12 June 2023: Clinical Research
Toward Integration of Pharmacogenomic Tests into Daily Clinical Practice: A General Survey for Polish Healthcare Workers
Alireza TafazoliDOI: 10.12659/MSM.940119
Med Sci Monit 2023; 29:e940119
Abstract
BACKGROUND: Pharmacogenomics (PGx) has a direct influence on personalized drug therapy for various types of disorders and has been proven to have an important role in the future of medicine. The present study evaluated the awareness of PGx testing of clinicians and healthcare workers in the Republic of Poland. To the best of our knowledge, this is the first direct assessment of Polish healthcare professionals’ attitudes toward introducing PGx tests into daily clinical practice.
MATERIAL AND METHODS: We used a comprehensive anonymous questionnaire with queries on level of education, background knowledge of PGx tests, advantages and barriers for implementation of such tests, and clinicians’ desire to order the test that was distributed online to doctors, healthcare workers, related students/Ph.D. students, and administrative staff managing healthcare units.
RESULTS: We gathered 315 responses. According to the answers, two-thirds of participants had heard about PGx before (64.4%). An overwhelming majority of respondents appreciated the benefits of PGx (93.3%). Indeed, prior knowledge and level of education showed significant associations with positive attitudes toward PGx clinical testing (P≤0.05). However, all participants agreed there are major challenges for including such tests as part of routine clinical practice.
CONCLUSIONS: While the awareness and interest in PGx clinical testing in Polish healthcare providers are rising, some main barriers for implementation of these tests still need to be addressed in Poland.
Keywords: Pharmacogenetics, Poland, Knowledge, Attitude, Physicians, Patient Care Team, Humans, Health Personnel, Educational Status
Background
Pharmacogenomics (PGx) is an interdisciplinary translational science for primary care that focuses on the utilization of an individual’s genomic profile to predict therapy response and prevention of adverse drug reactions (ADRs) or severe adverse effects in patients, while improving therapeutic outcomes [1]. PGx modifies patients’ prescriptions to adjust treatment decisions while considering the patients’ genomic profiles. In recent years, there were rapid developments in preparing PGx guidelines and annotations for many gene-drug interactions [2].
However, while PGx tests have been described as an important area in clinical centers, still there are many challenges for the incorporation of such tests into the routine clinical setting [3,4]. There are many obstacles for PGx tests to reach clinical utility and validity. These include, but are not limited to, a lack of background knowledge of general practitioners and physicians on the interpretation of a given PGx test result, absence of a comprehensive standardized program and/or recommendation to run PGx test in clinics (how the target patients must be selected, for example), small number of insurance providers for supporting the costs of such tests, and the need to provide a special infrastructure for cutting-edge panel-based PGx testing that can be implemented through any clinical center with the need for it [5–7]. Notably, an interactive discussion and exchange on personalized medicine through clinicians and other healthcare providers and also researchers would help fill the gap between the current efforts in investigations and the requirements in clinical setting to ensure that PGx testing with clinical utility could be brought to every patient in need [8,9].
Future research may emphasize preparation of more region-specific pharmacovariant (genomic variants in drug-related genes) evidences and target panel sequencing approaches aimed at distinct ethnic groups [10–12]. Furthermore, widespread and easy access to local genomic databases plus the PGx data for people in a portable format, such as a specific card with a QR code linked to the database (eg, Ubiquitous Pharmacogenomics Consortium. Ubiquitous Pharmacogenomics [U-PGx] project’s PGx passport [13]), might be essential. To deal with the above-mentioned topics, timely novel and original studies and evaluations would be required. However, initial steps seem to be the use of a general analytical survey that measures the primary knowledge of doctors and clinicians along with their desire to order clinical PGx tests that would be beneficial for both the community and individuals across populations.
The present research aimed to evaluate the level of awareness of PGx testing in clinicians and healthcare workers in the Republic of Poland. We aimed to demonstrate the associated difficulties and requirements for the implementation of such tests as part of the daily clinical practice through a specific questionnaire for the participants as well. Knowing about the main barriers of PGx testing is a fundamental phase for these genetic tests to be considered by the national healthcare system. To the best of our knowledge, this is the first direct assessment of the backgrounds and attitudes of Polish clinicians and healthcare workers toward the integration of PGx tests into a routine clinical context.
Material and Methods
STATISTICAL ANALYSIS:
Statistical analysis was performed in R v.4.1.2 (free open source,
Results
QUESTION 1: HAVE YOU EVER HEARD ABOUT PHARMACOGENOMIC TESTS?:
Table 1 also show the distribution of prior knowledge about pharmacogenomics. About two-thirds of participants had heard about PGx tests before. Fisher’s exact test did not detect any significant dependence between the group of respondents and the answer.
QUESTION 2: IS IT WORTH DOING PHARMACOGENOMIC TESTS?:
For this question, “yes” was the most common answer in all groups of participants (Figure 1). Only 3 respondents answered “no” to this question. Employment in healthcare services was not significantly connected with support for the use of PGx (Figure 1). Conversely, there was a significant association between the answer to the question and both the level of education and prior knowledge about PGx. In particular, the respondents who had not heard about PGx before were more likely to answer “I don’t know” to the question. The respondents at the medium educational level were more likely to give a negative answer to the question (although the uncertainty of this effect was big due to the small number of such participants and only 1 negative answer). To investigate the details, we repeated the tests after removing some groups of answers/respondents (Table 2). Removal of the answer “I don’t know” eliminated the dependence between the answer and the prior knowledge about PGx. The dependence on the education level disappeared after the removal of the group with medium education level.
QUESTION 3: BARRIERS TO IMPLEMENTATION OF PHARMACOGENETIC TESTS:
Table 3 and Figures 1, 2 show the frequency of particular answers to this question in different groups of participants. The most common answer in all the groups was “all of the above reasons”. The only association was found between education level and the answer. Namely, respondents with a higher education level were more likely to answer “all the barriers are important” than were other groups, and this difference was significant. However, students were more likely to give single answers (instead of “all the reasons”) for the third question, which asked about obstacles. Finally, we added 3 tests in the R script for the associations we found in the data. The result proved all the associations were significant as well (Table 4).
Discussion
LIMITATIONS:
To keep the data as private as possible and reduce participant hesitancy to share responses, we decided to not assess where the respondents were working or being educated. However, knowing about the workplace and place of study could also have been helpful in evaluating the depth and breadth of the responses. Also, the survey data collection was performed only for 4 months because we wanted to assess participants’ knowledge and attitudes in a very current timeframe. However, a longer period of time could have led to more collected data and a more meaningful result. Finally, the fact that only 1 question relied on background knowledge of participants and the depth of actual knowledge could not be assessed in a quick survey raises the issue of replacing the term “knowledge” with “awareness” of PGx.
Conclusions
This study revealed the positive trend and attitude among Poland’s clinicians and other healthcare system employees/students toward the integration of PGx tests in routine clinical practice. While the awareness and interest of the participants may encourage healthcare and legislative stakeholders to provide facilities with the necessities for wide scale clinical implementation of PGx tests, some main barriers remain. These need to be removed if the entire country wants to receive the benefits of personalizing drug therapy using genetic information.
Figures
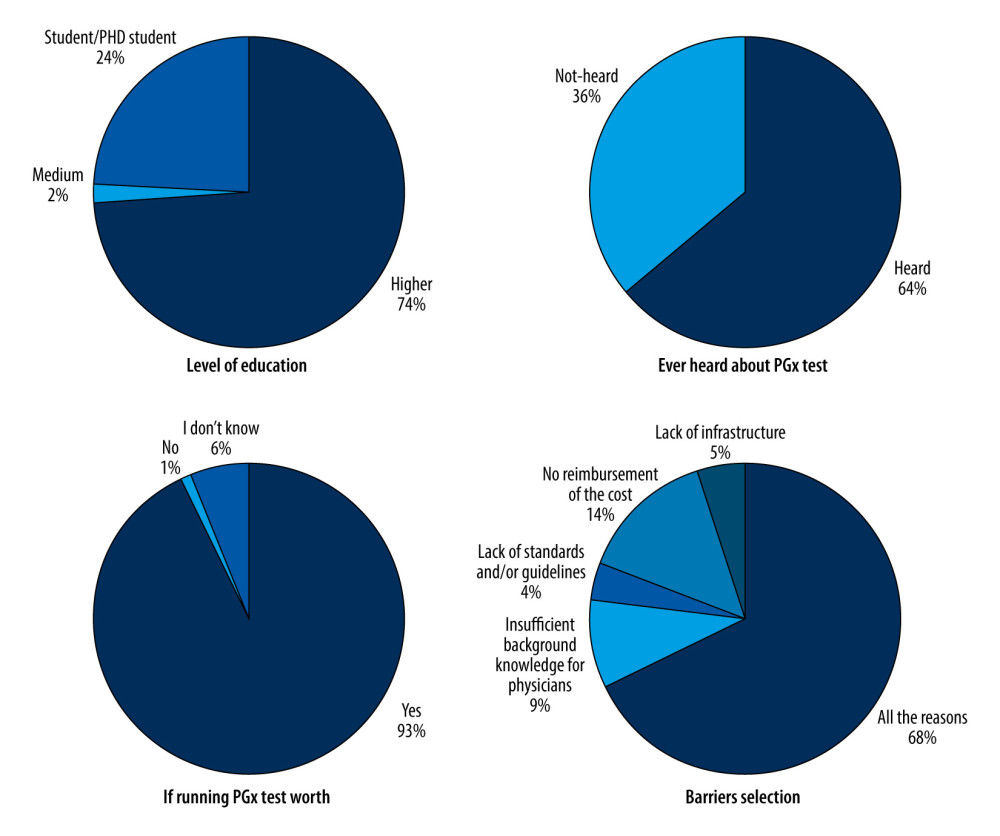 Figure 1. Pie plots displaying the independent proportion of each answer for the questions in the survey. Test benefits vs heard about pharmacogenomics (PGx). Test benefits vs the level of education. Barriers vs education. The level of education of the participants was provided separately. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.).
Figure 1. Pie plots displaying the independent proportion of each answer for the questions in the survey. Test benefits vs heard about pharmacogenomics (PGx). Test benefits vs the level of education. Barriers vs education. The level of education of the participants was provided separately. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.). 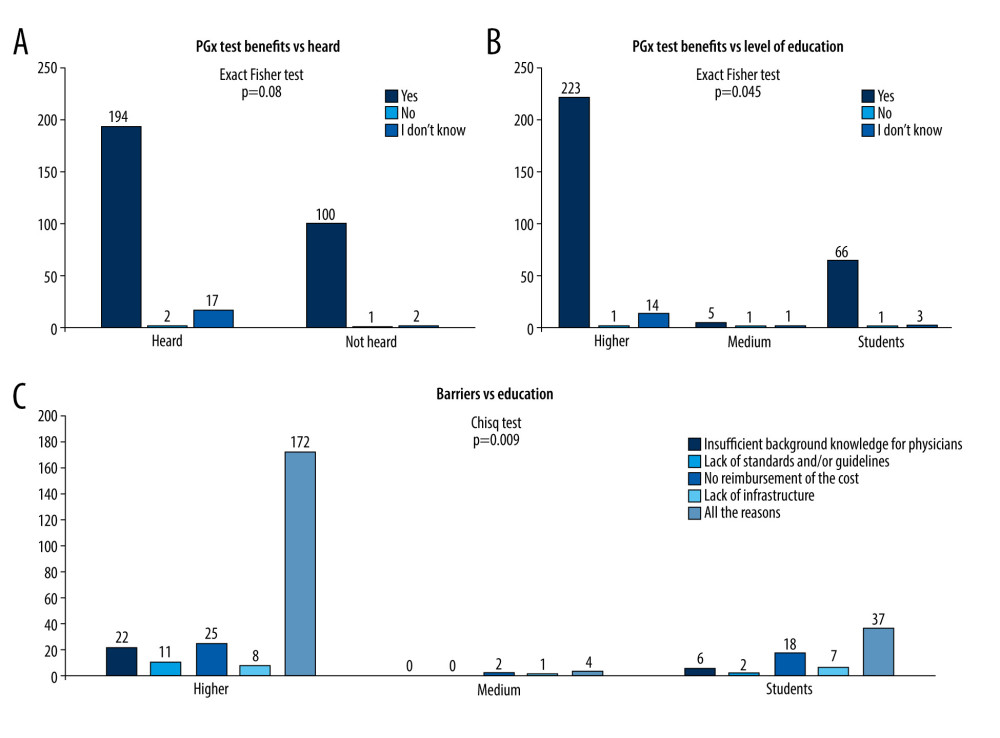 Figure 2. Bar plots for the association between answers and variables. (A) Answers to the question about benefits of pharmacogenomics (PGx) test vs prior knowledge. (B) Answers to the question about benefits of PGx test vs education level. (C) Distribution of answers to the third question which indicates the opinion about the main barriers for PGx test implementation vs different levels of education. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.).
Figure 2. Bar plots for the association between answers and variables. (A) Answers to the question about benefits of pharmacogenomics (PGx) test vs prior knowledge. (B) Answers to the question about benefits of PGx test vs education level. (C) Distribution of answers to the third question which indicates the opinion about the main barriers for PGx test implementation vs different levels of education. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.). Tables
Table 1. The question had the participant heard about the pharmacogenomics vs other descriptive variables.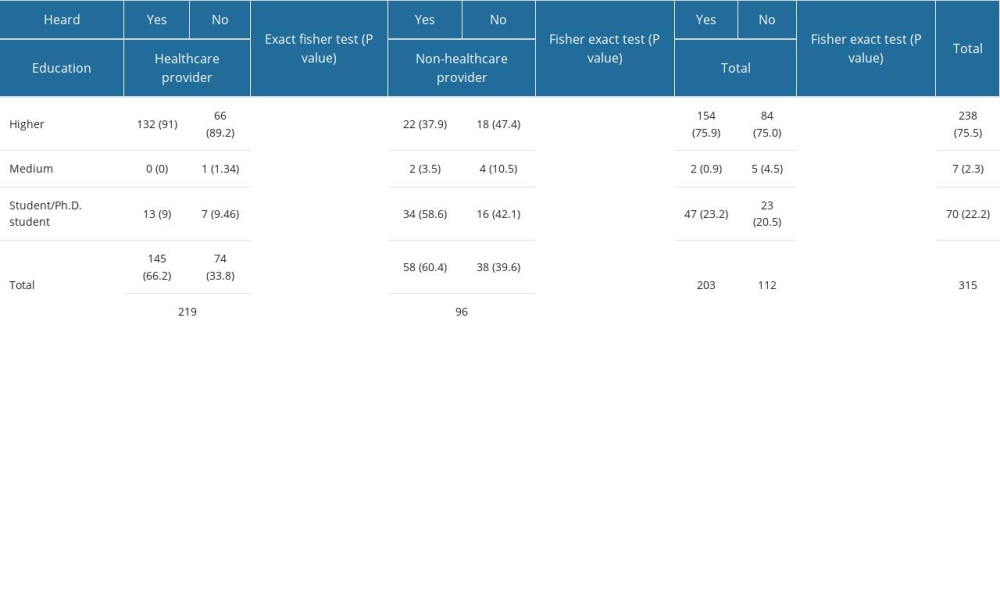 Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data.
Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data.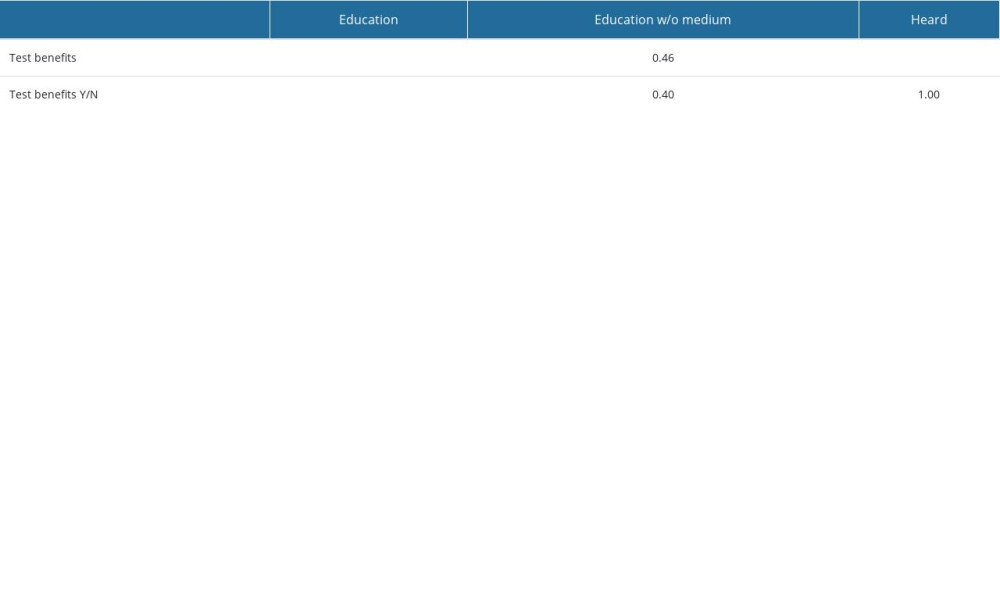 Table 3. Answer about barriers* vs other descriptive variables.
Table 3. Answer about barriers* vs other descriptive variables.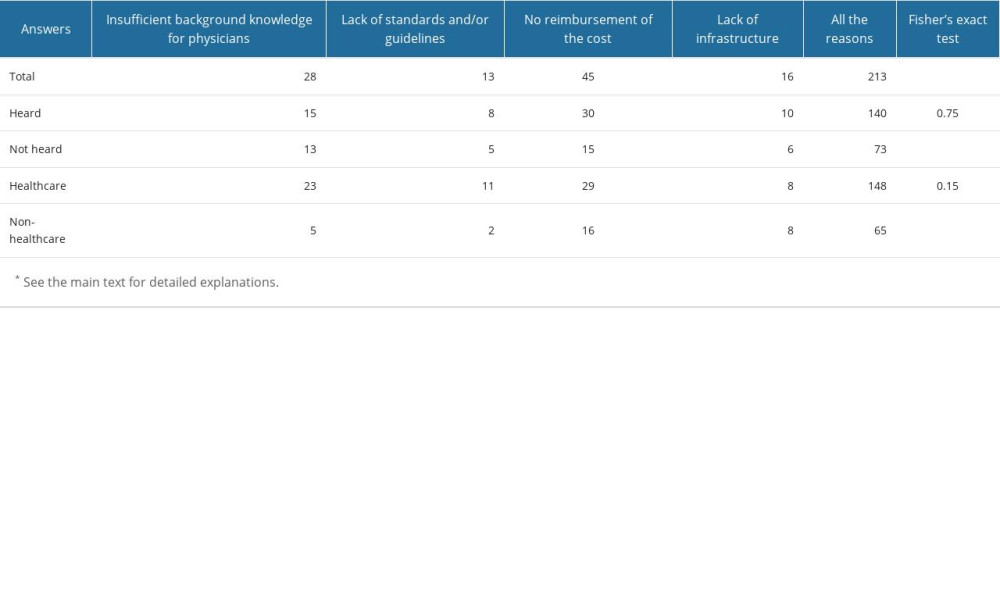 Table 4. Fisher’s exact test results for selected associations.
Table 4. Fisher’s exact test results for selected associations.
References
1. Hippman C, Nislow C, Pharmacogenomic testing: Clinical evidence and implementation challenges: J Pers Med, 2019; 9(3); 40
2. Abdullah-Koolmees H, van Keulen AM, Nijenhuis M, Deneer VHM, Pharmacogenetics guidelines: Overview and comparison of the DPWG, CPIC, CPNDS, and RNPGx guidelines: Front Pharmacol, 2021; 11; 595219
3. Lam YW, Scientific challenges and implementation barriers to translation of pharmacogenomics in clinical practice: ISRN Pharmacol, 2013; 2013; 641089
4. Abou Diwan E, Zeitoun RI, Abou Haidar L, Implementation and obstacles of pharmacogenetics in clinical practice: An international survey: Br J Clin Pharmacol, 2019; 85(9); 2076-88
5. Rafi I, Crinson I, Dawes M, Rafi D, The implementation of pharmacogenomics into UK general practice: A qualitative study exploring barriers, challenges and opportunities: J Community Genet, 2020; 11(3); 269-77
6. Chang WC, Tanoshima R, Ross CJD, Carleton BC, Challenges and opportunities in implementing pharmacogenetic testing in clinical settings: Annu Rev Pharmacol Toxicol, 2021; 61; 65-84
7. Hayward J, McDermott J, Qureshi N, Newman W, Pharmacogenomic testing to support prescribing in primary care: A structured review of implementation models: Pharmacogenomics, 2021; 22(12); 761-76
8. Peterson JF, Field JR, Shi Y, Attitudes of clinicians following large-scale pharmacogenomics implementation: Pharmacogenomics J, 2016; 16(4); 393-98
9. Ho TT, Gift M, Alexander E, Prioritizing pharmacogenomics implementation initiates: A survey of healthcare professionals: Per Med, 2022; 19(1); 15-23 [Erratum in: Per Med. 2022;19(2):165]
10. Nagar SD, Moreno AM, Norris ET, Population pharmacogenomics for precision public health in Colombia: Front Genet, 2019; 10; 241
11. Hernandez W, Danahey K, Pei X, Pharmacogenomic genotypes define genetic ancestry in patients and enable population-specific genomic implementation: Pharmacogenomics J, 2020; 20(1); 126-35
12. Ji X, Ning B, Liu J, Towards population-specific pharmacogenomics in the era of next-generation sequencing: Drug Discov Today, 2021; 26(8); 1776-83
13. van der Wouden CH, van Rhenen MH, Jama WOM, Development of the PGx-passport: A panel of actionable germline genetic variants for pre-emptive pharmacogenetic testing: Clin Pharmacol Ther, 2019; 106(4); 866-73
14. : MUB-Pharmacogenomics Available from: https://www.umb.edu.pl/farmakogenomika
15. Hashemi-Soteh MB, Nejad AV, Ataei G, Knowledge and attitude toward genetic diseases and genetic tests among pre-marriage individuals: A cross-sectional study in northern Iran: Int J Reprod Biomed, 2019; 17(8); 543-50
16. Guo C, Hu B, Guo C, A survey of pharmacogenomics testing among physicians, pharmacists, and researchers from China: Front Pharmacol, 2021; 12; 682020
17. Adesta F, Mahendra C, Junusmin KI, Pharmacogenomics implementation training improves self-efficacy and competency to drive adoption in clinical practice: Front Pharmacol, 2021; 12; 684907
18. Mustapa MAC, Amin L, Mahadi Z, Determinants of stakeholders’ intention to adopt pharmacogenomic: Pharmacogenomics J, 2020; 20(6); 801-12
19. Gurwitz D, Lunshof JE, Dedoussis G, Pharmacogenomics education: International Society of Pharmacogenomics recommendations for medical, pharmaceutical, and health schools deans of education: Pharmacogenomics J, 2005; 5(4); 221-25
20. Karas K, Prodan Ž, Gurwitz D, European Society of Pharmacogenomics and Personalized Therapy (ESPT). Pharmacogenomics education in medical and pharmacy schools: Conclusions of a global survey: Pharmacogenomics, 2019; 20(9); 643-57
21. Frick A, Benton CS, Scolaro KL, Transitioning pharmacogenomics into the clinical setting: Training future pharmacists: Front Pharmacol, 2016; 7; 241
22. Shugg T, Pasternak AL, London B, Luzum JA, Prevalence and types of inconsistencies in clinical pharmacogenetic recommendations among major U.S. sources: NPJ Genom Med, 2020; 5; 48
23. Tafazoli A, Guggilla RK, Kamel-Koleti Z, Miltyk W, Strategies to improve the clinical outcomes for direct-to-consumer pharmacogenomic tests: Genes (Basel), 2021; 12(3); 361
24. Johansen Taber KA, Dickinson BD, Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties: Pharmgenomics Pers Med, 2014; 7; 145-62
25. Virelli CR, Mohiuddin AG, Kennedy JL, Barriers to clinical adoption of pharmacogenomic testing in psychiatry: A critical analysis: Transl Psychiatry, 2021; 11(1); 509
26. Tata E, Ambele M, Pepper M, Barriers to implementing clinical pharmacogenetics testing in Sub-Saharan Africa. A critical review: Pharmaceutics, 2020; 12(9); 809
27. McKinnon RA, Ward MB, Sorich MJ, A critical analysis of barriers to the clinical implementation of pharmacogenomics: Ther Clin Risk Manag, 2007; 3(5); 751-59
28. Cecchin E, Roncato R, Guchelaar HJ, Toffoli GUbiquitous Pharmacogenomics Consortium, Ubiquitous Pharmacogenomics (U-PGx): The time for implementation is now. An Horizon2020 Program to Drive Pharmacogenomics into Clinical Practice: Curr Pharm Biotechnol, 2017; 18(3); 204-9
29. Swen JJ, van der Wouden CH, Manson LE, A 12-gene pharmacogenetic panel to prevent adverse drug reactions: An open-label, multicentre, controlled, cluster-randomised crossover implementation study: Lancet, 2023; 401(10374); 347-56
30. : SafePolyMed Available from: https://www.eurice.eu/news/2022/07/06/launch-of-new-eu-project-safepolymed-towards-safer-drug-treatment-and-enhanced-patient-empowerment
31. Tafazoli A, van der Lee M, Swen JJ, Development of an extensive workflow for comprehensive clinical pharmacogenomic profiling: Lessons from a pilot study on 100 whole exome sequencing data: Pharmacogenomics J, 2022; 22(5–6); 276-83
32. : KRAUM Available from: https://konferencje.umw.edu.pl/nowoczesnaedukacja/
33. Mela A, Poniatowski ŁA, Drop B, Overview and analysis of the cost of drug programs in Poland: Public payer expenditures and coverage of cancer and non-neoplastic diseases related drug therapies from 2015–2018 years: Front Pharmacol, 2020; 11; 1123
34. Lemke AA, Hutten Selkirk CG, Glaser NS, Primary care physician experiences with integrated pharmacogenomic testing in a community health system: Per Med, 2017; 14(5); 389-400
35. Just KS, Steffens M, Swen JJ, Medical education in pharmacogenomics-results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U-PGx): Eur J Clin Pharmacol, 2017; 73(10); 1247-52
Figures
 Figure 1. Pie plots displaying the independent proportion of each answer for the questions in the survey. Test benefits vs heard about pharmacogenomics (PGx). Test benefits vs the level of education. Barriers vs education. The level of education of the participants was provided separately. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.).
Figure 1. Pie plots displaying the independent proportion of each answer for the questions in the survey. Test benefits vs heard about pharmacogenomics (PGx). Test benefits vs the level of education. Barriers vs education. The level of education of the participants was provided separately. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.). Figure 2. Bar plots for the association between answers and variables. (A) Answers to the question about benefits of pharmacogenomics (PGx) test vs prior knowledge. (B) Answers to the question about benefits of PGx test vs education level. (C) Distribution of answers to the third question which indicates the opinion about the main barriers for PGx test implementation vs different levels of education. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.).
Figure 2. Bar plots for the association between answers and variables. (A) Answers to the question about benefits of pharmacogenomics (PGx) test vs prior knowledge. (B) Answers to the question about benefits of PGx test vs education level. (C) Distribution of answers to the third question which indicates the opinion about the main barriers for PGx test implementation vs different levels of education. Medium education level indicates education up to high school. Higher education means an academic degree (educated in university, with a degree such as B.Sc., M.Sc., M.D., Ph.D., or Pharm.D.). Tables
 Table 1. The question had the participant heard about the pharmacogenomics vs other descriptive variables.
Table 1. The question had the participant heard about the pharmacogenomics vs other descriptive variables. Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data.
Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data. Table 3. Answer about barriers* vs other descriptive variables.
Table 3. Answer about barriers* vs other descriptive variables. Table 4. Fisher’s exact test results for selected associations.
Table 4. Fisher’s exact test results for selected associations. Table 1. The question had the participant heard about the pharmacogenomics vs other descriptive variables.
Table 1. The question had the participant heard about the pharmacogenomics vs other descriptive variables. Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data.
Table 2. Fisher’s exact test results for answers to the question about the benefits of the pharmacogenomics test for truncated data. Table 3. Answer about barriers* vs other descriptive variables.
Table 3. Answer about barriers* vs other descriptive variables. Table 4. Fisher’s exact test results for selected associations.
Table 4. Fisher’s exact test results for selected associations. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








