30 June 2020: Animal Study
Effects of 2D-Shear Wave Elastography on Brain-Derived Neurotrophic Factor (BDNF) in the Brains of Neonatal Mice and Exploration of the Mechanism
Cheng Zhang1BCDEF, Junlai Li1A*, Changtian Li1AEFGDOI: 10.12659/MSM.924832
Med Sci Monit 2020; 26:e924832
Abstract
BACKGROUND: The aim of this study was to explore the effect and duration of 2-dimensional shear wave elastography (2D-SWE) irradiation on the expression of brain-derived neurotrophic factor (BDNF) in the brains of neonatal mice and to preliminarily investigate whether its mechanism is neuronal apoptosis.
MATERIAL AND METHODS: Neonatal mice (within 48 hours of birth) were subjected to 2D-SWE irradiation of the brain for 10 minutes (group S1), 20 minutes (group S2), and 30 minutes (group S3). The mice were sacrificed immediately after irradiation or 24 hours after irradiation. Brains were collected for real-time polymerase chain reaction (RT-PCR) and western blot experiments to determine the expression of BDNF in each group. TdT-mediated dUTP nick-end labeling (TUNEL) was performed to observe neuronal apoptosis in the brain.
RESULTS: The results of PCR and western blots from the brains of neonatal mice that were sacrificed immediately after irradiation show that S1, S2, and S3 were significantly different from those in the control group. The PCR and western blot results of brain tissues from neonatal mice sacrificed at 24 hours after irradiation showed that there was no significant difference between the S1, S2, S3, and control groups. The results of TUNEL experiments showed that there was no statistically significant difference in the number of apoptotic neurons between the S1, S2, S3, and control groups.
CONCLUSIONS: 2D-SWE irradiation of neonatal mice for more than 10 minutes downregulated the expression of BDNF. This effect disappeared within 24 hours after the irradiation, and the 2D-SWE scan seemed not to induce neuronal apoptosis.
Keywords: Brain-Derived Neurotrophic Factor, Elasticity Imaging Techniques, Neonatal Screening, Animals, Newborn, Brain, Gene Expression Regulation, Neurons, Ultrasonography, Doppler, Transcranial
Background
Transcranial ultrasound has become one of the preferred imaging methods for assessing craniocerebral diseases in newborns because of its non-ionizing radiation, non-invasiveness, economic advantages, convenience, and excellent repeatability. In the diagnosis of neonatal hypoxic-ischemic encephalopathy (HIE), periventricular-ventricular hemorrhage, and intracranial hemorrhage of the newborn, transcranial ultrasound has good sensitivity and specificity. In recent years, some researchers have begun to use ultrasound elastography for the differential diagnosis of neonatal craniocerebral diseases, indicating that ultrasound elastography has good application prospects in the diagnosis of neonatal craniocerebral diseases, especially HIE [1,2].
When ultrasound is widely used, however, people tend to overlook the potential biological impacts, which mainly include thermal and mechanical effects. A wide range of animal experiments have confirmed that ultrasound exposure can affect the central nervous system, but these experiments have focused on B-mode ultrasound and Doppler ultrasound [3,4]. There are few studies of the biological effects of 2-dimensional shear wave elastography (2D-SWE) exposure on the central nervous system. In our previous studies, we found that mTOR expression in the brain tissue of newborn rats was affected when the brain was irradiated for 30 minutes with 2D-SWE [5]. Although this change in mTOR returned to normal in mice when they reached adulthood, changes in expression and regulation of mTOR in the neonatal period may irreversibly damage neurons or their functions. Synaptic plasticity is an essential basis for learning and memory, and this process is partially regulated by mTOR. Therefore, it is of great significance to study the effect of this technique on the synaptic plasticity of hippocampal neurons in newborns and determine the relatively safe scanning duration when applying this technique to neonatal brains.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family, and it plays a vital role in the survival, growth, and maintenance of neurons during the development of neurons [6]. BDNF also affects synaptic plasticity by regulating axons, dendritic branching and reconstruction, the formation of axon dendritic synapses, and the effectiveness of synaptic transmission, excitability, and inhibition [7]. In view of the importance of BDNF expression in the brain during development, this study used real-time polymerase chain reaction (RT-PCR) and western blotting to explore the effect and duration of 2D-SWE craniocerebral irradiation on the expression of BDNF in the brain of neonatal mice and used TdT-mediated dUTP nick-end labeling (TUNEL) to investigate whether the mechanism that produces this effect is neuronal apoptosis.
Material and Methods
ANIMALS:
Female and male C57BL/6 mice (Sibeifu Experimental Animal Science and Technology, Beijing, China) were housed in 330×215×170 mm cages and were maintained in a controlled environment (temperature: 22–25°C) under a 12: 12-hours light: dark cycle (light period: 07: 00–19: 00). Male and female rats were kept in cages at a ratio of 1: 2. We used the offspring of these animals in our experiments. All animal experiments were approved by the Animal Ethics Committee of Chinese PLA General Hospital in Beijing, China.
ULTRASOUND EQUIPMENT AND IRRADIATION SCHEME:
We set the experimental model with reference to research by Li et al. [5]. An Aixplorer ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) equipped with a 4–15 MHz linear-array transducer was used in this study to generate 2D-SWE. Three neonatal mice born within 48 hours of the start of the experiment were fixed to a homemade board (Figure 1A), with a 3 cm water bag placed between the probe and the neonatal mice to improve the focus of the ultrasound on the mice. A standard image shows the mouse head, color-coded by 2D-SWE (Figure 1B). Neonatal mice were divided into a control group or into groups exposed to 2D-SWE for 10 minutes (group S1), 20 minutes (group S2), or 30 minutes (group S3). The control group was sham-irradiated for 30 minutes; that is, the fixed placement method and ultrasound probe placement were the same as the 2D-SWE irradiation group, but the ultrasound instrument was turned off. Some of the mice in each group were sacrificed immediately after irradiation. Brains were collected and then analyzed by western blot, PCR, and TUNEL tests. The remaining members of the groups were sacrificed 24 hours after the end of irradiation. Western blot and PCR experiments were performed to study the duration of the effect on BDNF.
REAL-TIME QUANTITATIVE POLYMERASE CHAIN REACTION (PCR):
According to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA), total ribonucleic acid (RNA) was extracted with TRIzol reagent, and a SuperScript III reverse transcriptase reagent kit (Invitrogen) was used to reverse transcribe total RNA (500 ng) into complementary DNA with random hexamer primers (Invitrogen). Real-time PCR was carried out using an ABI 7900 Real-Time PCR System (Thermo Fisher Scientific, USA) with Power SYBR® Green PCR Master Mix (Applied Biosystems USA). The sample results were compared against a standard curve, normalized to expression levels of actin to determine the messenger RNA (mRNA) expression levels. The control expression level was set to 1. The sequences of the primers used were as follows:
All primers were purchased from RiboBio (RiboBio, Guangzhou, China). All experiments were repeated 3 times, with at least 3 replicates in each group; mRNA expression levels were calculated using the comparative CT method (2−ΔΔCT) [8].
WESTERN BLOTTING:
Protein was extracted from the brains of animals from each group with radioimmunoprecipitation assay (RIPA) lysis buffer (pH 7.4; 50 mM Tris HCl, 150 mM NaCl, 1% NP-40 [nonionic polyoxymethylene surfactant], and 0.1% SDS [sodium dodecyl sulfate]), and it was incubated on ice for 30 minutes. Protein fractions in the pellet were collected following centrifugation at 10 000 g at 4°C for 10 minutes and then were quantified using Bradford protein assay reagent (Bio-Rad, USA). For each lane, 30 mg of total protein was separated by 12% SDS-PAGE (polyacrylamide gel electrophoresis) and then transferred to Trans-Blot polyvinylidene fluoride (PVDF) membranes (Applied Biosystems, USA). After blocking with 5% nonfat milk in a Tris-buffered saline (140 mM NaCl, 20 mM Tris, pH 7.4) solution containing 0.1% Tween (1×TBST) for 2 hours at room temperature; membranes were incubated overnight at 4°C with a primary antibody (anti-BDNF, 1: 1000, ab108319; Abcam, Cambridge, UK). Then, membranes were incubated with a goat anti-mouse secondary antibody conjugated to horseradish peroxidase (1: 3000, Servicebio, Wuhan, China) for 1 hour. Protein bands were visualized using an enhanced chemiluminescence assay (ECL, Thermo Scientific, USA) and captured by Alpha Innotech (Alpha, USA). Actin was used as the loading control. Densitometry was performed by using ImageJ software (NIH, Bethesda, MA, USA).
TUNEL METHOD:
Referring to the instructions of an In-Situ Cell Death Detection kit (Roche, Penzberg, Germany), 10 μm sections were cut from paraffin-embedded brains from each group. Deparaffinization and hydration of paraffin-embedded sections was performed. The solution of proteinase K was added dropwise to the sections, and the sections were incubated at 37°C for 25 minutes. Then, a solution was added the sections to rupture the cell membranes, and then they were incubated at room temperature for 20 minutes. Then 5 μL TdT and 45 μL of dUTP were mixed and added to the sections, and they were incubated at 37°C for 3 hours. DAPI (4′,6-diamidino-2-phenylindole) staining solution was added dropwise, which was followed by incubation at room temperature for 10 minutes in the dark. The sections were observed under a fluorescence microscope.
STATISTICS:
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the means±standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA), followed by Newman-Keuls post hoc testing for multiple comparisons.
Results
QUANTIFICATION OF BDNF EXPRESSION:
To investigate whether BDNF was affected following 2D-SWE, we quantified the expression of proteins and mRNA by western blot and PCR, respectively. A statistically significant decrease was noted between the experimental groups and the control group at both the mRNA level (Figure 2A) and the protein level (Figure 2B), though there were no significant differences between the 3 experimental groups. To assess whether the effect of 2D-SWE exposure on the expression of BDNF was transient or long-lasting, we also quantified expression levels by western blot and PCR in mice sacrificed 24 hours after exposure to 2D-SWE (Figure 3A, 3B). No statistically significant differences were observed between the experimental groups and the control group, confirming that the changes in BDNF mRNA and protein expression did not last more than 24 hours.
TUNEL TEST:
Figure 4 shows the results of the TUNEL images. The green dot-like spots of fluorescence indicated apoptotic cells. To further quantify apoptotic cells in hippocampal neuron cells, we randomly selected 3 visual fields of hippocampal neurons from one TUNEL-stained section per 5 neonatal mice in each group to count the number of apoptotic cells. The number of apoptotic cells per field in TUNEL sections of the 4 groups was as follows: the control group: 1.22±0.97; group S1: 1.11±0.93; group S2: 1.33±1.12; and group S3: 1.22±1.09. One-way ANOVA showed that P=0.829. There was no significant difference in the number of apoptotic neurons in the hippocampus among the 4 groups.
Discussion
As a new ultrasound technology, 2D-SWE uses dynamic radiating force to generate transverse shear waves in deep tissues, and imaging of the medium during propagation is at a very high frame rate. The elasticity of the measured tissue can be calculated based on the propagation of the captured shear wave [9]. The final data are displayed in units of shear wave propagation speed (m/sec) and can also be converted into Young’s modulus (kPa) [10]. This technique has been widely used clinically. In recent years, some scholars have begun to study the application of ultrasound elastography in the examination of neonatal brain diseases. Kim et al. measured the elasticity of healthy neonatal brain tissue using ultrasound elastography [2]. They found that the elasticity of gray matter in the cerebral cortex was higher than in other regions, the elasticity of the caudate nucleus was lower than in different areas; they found no significant gender difference in the elasticity of the brain region. Albayrak and Kasap found that using 2D-SWE could show differences in brain elasticity values between preterm and term neonates [11].
Ultrasound has potential biological effects. Previous animal experiments have confirmed that ultrasound can have different effects on different tissues of animals, especially the central nervous system of fetuses and newborns, which are susceptible to interference. Studies have shown that ultrasound irradiation for longer than 30 minutes affects neural migration in the fetal stage of mice, and this effect will be greater with increasing irradiation time [12]. Pregnant rats receiving ultrasound irradiation for more than 20 minutes can cause fetal rat cortical neuron apoptosis, and the apoptosis rate is related to the irradiation time. However, to date, related research reports on the safety of ultrasonic elastography in the central nervous system are still scarce. In a previous study, our research group used 2D-SWE to irradiate the brains of neonatal mice and found that when 2D-SWE was used to irradiate for 10 minutes, the expression of p-PKCa protein in brain tissue decreased; furthermore, the P13K/AKT/mTOR signaling pathway in brain tissue was affected when the scanning lasted for 30 minutes [5]. Although these changes may be self-repaired as the mice grew, however, whether the alterations in P13K/AKT/mTOR expression could affect other physiological processes is not known.
The Food and Drug Administration (FDA) has proposed output display standards (ODSs) to indicate the potential biological effects of ultrasound [13]. ODSs mainly include 2 critical indicators: thermal index (TI) and mechanical index (MI). Relevant experiments have confirmed that the thermal and mechanical effects of ultrasonic elastography in soft tissue are within the safety limits of TI and MI [14]. Compared with B-mode ultrasound and Doppler ultrasound, the output power and pulse duration of elastography are different [15]. Church et al. believe that the current safety indicator parameters, such as TI and MI, are based on ultrasound with a short duration of the pulse, such as B-mode ultrasound, but compared with B-mode ultrasound, the pulse duration of shear wave elastography can reach hundreds of μs [14]. However, to date, the relationship between the relevant safety parameters of shear wave elastography and their exact biological effects has not been clearly defined [16,17].
Infants and young children are in the stage of the synaptic bursts of the central nervous system, which is the critical period of rapid differentiation and migration of neuronal cells and the rapid formation of neural networks by dendrites. If this process is disturbed, it will produce significant effects on brain structure and function [12]. Exploring the biological impacts of 2D-SWE on neonatal brain tissue still has vital clinical significance [3].
BDNF is a member of the neurotrophin family, and it plays an essential role in modulating neuronal survival, growth, and maintenance during neuronal development [6]. BDNF also affects synaptic plasticity by regulating axonal and dendritic branching and remodeling, synapse formation, and the effectiveness of synaptic transmission, excitability, and inhibition [7,18,19]. Long-term potentiation (LTP) is an electrophysiological process that is necessary for learning and memory. If the BDNF gene is deleted or inhibited, LTP is adversely affected [20].
Based on the experimental results, we found that 2D-SWE exposure led to the downregulation of BDNF expression at both the mRNA and protein levels when the scanning lasted for more than 10 minutes, however, these changes in expression, did not differ between the experimental groups. Our study of mice at 24 hours after the exposure revealed no difference in the expression of BDNF between the experimental groups and the control group, demonstrating that the duration of the induced changes in expression may not exceed 24 hours.
We used TUNEL experiments to determine whether the mechanism of the effect of 2D-SWE craniocerebral irradiation on BDNF occurred because of an induction of neuronal apoptosis. The results of TUNEL experiments showed that there was no significant difference in the number of apoptotic neurons in the hippocampus of the S1, S2, and S3 groups, confirming that the effect of 2D-SWE craniocerebral irradiation on BDNF expression was not caused by neuronal apoptosis. The biological impact of 2D-SWE is essentially a physical factor, and it does not have the function of targeted regulation of specific gene expression. However, our previous studies found that within 30 minutes of craniocerebral irradiation via 2D-SWE, the expression of specific proteins, such as iNOS, CC3, and Bcl-2, was not affected, and mTOR expression was downregulated at 30 minutes after irradiation [5]. This experiment found that 2D-SWE irradiation of the brains of neonatal mice for 10 minutes affected the expression of BDNF. The effects of 2D-SWE on different genes are not consistent. It is speculated that the sensitivity of varying mRNA and proteins to the thermal and mechanical effects of ultrasound may be different.
Our experiment had some limitations. We did not study the long-term effects of 2D-SWE brain irradiation on mice. Whether the short-term downregulation of BDNF expression in childhood will lead to changes in learning and memory function in adult mice is still worthy of continued research. In addition, considering the differences in brain volume, skull hardness, etc. between mice and human neonates, it is still necessary to further study whether the same effect will appear in neonates after 2D-SWE scanning. At this stage, there is no specific international safety regulation for the application of 2D-SWE in craniocerebral examinations of newborns. In this era of continuous innovation in ultrasound technology and increasing levels of acoustic energy output [21], useful scanning and minimal exposure to ultrasound are still an essential part of limiting potential biological effects [22]. Some scholars have found that there is a lack of training standards for personnel who perform craniocerebral ultrasound scans of newborns, and there is a lack of understanding of the safety issues of diagnostic ultrasound [23,24]. Therefore, we recommend that doctors follow the ALARA (as low as reasonably achievable) recommendations when using 2D-SWE for neonatal craniocerebral ultrasound and minimize exposure time until the biological effects of 2D-SWE on children’s brain tissue can be more clearly elucidated [25].
Conclusions
In this study, we observed that the craniocerebral exposure of neonatal mice to 2D-SWE for 10 minutes caused a downregulation in the expression of BDNF, although the magnitude of this change did not differ at longer exposure times within 30 minutes. The effect of 2D-SWE craniocerebral irradiation on the expression of BDNF in the brains of newborn mice does not continue past 24 hours after the end of irradiation. The irradiation of 2D-SWE did not cause apoptosis of hippocampal neuron cells, and its mechanism of affecting BDNF needs to be further explored.
Figures
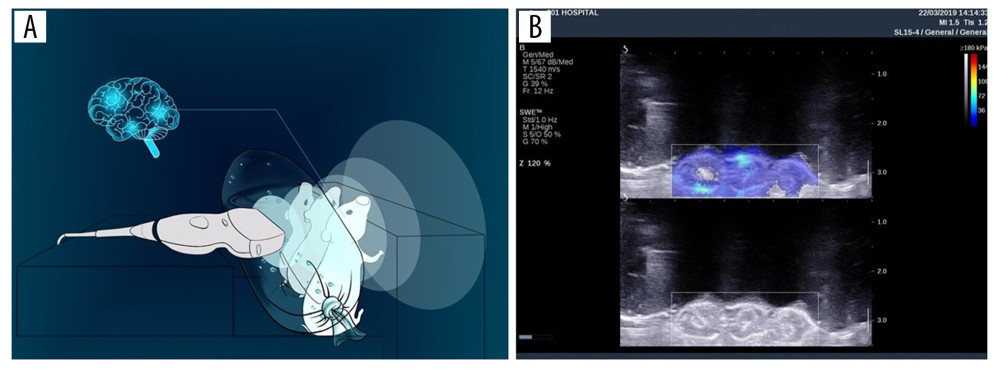 Figure 1. Preparation of animal models. (A) Schematic of neonatal rats exposed to 2D-SWE. (B) The standard image of neonatal rats subjected to 2D-SWE. 2D-SWE – 2-dimensional shear wave elastography.
Figure 1. Preparation of animal models. (A) Schematic of neonatal rats exposed to 2D-SWE. (B) The standard image of neonatal rats subjected to 2D-SWE. 2D-SWE – 2-dimensional shear wave elastography. 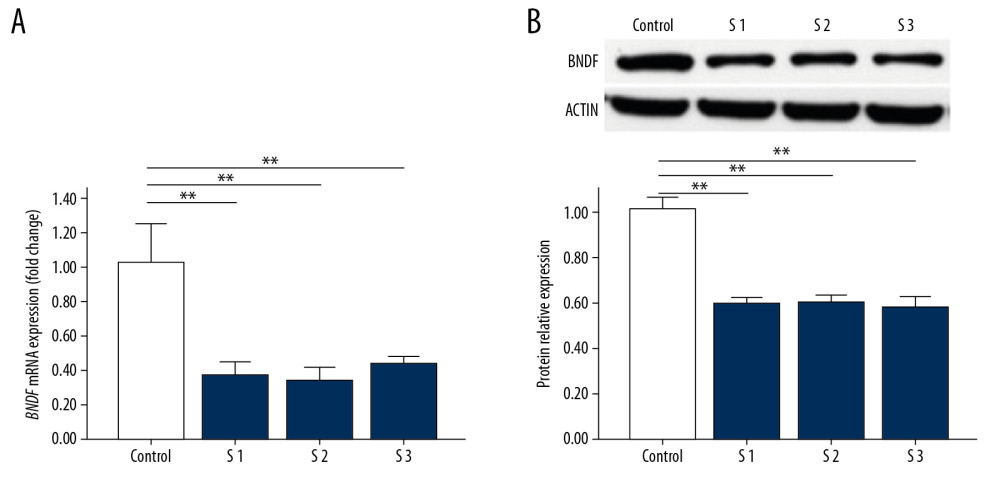 Figure 2. The expression of BDNF in the brains of mice sacrificed immediately after exposure to 2D-SWE. The relative mRNA expression of BDNF qRT-PCR (A). The protein levels of BDNF were assessed by western blot (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and protein expression levels were normalized to actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation.
Figure 2. The expression of BDNF in the brains of mice sacrificed immediately after exposure to 2D-SWE. The relative mRNA expression of BDNF qRT-PCR (A). The protein levels of BDNF were assessed by western blot (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and protein expression levels were normalized to actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation. 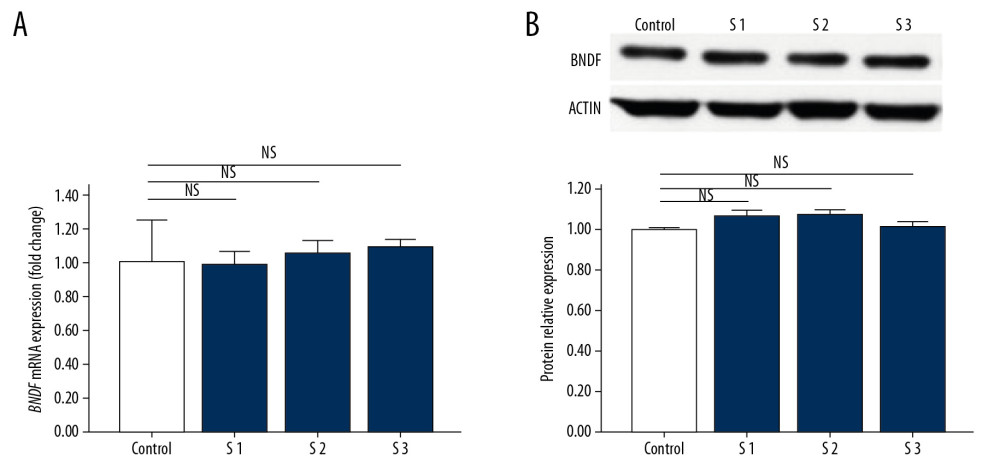 Figure 3. The expression of BDNF in the brains of mice sacrificed 24 hours after exposure to 2D-SWE. The relative mRNA expression levels of BDNF was detected by qRT-PCR (A). The protein expression levels of BDNF were quantified by western blots (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and the protein expression levels were normalized to those of actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA method. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation.
Figure 3. The expression of BDNF in the brains of mice sacrificed 24 hours after exposure to 2D-SWE. The relative mRNA expression levels of BDNF was detected by qRT-PCR (A). The protein expression levels of BDNF were quantified by western blots (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and the protein expression levels were normalized to those of actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA method. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation. 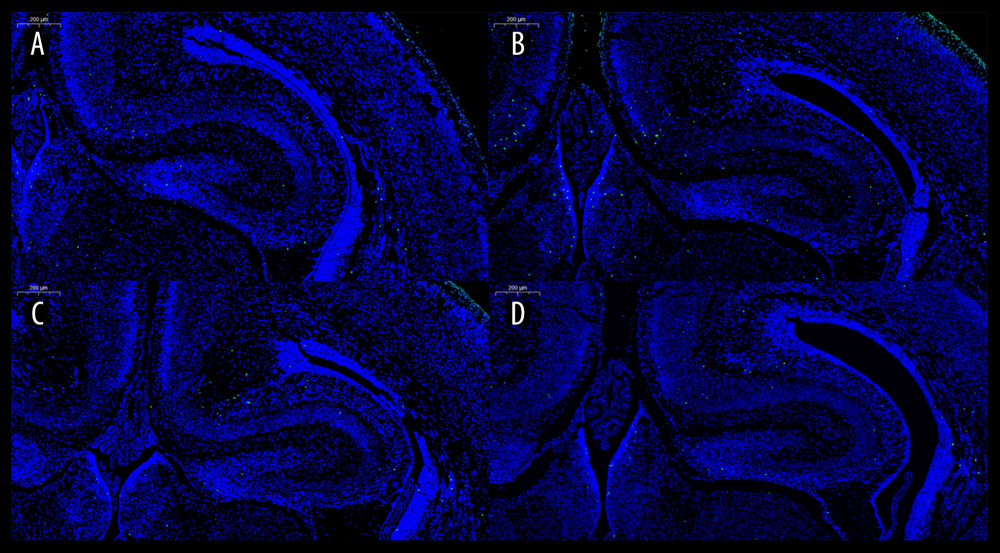 Figure 4. Image of the TUNEL test. The control group (A), Group S1 (B), Group S2 (C), and Group S3 (D). TUNEL – TdT-mediated dUTP nick-end labeling.
Figure 4. Image of the TUNEL test. The control group (A), Group S1 (B), Group S2 (C), and Group S3 (D). TUNEL – TdT-mediated dUTP nick-end labeling. References
1. Su Y, Ma J, Du LF, Evaluation of neonatal brain development using acoustic radiation force impulse imaging (ARFI): Neurophysiology, 2015; 47(4); 322-25
2. Kim HG, Park MS, Lee JD, Park SY, Ultrasound elastography of the neonatal brain: preliminary study: J Ultrasound Med, 2017; 36(7); 1313-19
3. Lalzad A, Wong F, Schneider M, Neonatal cranial ultrasound: Are current safety guidelines appropriate?: Ultrasound Med Biol, 2017; 43(3); 553-60
4. Unsworth T, Proceedings of the Institution of Mechanical Engineers Part H: Proc Inst Mech Eng H, 2008; 222(7); i
5. Li C, Zhang C, Li J, An experimental study of the potential biological effects associated with 2-D shear wave elastography on the neonatal brain: Ultrasound Med Biol, 2016; 42(7); 1551-59
6. Barde YA, Neurotrophins: A family of proteins supporting the survival of neurons: Prog Clin Biol Res, 1993; 390(390); 45-56
7. Alsina B, Vu T, Cohen-Cory S: Nat Neurosci, 2001; 4(11); 1093-101
8. Schmittgen TD, Livak KJ, Analyzing real-time PCR data by the comparative CT method: Nat Protoc, 2008; 3(6); 1101-8
9. Fatemi M, Greenleaf JF, Probing the dynamics of tissue at low frequencies with the radiation force of ultrasound: Phys Med Biol, 2000; 45(6); 1449-64
10. Bercoff J, Tanter M, Fink M, Supersonic shear imaging: A new technique for soft tissue elasticity mapping: IEEE Trans Ultrason Ferroelectr Freq Control, 2004; 51(4); 396-409
11. Albayrak E, Kasap T, Evaluation of neonatal brain parenchyma using 2-dimensional shear wave elastography: J Ultrasound Med, 2018; 37(4); 959-67
12. Ang ES, Gluncic V, Duque A, Prenatal exposure to ultrasound waves impacts neuronal migration in mice: Proc Natl Acad Sci USA, 2006; 103(34); 12903-10
13. Meltzer RS, Food and Drug Administration ultrasound device regulation: The output display standard, the “mechanical index,” and ultrasound safety: J Am Soc Echocardiogr, 1996; 9(2); 216-20
14. Church CC, Labuda C, Nightingale K, A theoretical study of inertial cavitation from acoustic radiation force impulse imaging and implications for the mechanical index: Ultrasound Med Biol, 2015; 41(2); 472-85
15. Friedrich-Rust M, Romenski O, Meyer G, Acoustic radiation force impulse-imaging for the evaluation of the thyroid gland: A limited patient feasibility study: Ultrasonics, 2012; 52(1); 69-74
16. Cho SH, Lee JY, Han JK, Choi BI, Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: Preliminary findings: Ultrasound in Med Biol, 2009; 36(2); 202-8
17. Fahey BJ, Palmeri ML, Trahey GE, Frame rate considerations for real-time abdominal acoustic radiation force impulse imaging: Ultrason Imaging, 2006; 28(4); 193-210
18. Yacoubian TA, Lo DC, Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth: Nat Neurosci, 2000; 3(4); 342-49
19. Seil FJ, Drake-Baumann R, TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis: J Neurosci, 2000; 20(14); 5367-73
20. Korte M, Carroll P, Wolf E, Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor: Proc Natl Acad Sci USA, 1995; 92(19); 8856-60
21. Duck FA, Martin K, Trends in diagnostic ultrasound exposure: Phys Med Biol, 1991; 36(11); 1423-32
22. Barnett SB, Current status of safety of diagnostic ultrasound: Hosp Med, 2002; 62(12); 726-27
23. Lalzad A, Wong F, Singh N, Knowledge of safety, training, and practice of neonatal cranial ultrasound: A survey of operators: J Ultrasound Med, 2018; 37(6); 1411-21
24. Davis PJ, Cox RM, Brooks J, Training in neonatal cranial ultrasound: A questionnaire survey: Br J Radiol, 2005; 78(925); 55-56
25. Cibull SL, Harris GR, Nell DM, Trends in diagnostic ultrasound acoustic output from data reported to the US Food and Drug Administration for device indications that include fetal applications: J Ultrasound Med, 2013; 32(11); 1921-32
Figures
 Figure 1. Preparation of animal models. (A) Schematic of neonatal rats exposed to 2D-SWE. (B) The standard image of neonatal rats subjected to 2D-SWE. 2D-SWE – 2-dimensional shear wave elastography.
Figure 1. Preparation of animal models. (A) Schematic of neonatal rats exposed to 2D-SWE. (B) The standard image of neonatal rats subjected to 2D-SWE. 2D-SWE – 2-dimensional shear wave elastography. Figure 2. The expression of BDNF in the brains of mice sacrificed immediately after exposure to 2D-SWE. The relative mRNA expression of BDNF qRT-PCR (A). The protein levels of BDNF were assessed by western blot (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and protein expression levels were normalized to actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation.
Figure 2. The expression of BDNF in the brains of mice sacrificed immediately after exposure to 2D-SWE. The relative mRNA expression of BDNF qRT-PCR (A). The protein levels of BDNF were assessed by western blot (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and protein expression levels were normalized to actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation. Figure 3. The expression of BDNF in the brains of mice sacrificed 24 hours after exposure to 2D-SWE. The relative mRNA expression levels of BDNF was detected by qRT-PCR (A). The protein expression levels of BDNF were quantified by western blots (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and the protein expression levels were normalized to those of actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA method. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation.
Figure 3. The expression of BDNF in the brains of mice sacrificed 24 hours after exposure to 2D-SWE. The relative mRNA expression levels of BDNF was detected by qRT-PCR (A). The protein expression levels of BDNF were quantified by western blots (B). The densitometric analysis of each band was performed using Image-Pro Plus 6.0, and the protein expression levels were normalized to those of actin. Data are presented as the mean±SD from 3 independent experiments. Differences in measurement data were compared with one-way ANOVA method. * P<0.05; ** P<0.01, and NS – no significance (P>0.05). BDNF – brain-derived neurotrophic factor; 2D-SWE – 2-dimensional shear wave elastography; mRNA – messenger RNA; qRT-PCR – quantitative real-time polymerase chain reaction; SD – standard deviation. Figure 4. Image of the TUNEL test. The control group (A), Group S1 (B), Group S2 (C), and Group S3 (D). TUNEL – TdT-mediated dUTP nick-end labeling.
Figure 4. Image of the TUNEL test. The control group (A), Group S1 (B), Group S2 (C), and Group S3 (D). TUNEL – TdT-mediated dUTP nick-end labeling. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








