23 August 2020: Lab/In Vitro Research
Octreotide-Paclitaxel Conjugate Reverses Paclitaxel Resistance by p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in A2780/Taxol Human Ovarian Cancer Cells
Li-li Fan1ABCDEF, Xi Chen1A, Xiao-yu Zhang1A, Ze-min Li1BG, Xue-mei Fan2G, Yang Shen1AEG*DOI: 10.12659/MSM.922612
Med Sci Monit 2020; 26:e922612
Abstract
BACKGROUND: Platinum plus paclitaxel is a first-line chemotherapy for ovarian cancer. Platinum resistance is a hot topic for many scholars, but drug resistance caused by paclitaxel is also a topic of concern. Currently, scholars believe that inhibition of MAPK signaling pathway may be an effective way to reverse the drug resistance of tumor paclitaxel.
MATERIAL AND METHODS: A2780/Taxol cells or nude mice were divided into 8 groups: control group, OCT (octreotide) group, OC (octreotide+cyclosomatostatin) group, PTX (paclitaxel) group, PO (paclitaxel+octreotide) group, POC (paclitaxel+octreotide+cyclosomatostatin) group, P-O (octreotide-paclitaxel conjugate) group, and P-OC (octreotide-paclitaxel conjugate+cyclosomatostatin) group. The phosphorylation level of p38 MAPK and the expression level of vascular endothelial growth factor (VEGF) were determined by western blot. Flow cytometry was used to discover the apoptosis of A2780/Taxol cells and xenografts. The expression of class III beta-tubulin was detected by immunohistochemistry.
RESULTS: Octreotide-paclitaxel conjugate inhibited phosphorylation of the p38MAPK signal pathway, decreased the expression of downstream VEGF, and increased the apoptosis of drug-resistant cancer cells. In addition, it reduced the expression of class III beta-tubulin protein and increase the sensitivity of drug-resistant cells to paclitaxel. All these effects of octreotide-paclitaxel conjugate were cancelled by cyclosomatostatin.
CONCLUSIONS: Octreotide-paclitaxel conjugate can reverse the paclitaxel resistance of A2780/Taxol human ovarian cancer cells by inhibiting the activity of p38 MAPK signaling pathway.
Keywords: Linoleic Acids, Conjugated, Ovarian Neoplasms, paclitaxel, somatostatin, Antineoplastic Agents, Hormonal, Antineoplastic Agents, Phytogenic, octreotide, Peptides, Cyclic, Phosphorylation, Taxoids, tubulin, Tumor Burden, Vascular Endothelial Growth Factor A
Background
Ovarian cancer is one of the 3 most common malignant tumors of female reproductive organs, the mortality rate of which ranks first among malignant tumors of the female reproductive system. The clinical treatment for ovarian cancer is mainly surgery, supplemented by chemotherapy and radiation therapy [1]. As a matter of fact, approximately 70% to 80% of patients with advanced ovarian cancer will still relapse even if they have accepted a satisfactory tumor cell reduction surgery accompanied by a full course of chemotherapy. The 5-year survival rate is only 20% to 30% [2].
Mitogen-activated protein kinase (MAPK) is a type of serine/threonine protein kinase and widely exists in mammalian cells. There are 4 major subtypes of MAPK signaling pathway: ERK, JNK, p38 MAPK and ERK5 [3]. The p38 MAPK signaling pathway represents by far the most investigated class of signal transduction in tumor development. In multicourse chemotherapy, p38 MAPK and JNK signaling pathways can balance the autophagy and apoptosis of tumor cells [4]. P38 MAPK inhibitor SB203580 can obviously promote the apoptosis of ovarian cancer cell lines A2780/T and showed time-dependent inhibitory effect [5,6]. Studies have shown that multiple courses of chemotherapy can inhibit p38 MAPK pathway and increase the expression of survivin, ERCC1, and LRP, then lead to the generation of platinum-resistant epithelial ovarian cancer. Activating the p38 MAPK pathway may ameliorate the prognosis of patients with platinum resistance [7]. However, most scholars believe that the p38 MAPK signaling pathway plays an important role in promoting apoptosis in A2780/Taxol cells and that blocking this pathway can enhance the apoptosis of drug-resistant cells in ovarian cancer [8].
In recent years, the inhibitory effect of somatostatin (SST) and its analogues (SSTAs) on tumor cells has attracted more and more attention. Most ovarian tissues highly express somatostatin receptor 2 (SSTR2). Octreotide as the most commonly used somatostatin analogue (SSTA), can effectively inhibit ovarian cancer cell proliferation and improve the apoptosis rate of ovarian cancer cells through targeted treatment of ovarian cancer combined with SSTR2 on the surface of ovarian tissue
We preliminary synthesized octreotide-paclitaxel conjugate (POC, patent No.: ZL 2013 1 2013 X), and experimental studies of the resistance to A2780/Taxol cells and tumor-burdened nude mice confirmed that the octreotide-paclitaxel conjugate has a highly property of targeting [11]. Tumor-burdened nude mice experiments
Based on the aforementioned research studies, we hypothesized that octreotide has a synergistic sensitization effect with the conjugated paclitaxel, and its mechanism is related to the p38 MAPK signaling pathway. We aimed to explore the drug resistance reversal mechanism of the octreotide-paclitaxel conjugate at the molecular level, to confirm the improvement in the response of ovarian cancer cells to paclitaxel, so as to increase the overall survival rate of recurrent ovarian cancer patients and improve the prognosis.
Material and Methods
CELL CULTURE:
The human ovarian cancer paclitaxel-resistant cell line A2780/Taxol was purchased from Shanghai Bogu Biotechnology Company; the drug resistance index was 30, and the concentration of the culture solution to maintain paclitaxel resistance was 800 ng/mL). Cells were cultured in incubators at 37°C, 5% CO2 with RPMI-1640 medium with 10% fetal bovine serum. Cells grew adherently. The morphology and growth of cells were observed under an inverted phase-contrast microscope daily, and the cells were passaged every 2 days. The experiment began when the cells grew vigorously.
Then 8 groups were set: 1) control group: RPMI-l640 complete culture medium; 2) OCT group: RPMI-1640 with 10 μg/mL OCT; 3) OC group: RPMI-l640 with 10 μg/mL OCT and 0.234 μg/mL cyclosomatostatin (CSS); 4) PTX group- RPMI-l640 with 8 μg/mL PTX; 5) PO group: RPMI-l640 with 10 μg/mL OCT and 8 μg/mL PTX; 6) POC group: RPMI-l640 with 10 μg/mL OCT, 8 μg/mL PTX, and 0.234 μg/mL CSS; 7) P-O group: RPMI-l640 with 20 μg/mL octreotide-paclitaxel conjugate; 8) P-OC group: RPMI-l640 with 20 μg/mL octreotide-paclitaxel conjugate and 0.234 μg/mL CSS. At 48 hours after incubation, the cell samples were collected and expression levels of p38 MAPK, P-p38 MAPK, and vascular endothelial growth factor (VEGF) in the A2780/Taxol cells was detected. The experiments were repeated 3 times. For apoptosis analysis, the cells were treated with agents for 36 hours. For detection of class III beta-tubulin protein expression, the cells were treated with agents for 48 hours.
ANIMALS AND XENOGRAFTS:
Female athymic nude mice (BALB/c-nu/nu, approximately 7 weeks old, weighing 18 to 20 g) were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Science and raised under specific pathogen free (SPF) conditions. A xenograft model was established by subcutaneously injecting 5×106 A2780/Taxol ovarian cancer cells into the right armpit of 40 nude mice. The behavior of mice and tumor growth were detected daily. All animal experiments were approved by the Laboratory Animal Ethics Committee of Southeast University.
After 1 week of inoculation, tumor formation was obvious and nude mice were randomly divided into 8 groups (n=5). Nude mice were injected once weekly with different agents via the tail vein for 3 weeks. The 8 groups were as follows: control group: saline; OCT group: 150 nmol/kg OCT; OC group: 150 nmol/kg OCT and cyclosomatostatin 1.282 nmol/kg; PTX group: 150 nmol/kg PTX; PO group: 150 nmol/kg PTX+150 nmol/kg OCT; POC group: 150 nmol/kg PTX+150 nmol/kg OCT+cyclosomatostatin 1.282 nmol/kg; P-O group: octreotide-paclitaxel conjugate 150 nmol/kg; P-OC group: octreotide-paclitaxel conjugate 150 nmol/kg+cyclosomatostatin 1.282 nmol/kg. The mice were sacrificed 21 days after their first injection. Tumor tissues were collected and divided into 2 halves. One half was fixed in 4% buffered formalin-saline at room temperature for 24 hours for histological experiments and the other half was stored for western blot and apoptosis analysis.
WESTERN BLOT ANALYSIS:
For the
For the
APOPTOSIS OF A2780/TAXOL CELLS:
For the
For the
IMMUNOHISTOCHEMICAL DETECTION OF CLASS III BETA-TUBULIN PROTEIN EXPRESSION:
For the
For the
STATISTICAL ANALYSIS:
SPSS 17.0 was used for data processing. Data were presented as mean ± standard deviation (SD). The
Results
OCTREOTIDE-PACLITAXEL CONJUGATE SUPPRESSED TUMOR GROWTH IN HUMAN OVARIAN CANCER PACLITAXEL-RESISTANT A2780/TAXOL CELL NUDE MOUSE XENOGRAFT MODEL:
In this experiment, 40 nude mice were used to establish a model of human A2780/Taxol nude mouse transplantation tumor. Then 8 groups were randomly divided to examine the efficacy in a human cancer xenograft model carrying paclitaxel-resistant A2780/Taxol cell, namely A2780/Taxol cell nude mouse xenograft model. When dosed for 3 weeks with tail vein injection after tumor formation, there was an obvious mass in the control group. As shown in Figure 1, the OCT group, PO group, and P-O group resulted in statistically significant tumor growth inhibition in the A2780/Taxol cell nude mouse xenograft model. But correspondingly, the OC, PTX, POC, and P-OC groups did not show a similar effect, and the nude mice in these groups still grew a clear mass. Significantly, the mice treated with PTX at the same dose as OCT displayed negligible inhibition effect on tumor growth, which is a direct proof that the human cancer xenograft model is paclitaxel-resistant. The inhibitory effects were strengthened by tumor weight and tumor volume measurements. At the same time, the weight of the mice showed no significant change, suggesting that drugs have little side effect with respect to mouse growth.
OCTREOTIDE-PACLITAXEL REGULATED EXPRESSION OF P-P38MAPK AND VEGF:
In order to verify our speculation, we used the A2780/Taxol cells and the tumor tissue sections to test the protein expression level and assessed the biological activity of the agents. A2780/Taxol cells were treated with different agents for 48 hours, and then p38 MAPK, P-p38 MAPK, and VEGF expression levels were detected via western blot. The OCT, PO and P-O groups had decreased P-p38MAPK expression compared to the control group (Figure 2) (P<0.05). Notably, the expression was downregulated most obviously with octreotide-paclitaxel conjugate treatment compared to OCT or PO (P<0.05). The OC, PTX, POC, and P-OC groups showed negligible effects compared to the control group (P > 0.05). The activation of p38 by the OCT, PO, and P-O groups was also demonstrated with the induction of the expression of VEGF (P<0.05). Direct evidence of P-p38 MAPK inhibition by P-O came from the significant downregulation of VEGF in A2780/Taxol cells after treatment (P<0.05),which further proved that the deactivation of p38 could play an important role in the proliferation of A2780/Taxol cells. Similar changes were observed in the tumor tissue sections in the 8 different groups. In summary, octreotide-paclitaxel conjugate served as an efficient modulator that regulated expression of P-p38 MAPK and VEGF (Figure 3).
APOPTOSIS OF DRUG-RESISTANT CELL A2780/TAXOL AND NUDE MICE:
Cell apoptosis was detected by flow cytometry after the cells were exposed to different drugs for 36 hours respectively and stained with Annexin V-FITC. Annexin-V/PI assays were used to quantify the apoptotic effect. The apoptosis rate of the P-O group was up to 9.65% and the P-O, PO, OCT, PTX, POC, and P-OC groups compared to the control group all showed a higher apoptosis rates (P<0.05). As shown in Figure 4, only the apoptotic effect of the OC group was less pronounced in A2780/Taxol cells than in the control group. The apoptotic trend was not consistent with the previous results in A2780/Taxol cells, and this may be related to the targeting of octreotide and complex intracellular signal-crosstalk mechanism (Figure 4).
In the meantime, Annexin V/PI double dye flow cytometry was used to detect the apoptosis of each group of nude mice with A2780/Taxol transplanted tumor tissues. As shown in Figure 5, apoptosis was induced to different extents by the other 7 groups, which were much larger than the control group. Group P-O showed the strongest inducing apoptosis effect. These results were all suggested that P-O conjugate deactivated the p38 MAPK pathway and induced cell apoptosis.
IMMUNOHISTOCHEMICAL DETECTION OF CLASS III BETA-TUBULIN PROTEIN EXPRESSION:
To measure the protein expression level of class III beta-tubulin protein and assess the biological activity of 8 different groups, we use immunohistochemical detection method and observe the difference in intracellular staining. Notably, lower expression levels of class III beta-tubulin protein were detected in P-O conjugate group. As shown in Figure 6, the optical signal intensity of the P-O, PO and OCT in A2780/Taxol cells showed an obviously reduced density of staining granules in brown-yellow of cytoplasm compared with the control group. Furthermore, the results of groups OC, POC, P-OC were consistent with previous reports on the inhibition effect of cyclosomatostatin (magnification, 400×)
According to the in vitro and in vivo experiments, P-O conjugate efficiently inhibited cancer growth in A2780/Taxol transplanted tumor tissues. Immunohistochemical detection showed similar results with the in vitro experiments (Figure 7). The P-O group had more tumor inhibition effect over other groups. Therefore, these in vivo assessments strongly indicated P-O conjugate as a biocompatible modulator for cancer therapy.
Discussion
This is the first study to confirm the anti-tumor effect of the original synthetic octreotide-paclitaxel conjugate in a human ovarian cancer paclitaxel-resistant cell A2780/Taxol xenograft mouse model and explore the possible mechanisms involved in the anti-tumor effect of octreotide-paclitaxel conjugate. The data demonstrated that octreotide-paclitaxel conjugate displayed a significant inhibition of tumor growth in xenograft mouse model, accelerated apoptosis, decreased the phosphorylation of p-38 MAPK, and the expression of its downstream product VEGF A2780/Taxol. Meanwhile, octreotide-paclitaxel conjugate also inhibited the expression of class III beta-tubulin. Octreotide-paclitaxel conjugate was evidently superior to octreotide, paclitaxel or their combination scheme. It did not affect the normal growth of nude mice.
Paclitaxel plays an important role in regulating the function of cancer cells, which makes cancer cells accumulate a large number of microtubules. The accumulation of microtubules interferes with cell function, especially prevents mitosis, and finally inhibits the growth of cancer cells [13]. Platinum compound plus paclitaxel is the first-line treatment for ovarian cancer chemotherapy scheme. In addition to the platinum-resistance, resistance of cancer cells to paclitaxel also significantly affects survival of patients with late recurrence of ovarian cancer. To enhance drug targeting and reduce drug resistance, we synthesized the octreotide-paclitaxel conjugate based on the ability and mechanisms which octreotide and paclitaxel have shown in previous research.
In our previous studies, we found that in A2780/Taxol cells which were treated with octreotide-paclitaxel conjugate, the expression of VEGF gene and protein were both downregulated, and the reversal of paclitaxel-resistance was associated with the inhibitory effect of SSTR on synthesis and secretion of VEGF. The results of current study further indicated that the expression of VEGF was related to the activity of the p38 MAPK signaling pathway, and octreotide-paclitaxel conjugate downregulated the phosphorylation level of p38MAPK signaling pathway, which consequently decreased and the expression of VEGF. When CSS, which is an SSR antagonist, competitively inhibited the effect of octreotide-paclitaxel conjugate, p38 MAPK signaling pathway was activated and the expression of VEGF was regulated (
The p38 MAPK signaling pathway is a branch of the MAPK family and has cancer-causing potential [14]. It enhances self-proliferation and anti-apoptosis, promotes angiogenesis, increases the ability of unlimited replication, tissue invasion and metastasis [15]. Hypoxia-inducible factors (HIF) induced by hypoxia can activate p38MAPK to increase the expression of VEGF [16] and can also play a role in the downstream signal of VEGF, thereby promotes the regeneration of blood vessels [17].
As the downstream product of activation of the p38 MAPK signaling pathway, VEGF is associated with progression and poor prognosis of different tumors, including lung cancer, colorectal cancer, breast cancer, and ovarian cancer. According to the literature, high expression level of VEGF-A is closely related to the low survival rate of patients with ovarian cancer [18], VEGF-D is involved in lymph node metastases of epithelial ovarian cancer, and VEGF receptor (VEGFR) in subgroups of ovarian cancer is abnormally activated [19]. Interestingly, we found in the current study that the expression of P-p38MAPK was not positively correlated with the expression of VEGF. The expression of VEGF was regulated by the p38MAPK signaling channel, but the downregulated expression of P-p38MAPK protein inhibited the activity of VEGF, which may result in the discrepancy.
In the analysis of anti-tumor agents induced apoptosis in A2780/Taxol cells, octreotide-paclitaxel conjugate showed the strongest inducing apoptosis effect. These results were all suggested that octreotide-paclitaxel conjugate deactivated the p38 MAPK pathway and induced cell apoptosis. It is worth noting that the increase trend of apoptosis in the xenograft mouse model and the P-p38MAPK signaling pathway increase trend of the expression level was not very consistent. It may be associated with targeting property of octreotide, or it may be linked to “signal interaction” with other cell signaling pathways. More research needs to be done to explain this result.
The main target of paclitaxel is microtubules. In A2780/Taxol cells, paclitaxel did not affect the expression of class III beta-tubulin. However, octreotide-paclitaxel conjugate showed significant inhibitory effect on class III beta-tubulin expression compared to octreotide, paclitaxel, or the combination of octreotide and paclitaxel, which indicated that the paclitaxel-resistant cells were still sensitive to octreotide-paclitaxel conjugate. Kavallaris reported that expression level of subtypes of class III β-tubulin was highly increased in ovarian epithelial tumors [20], and expression of class III β-tubulin was significantly decreased after increasing the sensitivity of the cells to paclitaxel. The research results of Gan et al. [21], which were consistent with Kavallaris et al. [20], considered that the cancer cells with lower expression of class III beta-tubulin were more sensitive to paclitaxel [21]. Pascal et al. found that the over-expression of class III beta-tubulin resulted in a decrease in the sensitivity cancer cells to paclitaxel [14]. The expression level of class III beta-tubulin may be an indicator of the efficacy of paclitaxel.
It is not entirely clear how p38 MAPK regulates cell proliferation and apoptosis in the course of ovarian cancer. The specific mechanism by which different cytokines act on ovarian cancer cells to activate the p38MAPK signaling pathway and whether there is “signal interaction” between the p38MAPK pathway and other intracellular signaling pathways remain to be further clarified. The limitation of this study was that the specific inhibitors of p-38 MAPK were not utilized to prevent the phosphorylation of p38 MAPK. The p38 MAPK knockout mouse model is also a good alternative to test whether p38 MAPK is the specific target of octreotide- paclitaxel conjugate.
Conclusions
Octreotide-paclitaxel conjugate can reverse the paclitaxel resistance of A2780/Taxol human ovarian cancer cells by inhibiting the activity of p38MAPK signaling pathway.
Figures
 Figure 1. Comparison of OCT, OC, PTX, PO, POC, P-O and P-OC on tumor growth in xenograft mice model. OCT – octreotide; OC – octreotide+cyclosomatostatin; PTX – paclitaxel; PO – paclitaxel+octreotide; POC – paclitaxel+octreotide+cyclosomatostatin; P-O – octreotide-paclitaxel conjugate; P-OC – octreotide-paclitaxel conjugate+cyclosomatostatin.
Figure 1. Comparison of OCT, OC, PTX, PO, POC, P-O and P-OC on tumor growth in xenograft mice model. OCT – octreotide; OC – octreotide+cyclosomatostatin; PTX – paclitaxel; PO – paclitaxel+octreotide; POC – paclitaxel+octreotide+cyclosomatostatin; P-O – octreotide-paclitaxel conjugate; P-OC – octreotide-paclitaxel conjugate+cyclosomatostatin. 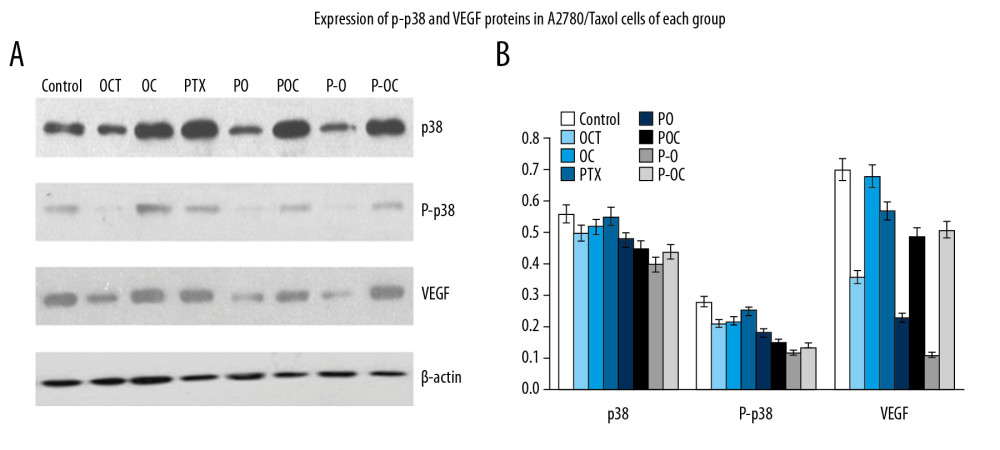 Figure 2. (A, B) Western blot analysis of p38, P-p38 MAPK, and VEGF in A2780/Taxol cells.
Figure 2. (A, B) Western blot analysis of p38, P-p38 MAPK, and VEGF in A2780/Taxol cells. 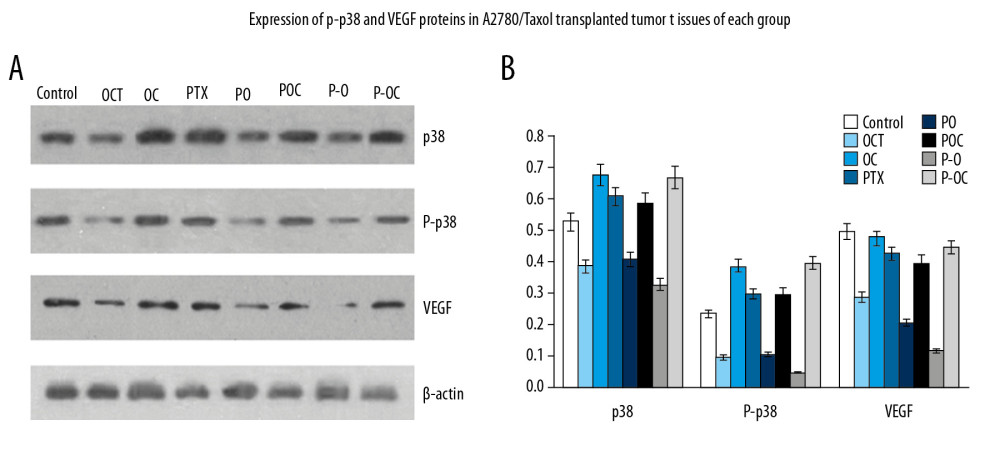 Figure 3. (A, B) Western blot analysis of p38, P-p38 MAPK and VEGF in tumor tissue of xenograft mice model.
Figure 3. (A, B) Western blot analysis of p38, P-p38 MAPK and VEGF in tumor tissue of xenograft mice model. 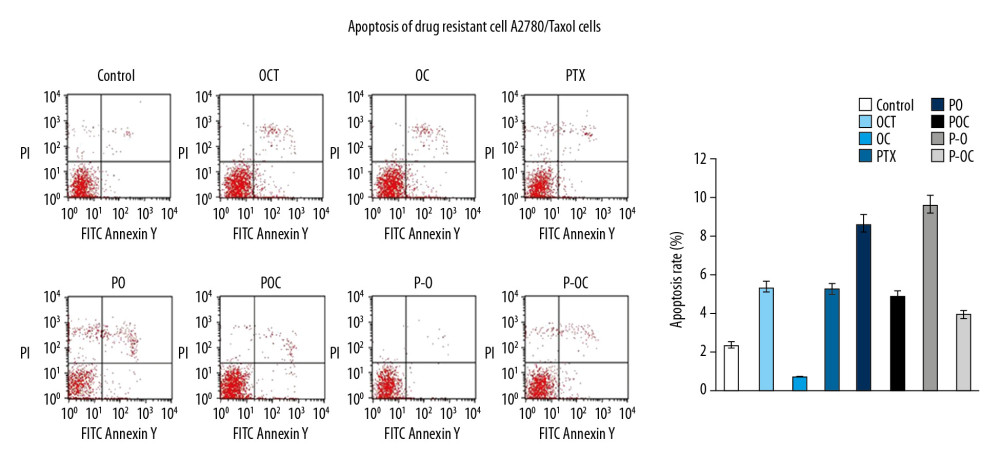 Figure 4. Apoptosis analysis in A2780/Taxol cells.
Figure 4. Apoptosis analysis in A2780/Taxol cells. 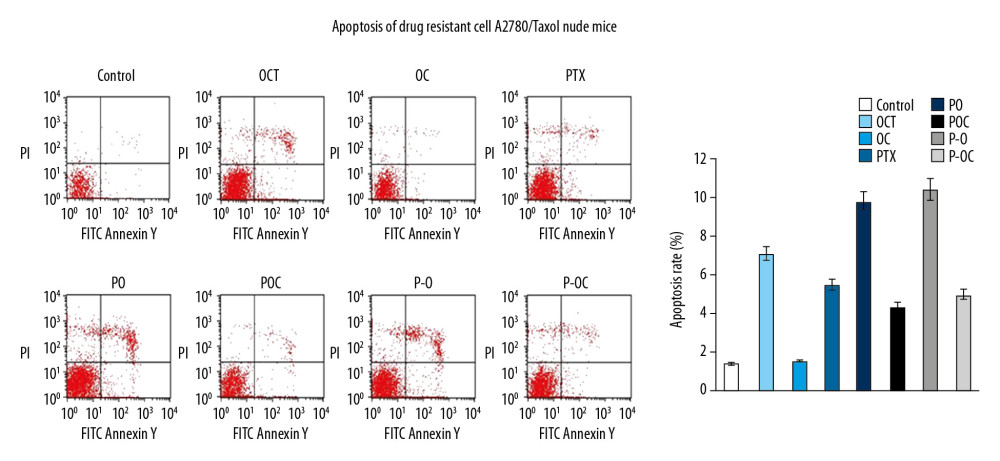 Figure 5. Apoptosis analysis in tumor tissue of xenograft mice model.
Figure 5. Apoptosis analysis in tumor tissue of xenograft mice model. 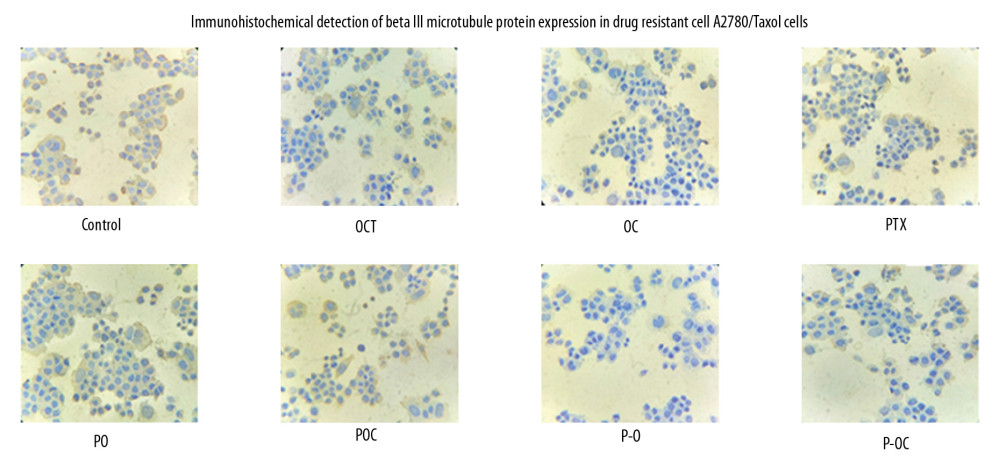 Figure 6. Class III beta-tubulin in A2780/Taxol cells.
Figure 6. Class III beta-tubulin in A2780/Taxol cells. 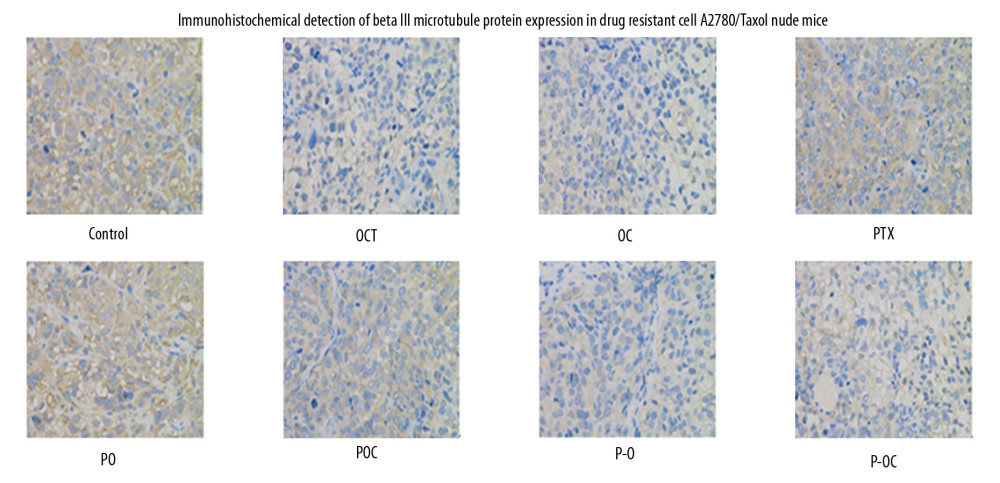 Figure 7. Class III beta-tubulin in tumor tissue of xenograft mice model.
Figure 7. Class III beta-tubulin in tumor tissue of xenograft mice model. References
1. Lokadasan R, James FV, Naranayan G, Prabhakaran PK, Targeted agents in epithelial ovarian cancer: Review on emerging therapies and future developments: Ecancermedicalscience, 2016; 10; 626
2. Yeung TL, Leung CS, Li F, Targeting stromal-cancer cell crosstalk networks in ovarian cancer treatment: Biomolecules, 2016; 6(1); 3-19
3. Achkar IW, Abdulrahman N, Al-Sulaiti H, Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway: J Transl Med, 2018; 16(1); 96
4. Sui X, Kong N, Ye L, p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents: Cancer Lett, 2014; 344(2); 174-79
5. Lu M, Xiao L, Hu J, Targeting of p38 mitogen-activated protein kinases to early growth response gene 1 (EGR-1) in the human paclitaxel-resistance ovarian carcinoma cells: J Huazhong Univ Sci Technol Med Sci, 2008; 28(4); 451-55
6. Cai Q-H, Tang Y, Fan S-H: Biomed Pharmacother, 2017; 95; 1830-37
7. Jiao JW, Wang L, Wen F, Pang XY, Expression and clinical significance of p38 MAPK in ovarian carcinoma of different cycles of chemotherapy: China Journal of Modern Medicine, 2011; 21(15); 1828-364
8. Mei-Song LU, Deng S, Xiao L, Study on the relationship between p38 MAPK activity and apoptosis in drug-resistant cell line of ovarian carcinoma: Journal of Harbin Medical University, 2007; 27(6); 725-28
9. Shen Y, Ren M, Shi Y: Exp Ther Med, 2011; 2(6); 1171-76
10. Li W, Shen Y, Zhang Y, Ren M, Research of octreotide on inhibiting the growth of human ovarian cancer cell line: Journal of Southeast University (Medical Science Edition), 2010; 29(4); 427-29
11. Shen Y, Zhang XY, Chen X, Synthetic paclitaxel-octreotide conjugate reverses the resistance of paclitaxel in A2780/Taxol ovarian cancer cell line: Oncol Rep, 2017; 37(1); 219-26
12. Chen X, Zhang XY, Shen Y, Synthetic paclitaxel-octreotide conjugate reversing the resistance of A2780/Taxol to paclitaxel in xenografted tumor in nude mice: Oncotarget, 2016; 7(50); 83451-561
13. Hong X, Li S, Li W, Disruption of protein neddylation with MLN4924 attenuates paclitaxel-induced apoptosis and microtubule polymerization in ovarian cancer cells: Biochem Biophys Res Commun, 2019; 508(3); 986-90
14. Sève P, Mackey J, Isaac S, Class III β-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel: Mol Cancer Ther, 2005; 4(12); 2001-7
15. Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation: Cell, 2011; 144(5); 646-74
16. Pages G, Berra E, Milanini J, Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability: J Biol Chem, 2000; 275(34); 26484-91
17. Houle F, Huaot J, Dysregulation of the endothelial cellular response to oxidative stress in cancer: Mol Carcinog, 2006; 45(6); S952-58
18. van der Bilt AR, Terwisscha van Scheltinga AG, Timmer-Bosscha H, Measurement of tumor VEGF-A levels with 89Zr-bevacizumab PET as an early biomarker for the antiangiogenic effect of everolimus treatment in an ovarian cancer xenograft model: Clin Cancer Res, 2012; 18(22); 6306-14
19. Becker MA, Farzan T, Harrington SC: Mol Cancer Ther, 2013; 12(12); 2909-16
20. Kavallaris M, Kuo YS, Burkhart CA, Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific-tubulin isotypes: J Clin Invest, 1997; 100(5); 1282-93
21. Gan PP, Pasquier E, Kavallaris M, Class III β-tubulin mediates sensitivity to chemotherapeutic drugs in non-small cell lung cancer: Cancer Res, 2007; 67(19); 9356-63
Figures
 Figure 1. Comparison of OCT, OC, PTX, PO, POC, P-O and P-OC on tumor growth in xenograft mice model. OCT – octreotide; OC – octreotide+cyclosomatostatin; PTX – paclitaxel; PO – paclitaxel+octreotide; POC – paclitaxel+octreotide+cyclosomatostatin; P-O – octreotide-paclitaxel conjugate; P-OC – octreotide-paclitaxel conjugate+cyclosomatostatin.
Figure 1. Comparison of OCT, OC, PTX, PO, POC, P-O and P-OC on tumor growth in xenograft mice model. OCT – octreotide; OC – octreotide+cyclosomatostatin; PTX – paclitaxel; PO – paclitaxel+octreotide; POC – paclitaxel+octreotide+cyclosomatostatin; P-O – octreotide-paclitaxel conjugate; P-OC – octreotide-paclitaxel conjugate+cyclosomatostatin. Figure 2. (A, B) Western blot analysis of p38, P-p38 MAPK, and VEGF in A2780/Taxol cells.
Figure 2. (A, B) Western blot analysis of p38, P-p38 MAPK, and VEGF in A2780/Taxol cells. Figure 3. (A, B) Western blot analysis of p38, P-p38 MAPK and VEGF in tumor tissue of xenograft mice model.
Figure 3. (A, B) Western blot analysis of p38, P-p38 MAPK and VEGF in tumor tissue of xenograft mice model. Figure 4. Apoptosis analysis in A2780/Taxol cells.
Figure 4. Apoptosis analysis in A2780/Taxol cells. Figure 5. Apoptosis analysis in tumor tissue of xenograft mice model.
Figure 5. Apoptosis analysis in tumor tissue of xenograft mice model. Figure 6. Class III beta-tubulin in A2780/Taxol cells.
Figure 6. Class III beta-tubulin in A2780/Taxol cells. Figure 7. Class III beta-tubulin in tumor tissue of xenograft mice model.
Figure 7. Class III beta-tubulin in tumor tissue of xenograft mice model. In Press
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








