07 June 2023: Database Analysis
Influenza and Influenza-Like Virus Infection in Children Under 14 Years of Age: An Investigation of 725 Cases in the 2021/2022 Influenza Epidemic Season in Poland
Katarzyna Kondratiuk1ABCDEF, Ewelina Hallmann1ABCDEF*, Katarzyna Łuniewska1ABCDEF, Karol SzymańskiDOI: 10.12659/MSM.940368
Med Sci Monit 2023; 29:e940368
Abstract
BACKGROUND: Influenza in children poses a significant health problem worldwide. In this study we investigated 725 cases of influenza and influenza-like virus infection in children under 14 years of age in the 2021/2022 influenza epidemic season in Poland.
MATERIAL AND METHODS: The material for the study (nose and throat swabs) was collected during the 2021/2022 epidemic season. We analyzed 725 samples from the National Influenza Center, Department of Influenza Research at the National Institute of Public Health NIH-NRI or at 16 Voivodship Sanitary Epidemiological Stations across Poland. Quantitative polymerase chain reaction (qRT-PCR) was applied to determine the influenza virus type and subtype (in RNA isolated from positive samples).
RESULTS: This study shows the high incidence of influenza among children under the age of 14. Most confirmed infections were caused by influenza A. The genetic material of the A/H1N1/pdm09 subtype was not found among the analyzed samples. The highest number of influenza A infections was among the youngest children (the 0-4 years age group). The most common influenza-like virus was respiratory syncytial virus (RSV). The greatest number of cases caused by this respiratory virus was registered among the youngest children (0-4 years).
CONCLUSIONS: This study, which shows the high incidence of influenza among children under the age of 14, highlights the importance of regular influenza vaccination. Since children often play a dominant role in spreading influenza virus in the community, regular vaccination can have both health and economic benefits for all age groups.
Keywords: Influenza A virus, Influenza B virus, Epidemiology, Respiratory Syncytial Virus Infections, Influenza, Human, Child, Humans, Infant, Influenza A Virus, H1N1 Subtype, Poland, Seasons, Communicable Diseases
Background
Influenza in children is one of the most common viral respiratory diseases. The current classification of influenza viruses is based on their biological and molecular properties. The influenza virus is an RNA virus of the
Influenza is characterized by a sudden onset of intense symptoms. Initially, the following symptoms are observed: fever (usually high, reaching 40°C), muscle and joint pain, headache, chills, fatigue, and weakness. With the development of the infection, other respiratory symptoms may occur: cough, runny nose, or sore throat. Unlike adults, children are more likely to experience gastrointestinal manifestations such as nausea, vomiting, and diarrhea. Flu symptoms in children usually last about 7 days, but a child can be contagious for up to 10 days (usually up to 3 days after the fever subsides). The first symptoms of flu in a child can be easily mistaken for a cold, but compared with the common cold, the flu lasts longer and its course is more severe [5]. Quickly detecting influenza virus in the body, especially in children, enables immediate anti-influenza treatment, thus reducing the risk of serious post-influenza complications and death. It needs to be emphasized that the use of anti-influenza drugs (so-called neuraminidase inhibitors) is up to the doctor if possible after laboratory confirmation of an influenza infection. The fundamental and most reliable methods of detecting influenza viruses in material collected from patients are molecular biology methods. It is estimated that 10–30% of children become infected with influenza each flu season [4]. Although influenza virus infection in healthy children is usually self-limiting and uncomplicated, it can be dangerous and fulminant. The younger the child and the less efficient the immune system, the more serious flu complications can be, which can even lead to the child’s death.
Influenza prevention is mainly based on vaccination. Since 2000, the Advisory Committee on Immunization Practices (ACIP) has advised that it is best to become vaccinated against influenza before the start of the influenza epidemic season, as soon as the vaccine appears on the pharmaceutical market. This recommendation applies especially to patients from high-risk groups, which may include young children [6]. The youngest age when the influenza vaccine can be administered is 6 months old, depending on the vaccine type. Even if a child gets the flu, it will be easier for them to cope with the illness and they will be less likely to suffer from flu-related complications. Optimally, vaccination should be carried out at the beginning of the flu season, which falls on October 1 each year. In accordance with the recommendations of the ACIP and the American Academy of Pediatrics (AAP), vaccination against influenza is recommended for everyone above the age of 5 years, depending on the vaccine type, unless there are medical contraindications [7]. In Poland, in the 2021/2022 epidemic season, a 4-component influenza vaccine is available: an inactivated split vaccine (with a split virion, recommended from the age of 6 months) and an inactivated subunit vaccine (ie, only glycoprotein proteins, containing hemagglutinin and neuraminidase) administered from the age of 3 years. Inactivated split vaccines and subunit influenza vaccines are immunologically equivalent and are administered deep intramuscularly. From November 2019, the so-called “live” vaccine, ie, attenuated (prepared from viruses adapted to a reduced replication temperature) has been used. The so-called “viva” vaccine is intended to actively immunize healthy children and adolescents aged 24 months to 17 years of age. The preparation is in the form of aerosol used intranasally [6,8]. Vaccinating school-aged children against influenza protects them while reducing deaths in the older population and in high-risk patients [9,10].
Children are at risk not only of influenza but also of other respiratory viruses (so-called flu-like viruses) that are circulating at the same time, with symptoms similar to those of a flu infection.
The World Health Organization (WHO) global influenza surveillance standards define the surveillance case definitions for influenza-like illness (ILI) as an acute respiratory infection with fever ≥38°C and cough, with onset within the last 10 days (WHO). The most commonly diagnosed flu-like viruses include: parainfluenza 1–4 (PIV-1, PIV-2, PIV-3, PIV-4), human respiratory syncytial virus (RSV), human corona virus (HCoV), human rhinovirus (RV), human adenovirus (ADV), and human metapneumovirus (hMPV) [5,11–14].
Emerging infectious diseases are a major threat to global health security, which was clearly shown by the recent COVID-19 pandemic. The ease of transmission makes respiratory pathogens especially suited for epidemic spread [15]. Children constitute a very important group in spreading the flu virus throughout the community. A higher fraction of vaccinated children would most likely reduce the number of infections in all age groups.
Material and Methods
MATERIAL COLLECTION:
The samples (nasal and throat swabs) were taken from children up to 14 years of age in the whole of Poland under the SENTINEL and Non-SENTINEL surveillance systems. These samples were analyzed at the Department of Influenza Research, National Influenza Center at National Institute of Public Health NIH – National Research Institute (NIPH NIH – NRI) and Voivodship Sanitary Epidemiological Stations (VSES). Tests for the presence of genetic material of influenza viruses and influenza-like viruses were performed on 725 samples.
ISOLATION OF RNA:
Nasal and throat swabs were suspended in 1 mL of saline. RNA isolation from these materials was performed using the Maxwell 16 Viral Total Nucleic Acid Purification Kit (Promega Corporation, Madison, WI) according to the manufacturer’s instructions; 200 μL of clinical sample suspended in saline was used and 50 μL of RNase-free water was obtained.
REAL-TIME POLYMERASE CHAIN REACTION:
Real-time reverse transcription polymerase chain reaction (rRT-PCR) was used to determine the influenza virus type and subtype. In the Department of Influenza Research, National Influenza Center, the Rotor-Gene Q thermal cycler (Qiagen) and a set of reagents – SuperScript Platinum III kit (Invitrogen) – were used for this purpose. Primer sets for each subtype of influenza viruses (influenza A, influenza A/H3N2/, influenza A/H1N1/pdm09, and influenza B) were received from International Reagent Resource (IRR) of the Centers for Disease Control and Prevention. The 20 μL reaction mixture consisted of 5 μL of RNA, 0.4 μM of each primer, 0.4 μM of each probe, 10 μL of 2×reaction buffer mix, 0.4 μL of SuperScript III/Platinum Taq Mix, and 3.4 μL of water. To obtain complementary DNA for isolated RNA, reverse transcription reaction was conducted in 50°C for 30 min. Next, initial denaturation was performed at 95°C for 2 min. Amplification was carried out under the following conditions: 45 cycles of denaturation (95°C for 15 s), annealing (55°C for 30 s), and elongation (72°C for 20 s). The positive controls for the reactions were isolated from viruses derived for the vaccine from the 2021/2022 epidemic season: A/Victoria/2570/2019 (H1N1)pdm09, A/Cambodia/e0826360/2020 (H3N2), B/Washington/02/2019 (B/Victoria lineage), and B/Phuket/3073/2013 (B/Yamagata lineage).
Results
CASES OF INFLUENZA IN TESTED SAMPLES FROM CHILDREN:
The presence of genetic material of influenza viruses was confirmed in 314 samples (45.5% of all tested samples). As in the epidemic season 2019/2020 [16], the vast majority of confirmed cases were influenza A infections – 272 cases (82.4% of all confirmed influenza infections), while 42 cases of influenza B were confirmed (12.7% of all confirmed influenza cases). Out of the confirmed influenza A cases, 114 were subtype A/H3N2/ infections. Among influenza A, the remaining cases were unsubtyped influenza A viruses. The genetic material of the A/H1N1/pdm09 subtype was not found among the analyzed samples. It was different in the epidemic season 2019/2020 in that influenza A/H1N1/pdm09 viruses dominated among the types of influenza A viruses in this season [16]. The course of the 2020/2021 epidemic season was clearly different from the trend in the previous seasons. No positive sample was recorded this season in the age group 0–14 years.
Figure 1 shows the number of confirmed cases of influenza virus in the age groups 0–4, 5–9, 10–14 in the 2021/2022 epidemic season in Poland.
In accordance with the innovation in influenza supervision introduced by the National Influenza Center at the Department of Influenza Research at NIPH NIH-NRI, the results were also analyzed in 3 smaller age groups (0–4, 5–9, 10–14) [11]. The highest number of influenza A infections was confirmed among the youngest children, in the 0–4 years group (116 confirmed cases) and in the 5–9 years group (114 confirmed cases). In the 10–14 age group, 42 infections caused by influenza type A were recorded. Most confirmed cases of influenza type B infections were recorded among children aged 5–9 years (25 confirmed cases). In the youngest children, aged 0–4, there were 10 such infections, while in the 10–14 group there were 7 infections.
CASES OF INFLUENZA-LIKE INFECTIONS IN TESTED SAMPLES FROM CHILDREN:
Influenza-like infections are also very common in children under the age of 14 years. This has been confirmed by the analyzed results of infection studies in children. As in the epidemic season 2019/2020 [16], the highest number of RSV infections was recorded, with 12 confirmed in children aged 0–14, which accounted for 80.0% of all influenza-like virus infections confirmed in the laboratory using the molecular biology method. In this age group, 3 cases of ADV infection were also confirmed. After analyzing the research results, it was found that the largest number of infections with influenza-like viruses was confirmed among the youngest children, aged 0–4. Similar results were shown by a study conducted by Pellegrinelli et al [17]. The risk of infection from RSV in the 0–5 years age group was more than 18-fold greater than that observed in the 6–10 years age group, and 33-fold greater than that in the 11–14 years age, demonstrating the massive impact of RSV infection in the youngest age group [17].
The proportion of positive samples in relation to all the tested samples in the 0–14 age group in the 2020/2021 epidemic season in Poland is shown in Figure 2.
CASES OF CO-INFECTION IN TESTED SAMPLES FROM CHILDREN:
In the 2021/2022 epidemic season, among the tested samples taken from children aged up to 14, 1 co-infection was also registered (simultaneous infection with 2 or more respiratory viruses). In a 10-year-old child, genetic material of both influenza A virus (subtype A/H3N2/) and influenza B virus was detected.
STATISTICAL ANALYSIS:
Fisher’s exact tests were performed to check for differences between age groups in terms of their vulnerability to the 2 virus types: A and B. Since there were 3 age groups, 3 tests were conducted, checking whether the ratio of A cases to B cases varied significantly between each pair of the age groups. We found that in neither of the pairs was the difference significant at the 1% significance level: the
Discussion
Infectious diseases, including influenza and influenza-like viruses, pose a serious threat to global health security. This has been manifested by the recent COVID-19 pandemic, which was declared a pandemic by the WHO on March 11, 2020 [18]. This has prompted governments around the world to introduce a variety of non-pharmaceutical solutions to limit the spread of the virus and thus reduce the burden on healthcare systems. Precautions such as wearing protective masks, disinfecting hands and shared surfaces, social distancing, and bans on gatherings have made past flu seasons unprecedentedly mild. The emergence of the new SARS-CoV-2 virus has initiated an interesting trend in many countries around the world associated with a decrease in the number of upper respiratory tract viral infections, eg, influenza and RSV [19]. This trend continued, to varying degrees, during the further waves of infections caused by the SARS-CoV-2 virus. Data from the European Center for Disease Prevention and Control (ECDC) showed a 99.5% decrease in confirmed influenza cases in the 2020/2021 epidemic season, across all age groups [15,16]. This was often due to the health policy of a given country. During the COVID-19 pandemic, a record small number of cases of influenza was also observed in Poland. In the 2020/2021 epidemic season, in the SENTINEL and Non-SENTINEL systems, no infection caused by influenza viruses was confirmed among children under the age of 14 [20].
In Poland, biological material for testing for the possible presence of influenza virus is collected from sick people too rarely. A huge decrease in the number of samples tested for the presence of genetic material of influenza viruses was recorded during the recent SARS-CoV-2 virus pandemic. The period of the COVID-19 pandemic was marked by diagnoses made mainly for SARS-CoV-2. Research on other respiratory viruses has been limited, but this does not mean that other respiratory viruses have suddenly stopped circulating. This is a worrying phenomenon, because if patients are not tested for influenza, it will not be possible to develop an influenza vaccine for the next influenza epidemic season or to prevent post-influenza complications with new-generation anti-influenza drugs, such as neuraminidase [19,21]. During the SARS-CoV-2 pandemic in Poland, in the 2020/2021 epidemic season, a much smaller number of samples was also tested among children than in other epidemic seasons. Figure 3 shows the number of samples taken from children aged 0–14 and tested for the presence of genetic material of influenza viruses and influenza-like viruses in the epidemic seasons 2019/2020, 2020/2021, and 2021/2022 in Poland.
The circulation of respiratory (and therefore influenza) viruses in the community is believed to be largely driven by household transmission of influenza viruses. Therefore, young children play a significant role in the transmission of such infections [19]. Stay-at-home orders during the pandemic have reduced social interaction outside the household, thereby reducing the circulation of respiratory viruses in pediatric populations and thus the further spread of respiratory viruses to other groups, including those at an increased risk of infection, such as the elderly [30].
All the measures taken to limit the transmission of COVID-19, such as wearing masks, limiting mobility, and remote learning, inhibit the spread of both SARS-CoV-2 and the flu virus. However, since all these measures were lifted, we have been seeing a resurgence in the activity of respiratory viruses, including the influenza virus. The analyzed results of tests on samples taken from children aged up to age 14 years indicate numerous infections with influenza and influenza-like viruses in the 2021/2022 epidemic season in Poland.
Studies have shown that fewer cases of severe influenza were observed during certain periods of the SARS-CoV-2 pandemic. In the USA, during the 2020/2021 epidemic season, at the time of the SARS-CoV-2 pandemic, only 1 death of a child caused by post-influenza complications was recorded. In the seasons preceding the pandemic, 144 deaths were recorded in the 2018/2019 epidemic season and 199 deaths in the 2019/2020 epidemic season [21]. In Poland, in the 2021/2022 epidemic season, no deaths of children under 14 years of age were recorded. In the period before the SARS-CoV-2 pandemic, deaths due to post-influenza complications among children were observed in Poland. In the 2018/2019 epidemic season, 1 death due to influenza complications was recorded in the 0–4 age group, while in the 2019/2020 season 5 deaths were recorded in the 5–14 age group [20,23,24].
Although it was thought that our newest enemy (COVID-19) would cause more serious consequences than other respiratory tract infections, recent studies have revealed that influenza is not such a benign virus in children [25]. Tasar et al demonstrated that influenza caused more frequent admissions to intensive care units and a greater need for respiratory support in hospitalized pediatric patients than did COVID-19. Approximately 88.7% of COVID-19 patients did not require respiratory support, while 59.4% of influenza patients did require respiratory support [24]. It is therefore very important to quickly diagnose and identify the virus causing infection in young children. This will allow doctors to implement appropriate treatment as soon as possible to make it as effective as possible. Based on the study by Tasar et al, it can be concluded that influenza can be a serious and frequent cause of complications in children [25].
Despite recommendations and solid, multidirectional educational activities, only 112 285 children aged 0–14 were vaccinated against influenza in Poland in 2020, which is 1.91% of the population of all children this age. This is a very low level compared to analogous data in other European countries [26,27]. Regrettably, there are no data on influenza vaccination in the 6–14 age during influenza epidemic seasons. The proportion of the vaccinated pediatric population in Poland in the years 2006–2014 is shown in Figure 4.
Optimizing the number of vaccinations in this vulnerable population is extremely important, as it is the best way to prevent severe and complicated infections and even deaths from vaccine-preventable diseases. Regular vaccination is one of few things that can be done to protect those at high risk (including children from the age of 6 months to 18 years) from the potential risk of serious complications from influenza and should therefore be part of sound medical practice. Campbell et al [28] conducted a case-control study that showed that influenza vaccination was effective even when the composition of the vaccine had not been sufficiently matched to the strains circulating in the population [28]. They concluded that, in children, influenza vaccination provided marked protection against more severe influenza caused by the 2 dominant types of influenza virus. Most importantly, vaccination halved the risk of hospitalization or emergency department visits for influenza caused by influenza B virus that was genetically and antigenically different from the current influenza vaccine strain. The impact of influenza infection should be considered not only in terms of health loss and often dangerous complications, but also measurable, quantifiable economic costs incurred by the country due to influenza. A study bThe consulting company Ernst & Young, which is part of the National Influenza Control Program, states that the costs of influenza should include both direct and indirect costs of its treatment [29]. The direct costs of influenza treatment include: doctors’ appointments, expenses for medicines, treatment of post-influenza complications, hospitalizations, and specialist examinations. Indirect costs are losses to the economy as a result of sickness absence of employees (eg, dismissal of parents from work due to the child’s illness), long-term absence from work due to illness or caring for a sick person, and severe post-influenza complications, which in turn can lead to partial or complete unfitness for work. Economic analyses also suggest that influenza vaccination has both health and economic benefits [5,29]. Table 1 includes previous studies of the humoral response in high-risk groups.
Considering the very low percentage of the vaccinated population in Poland, the above information will certainly be helpful in promoting prevention and encouraging healthcare professionals to protect themselves, their patients, and their loved ones.
Conclusions
Among many diseases of the respiratory system, influenza is of great importance. The most vulnerable are children who are in high-risk groups that are particularly vulnerable to post-influenza complications: healthy children aged 6–59 months; children with chronic diseases of the cardiovascular or respiratory system, including asthma; and children who often require hospitalization due to various diseases. This study, which shows the high incidence of influenza among children under the age of 14 years, highlights the importance of regular influenza vaccination. It is the cheapest and most effective method of fighting dangerous flu complications. Since children play a very important role in spreading influenza virus in the community, regular vaccination can have both health and economic benefits for all age groups.
Limitations of the study
This work was based solely on the analyses of samples that were reported to the SENTINEL influenza surveillance system. Not all tested samples are reported to the system; therefore, the number of patients studied in Poland in given seasons could be much higher.
Figures
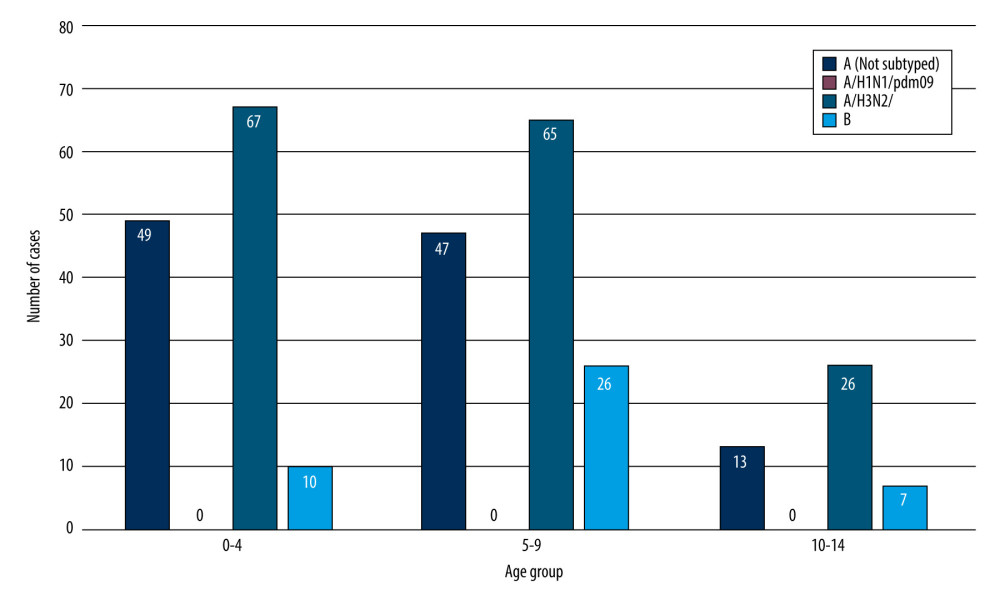 Figure 1. The number of confirmed cases of influenza virus in the age groups 0–4, 5–9, 10–14 in the 2021/2022 epidemic season in Poland.
Figure 1. The number of confirmed cases of influenza virus in the age groups 0–4, 5–9, 10–14 in the 2021/2022 epidemic season in Poland. 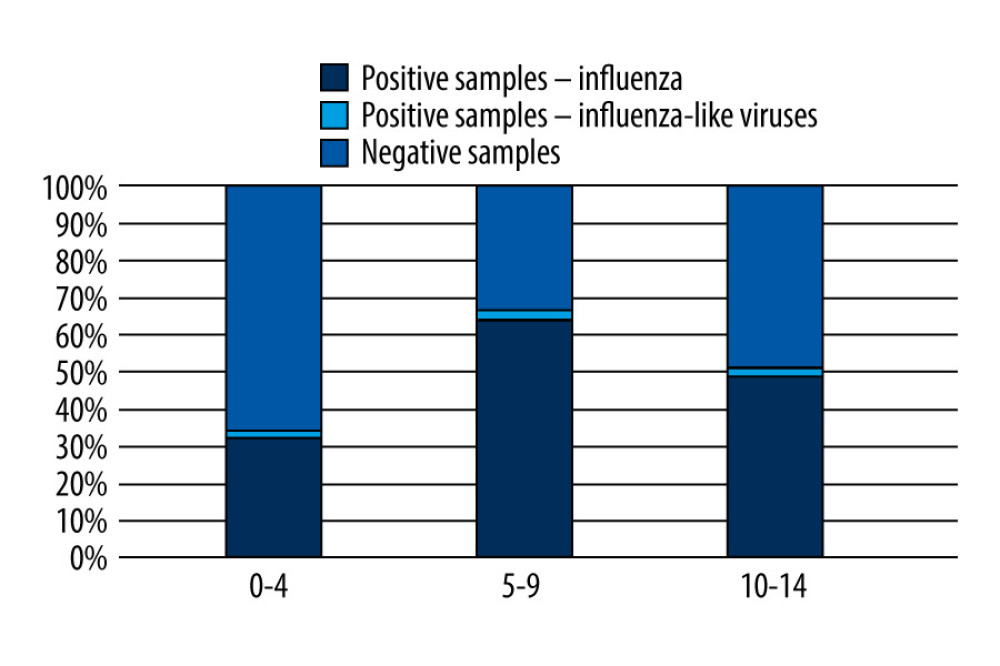 Figure 2. The proportion of positive samples in relation to all tested samples in the 0–14 age group in the 2021/2022 epidemic season in Poland.
Figure 2. The proportion of positive samples in relation to all tested samples in the 0–14 age group in the 2021/2022 epidemic season in Poland. 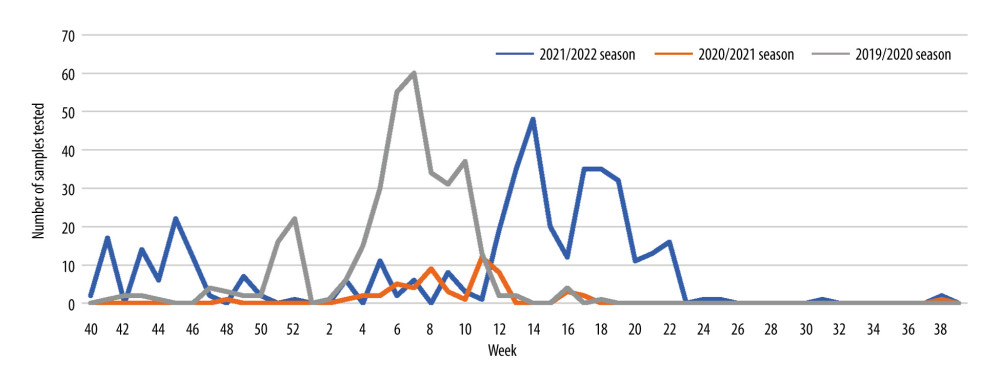 Figure 3. The number of samples taken from children aged 0–14 and tested for the presence of genetic material of influenza viruses and influenza-like viruses in the epidemic seasons 2019/2020, 2020/2021, and 2021/2022 in Poland.
Figure 3. The number of samples taken from children aged 0–14 and tested for the presence of genetic material of influenza viruses and influenza-like viruses in the epidemic seasons 2019/2020, 2020/2021, and 2021/2022 in Poland. 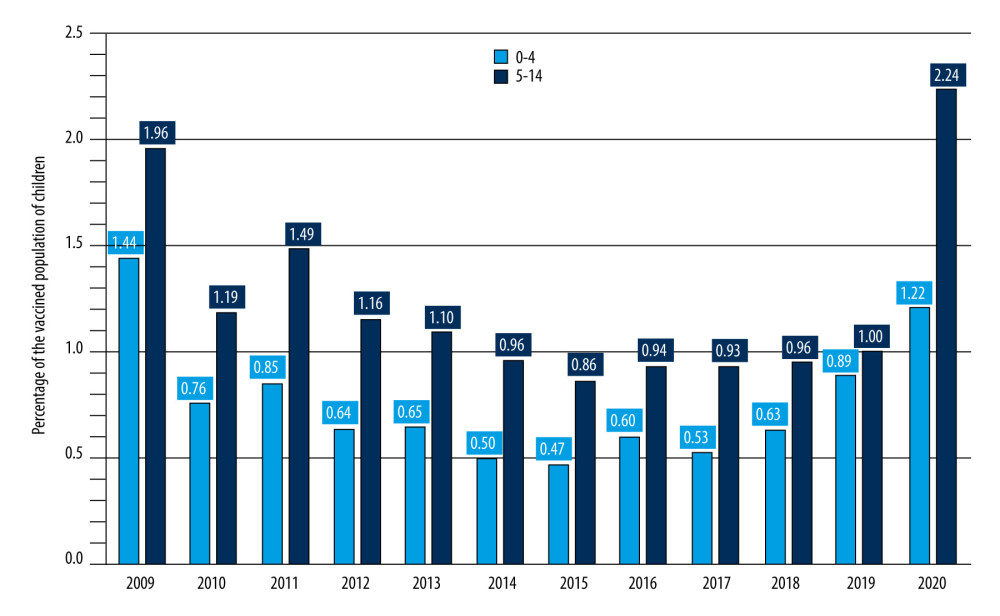 Figure 4. Percentage of vaccinated children in Poland.
Figure 4. Percentage of vaccinated children in Poland. References
1. Li Q, Sun X, Li Z, Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza A virus: Proc Natl Acad Sci USA, 2012; 109(46); 18897-902
2. Tong S, Shu X, Shi M, New World bats harbor diverse influenza A viruses: PLoS Pathog, 2013; 9(10); 1-12
3. Fraaij PLA, Heikkinen T, Seasonal influenza: The burden of disease in children: Vaccine, 2011; 29(43); 7524-28
4. Boddington NL, Mangtani P, Zhao H, Live-attenuated influenza vaccine effectiveness against hospitalization in children aged 2–6 years, the first three seasons of the childhood influenza vaccination program in England, 2013/14–2015/16: Influenza Other Respir Viruses, 2022; 16(5); 897-905
5. Brydak LBInfluenza – prevention and treatment in children and young adolescents: Standardy Medyczne Pediatria, 2019; 2(16); 162-171 [in Polish]
6. Grohskopf LA, Alyanak E, Ferdinands JM, Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2021–22 Influenza Season: MMWR Recomm Rep, 2021; 70(5); 1-28
7. Committee on Infectious Diseases, Recommendations for Prevention and Control of Influenza in Children, 2021–2022: Pediatrics, 2021; 148(4); e2021053745
8. : Szczepienia.info [serial online] [cited 2022 Sept 20]; Available from: URL [in Polish]http://www.szczepienia.info/
9. Brydak LB, 2008; 193-252, Warsaw, Rytm [in Polish]
10. Reichert TA, Sugaya NS, Fedson DS, The Japanese experience with vaccinating schoolchildren against influenza: N Engl J Med, 2001; 43(12); 889-96
11. Bednarska K, Hallmann-Szelińska E, Kondratiuk KInnovations in flu surveillance: Prob Hig Epidemiol, 2016; 97(2); 101-5 [in Polish]
12. Al-Romaihi HE, Smatti MK, Al-Khatib HA, Molecular epidemiology of influenza, RSV, and other respiratory infections among children in Qatar: A six years report (2012–2017): Int J Infect Dis, 2020; 95; 133-41
13. Yoke LL, Wong SY, Hor Lee EK, Prevalence of respiratory viruses among paediatric patients in acute respiratory illnesses in Malaysia: PLoS One, 2022; 17(8); e0265288
14. Brydak LB, 2008; 9-34, Warsaw, Rytm [in Polish]
15. Kutter JS, Spronken MI, Fraaij PL, Transmission routes of respiratory viruses among humans: Current Opinion Virol, 2018; 28; 42-151
16. Kondratiuk K, Hallmann E, Łuniewska K, Influenza and Influenza-Like Respiratory Virus Infections in Children During the 2019/20 Influenza Seazon and the COVID-19 Pandemic in Poland: Data from the Department of Influenza Research, the National Influenza Center at the National Institute of Public Health, National Institute of Hygiene-National Research Institute and 16 Voivodeship Sanitary and Epidemiological Stations: Med Sci Monit, 2021; 27; e934862
17. Pellegrinelli L, Galli C, Bubba L, Respiratory syncytial virus in pediatric influenza-like illness cases in Lombardy, Northern Italy, during seven consecutive winter seasons (from 2014–2015 to 2020–2021): Influenza Other Respir Viruses, 2022; 16(3); 481-91
18. : WHO surveillance case definitions for ILI and SARI [serial online] [cited 2022 Sept 20];]; Available from: URLhttps://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari
19. Chow EJ, Uyeki TM, Chu HY, The effects of the COVID-19 pandemic on community respiratory virus activity: Nat Rev Microbiol, 2022; 17; 1-16
20. NIZP PZH-PIB [serial online] [cited 2022 Sept 20]; Available from: [in Polish]http://wwwold.pzh.gov.pl/oldpage/epimeld/grypa/index.htm
21. Nitsch-Osuch A, Brydak LB, Wardyn AKNeuraminidase inhibitors in the prevention and treatment of influenza: Polski Merkuriusz Lekarski, 2008; 25; 67-73 [in Polish]
22. Nitsch-Osuch A, Brydak LB, Treatment and prophylaxis of influenza and the problem of resistance to neuraminidase inhibitors: Postepy Hig Medy Dosw (Online), 2015; 69; 1087-95
23. European Centre for Disease Prevention and Control: Seasonal influenza. ECDC. Annual epidemiological report 2020, 2021, ECDC
24. Centers for Disease Control and Prevention: Weekly U.S. influenza surveillance report: FluView, 2022 https://www.cdc.gov/flu/weekly/index.htm
25. Tasar S, Karadag-Oncel E, Yilmaz-Ciftdogan D, Influenza is more severe than our newest enemy (COVID-19) in hospitalized children: Experience from a tertiary center: J Med Virol, 2022; 94(9); 4107-14
26. NIZP PZH-PIB [serial online] [cited 2022 Sept 20]; Available from: [in Polish]http://wwwold.pzh.gov.pl/oldpage/epimeld/2020/Sz_2020.pdf
27. , Influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States. 2018–2019, 2018; 67; 1-20
28. Campbell AP, Ogokeh C, Lively JY, Vaccine effectiveness against pediatric influenza hospitalizations and emergency visits: Pediatrics, 2020; 146(5); e20201368
29. Nowakowska E: Pharmacoeconomics in the management of health care resources, 2018; 274-283, Warsaw, Wolters Kluwer
30. Engels G, Sack J, Weissbrich B, Very low incidence of SARS-CoV-2, influenza and RSV but high incidence of rhino-, adeno- and endemic coronaviruses in children with acute respiratory infection in primary care pediatric practices during the second and third wave of the SARS-CoV-2 pandemic: Pediatr Infect Dis J, 2022; 41(4); e146-e48
Figures
 Figure 1. The number of confirmed cases of influenza virus in the age groups 0–4, 5–9, 10–14 in the 2021/2022 epidemic season in Poland.
Figure 1. The number of confirmed cases of influenza virus in the age groups 0–4, 5–9, 10–14 in the 2021/2022 epidemic season in Poland. Figure 2. The proportion of positive samples in relation to all tested samples in the 0–14 age group in the 2021/2022 epidemic season in Poland.
Figure 2. The proportion of positive samples in relation to all tested samples in the 0–14 age group in the 2021/2022 epidemic season in Poland. Figure 3. The number of samples taken from children aged 0–14 and tested for the presence of genetic material of influenza viruses and influenza-like viruses in the epidemic seasons 2019/2020, 2020/2021, and 2021/2022 in Poland.
Figure 3. The number of samples taken from children aged 0–14 and tested for the presence of genetic material of influenza viruses and influenza-like viruses in the epidemic seasons 2019/2020, 2020/2021, and 2021/2022 in Poland. Figure 4. Percentage of vaccinated children in Poland.
Figure 4. Percentage of vaccinated children in Poland. Tables
 Table 1. Research conducted at the Department of Influenza Research, National Influenza Center at NIPH NIH-NRI in collaboration with clinicians in high-risk groups where the humoral response to influenza vaccinations was assessed.
Table 1. Research conducted at the Department of Influenza Research, National Influenza Center at NIPH NIH-NRI in collaboration with clinicians in high-risk groups where the humoral response to influenza vaccinations was assessed. Table 1. Research conducted at the Department of Influenza Research, National Influenza Center at NIPH NIH-NRI in collaboration with clinicians in high-risk groups where the humoral response to influenza vaccinations was assessed.
Table 1. Research conducted at the Department of Influenza Research, National Influenza Center at NIPH NIH-NRI in collaboration with clinicians in high-risk groups where the humoral response to influenza vaccinations was assessed. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








