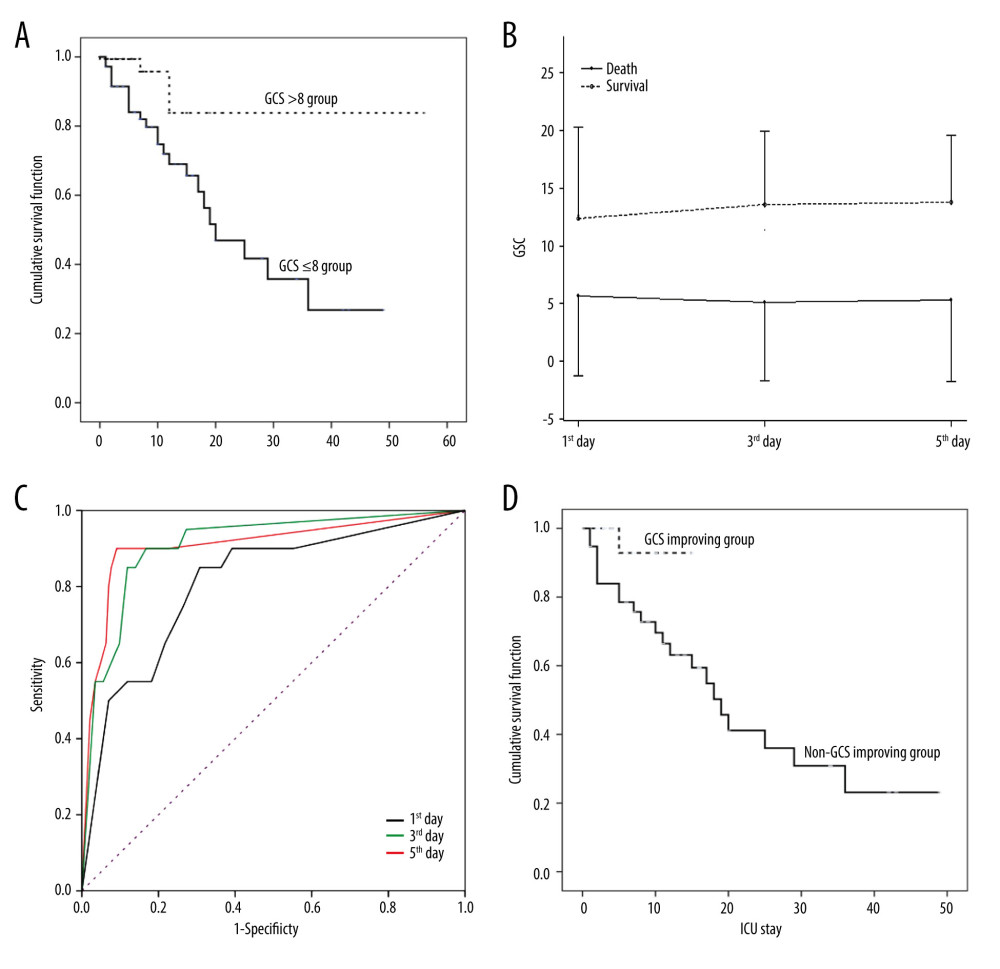 Figurre 2. Association of Glasgow Coma Scale (GCS) with outcomes in heatstroke(A). Kaplan-Meier Curve. Patients with GCS ≤8 at admission had poorer clinical outcomes. (B). Kinetics of GCS. GCSs in non-surviving patients were significantly lower than those in surviving patients. (C). Receiver operating characteristic curve (ROCs). GCSs on the 1st, 3rd, and 5th days after admission predicted mortality. (D). Improved GCS predicted better clinical outcomes. The figures were drawn using the SPSS 20.0 for windows and EmpowerStats 2.0 software.
Figurre 2. Association of Glasgow Coma Scale (GCS) with outcomes in heatstroke(A). Kaplan-Meier Curve. Patients with GCS ≤8 at admission had poorer clinical outcomes. (B). Kinetics of GCS. GCSs in non-surviving patients were significantly lower than those in surviving patients. (C). Receiver operating characteristic curve (ROCs). GCSs on the 1st, 3rd, and 5th days after admission predicted mortality. (D). Improved GCS predicted better clinical outcomes. The figures were drawn using the SPSS 20.0 for windows and EmpowerStats 2.0 software. 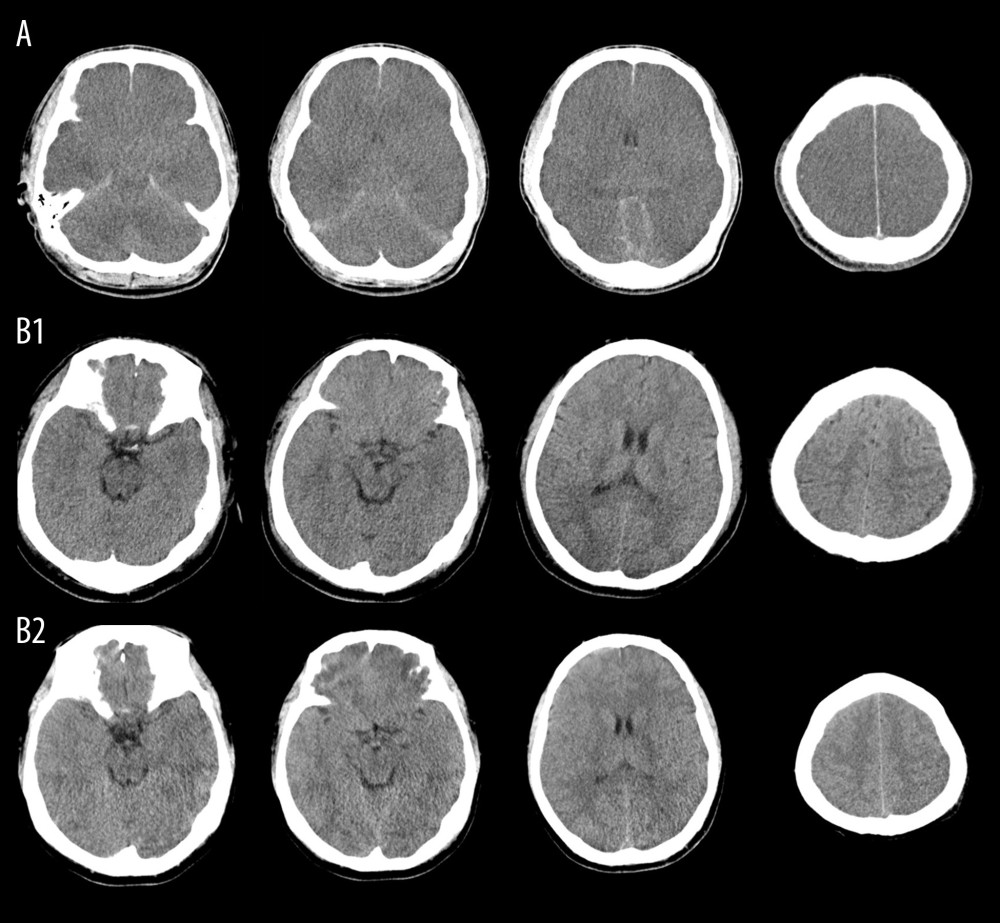 Figure 3. Representative Images of brain CT scanBrain computed tomography (CT) scan of a 22-year-old male heatstroke patient admitted with persistent coma showed diffuse encephalic swelling, compressed or occluded ventricles, sulci, and cisterna, and extensive subarachnoid hemorrhage on the 3rd day after admission (A). Another 27-year-old male heatstroke patient admitted with GCS of 5 survived; although his brain CT scan did not show swelling or edema at admission (B1), and a light encephalic swelling was shown by a CT scan 6 days later (B2), his GCS improved after admission and he survived.
Figure 3. Representative Images of brain CT scanBrain computed tomography (CT) scan of a 22-year-old male heatstroke patient admitted with persistent coma showed diffuse encephalic swelling, compressed or occluded ventricles, sulci, and cisterna, and extensive subarachnoid hemorrhage on the 3rd day after admission (A). Another 27-year-old male heatstroke patient admitted with GCS of 5 survived; although his brain CT scan did not show swelling or edema at admission (B1), and a light encephalic swelling was shown by a CT scan 6 days later (B2), his GCS improved after admission and he survived. References
1. Hunter GD, Notes on heat and “heat-stroke” at Assouan in the Summer of 1886: Br Med J, 1887; 2(1384); 65
2. , Heat-stroke: Hospital (Lond 1886), 1888; 4(99); 319
3. , Heatstroke: Hospital (Lond 1886), 1903; 33(861); 442-43
4. Liu SY, Song JC, Mao HDExpert Group of Heat Stroke Prevention and Treatment of the People’s Liberation Army, and People’s Liberation Army Professional Committee of Critical Care Medicine, Expert consensus on the diagnosis and treatment of heat stroke in China: Mil Med Res, 2020; 7(1); 1
5. Asmara IGY, Diagnosis and management of heatstroke: Acta Med Indones, 2020; 52(1); 90-97
6. Sharma A, Deng L, Wang YC, Estimation of effects of extreme temperature on the risk of hospitalisation in Taiwan: J Epidemiol Community Health, 2023 [Online ahead of print]
7. Wu X, Brady JE, Rosenberg H, Li G, Emergency Department visits for heat stroke in the United States, 2009 and 2010: Inj Epidemiol, 2014; 1(1); 8
8. Pan MZ, Xu HH, Dong CYAnalysis on influencing factors of deaths from severe heatstroke in Shanghai, 2013–2017: Zhonghua Yu Fang Yi Xue Za Zhi, 2019; 53(1); 93-96 [in Chinese]
9. Wen YT, Liu TT, Lin YF, Heatstroke effect on brain heme oxygenase-1 in rats: Int J Med Sci, 2015; 12(9); 737-41
10. Shimazaki J, Hifumi T, Shimizu K, Clinical characteristics, prognostic factors, and outcomes of heat-related illness (Heatstroke Study 2017–2018): Acute Med Surg, 2020; 7(1); e516
11. Young GB, Coma: Ann N Y Acad Sci, 2009; 1157; 32-47
12. Zhang XY, Li J, Susceptibility-weighted imaging in heat stroke: PLoS One, 2014; 9(8); e105247
13. Matis G, Birbilis T, The Glasgow Coma Scale – a brief review. Past, present, future: Acta Neurol Belg, 2008; 108(3); 75-89
14. Jain S, Iverson LM, Glasgow Coma Scale: StatPearls [Internet] Jun 21, 2022, Treasure Island (FL), StatPearls Publishing
15. Chun JK, Choi S, Kim HH, Predictors of poor prognosis in patients with heat stroke: Clin Exp Emerg Med, 2019; 6(4); 345-50
16. Yang MM, Wang L, Zhang Y, Establishment and effectiveness evaluation of a scoring system for exertional heat stroke by retrospective analysis: Mil Med Res, 2020; 7(1); 40
17. Li P, Yang L, Liu R, Chen RL, The value of the exertional heat stroke score for the prognosis of patients with exertional heat stroke: Am J Emerg Med, 2021; 50; 352-55
18. Shimazaki J, Hifumi T, Shimizu K, Clinical characteristics, prognostic factors, and outcomes of heat-related illness (Heatstroke Study 2017–2018): Acute Med Surg, 2020; 7(1); e516
19. de Castro RRT, Filho RC, Nóbrega ACL, Fulminant liver failure in a street runner: Effects of heat stroke: Rev Assoc Med Bras (1992), 2018; 64(3); 208-11
20. Niu KC, Lin MT, Chang CP, Hyperbaric oxygen improves survival in heatstroke rats by reducing multiorgan dysfunction and brain oxidative stress: Eur J Pharmacol, 2007; 569(1–2); 94-102
21. Liu Y, Subedi K, Baride A, Peripherally misfolded proteins exacerbate ischemic stroke-induced neuroinflammation and brain injury: J Neuroinflammation, 2021; 18(1); 29
22. Yi J, He G, Yang J, Heat acclimation regulates the autophagy-lysosome function to protect against heatstroke-induced brain injury in mice: Cell Physiol Biochem, 2017; 41(1); 101-14
23. Liu TT, Hu CH, Tsai CD, heatstroke induces autophagy as a protection mechanism against neurodegeneration in the brain: Shock, 2010; 34(6); 643-48
24. Lo YC, Yen DH, Guo WY, Yang CC, Diffuse cerebral cortex, cerebellar cortex and basal ganglia injury: A rare MR imaging manifestation of heatstroke: Neuroradiol J, 2007; 20(1); 37-40
25. Du Y, Xu JT, Jin HN, Increased cerebral expressions of MMPs, CLDN5, OCLN, ZO1 and AQPs are associated with brain edema following fatal heatstroke: Sci Rep, 2017; 7(1); 1691
26. Kreitzer N, Lindsell CJ, Hart K, Adeoye O, In reply: GCS in prognostication after traumatic brain injury: Am J Emerg Med, 2017; 35(8); 1191
27. Jolobe OMP, Glasgow coma scale versus computed tomography in prognostication: Am J Emerg Med, 2017; 35(8); 1190
28. Qin W, Wang S, Yang L, Correlation between bispectral index and prognosis of patients with acute cerebral infarction: Curr Neurovasc Res, 2021; 18(4); 389-94
29. Abouhashem S, Eldawoody H, Functional outcome after primary decompressive craniectomy for acute subdural hematoma in severe traumatic brain injury: Turk Neurosurg, 2022; 32(2); 211-20
30. Abeytunge K, Miller MR, Cameron S, Development of a mortality prediction tool in pediatric severe traumatic brain injury: Neurotrauma Rep, 2021; 2(1); 115-22
31. Yang M, Li Z, Zhao Y, Outcome and risk factors associated with extent of central nervous system injury due to exertional heatstroke: Medicine (Baltimore), 2017; 96(44); e8417
32. Yang MM, Wang L, Zhang Y, Establishment and effectiveness evaluation of a scoring system for exertional heatstroke by retrospective analysis: Mil Med Res, 2020; 7(1); 40
33. Epstein Y, Yanovich R, Heatstroke: N Engl J Med, 2019; 380(25); 2449-59
Figures
 Figure 1. Sub-group analysis of co-effects of coma on other mortality-associated risk factorsAll patients were grouped according to whether they were comatose or not. Coma (GCS ≤8) was applied as a stratification factor. Among the coma patients, older age and higher RR, HCT, PLT, and CKMB were all harmful factors (OR≥1, and P value less than 0.05) (all P≤0.05). These results indicated that an additional 1 year of age, 1/min of RR, 1% of HCT, and 1×109/L of PLT increased the risks of death by 3%, 6%, 4%, and 0.3%, respectively (all P≤0.05). GCS – Glasgow Coma Scale; APACHE II score – Acute Physiology and Chronic Health Evaluation II score; SOFA score – Sequential Organ Failure Assessment Score; HR – heart rate; RR – respiratory rate; MAP – mean arterial pressure; Tmax – the max body temperature; Tadmit – the body temperature at admission; HCT – hematocrit; PLT – platelets; TBIL – total bilirubin; ALB – albumin; SCr – serum creatinine; CK-MB – creatine kinase isoenzyme. The data of the figure were calculated and compared using R language with EmpowerStats 2.0 software, and the forest plot was drawn using Python 3.8.
Figure 1. Sub-group analysis of co-effects of coma on other mortality-associated risk factorsAll patients were grouped according to whether they were comatose or not. Coma (GCS ≤8) was applied as a stratification factor. Among the coma patients, older age and higher RR, HCT, PLT, and CKMB were all harmful factors (OR≥1, and P value less than 0.05) (all P≤0.05). These results indicated that an additional 1 year of age, 1/min of RR, 1% of HCT, and 1×109/L of PLT increased the risks of death by 3%, 6%, 4%, and 0.3%, respectively (all P≤0.05). GCS – Glasgow Coma Scale; APACHE II score – Acute Physiology and Chronic Health Evaluation II score; SOFA score – Sequential Organ Failure Assessment Score; HR – heart rate; RR – respiratory rate; MAP – mean arterial pressure; Tmax – the max body temperature; Tadmit – the body temperature at admission; HCT – hematocrit; PLT – platelets; TBIL – total bilirubin; ALB – albumin; SCr – serum creatinine; CK-MB – creatine kinase isoenzyme. The data of the figure were calculated and compared using R language with EmpowerStats 2.0 software, and the forest plot was drawn using Python 3.8. Figurre 2. Association of Glasgow Coma Scale (GCS) with outcomes in heatstroke(A). Kaplan-Meier Curve. Patients with GCS ≤8 at admission had poorer clinical outcomes. (B). Kinetics of GCS. GCSs in non-surviving patients were significantly lower than those in surviving patients. (C). Receiver operating characteristic curve (ROCs). GCSs on the 1st, 3rd, and 5th days after admission predicted mortality. (D). Improved GCS predicted better clinical outcomes. The figures were drawn using the SPSS 20.0 for windows and EmpowerStats 2.0 software.
Figurre 2. Association of Glasgow Coma Scale (GCS) with outcomes in heatstroke(A). Kaplan-Meier Curve. Patients with GCS ≤8 at admission had poorer clinical outcomes. (B). Kinetics of GCS. GCSs in non-surviving patients were significantly lower than those in surviving patients. (C). Receiver operating characteristic curve (ROCs). GCSs on the 1st, 3rd, and 5th days after admission predicted mortality. (D). Improved GCS predicted better clinical outcomes. The figures were drawn using the SPSS 20.0 for windows and EmpowerStats 2.0 software. Figure 3. Representative Images of brain CT scanBrain computed tomography (CT) scan of a 22-year-old male heatstroke patient admitted with persistent coma showed diffuse encephalic swelling, compressed or occluded ventricles, sulci, and cisterna, and extensive subarachnoid hemorrhage on the 3rd day after admission (A). Another 27-year-old male heatstroke patient admitted with GCS of 5 survived; although his brain CT scan did not show swelling or edema at admission (B1), and a light encephalic swelling was shown by a CT scan 6 days later (B2), his GCS improved after admission and he survived.
Figure 3. Representative Images of brain CT scanBrain computed tomography (CT) scan of a 22-year-old male heatstroke patient admitted with persistent coma showed diffuse encephalic swelling, compressed or occluded ventricles, sulci, and cisterna, and extensive subarachnoid hemorrhage on the 3rd day after admission (A). Another 27-year-old male heatstroke patient admitted with GCS of 5 survived; although his brain CT scan did not show swelling or edema at admission (B1), and a light encephalic swelling was shown by a CT scan 6 days later (B2), his GCS improved after admission and he survived. Tables
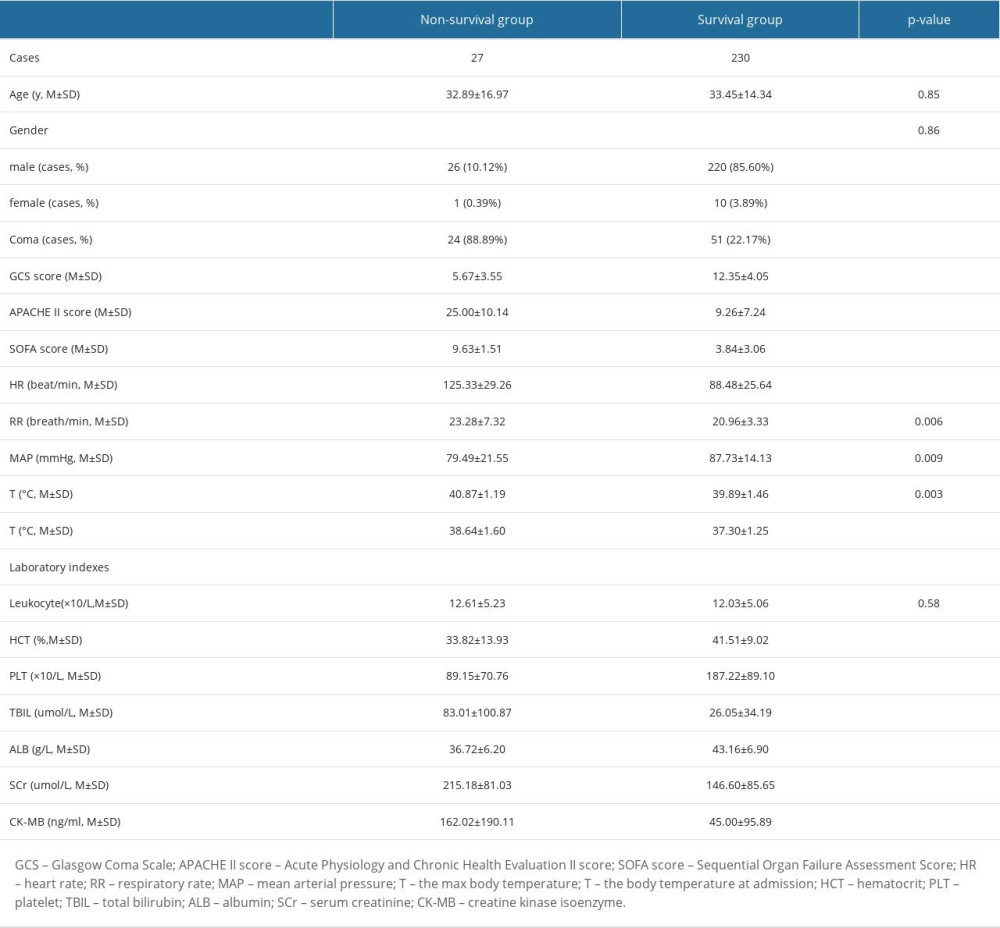 Table 1. Baseline demographic characteristics of non-surviving and surviving patients with heatstroke.
Table 1. Baseline demographic characteristics of non-surviving and surviving patients with heatstroke.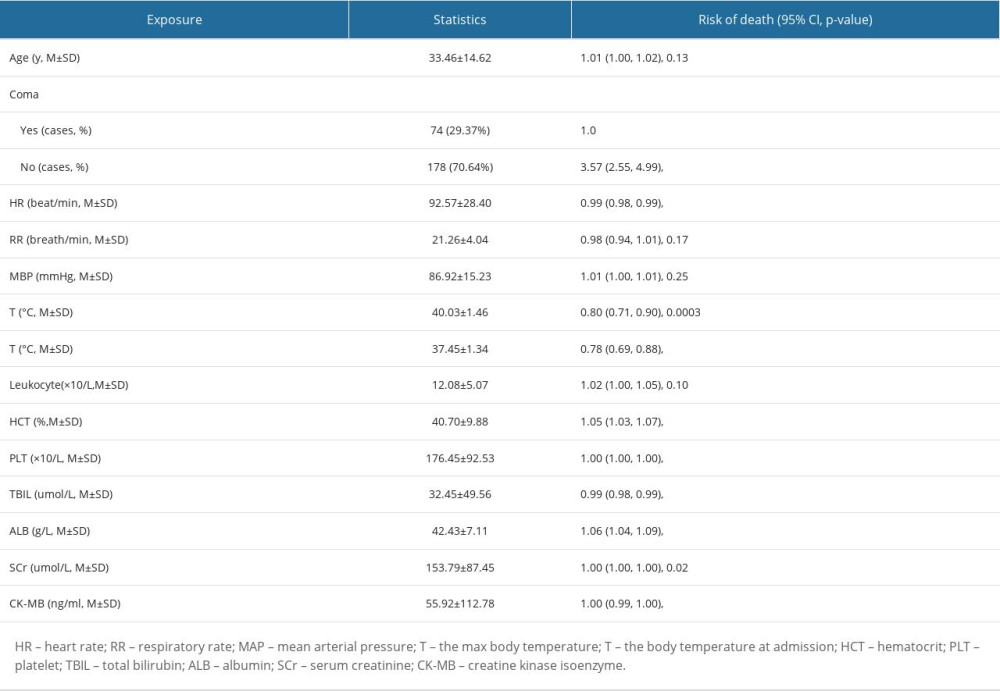 Table 2. Univariate analysis of mortality-associated risk factors in heatstroke.
Table 2. Univariate analysis of mortality-associated risk factors in heatstroke.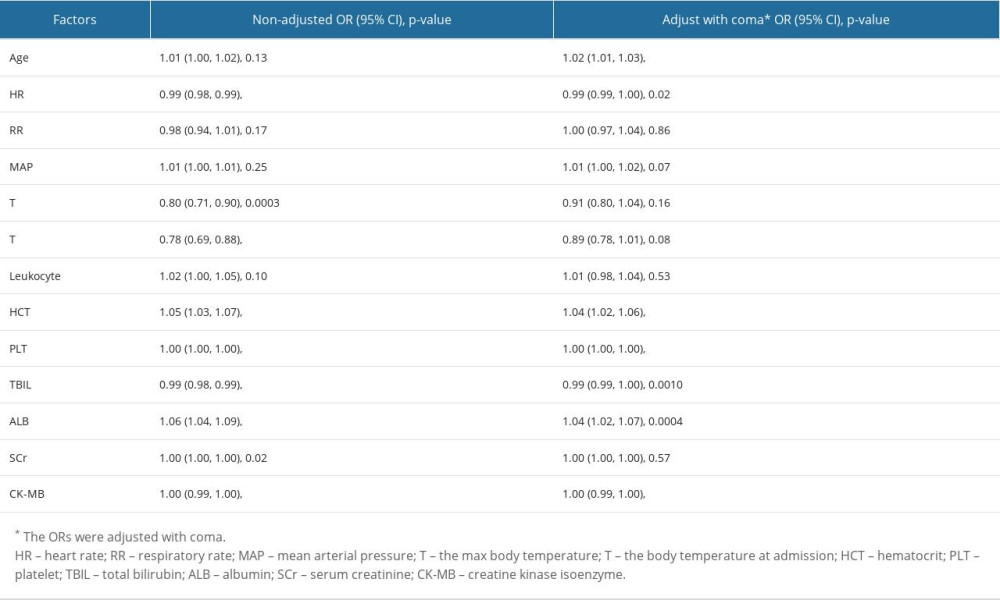 Table 3. Multivariate logistic regression analysis of risk factors.
Table 3. Multivariate logistic regression analysis of risk factors. Table 1. Baseline demographic characteristics of non-surviving and surviving patients with heatstroke.
Table 1. Baseline demographic characteristics of non-surviving and surviving patients with heatstroke. Table 2. Univariate analysis of mortality-associated risk factors in heatstroke.
Table 2. Univariate analysis of mortality-associated risk factors in heatstroke. Table 3. Multivariate logistic regression analysis of risk factors.
Table 3. Multivariate logistic regression analysis of risk factors. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








