25 June 2020: Database Analysis
Effects of Marital Status on Prognosis in Women with Infiltrating Ductal Carcinoma of the Breast: A Real-World 1: 1 Propensity-Matched Study
Tian Lan1ABCDEF*, Yunyan Lu2BCD, Hua Luo1CD, Junling He1BE, Jiawei He1CF, Zujian Hu1EF, Haibin Xu1ADOI: 10.12659/MSM.923630
Med Sci Monit 2020; 26:e923630
Abstract
BACKGROUND: The effects of marital status on infiltrating ductal carcinoma of breast cancer (IDC) have not been studied in detail. This study investigated the impact of marital status on IDC patients.
MATERIAL AND METHODS: SEER databases were searched from 2010 to 2015 for subjects who were married, divorced, single, and widowed. The influence of marital status on breast cancer-specific survival (BCSS) and overall survival (OS) of IDC patients was investigated through multivariate Cox regression analysis and Kaplan-Meier analysis. To prevent bias, propensity score matching (PSM) analysis was performed.
RESULTS: The 5-year OS was 89.6%in married patients, 84.9% in divorced patients, 83.5% in single patients, and 71.3% in widowed patients (p<0.001). The 5-year BCSS were 92.9%, 90.2%, 87.6%, and 86.4%, respectively (p<0.001). Multivariate Cox regression analysis revealed that marriage was a protective factor for patients with IDC in terms of OS (divorced: HR, 1.27; 95% CI, 1.21–1.32; p<0.001; single: HR, 1.36; 95% CI, 1.31–1.42; p<0.001; widowed: HR, 1.42; 95% CI, 1.36–1.48; p<0.001) and BCSS (divorced: HR, 1.15; 95% CI, 1.09–1.21; p<0.001; single: HR, 1.27; 95% CI, 1.21-1.33; p<0.001; widowed: HR, 1.32; 95% CI, 1.25–1.40; p<0.001). Following subgroup and PSM analysis, married patients were shown to have better OS and BCSS as opposed to divorced, single, or widowed patients.
CONCLUSIONS: We identify marital status as a predictor of survival in those with IDC. Widowed patients showed the highest mortality risk.
Keywords: Breast Neoplasms, Marital Status, SEER Program, Breast, Carcinoma, Ductal, Carcinoma, Ductal, Breast, Multivariate Analysis, propensity score, Proportional Hazards Models, protective factors, Risk Factors, United States
Background
Breast cancer is a common tumor in women, with ~279 100 new cases and 42 690 deaths in 2020 alone [1]. Infiltrating ductal carcinoma of breast cancer (IDC) accounts for ~70–80% of breast cancers globally [2,3]. Although advances in treatment have reduced the mortality rate of IDC, the increasing incidence of IDC is still a serious problem [4]. Therefore, it is urgent to explore potential risk factors contributing to IDC development. The risk factors for breast cancer include reproductive risk factors [5], lifestyle [6], family history [7], and genetic predisposition [8]. Psychological and social factors are also emerging as key indicators of cancer development [9].
Marital status is a key sociocultural variable that influences cancer patients. Marital status can predict the outcomes of rectal cancer [10], ovarian serous carcinoma [11], pancreatic cancer [12], and non-small cell lung cancer [13]. Similarly, marital status has been suggested as a predictive factor for breast cancer survival [14–17]. Breast cancer is highly heterogeneous, with a range of pathologies, biological behavior, and prognosis that differ from other histological subtypes [18,19]. However, most previous reports did not distinguish histologic subtypes or molecular subtypes. In addition, significant imbalances in baseline characteristics exist amongst the studied groups based on marital status. The effects of marital status on the prognosis of IDC patients therefore require assessment.
In this study, 1: 1 propensity score matching (PSM) was performed to explore the influence of marital status on IDC prognosis in the Surveillance, Epidemiology, and End Results (SEER) database.
Material and Methods
PATIENTS:
The SEER 18 regions database [Incidence-SEER 18 Regs Research Data (with additional treatment fields), Nov 2017 Sub (1975–2016 varying)] was used, encompassing ~28% of the U.S. population. Patients diagnosed from 2010 to 2015 were collected due to the lack of availability of Her2 information prior to 2010. Inclusion criteria were as follows: (1) age ≥18 years at diagnosis; (2) accessible marital information; (3) histology ICD-O-3 (International Classification of Diseases for Oncology, 3rd edition) limited to infiltrating duct carcinoma (8500/3); and (4) survival times ≥1 month. Patients with missing or incomplete demographic, clinicopathological, treatment, or follow-up information were excluded.
CLINICOPATHOLOGICAL VARIABLES:
Marital status, gender, age at diagnosis, ethnicity, median household income, insurance status, tumor grade, tumor size, lymph node, metastasis, TNM stage, ER, PR, Her2, molecular subtype, treatment regimens, and prognostic information were assessed. Patients were divided into those who were married, single, divorced, and widowed based on marital status. Age was categorized as 18–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥80 years. Ethnicity was classified into white, black, American Indian/Alaska Native (AI), and Asian or Pacific Islander (API). Socioeconomic status was divided into Quartile 1 (<$52 620), Quartile 2 ($52 621–$60 890), Quartile 3 ($60 891–$74 440), and Quartile 4 (>$74 441). Tumor grade IV was combined with grade III. TNM staging was performed according to the 7th edition of the American Joint Committee on Cancer (AJCC) and classed into stage I to stage IV. Radiation and chemotherapy were categorized as “yes” and “no/unknown”.
STATISTICAL ANALYSES:
Baseline features were compared using the chi-square test. The primary endpoints were overall survival (OS) and breast cancer-specific survival (BCSS). Kaplan-Meier (KM) curves were used to investigate survival differences amongst the groups. Log-rank tests were applied for group comparisons. Prognostic factors were identified using multivariate Cox proportional hazard assessments.
PSM can reduce selection bias and mimic randomized controlled trials [20,21], and was employed to reassess the influence of marital status. PSM was performed using 1: 1 nearest neighbor matching with a caliper of 0.01. Standardized differences (SD) were used to assess the changes in variables before and after PSM. SD ≤0.1 were employed to denote significant balances in the baseline covariate [22].
Statistical analyses were performed using R (version 3.5.2,
Results
CLINICOPATHOLOGICAL CHARACTERISTICS:
From 2010 to 2015, 183 260 patients with IDC were included. Clinicopathological characteristics in each group are presented in Table 1. Those who were widowed tended to be in the older age groups of 60–69 (22.8%), 70–79 (33.9%), and ≥80 years (33.8%). The single group had more black patients (23.4%), while the married group had more Asian/Pacific Islander patients (7.1%). Compared to those who were divorced, single, and widowed, married patients tended to have earlier stage (53.4%), smaller tumor sizes (62.6%), negative lymph nodes (67.5%), and no metastasis (97.1%). Patients in the widowed group were least likely to have received radiation (45.3%) or chemotherapy (25.4%).
EFFECTS OF MARITAL STATUS ON OS AND BCSS:
The OS and BCSS of patients with IDC were assessed using Kaplan-Meier analysis. Significant differences in OS were observed based on marital status (p<0.0001) (Figure 1A). The 5-year OS was 89.6% in married patients, and 71.3%, 84.9%, and 83.5% in those who were widowed, single, and divorced, respectively (Table 2). The BCSS of the 4 marital subgroups also differed (Figure 1B). The 5-year BCSS was 92.9% in the married group, 90.2% in the divorced group, 87.6% the single group, and 86.4% in the widowed group (p<0.001) (Table 3).
Univariate analysis demonstrated that ethnicity, age, gender, income, insurance status, tumor grade, stage, subtype, surgical therapy, radiation therapy, and chemotherapy were significantly associated with OS (Table 2) and BCSS (Table 3) (all p<0.001).
Results from multivariate Cox regression analysis revealed marriage as a protective factor for OS (divorced: HR, 1.27; 95% CI, 1.21–1.32; p<0.001; single: HR, 1.36; 95% CI, 1.31–1.42; p<0.001; and widowed: HR, 1.42; 95% CI, 1.36–1.48; p<0.001) (Table 2) and BCSS (divorced: HR, 1.15; 95% CI, 1.09–1.21; p<0.001; single: HR, 1.27; 95% CI, 1.21–1.33; p<0.001; and widowed: HR, 1.32; 95% CI, 1.25–1.40; p<0.001) (Table 3) in patients with IDC. Molecular subtype, insurance, surgery, radiation therapy, and chemotherapy showed a highly significant association with OS and BCSS.
To reduce the effect of confounders, IDC patients were stratified according to clinical features. We also identified marital status as an independent prognostic indicator of OS (Figure 2) and BCSS (Figure 3) in all subgroups.
SURVIVAL ANALYSIS AFTER 1: 1 PSM:
To minimize the confounding factors and assess the impact of marital status, we performed 1: 1 PSM. Three 1: 1 matched cohorts were obtained: a divorced and married cohort, a single and married cohort, and a widowed and married cohort. The demographic and clinicopathological features between 2 groups in the 2 cohorts were balanced (Table 4). The absolute mean differences in all variables across the groups were less than 0.1 following PSM assessment (Figure 4). Married patients showed better BCSS and OS in the divorced-married cohort (Figure 5A, 5B), the single-married cohort (Figure 5C, 5D), and the widowed-married cohort (Figure 5E, 5F).
Discussion
This is the first study to investigate the influence of marital status on IDC prognosis using PSM in the SEER database. In comparison to previous SEER-based studies, we particularly assessed significant covariates, including molecular subtype, household income, and insurance. We found that 4 marital subgroups showed different survival outcomes for OS and BCSS. In multivariate Cox analysis encompassing an integrated range of variables, we demonstrated that marriage was an independent prognostic and protective factor for OS and BCSS, and widowed patients were the most likely to die of IDC. After PSM, we further confirmed that those who married showed better OS and BCSS compared to the divorced, single or widowed patients.
These findings raise the intriguing question of why married patients showed better clinical outcomes. One hypothesis is the higher likelihood for early diagnosis in those who are married. Studies have shown that delayed diagnosis can lead to poor survival of unmarried patients [17,23,24]. In the present study, widowed patients tended to be older than married patients. The incidence of metastasis was lower in the married group (2.9%) compared to the divorced (4.2%), single (5.3%), and widowed (4.0%) groups. Spouses might facilitate early IDC diagnosis, leading to better prognosis.
Secondly, married patients tended to have more financial resources and better access to effective treatment [25,26]. Spouses and their children may provide financial assistance that is unavailable to single, divorced, or widowed patients [27]. Our research indicated that compared with married patients, those who were widowed, single, or divorced tended to be undertreated, which may have contributed to their worse prognosis [28].
Thirdly, married patients might obtain extra psychological and emotional support from their spouse and children, which can improve disease outcomes [29]. A cancer diagnosis was reported to cause higher levels of psychological distress than that of other chronic diseases [30]. In addition, compared to married patients, the single, divorced, and widowed patients were more likely to have depression and anxiety after a diagnosis of cancer [31]. Stress and depression combined had an association with immune dysfunction, nonadherence to medical advice, and tumor progression [32,33]. Emotional assistance can improve the quality of life, thereby preventing disease-associated decline in breast cancer patients [34,35]. Therefore, the benefits of psychosocial support should not be underestimated for single, divorced, and widowed populations. It is vital that physicians screen for such distress and provide psychosocial support interventions as required.
Fourthly, it was reported that married people have healthier lifestyle behaviors [36]. The single, divorced, and widowed patients were more likely have unhealthy lifestyles, such as heavy drinking and smoking, which can adversely affect overall survival of breast cancer patients [37,38]. This may partly explain the better prognosis in those who are married.
Some limitations of the present study should be discussed. Firstly, reproductive history and comorbidities were not included in the SEER database. These missing factors associated with prognosis may lead to potential bias. Secondly, the SEER database records marital status at diagnosis, but we lacked detailed information on the quality of marriage, the subsequent changes in marital status, and other marital statuses, including gay, lesbian, bisexual, and transgender. Finally, given the retrospective nature of our analysis, further prospective studies are required.
Conclusions
This study, which had a large sample and used ingenious statistical analyses, found that married status was a protective prognostic factor for IDC patients. The single, divorced, and widowed patients were at higher risk of undertreatment, metastasis, and poor outcomes. Widowed patients had the highest mortality rates. Targeted psychosocial support should now be provided to these IDC patient subsets.
Figures
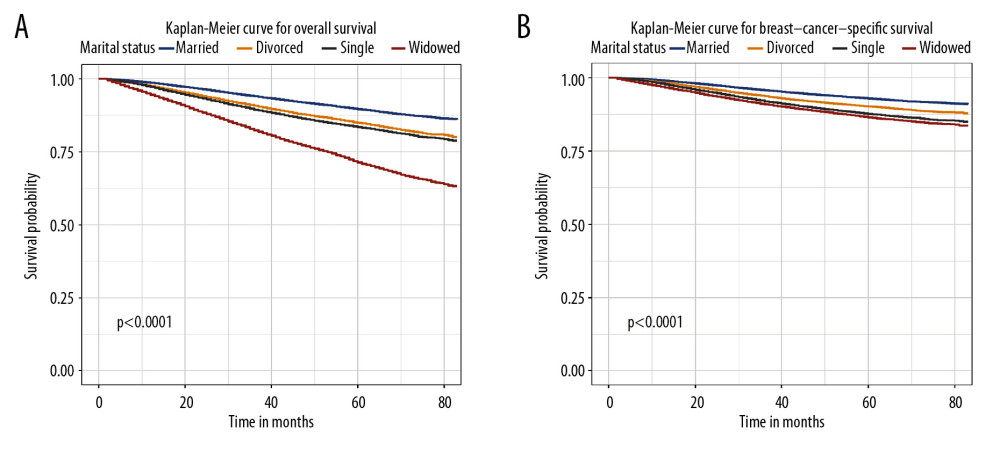 Figure 1. Overall survival (A) and breast cancer-specific survival (B) curve of breast cancer patients based on marital status (married, divorced, widowed, and single).
Figure 1. Overall survival (A) and breast cancer-specific survival (B) curve of breast cancer patients based on marital status (married, divorced, widowed, and single). 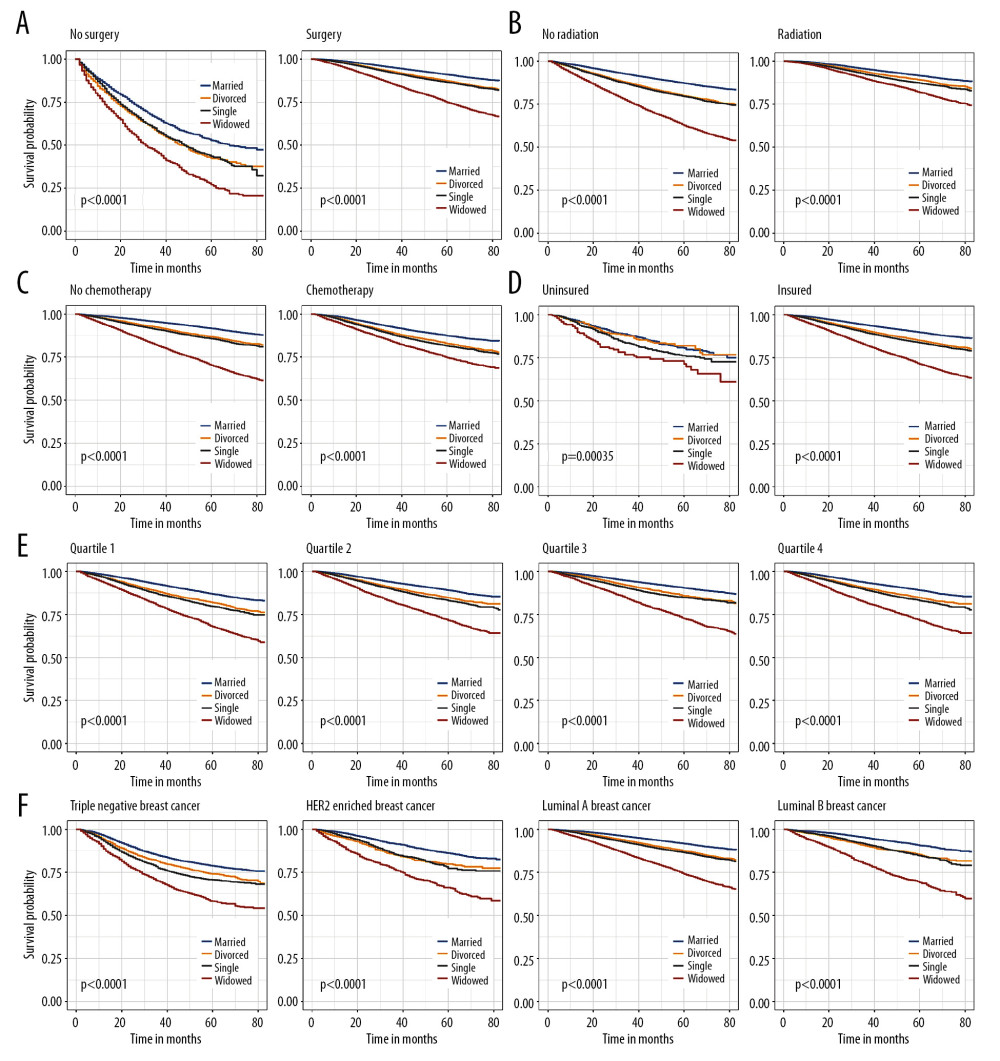 Figure 2. Kaplan-Meier analysis for overall survival in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F).
Figure 2. Kaplan-Meier analysis for overall survival in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F). 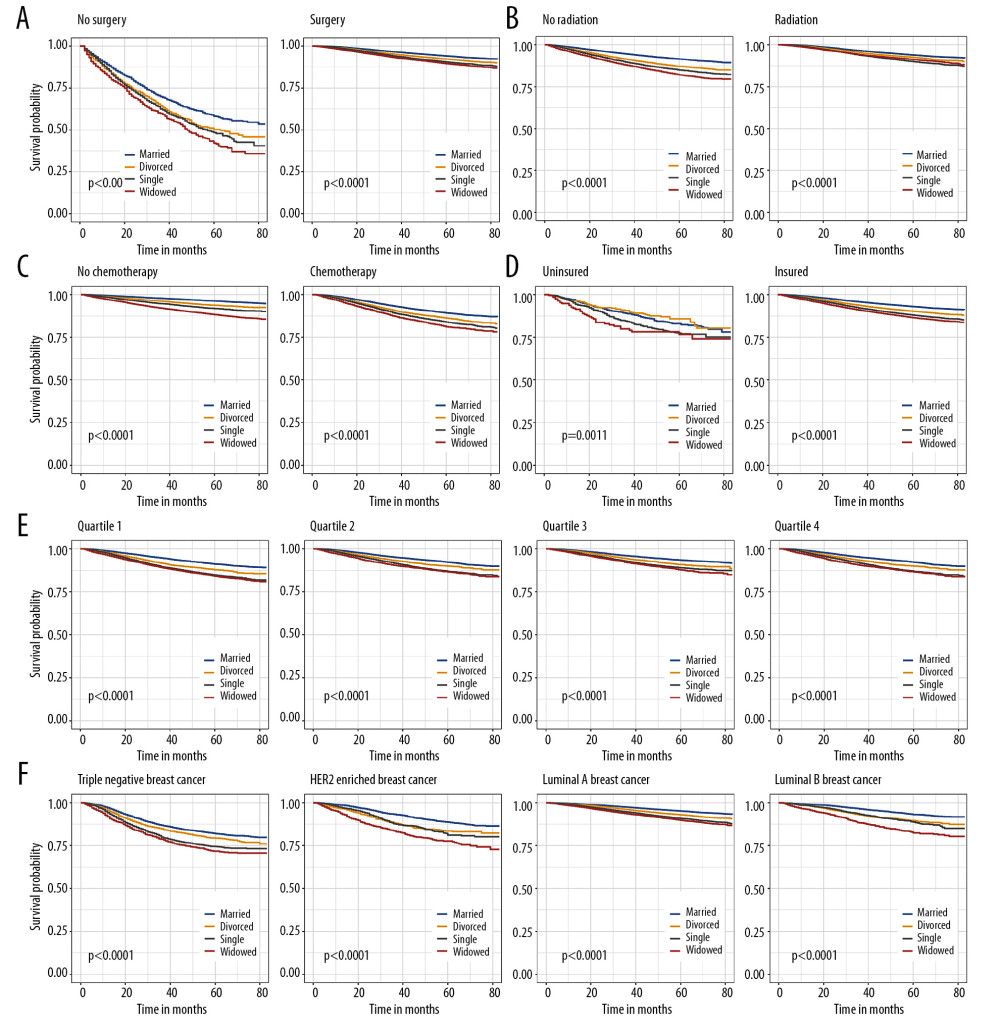 Figure 3. Breast cancer-specific survival curves in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F).
Figure 3. Breast cancer-specific survival curves in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F). 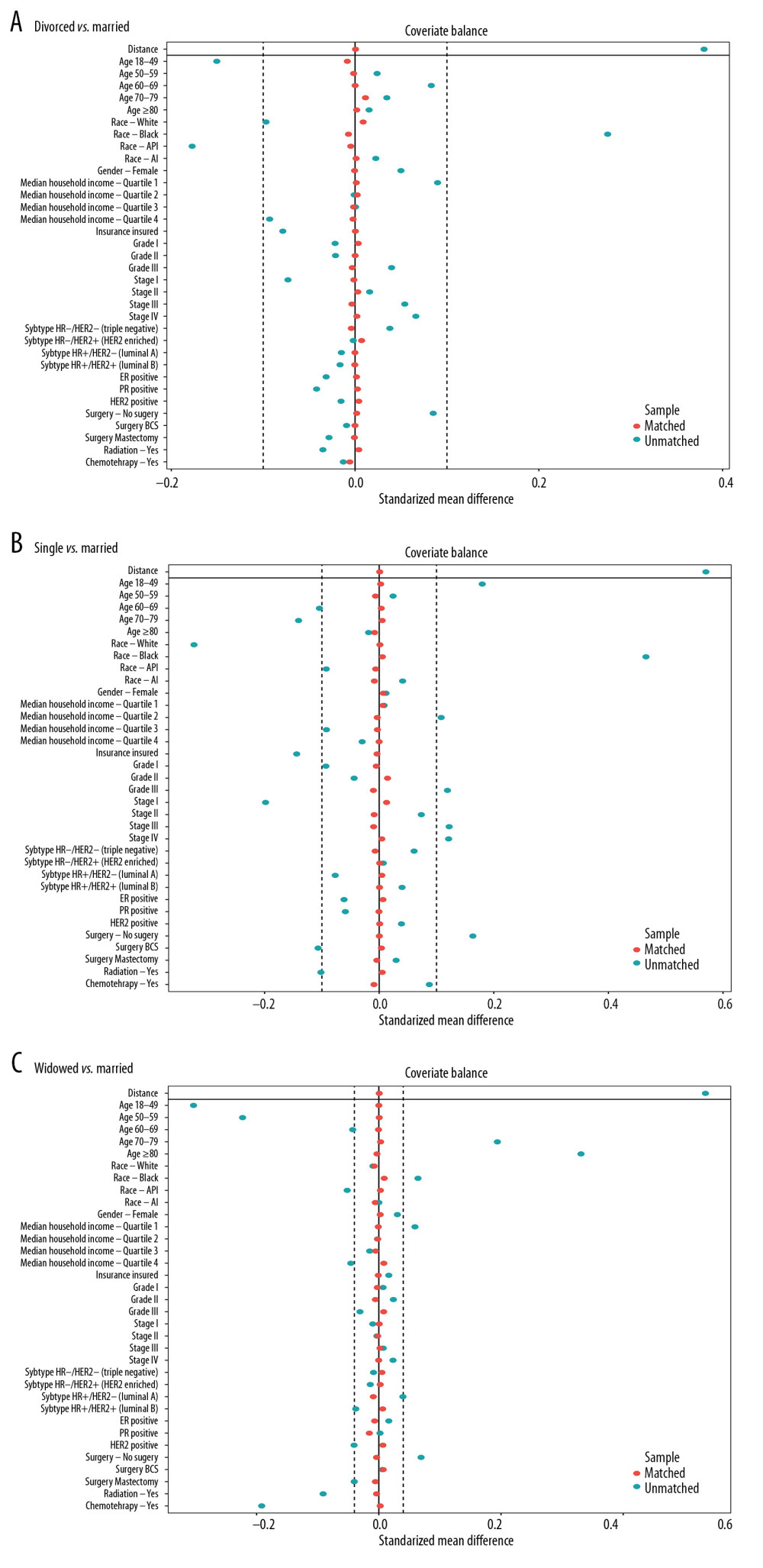 Figure 4. (A) The mean difference in all variables before and after PSM between divorced and married groups. (B) The mean difference between single and married groups. (C) The mean difference between widowed and married groups.
Figure 4. (A) The mean difference in all variables before and after PSM between divorced and married groups. (B) The mean difference between single and married groups. (C) The mean difference between widowed and married groups. 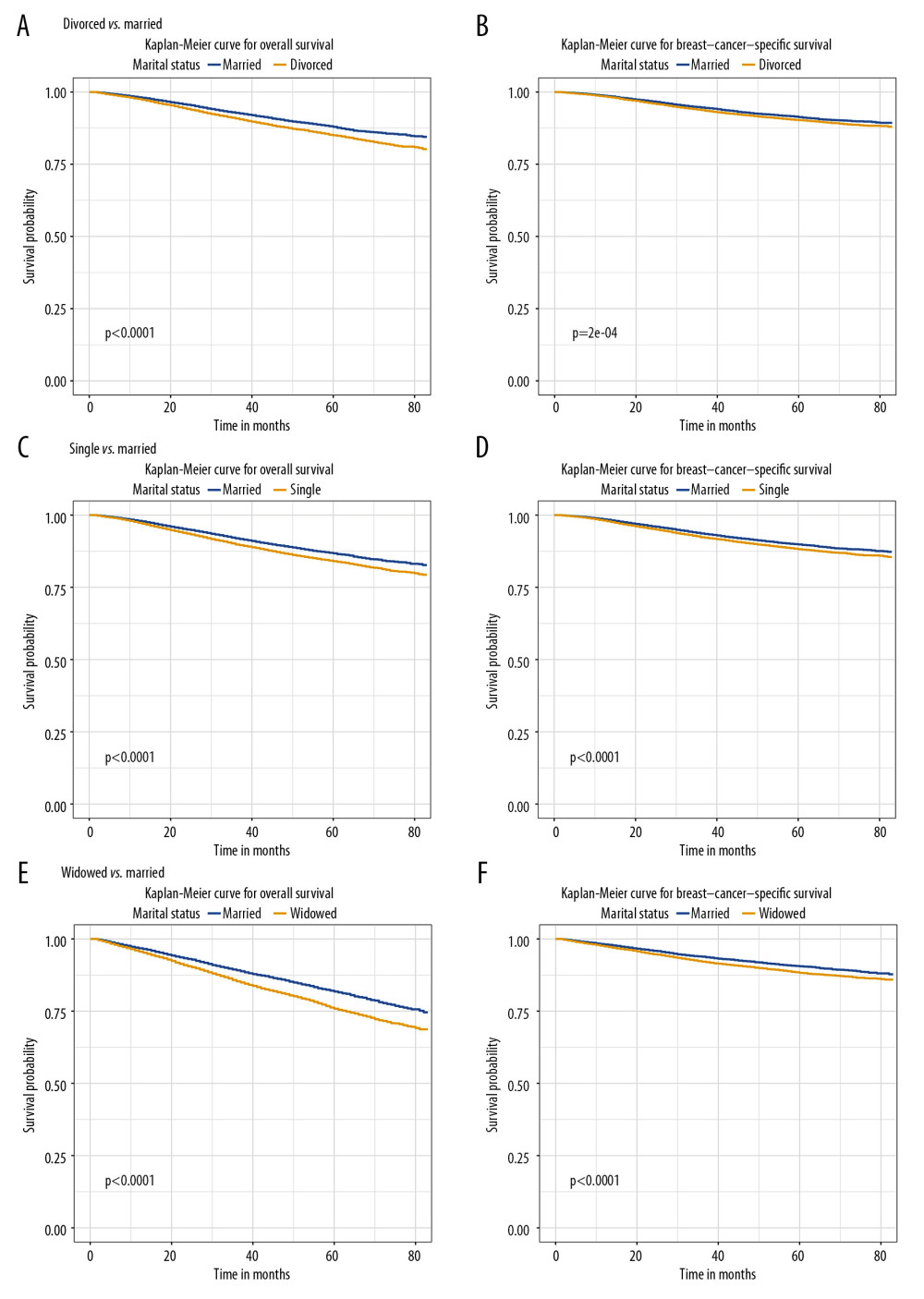 Figure 5. The overall survival (A, C, E) and breast cancer-caused special survival (B, D, F) of patients with breast cancer according to marital status after PSM.
Figure 5. The overall survival (A, C, E) and breast cancer-caused special survival (B, D, F) of patients with breast cancer according to marital status after PSM. Tables
Table 1. The characteristics of patients with breast cancer according to marital status in the SEER database.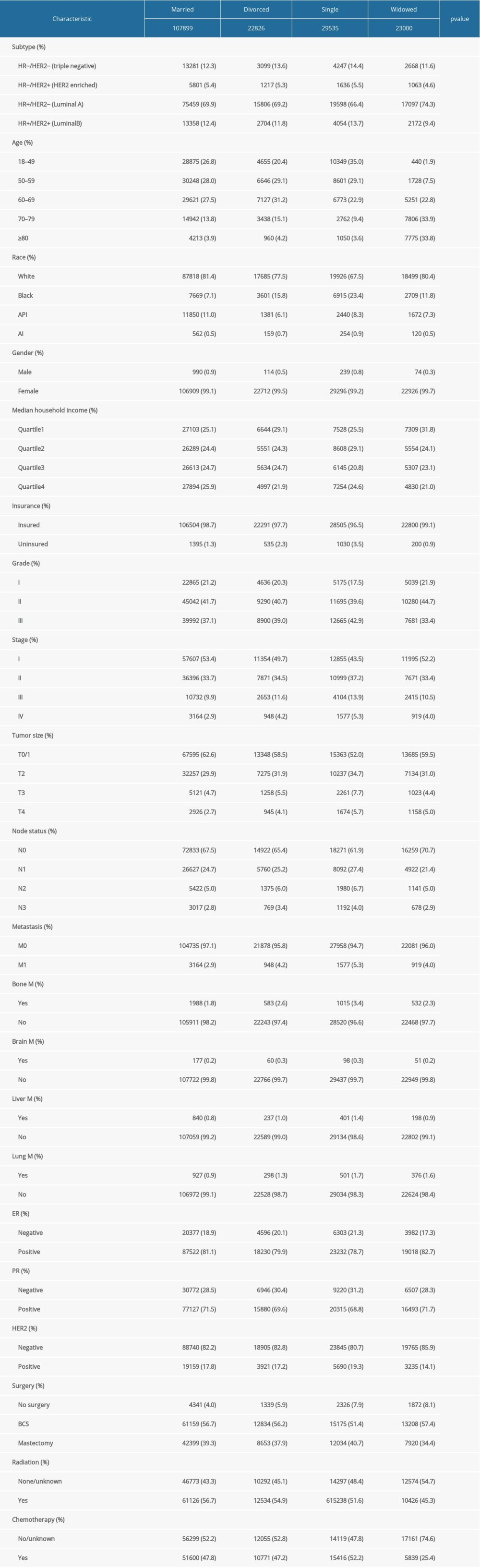 Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM.
Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM.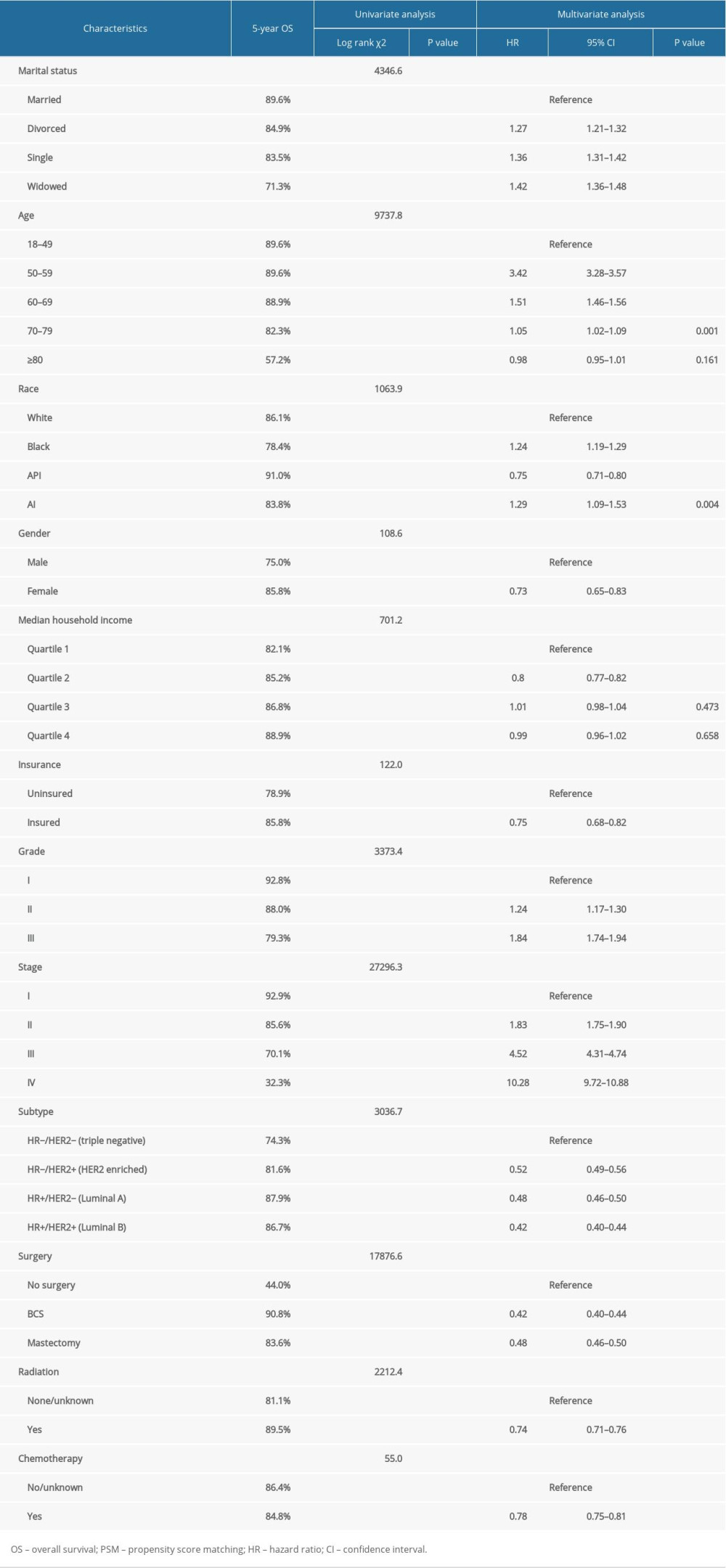 Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM.
Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM.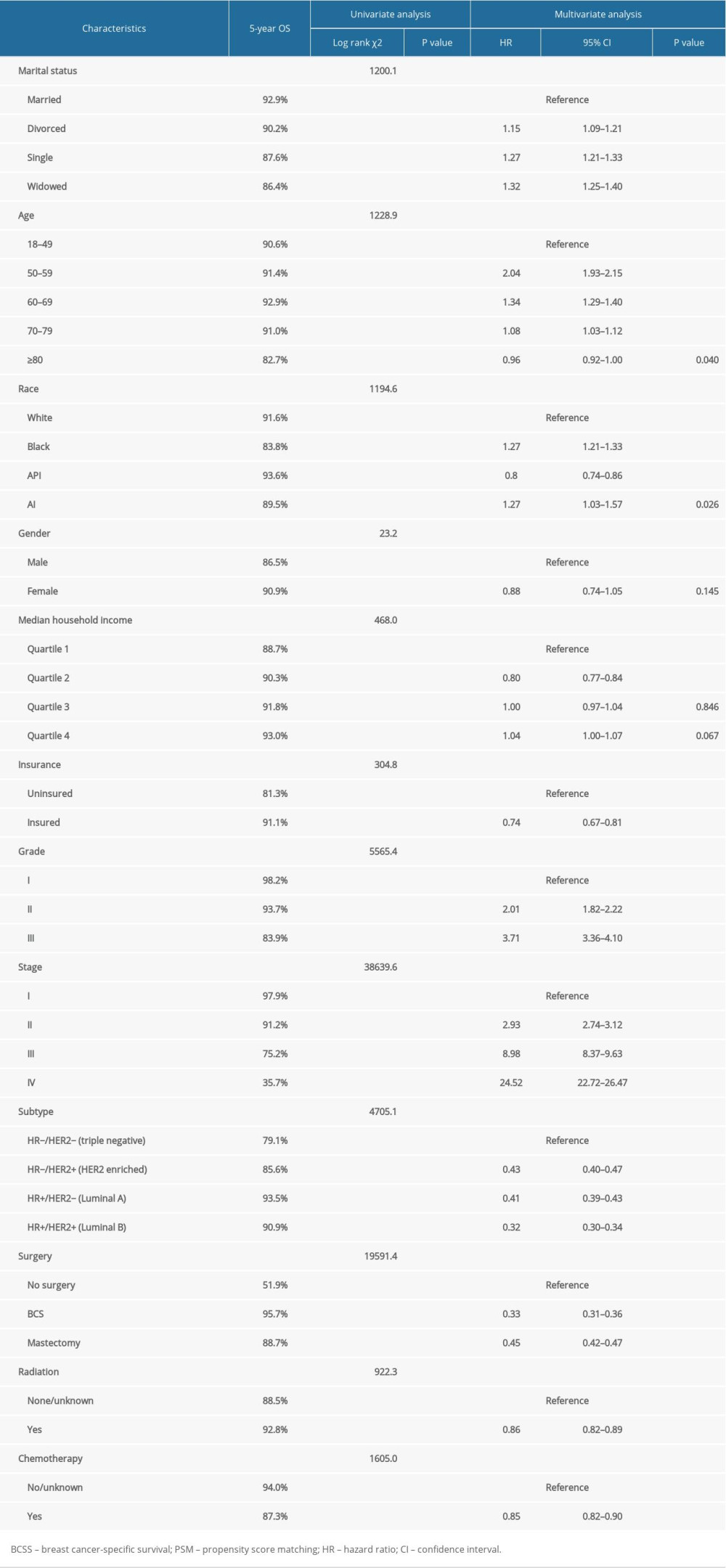 Table 4. Patient baseline characteristics after PSM.
Table 4. Patient baseline characteristics after PSM.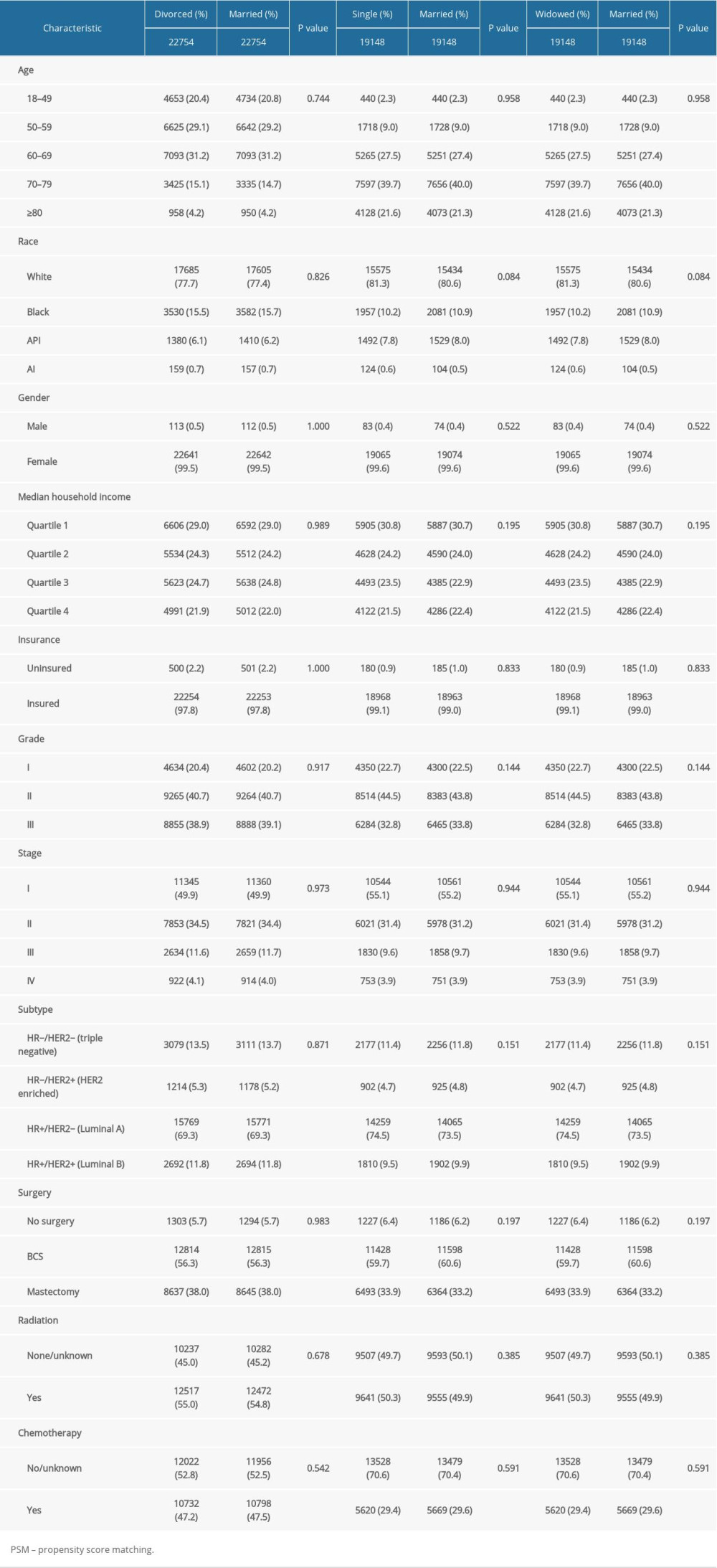
References
1. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020: Cancer J Clin, 2020; 70(1); 7-30
2. Li CI, Anderson BO, Daling JR, Moe RE, Trends in incidence rates of invasive lobular and ductal breast carcinoma: JAMA, 2003; 289(11); 1421-24
3. van Netten JP, Cann SH, Thornton IG, Finegan RP, The lymphatics in infiltrating ductal carcinoma (IDC) of the breast: Cancer Treat Rev, 2018; 62; 97
4. Bandyopadhyay S, Bluth MH, Ali-Fehmi R, Breast carcinoma: Updates in Molecular Profiling 2018: Clin Lab Med, 2018; 38(2); 401-20
5. Thorbjarnardottir T, Olafsdottir EJ, Valdimarsdottir UA, Oral contraceptives, hormone replacement therapy and breast cancer risk: A cohort study of 16 928 women 48 years and older: Acta Oncol, 2014; 53(6); 752-58
6. Ghosn B, Benisi-Kohansal S, Ebrahimpour-Koujan S, Association between healthy lifestyle score and breast cancer: Nutr J, 2020; 19(1); 4
7. Brewer HR, Jones ME, Schoemaker MJ, Family history and risk of breast cancer: An analysis accounting for family structure: Breast Cancer Res Treat, 2017; 165(1); 193-200
8. Owens DK, Davidson KW, Krist AHUS Preventive Services Task Force, Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation Statement: JAMA, 2019; 322(7); 652-65
9. Ozkan M, Yildirim N, Disci R, Roles of biopsychosocial factors in the development of breast cancer: Eur J Breast Health, 2017; 13(4); 206-12
10. Wang X, Cao W, Zheng C, Marital status and survival in patients with rectal cancer: An analysis of the Surveillance, Epidemiology and End Results (SEER) database: Cancer Epidemiol, 2018; 54; 119-24
11. Luo P, Zhou J-G, Jin S-H, Influence of marital status on overall survival in patients with ovarian serous carcinoma: Finding from the surveillance epidemiology and end results (SEER) database: J Ovarian Res, 2019; 12(1); 126
12. Reyngold M, Winter KA, Regine WF, Marital status and overall survival in patients with resectable pancreatic cancer: Results of an ancillary analysis of NRG Oncology/RTOG 9704: Oncologist, 2020; 25(3); e477-83
13. Chen Z, Yin K, Zheng D, Marital status independently predicts non-small cell lung cancer survival: A propensity-adjusted SEER database analysis: J Cancer Res Clin Oncol, 2020; 146(1); 67-74
14. Osborne C, Ostir GV, Du X, The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer: Breast Cancer Res Treat, 2005; 93(1); 41-47
15. Zhai Z, Zhang F, Zheng Y, Effects of marital status on breast cancer survival by age, race, and hormone receptor status: A population-based Study: Cancer Med, 2019; 8(10); 4906-17
16. Hinyard L, Wirth LS, Clancy JM, Schwartz T, The effect of marital status on breast cancer-related outcomes in women under 65: A SEER database analysis: Breast, 2017; 32; 13-17
17. Martínez ME, Unkart JT, Tao L, Prognostic significance of marital status in breast cancer survival: A population-based study: PLoS One, 2017; 12(5); e0175515
18. Yeo SK, Guan J-L, Breast cancer: Multiple subtypes within a tumor?: Trends Cancer, 2017; 3(11); 753-60
19. Biglia N, Maggiorotto F, Liberale V, Clinical-pathologic features, long term-outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC): Eur J Surg Oncol, 2013; 39(5); 455-60
20. Austin PC, An introduction to propensity score methods for reducing the effects of confounding in observational studies: Multivariate Behav Res, 2011; 46(3); 399-424
21. Shah BR, Laupacis A, Hux JE, Austin PC, Propensity score methods gave similar results to traditional regression modeling in observational studies: A systematic review: J Clin Epidemiol, 2005; 58(6); 550-59
22. Austin PC, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples: Stat Med, 2009; 28(25); 3083-107
23. Goodwin JS, Hunt WC, Key CR, Samet JM, The effect of marital status on stage, treatment, and survival of cancer patients: JAMA, 1987; 258(21); 3125-30
24. Sharon CE, Sinnamon AJ, Ming ME, Association of marital status with T stage at presentation and management of early-stage melanoma: JAMA Dermatol, 2018; 154(5); 574-80
25. Xie J-C, Yang S, Liu X-Y, Zhao Y-X, Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status: Cancer Med, 2018; 7(8); 3722-42
26. Woods LM, Rachet B, Coleman MP, Origins of socio-economic inequalities in cancer survival: A review: Ann Oncol, 2006; 17(1); 5-19
27. Cheng Y-P, Birditt KS, Zarit SH, Fingerman KL, Young adults’ provision of support to middle-aged parents: J Gerontol B Psychol Sci Soc Sci, 2015; 70(3); 407-16
28. Stephens MAP, Fekete EM, Franks MM, Spouses’ use of pressure and persuasion to promote osteoarthritis patients’ medical adherence after orthopedic surgery: Health Psychol, 2009; 28(1); 48-55
29. Aizer AA, Chen M-H, McCarthy EP, Marital status and survival in patients with cancer: J Clin Oncol, 2013; 31(31); 3869-76
30. Kaiser NC, Hartoonian N, Owen JE, Toward a cancer-specific model of psychological distress: population data from the 2003–2005 National Health Interview Surveys: J Cancer Surviv, 2010; 4(4); 291-302
31. Goldzweig G, Andritsch E, Hubert A, Psychological distress among male patients and male spouses: What do oncologists need to know?: Ann Oncol, 2010; 21(4); 877-83
32. DiMatteo MR, Lepper HS, Croghan TW, Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence: Arch Intern Med, 2000; 160(14); 2101-7
33. Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW, The immune system in cancer metastasis: friend or foe?: J Immunother Cancer, 2017; 5(1); 79
34. Tsimopoulou I, Pasquali S, Howard R, Psychological prehabilitation before cancer surgery: A systematic review: Ann Surg Oncol, 2015; 22(13); 4117-23
35. Brandão T, Schulz MS, Matos PM, Psychological intervention with couples coping with breast cancer: A systematic review: Psychol Health, 2014; 29(5); 491-516
36. Sommerlad A, Ruegger J, Singh-Manoux A, Marriage and risk of dementia: Systematic review and meta-analysis of observational studies: J Neurol Neurosurg Psychiatry, 2018; 89(3); 231-38
37. Keenan K, Ploubidis GB, Silverwood RJ, Grundy E, Life-course partnership history and midlife health behaviours in a population-based birth cohort: J Epidemiol Community Health, 2017; 71(3); 232-38
38. Warren GW, Kasza KA, Reid ME, Smoking at diagnosis and survival in cancer patients: Int J Cancer, 2013; 132(2); 401-10
Figures
 Figure 1. Overall survival (A) and breast cancer-specific survival (B) curve of breast cancer patients based on marital status (married, divorced, widowed, and single).
Figure 1. Overall survival (A) and breast cancer-specific survival (B) curve of breast cancer patients based on marital status (married, divorced, widowed, and single). Figure 2. Kaplan-Meier analysis for overall survival in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F).
Figure 2. Kaplan-Meier analysis for overall survival in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F). Figure 3. Breast cancer-specific survival curves in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F).
Figure 3. Breast cancer-specific survival curves in subgroups stratified by surgery (A), radiation (B), chemotherapy (C), insurance status (D), median household income (E), and subtype (F). Figure 4. (A) The mean difference in all variables before and after PSM between divorced and married groups. (B) The mean difference between single and married groups. (C) The mean difference between widowed and married groups.
Figure 4. (A) The mean difference in all variables before and after PSM between divorced and married groups. (B) The mean difference between single and married groups. (C) The mean difference between widowed and married groups. Figure 5. The overall survival (A, C, E) and breast cancer-caused special survival (B, D, F) of patients with breast cancer according to marital status after PSM.
Figure 5. The overall survival (A, C, E) and breast cancer-caused special survival (B, D, F) of patients with breast cancer according to marital status after PSM. Tables
 Table 1. The characteristics of patients with breast cancer according to marital status in the SEER database.
Table 1. The characteristics of patients with breast cancer according to marital status in the SEER database. Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM.
Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM. Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM.
Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM. Table 4. Patient baseline characteristics after PSM.
Table 4. Patient baseline characteristics after PSM. Table 1. The characteristics of patients with breast cancer according to marital status in the SEER database.
Table 1. The characteristics of patients with breast cancer according to marital status in the SEER database. Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM.
Table 2. Impact of marital status on the OS by univariate and multivariate survival analysis before PSM. Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM.
Table 3. Impact of marital status on the BCSS by univariate and multivariate survival analysis before PSM. Table 4. Patient baseline characteristics after PSM.
Table 4. Patient baseline characteristics after PSM. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








