04 October 2020: Animal Study
Z-Ligustilide Ameliorates Diabetic Rat Retinal Dysfunction Through Anti-Apoptosis and an Antioxidation Pathway
Bing Yang1ABCE*, Guobin Ma1BCE, Yang Liu1DEFDOI: 10.12659/MSM.925087
Med Sci Monit 2020; 26:e925087
Abstract
BACKGROUND: Diabetic retinopathy (DR) is one of the major causes of vision impairment. Z-ligustilide (3-butylidene-4,5-dihydrophthalide; Z-LIG) is an important volatile oil from the Chinese herb Angelica sinensis (Oliv.) Diels. It has been extensively studied and reportedly has anti-inflammatory, antioxidant, antitumor, analgesic, vasodilatory, and neuroprotective effects. Its effects on DR, however, remain obscure. In this study, we attempted to explore the protective effects of Z-LIG on retinal dysfunction and the potential underlying mechanisms.
MATERIAL AND METHODS: A diabetic rat model was constructed with streptozotocin injection. Three study groups were constituted: control (CON), diabetic model (DM), and DM+Z-LIG. The DM+Z-LIG group was injected intraperitoneally with 10 mg/kg of Z-LIG. The other groups received the same volume of 3% solution of polysorbate 80. After a 12-week intervention, a series of assessments were performed, including tests for retinal function, morphology, and molecular biology.
RESULTS: Z-LIG treatment significantly elevated b-wave and OPs2-wave amplitude and thickened the inner layer of the nucleus of the retina, and the outer plexiform and nuclear layers (INL+OPL+ONL). Moreover, the rate of apoptosis and expression of bcl-2- associated X protein (BAX) and cleaved-Caspase-3 were clearly reduced, and the expression of bcl-2 was raised by Z-LIG in retinas of diabetic mice. In addition, the levels of retinal proinflammatory cytokines interleukin-1 and tumor necrosis factor-α were downregulated by Z-LIG. Furthermore, Z-LIG inhibited expression of vascular endothelial growth factor-α (VEGF-α) at the mRNA and protein levels.
CONCLUSIONS: Z-LIG can inhibit inflammatory response and cell apoptosis in retinas of diabetic rats by repressing the VEGF-α pathway. Therefore, it may serve as a potential therapeutic agent for DR.
Keywords: Diabetic Retinopathy, Vascular Endothelial Growth Factor A, 4-Butyrolactone, Diabetes Mellitus, Experimental
Background
Diabetes mellitus (DM) is quickly becoming a global epidemic [1]. In 2017, there were more than 400 million patients with diabetes worldwide, and the number is expected to rise to 700 million by 2045 [2]. Diabetic retinopathy (DR), a neurovascular complication of DM, is one of the most prevalent causes of vision loss in elderly adults all over the world [3].
Clinically, DR is characterized by primary blood vessel impairment, which causes sight-threatening pathologic neovascularization, tissue ischemia, and hypoxia. Moreover, DR is seen in about one-third of patients with diabetes, and one-tenth of patients with DR are clinically diagnosed with significant vision impairment [4,5]. The pathogenesis of DR involves pathological processes and signaling pathways, such as ischemia, an inflammatory response to oxidative stress, accumulation of advanced glycation end products (AGEs), and overactivation of protein kinase C (PKC) [6–8]. These events upregulate the level of vascular endothelial growth factor (VEGF), a potent factor in angiogenesis, transforming VEGF from having a favorable effect on physiology to inducing negative processes such as, acceleration of neovascularization, survival of vascular endothelial cells, promotion of vascular diosmosis and pro-apoptotic molecules including BAX and Caspase-3, and activation of various other inflammatory factors [8,12]. A colossal inflammatory reaction induced by VEGF alone and combined with other inflammatory mediators that it elicits aggravates the endothelial dysfunction that arises during the progression of DR [13–16]. The inflammatory events, as one of the primary promoters of DR, play a vital role in transforming non-proliferative DR into proliferative DR [17]. Retinal neovascularization is the hallmark of proliferative DR. Furthermore, inflammatory mediators have been reported to lead to the morphological and functional pathological changes that characterize progression of DR [18,19].
Apoptosis, as one of the most pivotal pathological processes in DR, disrupts vascular homeostasis and inevitably accelerates the development of DR [20–25]. Therefore, effectively regulating inflammation and apoptosis in DR is an alternative approach to treating the condition. An intricate milieu of dysregulated proinflammatory mediators in the diabetic retina, such as interleukin (IL)-1, IL-6 and tumor necrosis factor-α (TNF-α) has been linked to both neurovascular dysfunction and formation of acellular capillaries [18,26]. Inflammation also may upregulate production of reactive oxygen species (ROS), triggering retinal cell apoptosis and giving rise to significant damage to the retina’s function and structure as a result of DNA injury, lipid peroxidation, and mitochondrial dysfunction [27,28]. This, in turn, damages the integrity of the blood retinal barrier, inducing retinal microvascular occlusion and causing ischemic retinal changes. In addition, the increased oxidative stress further perturbs the balance of redox reactions, stimulating recruitment of inflammatory cells and augmenting production of inflammatory cytokines and proteins, such as IL-1 and TNF-α, which in turn aggravates retinal neuron damage and causes cell death [29,30]. Moreover, the actions of these proinflammatory cytokines may culminate in activation of caspases, exacerbating apoptosis in the retina.
Z-ligustilide (3-butylidene-4,5-dihydrophthalide; Z-LIG), which is considered one of the most essential volatile oils in the rhizomes of the umbelliferous plants Diels, has various pharmacological effects, including conferring resistance to oxidation, protection from inflammation, and neuroprotection [31,32]. In a model of oxygen-glucose deprivation injury, Z-LIG has been shown to ameliorate the permeability of the blood-brain barrier through the VEGF pathway [33]. Studies have shown that Z-LIG can protect vascular endothelial cells damaged by oxidative stress and rescue high-fat-diet–related atherosclerosis by activating
In this paper, we explored the anti-apoptotic effect of Z-LIG on STZ-induced retinal dysfunction in a diabetic rat model, evaluated the mechanism related to it, and found evidence supporting a clinical pharmacological application for Z-LIG as an alternative treatment for DR.
Material and Methods
ANIMALS:
All animal experiments were accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Studies were approved by the Institutional Laboratory Animal Care and Use Ethics Committee of Xi’an Jiaotong University. Adult male Sprague-Dawley rats weighing 180 g to 220 g were purchased from Air Force Medical University Laboratory Animal Center (Xi’an, China). Animals were housed at a suitable temperature (22°C to 24°C) in a 12-hour light/dark cycle-controlled laboratory environment and free access to rat chow and water.
INDUCTION OF DIABETES AND DRUG INTERVENTION:
Male Sprague-Dawley rats were acclimated to the laboratory animal room for 5 days before the study began. Then, they were rendered diabetic with a single dose of intraperitoneally-injected streptozotocin (STZ; Sigma-Aldrich, UK; 60 mg/kg in citrate buffer, pH 4.4). A week later, blood glucose levels in the rats were assessed by Roche glucometer (ACCU-CHEK Performa, Germany) using tail-prick blood samples. Rats with blood glucose concentrations ranging from 16.7 mmol/L to 33.3 mmol/L were presumed to have DM and were used in this study. Moreover, Z-LIG (purity >98%, CAS number: 4431-01-0, Cat. L115724, Aladdin) was dissolved in a 3% (w/v) solution of polysorbate 80 (TW-80). Subsequently, 24 rats with successfully controlled DM were chosen and randomly divided into 2 groups: DM and DM+Z-LIG group (n=12 in each group). For 12 consecutive weeks after establishment of the diabetic rat model, the groups were administered equal volumes of 3% solution of TW-80 and 10 mg/kg Z-LIG, respectively. Simultaneously, 12 citrate-buffer-injected, age-matched normal rats were used as the control group (3% solution of TW-80, equal volume). The administration method was intraperitoneal injection, once a day.
FULL-FIELD ELECTRORETINOGRAMS:
Twelve weeks after the diabetes model was established, full-field electroretinograms (ffERGs) were performed with the EPIC-4000 Visual Electrodiagnostic Testing System (LKC Technologies, Gaithersburg, Maryland, United States). The rats were placed in a dark room overnight for dark adaptation and anesthetized with ketamine (150 mg/kg). After their pupils were dilated with atropine eye drops, ffERGs were recorded with a corneal electrode and steel needle electrode placed on the cheek and the tail. ERG testing was conducted with the previously described approach [38], which is in accordance with International Society for Clinical Electrophysiology of Vision guidelines. To prevent corneal infection, levofloxacin eye drops (Suzhou, China) were applied once daily after the ffERGs were performed.
HISTOPATHOLOGICAL EXAMINATION:
To evaluate the histologic characteristics of diabetic rat retinas, hematoxylin & eosin (HE) staining was performed. After intraperitoneal injection of a lethal dose of sodium pentobarbital, rats were euthanized by cervical dislocation under deep anesthesia, and their eyes were quickly removed. The eyes were immersed for 24 hours in a 4% paraformaldehyde solution and embedded in paraffin blocks, from which 5-μm sections were cut. The paraffin sections were stained using the HE staining protocol. The thicknesses of the internal limiting membranes (ILM), inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layers (ONL) were measured. Three measurements were made at adjacent locations (250 μm) in the optic nerve, and the average of 12 measurements from 4 eyes was recorded as the representative value. Microscopic images were digitally captured with a light microscope (Leica, Heidelberg, Germany).
ELISA:
To evaluate proinflammatory cytokine activity in the diabetic rat retina, an enzyme-linked immunosorbent assay (ELISA) was performed for estimation of retinal levels of IL-1 and TNF-α. Dissected retinas were homogenized in lysis buffer and centrifuged at 10 000 revolutions for 15 minutes to extract retinal proteins. Protein concentrations were detected with a bicinchoninic (BCA) protein assay (Beyotime, China). Aliquots (100 μL) of each supernatant were used to calculate the levels of proinflammatory cytokines IL-1 and TNF-α with specific ELISA kits (Sigma, United States), following the manufacturer’s instructions.
REAL-TIME QUANTITATIVE POLYMERASE CHAIN REACTION:
Total RNA was abstracted from the retinal tissues with a TRIzol reagent (Invitrogen, California, United States). Then, the RNA was reverse-transcribed into complementary DNA with an RTase kit (TaKaRa, Otsu, Japan), in accordance with the manufacturer’s instructions. Polymerase chain reaction (PCR) was amplified with the Applied Bio-system 7500 Fast Real-Time PCR System (Foster City, California, United States). Specifically, 1 μg of total cDNA was added per 20 μL of reaction buffer with ROX Reference Dye (50×) and SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (2×). The PCR primers employed were as follows. The primers of VEGF-α were 5′-GAGCAACGTCACTATGCAGATC-3′ (forward) and 5′-TTTCTCCGCTCTGAACAAGG-3′ (reverse). The primers of GAPDH were 5′-CAAGTTCAACGGCACAGTCAA-3′ (forward) and 5′-CGCCAGTAGACTCCACGACA-3′ (reverse). Cycling conditions were 15 seconds of polymerase activation at 95°C, followed by 30 cycles at 95°C for 7 seconds and at 55°C for 30 seconds. The 2−ΔΔCT method was applied to analyze the relative transcript levels. All experiments were repeated 3 times.
DETERMINATION OF RETINAL EXPRESSION OF VEGF-α, BAX, BCL-2, AND CASPASE-3 PROTEINS:
Western blotting was performed to assess VEGF-α, bcl-2, BAX and Caspase-3 protein expression. Protein was isolated from retinas and BCA assay was applied to measure the total protein concentration. Western blots were applied with 20 mg of protein in each lane and the gels were transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, Massachusetts, United States) after electrophoresis. The membranes were blocked with 5% nonfat dry milk for 1 hour and then incubated in rabbit VEGF-α (Millipore, Billerica, Massachusetts, United States; 1: 1000), BAX (Invitrogen, Carlsbad, California, United States; 1: 2000), bcl-2 (Abcam, Cambridge, Massachusetts, United States; 1: 500), Caspase-3 (Invitrogen, Carlsbad, California, United States; 1: 500) and GAPDH (Abcam, Cambridge, Massachusetts, United States; 1: 2000) antibody solution for 12 hours at 4°C. The blots were then washed with Tris Buffered Saline with Tween (TBST) buffer and then incubated with goat anti-rabbit immunoglobulin G secondary antibody (Zhuangzhi, Xi’an, China) solution (1: 10 000) for 1 hour. The membranes were than washed and exposed to Chemiluminescent Protein Detection System (Bio-Rad, United States) to determine levels of protein signaling. The protein load in the gels was evaluated by binding of glyceraldehyde 3-phosphate dehydrogenase. ImageJ analysis software was used for densitometric analysis of the protein.
TERMINAL DEOXYNUCLEOTIDYL TRANSFERASE DUTP NICK-END LABELING (TUNEL) STAINING:
At the end of the 12-week study, the level of retinal apoptotic cells was measured with TUNEL staining, using a fluorescence testing kit (Roche, United States). The sections were paraffinized, hydrated, and incubated with a 20-μg/mL proteinase K working solution at 37°C for 15 minutes following the manufacturer’s protocols. The sections were rinsed with PBS for 5 minutes and the process was repeated 3 times, and TUNEL testing was performed in accordance with the manufacturer’s instructions. Fluorescent cells were detected under a fluorescence microscope (BX-51, Olympus, Japan) and imaged at 400× magnification. Three measurements were made at locations 250 μm from each optic nerve, and the average of 12 measurements in 4 eyes was recorded as the representative value for each group.
STATISTICAL ANALYSIS:
Values were described as mean±significant deviation (SD). Statistical analyses were assessed with SPSS Software version 19.0 (SPSS, Inc., Chicago, Illinois, United States). One-way analysis of variance and Turkey’s test for post hoc analysis were performed to assess differences among the groups. The level of significance was set at
Results
BLOOD GLUCOSE LEVELS:
Changes in blood glucose concentrations were measured with ACCU-CHEK Performa every week up to Week 12 after diabetes had been induced in the rats. In diabetic rats injected with STZ (DM group and DM+Z-LIG group), statistically significant increases in levels of blood glucose were observed, compared with levels in the animals in the CON group that had been injected with vehicle control (Figure 1). Namely, high levels of circulating glucose were sustained in the rat model during the experimental period. However, there were no significant differences in blood glucose levels in the CON and DM+Z-LIG groups.
FULL-FIELD ELECTRORETINOGRAPHY:
After 12 weeks of treatment, alterations in ERG in rats in the different groups were measured, as previously described and shown in Figure 2. The amplitudes of dark-adapted b waves and OPs2 waves, and of light-adapted b waves were significantly depressed in diabetic rats (DM and DM+Z-LIG groups) compared with the control rats (CON group) (P<.05). However, the DM+Z-LIG group had notable elevations in amplitudes of dark-adapted b waves and OPs2 waves (P<.05 to.01) compared with those in the DM group. The difference between the DM and DM+Z-LIG groups in amplitudes of light-adapted b waves did not reach statistical significance.
HE STAINING OF MORPHOMETRIC STRUCTURE:
After the 12-week Z-LIG intervention, HE staining was performed on tissue sections from each group of rats. As shown in Figure 3, the retinal inner nuclear and outer plexiform, and nuclear layers (INL+OPL+ONL) in the DM and DM+Z-LIG groups were significantly thinner (P<.05 to.001) than in the CON group. Moreover, the retinal ILM in the DM group were thicker than in the CON group (P<.001), while there was no significant difference between the CON and DM+Z-LIG groups (P>.05). However, compared with the DM group, the DM+Z-LIG group had significantly thinner INL+OPL+ONL (P<.001) and little retinal ILM thickening (P<.001).
IL-1 AND TNF-α EXPRESSION:
After the 12-week Z-LIG intervention, expression of the proinflammatory molecules IL-1 and TNF-α was detected with ELISA. As is shown in Figure 4, IL-1 expression in the DM and DM+Z-LIG groups (P<.05 to.01) was notably higher than in the CON group. As for TNF-α, expression in the DM group (P<.01) was significantly higher than in the CON group. However, expression of IL-1 and TNF-α in the DM+Z-LIG group was lower than in the DM group (P<.01).
VEGF-α PROTEIN AND MRNA EXPRESSION:
To further evaluate the underlying anti-inflammatory and anti-angiogenic properties of Z-LIG, VEGF-α levels in both protein and mRNA were assessed by Western blotting and quantitative PCR (qPCR) after the 12-week treatment. As shown in Figure 5A and 5B, protein expression of VEGF-α in the DM and DM+Z-LIG groups was notably enhanced compared with that in the CON group (P<.01). In contrast, VEGF-α protein expression in the DM+Z-LIG group was lower than that in the DM group (P<.001). Moreover, as shown in Figure 5D, expression of VEGF-α in mRNA level was inconsistent with results obtained with Western blotting. The primers for VEGF-α are shown in Figure 5C.
CELL APOPTOSIS:
After the 12-week Z-LIG intervention, apoptosis was assessed by TUNEL experiment and levels of apoptosis-related proteins, including bcl-2, BAX, and Caspase-3, were detected with Western blotting. As shown in Figure 6A and 6B, apoptosis was remarkably elevated in the DM and DM+Z-LIG groups compared with the CON group (P<.001), particularly in retinal ONL and INL. In comparison with the DM group, the DM+Z-LIG group exhibited a significantly decreased rate of apoptosis (P<.001). As shown in Figures 6C–6F, expression of BAX and cleaved-Caspase-3 in the DM (P<.001) and DM+Z-LIG groups (P<.05 to.001) was notably higher than in the CON group. In addition, expression of bcl-2 in the DM group, but not in the DM+Z-LIG group, was less than in the CON group (P<.001). However, expression of BAX and cleaved-Caspase-3 in the DM+Z-LIG group was lower than in the DM group (P<.05 to.001). Moreover, the level of bcl-2 in the DM+Z-LIG group was increased compared with the DM group (P<.01). These results were in accordance with those obtained with TUNEL.
Discussion
In the present study, we found that Z-LIG could ameliorate DR-related damage to retina function and structure by inhibiting inflammation and apoptosis. Diabetes was induced in rats with a single 60-mg/kg STZ injection, which has been widely used to mimic DM in other animal models [39].
A related study showed a reduced b wave amplitude in rats 7 weeks after STZ induction of diabetes [40]. Declining ERG is widely regarded as a typical feature of DR. It is reported that the amplitudes of the OPs progressively decrease with the progression of DR, making it a useful clinical measurement in the diagnosis and the prediction of the progression of DR [41]. Declining b waves in both dark- and light-adapted 3.0 response are specific indicators of ERG that correlate with DR progression [42]. Moreover, ERG was used to evaluate functional deficits in diabetic retina at earlier stages of the disease [43,44]. After the 12-week intervention, retinal function in Z-LIG-treated diabetic rats was improved, both in b (dark-adapted 3.0 response) and in OPs2. Therefore, Z-LIG can ameliorate retinal dysfunction and delay progression of DR.
HE staining showed that retinal INL+OPL+ONL thickness was decreased, while retinal ILM thickness was increased after the 12-week study. Progressive retinal thinning is another crucial feature of DR in patients and in experimental animals [45–57]. In clinical research, thinning of the inner retina and thickening of the ILM have been observed in association with DM, even in earlier-stage retinopathy, indicating progression of DR [48–50]. Z-LIG may have a protective effect on retinal function and also act to rescue the retinal structure, possibly by mediating electrical activity in retina cells.
The deleterious functional and structural pathological alterations in the retina occurred at the same time as detrimental reactions to the regulators of apoptosis and inflammation. In our results, levels of BAX, a pro-apoptotic protein, and of cleaved-Caspase-3, an apoptosis effector protein, were significantly elevated in the retinas of diabetic rat. Moreover, bcl-2, an apoptosis suppressor protein, was notably downregulated. Accordingly, we speculated that a massive number of cells in the retinas of rats with DR underwent apoptosis. Our findings were similar to those of Oshitari et al., who observed increased expression of Caspase-9, Caspase-3, and BAX in sections of retina from patients with DM [24]. Using the TUNEL method, apoptosis also was identified in the present study. We found that apoptosis tends to be concentrated in the ONL and INL of diabetic retinas. We theorized that death in a series of cells endangered retinal function and structure, leading to progression of DR pathology. Our findings are consistent with other research, which showed that apoptosis is one of the most pivotal pathological reactions in DR [20,22–24]. Loss of retinal ganglion cells occurred at 6 weeks, and retinal INL and ONL thinning occurred at 10 weeks after onset of STZ-induced hyperglycemia in mice [51,52]. In our study, hyperglycemia-induced increases in apoptotic factors, such as BAX and cleaved-caspase-3, were all reversed by treatment with Z-LIG. Expression of the apoptosis suppressor protein bcl-2 was markedly increased. Thus, the anti-apoptotic pathway may play a role in Z-LIG’s therapeutic properties. Moreover, we observed significant upregulation of levels of the inflammatory factors IL-1 and TNF-α in the retinas of diabetic rats, which indicated that a significant inflammatory response had occurred. After 12-week Z-LIG treatment, the inflammatory response was ameliorated in retinas of diabetic rats, suggesting that Z-LIG has remarkable anti-inflammatory effects.
To further investigate how Z-LIG exerts its anti-inflammatory and anti-apoptotic actions, expression of VEGF-α, the key instigator in pathological neovascularization, was assessed. Many studies have demonstrated that overexpression of VEGF stimulates certain pro-apoptotic proteins, triggering a cascade of signaling events that leads to endothelial cells apoptosis [7,53]. Another study found that hyperglycemia-induced oxidative stress is responsible for overexpression of VEGF, which negates the positive role that the protein usually plays physiologically in upregulating cell survival, while simultaneously resulting in increased pathological events [15]. In our study, results confirmed by qPCR and Western blotting revealed that levels of expression of VEGF-α in the diabetic retina were significantly increased, suggesting that the VEGF-α signaling pathway was overactivated. We reckoned that this may be one of the possible causative factors of inflammation and apoptosis in retinas of diabetic mice. In addition, related studies have found that VEGF-α is quite a vicious inflammatory mediator, and overregulation of it results in a robust inflammatory response [54,55]. Moreover, several studies have confirmed that by modulating various inflammatory and apoptotic pathways, VEGF-related pathological pathways are involved in damage to retinal blood vessels and endothelial cells at the molecular level [15,53,56]. VEGF-α is known to play a significant role in disease progression, especially in retinal tissue neovascularization and macular edema, the 2 major lesions seen in patients with DR. Of note, Z-LIG clearly reduced expression of mRNA and protein by VEGF-α and had effects similar to those of an VEGF-α inhibitor. That indicates that Z-LIG effectively suppresses activation of the VEGF-α pathway in diabetic retinas.
Despite these findings, whether Z-LIG can modulate VEGF-α signaling directly or indirectly is unknown. In DR, hypoxia-inducible factor-1α (HIF-1α) is recognized as the transcription factor of VEGF and it is a pivotal contributor to diabetes- and ischemia-induced retinal inflammation, vascular leakage, and neovascularization [57–59]. Hyperglycemia has been reported to regulate HIF-1α protein stability by interfering with its proteasome degradation [60]. Extensive research has been done to investigate the mechanism of interaction between HIF-1α and VEGF, and the sites at which HIF-1α is combined with VEGF [61–63]. In addition, some studies have demonstrated that VEGF transcription is dynamically and strictly regulated by activation of extracellular signal-regulated protein kinases 1 and 2, CCAAT enhancer binding protein beta,
Ultimately, our convincing structural, functional, and molecular data have demonstrated that Z-LIG, a multifunctional lipophilic ingredient of the traditional Chinese medicine Radix Angelica Sinensis, helps protect against development of DR. Radix Angelica Sinensis is classically used in clinical treatment and prophylaxis of anemia, rheumatic arthralgia, and cardiovascular conditions. It has effects on the circulatory, blood, immune, and nervous systems, among others, and extensive reports exist about its use in therapy for conditions and diseases such as brain injury, diabetic nephropathy, and atherosclerosis [31,32]. The present study showed that Z-LIG is capable of protecting the structure, restoring the function, reducing cell apoptosis, and inhibiting proinflammatory factors IL-1 and TNF-α in the retinas of diabetic rats, and its activity is related to the VEGF pathway. Therefore, we have identified a new mechanism of action (MOA) by which Z-LIG helps prevent the damage caused to the retina by DM. To our knowledge, this is the first time such a MOA has been reported.
Conclusions
In conclusion, our study demonstrated that Z-LIG plays a significant role in protecting retina function and morphology from damage, and relieving retinal cell apoptosis by increasing expression of bcl-2 and suppressing expression of BAX and cleaved-Caspase3. Moreover, Z-LIG may combat inflammation by inhibiting expression of IL-1 and TNF-α and regulating expression of VEGF-α. Our study, therefore, shows that Z-LIG has potential for use in prevention and treatment of DR.
Figures
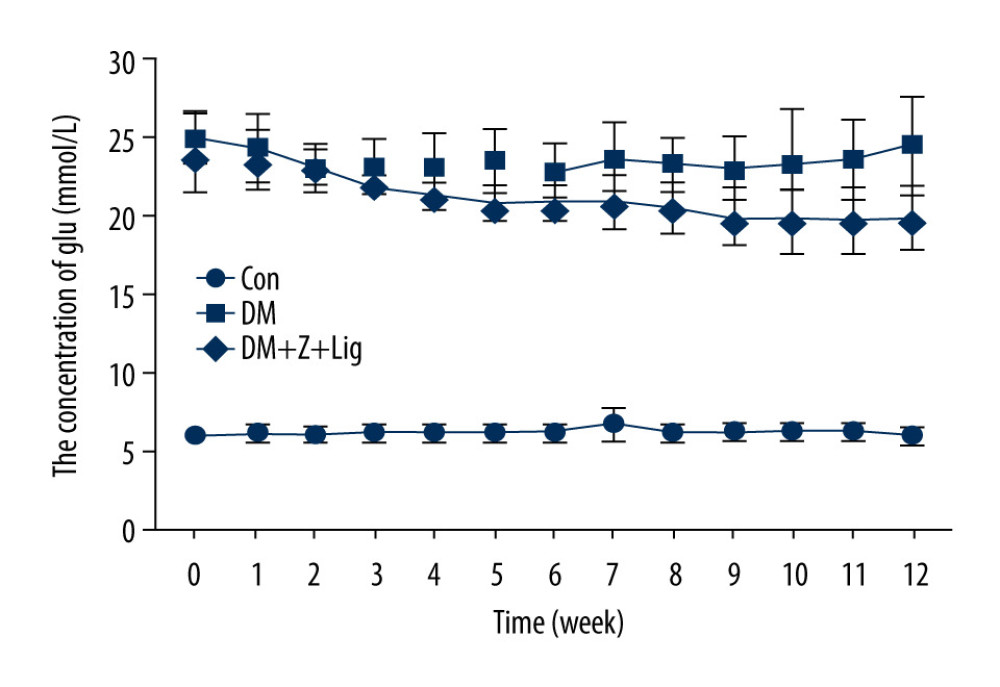 Figure 1. Change in blood glucose levels. A significant increase in blood glucose level is shown in the diabetic rats (DM and DM+Z-Lig groups). Values are presented mean±standard deviation, n=12.
Figure 1. Change in blood glucose levels. A significant increase in blood glucose level is shown in the diabetic rats (DM and DM+Z-Lig groups). Values are presented mean±standard deviation, n=12. 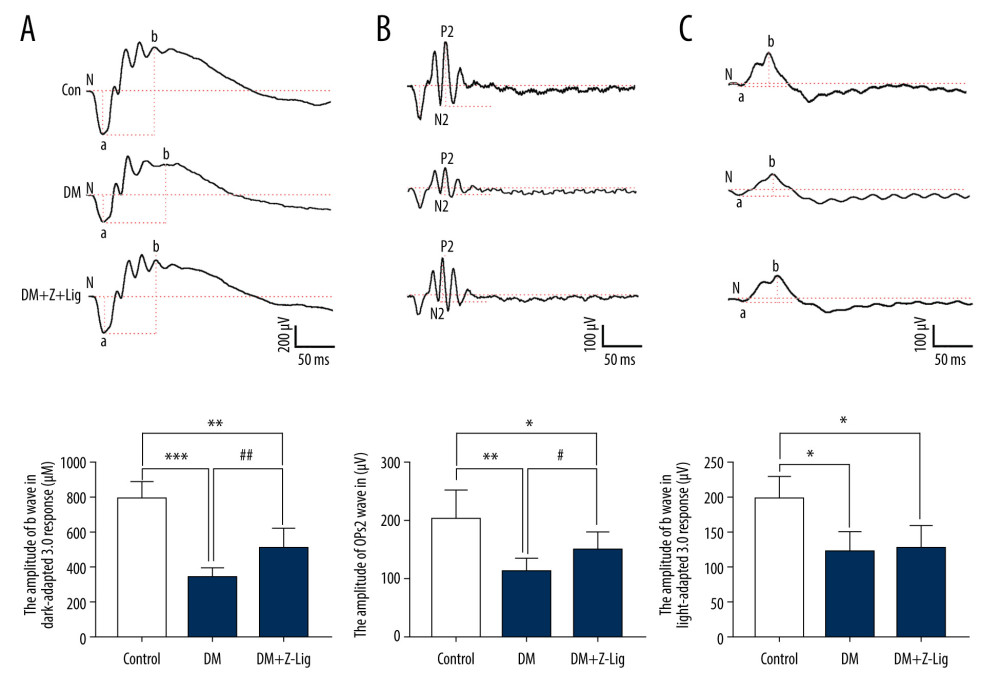 Figure 2. Z-LIG protects diabetic rat retinal function. (A) Amplitude of b wave (dark-adaptation 3.0 response). (B) Amplitude of OPs2 wave (dark-adaptation 3.0 response). (C) Amplitude of b-wave (light-adaptation 3.0 response). Values are presented as mean±standard deviation, n=12. * P<.05, ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; # P<.05, ## P<.01: DM+Z-LIG group vs. DM group.
Figure 2. Z-LIG protects diabetic rat retinal function. (A) Amplitude of b wave (dark-adaptation 3.0 response). (B) Amplitude of OPs2 wave (dark-adaptation 3.0 response). (C) Amplitude of b-wave (light-adaptation 3.0 response). Values are presented as mean±standard deviation, n=12. * P<.05, ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; # P<.05, ## P<.01: DM+Z-LIG group vs. DM group. 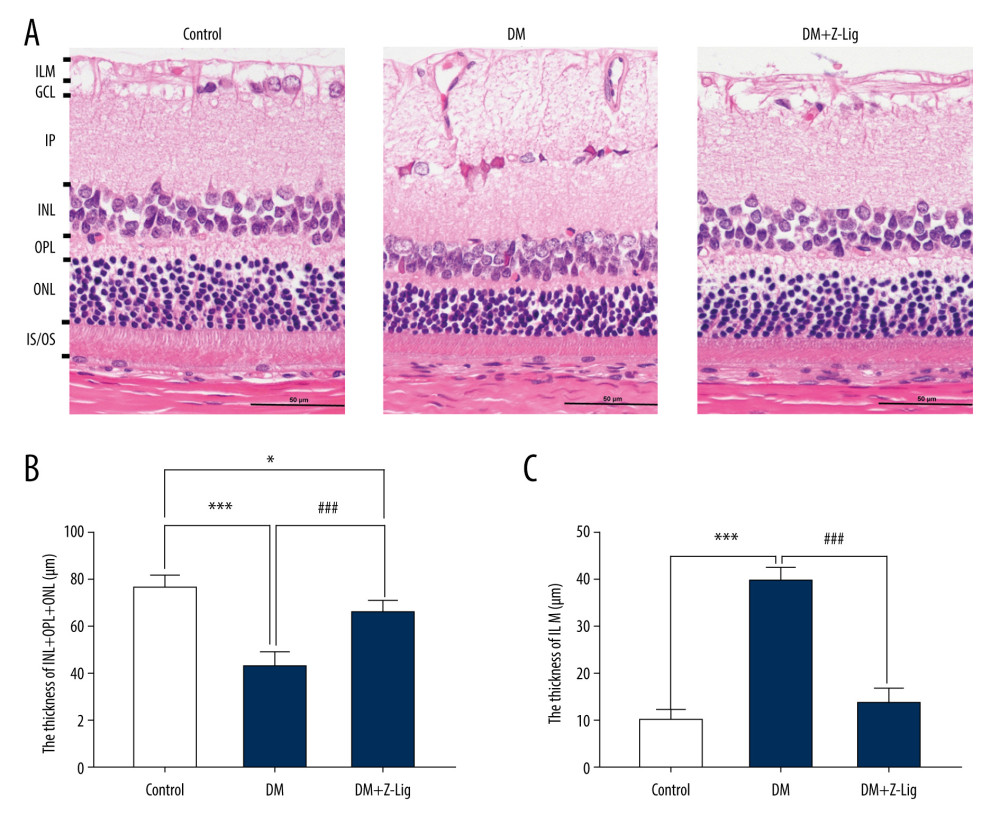 Figure 3. Z-LIG protects the morphometric structure of the retina in diabetic rats after 12 weeks of treatment. (A) Typical HE staining image of the retina. (B) Thickness of retinal INL+OPL+ONL. (C) Thickness of the retinal ILM. GCL – ganglion cell layer; ILM – internal limiting membrane; INL – inner nuclear layer; IPL – inner plexiform layer; IS/OS – photoreceptor inner segment/outer segment; ONL – outer nuclear layer; OPL – outer plexiform layer. Values are presented as mean±standard deviation, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group.
Figure 3. Z-LIG protects the morphometric structure of the retina in diabetic rats after 12 weeks of treatment. (A) Typical HE staining image of the retina. (B) Thickness of retinal INL+OPL+ONL. (C) Thickness of the retinal ILM. GCL – ganglion cell layer; ILM – internal limiting membrane; INL – inner nuclear layer; IPL – inner plexiform layer; IS/OS – photoreceptor inner segment/outer segment; ONL – outer nuclear layer; OPL – outer plexiform layer. Values are presented as mean±standard deviation, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group. 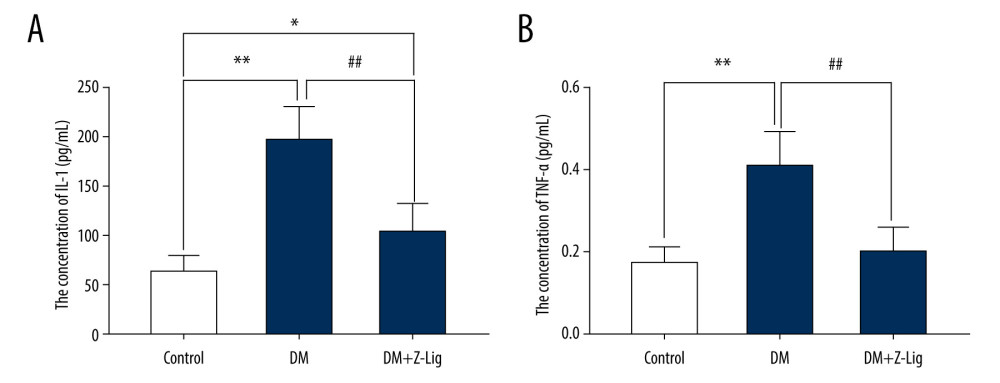 Figure 4. Z-LIG decreases retina IL-1 and TNF-α levels induced by diabetes after 12 weeks of treatment. (A) The concentration of IL-1. (B) The concentration of TNF-α. Values are presented as mean±SD, n=4. * P<.05, ** P<.01: DM group, DM+Z-LIG group vs. control group; ## P<.01: DM+Z-LIG group vs. DM group.
Figure 4. Z-LIG decreases retina IL-1 and TNF-α levels induced by diabetes after 12 weeks of treatment. (A) The concentration of IL-1. (B) The concentration of TNF-α. Values are presented as mean±SD, n=4. * P<.05, ** P<.01: DM group, DM+Z-LIG group vs. control group; ## P<.01: DM+Z-LIG group vs. DM group. 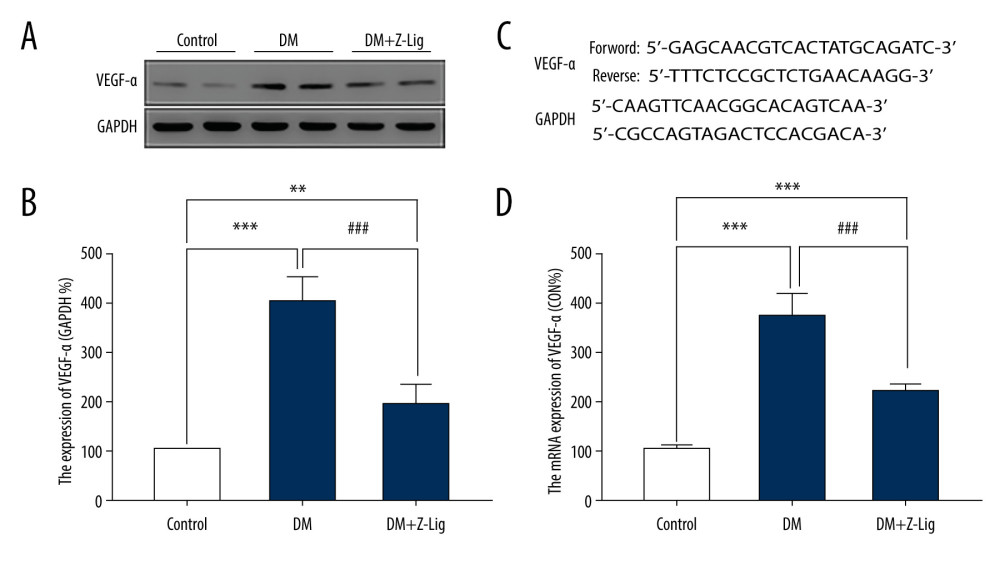 Figure 5. Z-LIG decreases diabetes-induced expression of VEGF-α in protein and mRNA in the retina after 12 weeks of treatment. (A) VEGF-α representative western blots with the respective loading control (GAPDH). (B) The protein expression of VEGF-α (GAPDH%). (C) The primers of VEGF-α. (D) The mRNA expression of VEGF-α (CON%). Data are presented as percentage of control and values are presented as mean ± standard deviation, n=4. ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group.
Figure 5. Z-LIG decreases diabetes-induced expression of VEGF-α in protein and mRNA in the retina after 12 weeks of treatment. (A) VEGF-α representative western blots with the respective loading control (GAPDH). (B) The protein expression of VEGF-α (GAPDH%). (C) The primers of VEGF-α. (D) The mRNA expression of VEGF-α (CON%). Data are presented as percentage of control and values are presented as mean ± standard deviation, n=4. ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group. 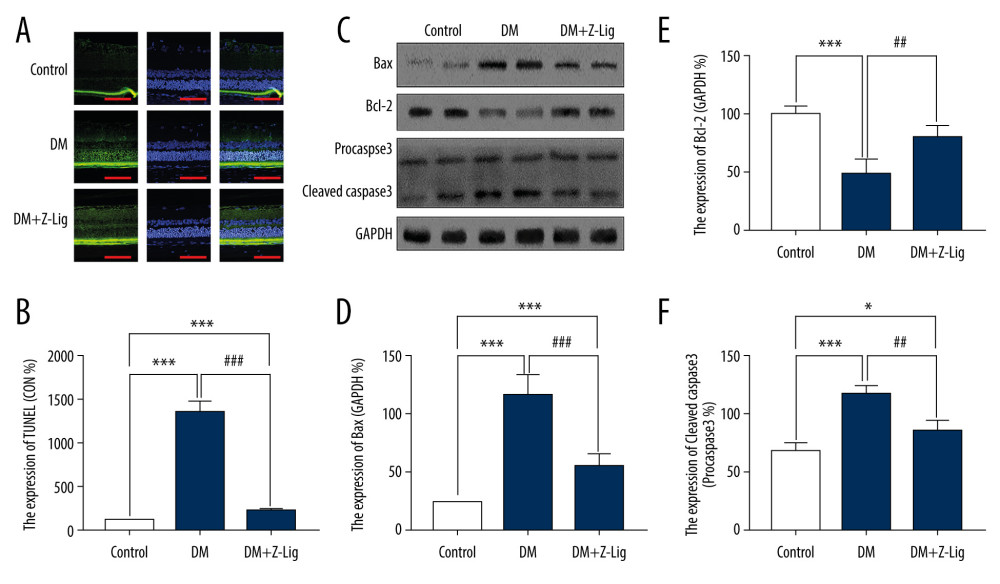 Figure 6. Z-LIG attenuates retinal cell apoptosis after 12 weeks of treatment. (A) Representative TUNEL images. (B) Expression of TUNEL (CON%). (C) Representative Western blots (BAX, bcl-2, procaspase and cleaved-Caspase-3), with their respective loading controls (GAPDH). (D) Relative density of immunoblot of BAX. (E) Relative density of immunoblot of bcl-2. (F) Relative density of immunoblot of cleaved-Caspase-3. Data are presented as percentage of control (or pro-Caspase-3) and values are presented as mean±SD, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ## P<.01, ### P<.001: DM+Z-LIG group vs. DM group.
Figure 6. Z-LIG attenuates retinal cell apoptosis after 12 weeks of treatment. (A) Representative TUNEL images. (B) Expression of TUNEL (CON%). (C) Representative Western blots (BAX, bcl-2, procaspase and cleaved-Caspase-3), with their respective loading controls (GAPDH). (D) Relative density of immunoblot of BAX. (E) Relative density of immunoblot of bcl-2. (F) Relative density of immunoblot of cleaved-Caspase-3. Data are presented as percentage of control (or pro-Caspase-3) and values are presented as mean±SD, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ## P<.01, ### P<.001: DM+Z-LIG group vs. DM group. References
1. Ting DS, Cheung GC, Wong TY, Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review: Clin Exp Ophthalmol, 2016; 44; 260-77
2. Cho NH, Shaw JE, Karuranga S, IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045: Diabetes Res Clin Pract, 2018; 138; 271-81
3. Das A, Stroud S, Mehta A, Rangasamy S, New treatments for diabetic retinopathy: Diabetes Obes Metab, 2015; 17; 219-30
4. Kempen JH, O’Colmain BJ, Leske MC, The prevalence of diabetic retinopathy among adults in the United States: Arch Ophthalmol, 2004; 122; 552-63
5. Yau JW, Rogers SL, Kawasaki R, Global prevalence and major risk factors of diabetic retinopathy: Diabetes Care, 2012; 35; 556-64
6. Cheung N, Mitchell P, Wong TY, Diabetic retinopathy: Lancet, 2010; 376; 124-36
7. Wu MY, Yiang GT, Lai TT, Li CJ, The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy: Oxid Med Cell Longev, 2018; 2018 3420187
8. Brownlee M, The pathobiology of diabetic complications: A unifying mechanism: Diabetes, 2005; 54; 1615-25
9. Nentwich MM, Ulbig MW, Diabetic retinopathy – ocular complications of diabetes mellitus: World J Diabetes, 2015; 6; 489-99
10. Zhang X, Bao S, Lai D, Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas: Diabetes, 2008; 57; 1026-33
11. Deissler HL, Deissler H, Lang GK, Lang GE, VEGF but not PlGF disturbs the barrier of retinal endothelial cells: Exp Eye Res, 2013; 115; 162-71
12. Geraldes P, King GL, Activation of protein kinase C isoforms and its impact on diabetic complications: Circ Res, 2010; 106; 1319-31
13. Rubsam A, Parikh S, Fort PE, Role of inflammation in diabetic retinopathy: Int J Mol Sci, 2018; 19(4); 942
14. El-Asrar AM, Role of inflammation in the pathogenesis of diabetic retinopathy: Middle East Afr J Ophthalmol, 2012; 19; 70-74
15. Behl T, Kotwani A, Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy: Pharmacol Res, 2015; 99; 137-48
16. Penn JS, Madan A, Caldwell RB, Vascular endothelial growth factor in eye disease: Prog Retin Eye Res, 2008; 27; 331-71
17. Kovacs K, Marra KV, Yu G, Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia: Invest Ophthalmol Vis Sci, 2015; 56; 6523-30
18. Tang J, Kern TS, Inflammation in diabetic retinopathy: Prog Retin Eye Res, 2011; 30; 343-58
19. Zheng L, Howell SJ, Hatala DA, Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy: Diabetes, 2007; 56; 337-45
20. Li W, Yanoff M, Liu X, Ye X, Retinal capillary pericyte apoptosis in early human diabetic retinopathy: Chin Med J (Engl), 1997; 110; 659-63
21. Barber AJ, Lieth E, Khin SA, Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin: J Clin Invest, 1998; 102; 783-91
22. Mizutani M, Kern TS, Lorenzi M, Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy: J Clin Invest, 1996; 97; 2883-90
23. Podesta F, Romeo G, Liu WH: Am J Pathol, 2000; 156; 1025-32
24. Oshitari T, Yamamoto S, Hata N, Roy S, Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy: Br J Ophthalmol, 2008; 92; 552-56
25. Abu EA, Dralands L, Missotten L, Geboes K, Expression of antiapoptotic and proapoptotic molecules in diabetic retinas: Eye (Lond), 2007; 21; 238-45
26. Joussen AM, Poulaki V, Le ML, A central role for inflammation in the pathogenesis of diabetic retinopathy: FASEB J, 2004; 18; 1450-52
27. Giacco F, Brownlee M, Oxidative stress and diabetic complications: Circ Res, 2010; 107; 1058-70
28. Kowluru RA, Mishra M, Oxidative stress, mitochondrial damage and diabetic retinopathy: Biochim Biophys Acta, 2015; 1852; 2474-83
29. Behl Y, Krothapalli P, Desta T, Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy: Am J Pathol, 2008; 172; 1411-18
30. Joussen AM, Doehmen S, Le ML, TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations: Mol Vis, 2009; 15; 1418-28
31. Donkor PO, Chen Y, Ding L, Qiu F: J Ethnopharmacol, 2016; 194; 530-48
32. Zuo AH, Wang L, Xiao HBResearch progress studies on pharmacology and pharmacokinetics of ligustilide: Zhongguo Zhong Yao Za Zhi, 2012; 37; 3350-53 [in Chinees]
33. Wu S, Wang N, Li J: J Cardiovasc Pharm, 2019; 73; 316-25
34. Yang WJ, Li YR, Gao H, Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin-induced diabetic nephropathy in mice: J Ethnopharmacol, 2018; 227; 166-75
35. Wang J, Du JR, Wang Y, Z-ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-kappaB pathway in primary rat microglia: Acta Pharmacol Sin, 2010; 31; 791-97
36. Chen D, Tang J, Khatibi NH, Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid hemorrhage in rats: J Pharmacol Exp Ther, 2011; 337; 663-72
37. Kuang X, Yao Y, Du JR, Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice: Brain Res, 2006; 1102; 145-53
38. Yan W, Long P, Chen T, A Natural occurring mouse model with Adgrv1 mutation of Usher Syndrome 2C and characterization of its recombinant inbred strains: Cell Physiol Biochem, 2018; 47; 1883-97
39. Olivares AM, Althoff K, Chen GF, Animal models of diabetic retinopathy: Curr Diab Rep, 2017; 17; 93
40. McDowell RE, Barabas P, Augustine J, Muller glial dysfunction during diabetic retinopathy in rats is reduced by the acrolein-scavenging drug, 2-hydrazino-4,6-dimethylpyrimidine: Diabetologia, 2018; 61; 2654-67
41. Bresnick GH, Korth K, Groo A, Palta M, Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report: Arch Ophthalmol, 1984; 102; 1307-11
42. McCulloch DL, Marmor MF, Brigell MG, ISCEV Standard for full-field clinical electroretinography (2015 update): Doc Ophthalmol, 2015; 130; 1-12
43. Ewing FM, Deary IJ, Strachan MW, Frier BM, Seeing beyond retinopathy in diabetes: Electrophysiological and psychophysical abnormalities and alterations in vision: Endocr Rev, 1998; 19; 462-76
44. Di Leo MA, Caputo S, Falsini B, Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy: Diabetologia, 1994; 37; 911-16
45. van Dijk HW, Verbraak FD, Stehouwer M, Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy: Vision Res, 2011; 51; 224-28
46. Barcelona PF, Sitaras N, Galan A, p75NTR and its ligand ProNGF activate paracrine mechanisms etiological to the vascular, inflammatory, and neurodegenerative pathologies of diabetic retinopathy: J Neurosci, 2016; 36; 8826-41
47. Araszkiewicz A, Zozulinska-Ziolkiewicz D, Meller M, Neurodegeneration of the retina in type 1 diabetic patients: Pol Arch Med Wewn, 2012; 122; 464-70
48. Ruiz-Ocana P, Espinoza RP, Alonso-Ojembarrena A, Decreased retinal thickness in Type 1 diabetic children with signs of nonproliferative diabetic retinopathy: Int J Endocrinol, 2018; 2018 1078531
49. Jonsson KB, Frydkjaer-Olsen U, Grauslund J, Vascular changes and neurodegeneration in the early stages of diabetic retinopathy: Which comes first?: Ophthalmic Res, 2016; 56; 1-9
50. Stitt AW, Curtis TM, Chen M, The progress in understanding and treatment of diabetic retinopathy: Prog Retin Eye Res, 2016; 51; 156-86
51. Yang Y, Hayden MR, Sowers S, Retinal redox stress and remodeling in cardiometabolic syndrome and diabetes: Oxid Med Cell Longev, 2010; 3; 392-403
52. Martin PM, Roon P, Van Ells TK, Death of retinal neurons in streptozotocin-induced diabetic mice: Invest Ophthalmol Vis Sci, 2004; 45; 3330-36
53. Mieno S, Boodhwani M, Robich MP, Effects of diabetes mellitus on VEGF-induced proliferation response in bone marrow derived endothelial progenitor cells: J Card Surg, 2010; 25; 618-25
54. Rusnak S, Vrzalova J, Sobotova M, The measurement of intraocular biomarkers in various stages of proliferative diabetic retinopathy using multiplex xMAP technology: J Ophthalmol, 2015; 2015 424783
55. Adamiec-Mroczek J, Oficjalska-Mlynczak J, Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes – role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy: Graefes Arch Clin Exp Ophthalmol, 2008; 246; 1665-70
56. Senger DR, Vascular endothelial growth factor: Much more than an angiogenesis factor: Mol Biol Cell, 2010; 21; 377-79
57. Gariano RF, Gardner TW, Retinal angiogenesis in development and disease: Nature, 2005; 438; 960-66
58. Lin M, Chen Y, Jin J, Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells: Diabetologia, 2011; 54; 1554-66
59. Arjamaa O, Nikinmaa M, Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors: Exp Eye Res, 2006; 83; 473-83
60. Catrina S, Okamoto K, Pereira T, Hyperglycemia regulates hypoxia-inducible factor-1 protein stability and function: Diabetes, 2004; 53; 3226-32
61. Kageyama Y, Sugiyama H, Ayame H, Suppression of VEGF transcription in renal cell carcinoma cells by pyrrole-imidazole hairpin polyamides targeting the hypoxia responsive element: Acta Oncol, 2006; 45; 317-24
62. Damert A, Ikeda E, Risau W, Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells: Biochem J, 1997; 327; 419-23
63. Li T, Hu J, Gao F, Transcription factors regulate GPR91-mediated expression of VEGF in hypoxia-induced retinopathy: Sci Rep, 2017; 7; 45807
64. Giuliani N, Lunghi P, Morandi F, Downmodulation of ERK protein kinase activity inhibits VEGF secretion by human myeloma cells and myeloma-induced angiogenesis: Leukemia, 2004; 18; 628-35
65. Zhang Y, Li F, Guo Y, (Z)-ligustilide increases ferroportin1 expression and ferritin content in ischemic SH-SY5Y cells: Eur J Pharmacol, 2016; 792; 48-53
Figures
 Figure 1. Change in blood glucose levels. A significant increase in blood glucose level is shown in the diabetic rats (DM and DM+Z-Lig groups). Values are presented mean±standard deviation, n=12.
Figure 1. Change in blood glucose levels. A significant increase in blood glucose level is shown in the diabetic rats (DM and DM+Z-Lig groups). Values are presented mean±standard deviation, n=12. Figure 2. Z-LIG protects diabetic rat retinal function. (A) Amplitude of b wave (dark-adaptation 3.0 response). (B) Amplitude of OPs2 wave (dark-adaptation 3.0 response). (C) Amplitude of b-wave (light-adaptation 3.0 response). Values are presented as mean±standard deviation, n=12. * P<.05, ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; # P<.05, ## P<.01: DM+Z-LIG group vs. DM group.
Figure 2. Z-LIG protects diabetic rat retinal function. (A) Amplitude of b wave (dark-adaptation 3.0 response). (B) Amplitude of OPs2 wave (dark-adaptation 3.0 response). (C) Amplitude of b-wave (light-adaptation 3.0 response). Values are presented as mean±standard deviation, n=12. * P<.05, ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; # P<.05, ## P<.01: DM+Z-LIG group vs. DM group. Figure 3. Z-LIG protects the morphometric structure of the retina in diabetic rats after 12 weeks of treatment. (A) Typical HE staining image of the retina. (B) Thickness of retinal INL+OPL+ONL. (C) Thickness of the retinal ILM. GCL – ganglion cell layer; ILM – internal limiting membrane; INL – inner nuclear layer; IPL – inner plexiform layer; IS/OS – photoreceptor inner segment/outer segment; ONL – outer nuclear layer; OPL – outer plexiform layer. Values are presented as mean±standard deviation, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group.
Figure 3. Z-LIG protects the morphometric structure of the retina in diabetic rats after 12 weeks of treatment. (A) Typical HE staining image of the retina. (B) Thickness of retinal INL+OPL+ONL. (C) Thickness of the retinal ILM. GCL – ganglion cell layer; ILM – internal limiting membrane; INL – inner nuclear layer; IPL – inner plexiform layer; IS/OS – photoreceptor inner segment/outer segment; ONL – outer nuclear layer; OPL – outer plexiform layer. Values are presented as mean±standard deviation, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group. Figure 4. Z-LIG decreases retina IL-1 and TNF-α levels induced by diabetes after 12 weeks of treatment. (A) The concentration of IL-1. (B) The concentration of TNF-α. Values are presented as mean±SD, n=4. * P<.05, ** P<.01: DM group, DM+Z-LIG group vs. control group; ## P<.01: DM+Z-LIG group vs. DM group.
Figure 4. Z-LIG decreases retina IL-1 and TNF-α levels induced by diabetes after 12 weeks of treatment. (A) The concentration of IL-1. (B) The concentration of TNF-α. Values are presented as mean±SD, n=4. * P<.05, ** P<.01: DM group, DM+Z-LIG group vs. control group; ## P<.01: DM+Z-LIG group vs. DM group. Figure 5. Z-LIG decreases diabetes-induced expression of VEGF-α in protein and mRNA in the retina after 12 weeks of treatment. (A) VEGF-α representative western blots with the respective loading control (GAPDH). (B) The protein expression of VEGF-α (GAPDH%). (C) The primers of VEGF-α. (D) The mRNA expression of VEGF-α (CON%). Data are presented as percentage of control and values are presented as mean ± standard deviation, n=4. ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group.
Figure 5. Z-LIG decreases diabetes-induced expression of VEGF-α in protein and mRNA in the retina after 12 weeks of treatment. (A) VEGF-α representative western blots with the respective loading control (GAPDH). (B) The protein expression of VEGF-α (GAPDH%). (C) The primers of VEGF-α. (D) The mRNA expression of VEGF-α (CON%). Data are presented as percentage of control and values are presented as mean ± standard deviation, n=4. ** P<.01, *** P<.001: DM group, DM+Z-LIG group vs. control group; ### P<.001: DM+Z-LIG group vs. DM group. Figure 6. Z-LIG attenuates retinal cell apoptosis after 12 weeks of treatment. (A) Representative TUNEL images. (B) Expression of TUNEL (CON%). (C) Representative Western blots (BAX, bcl-2, procaspase and cleaved-Caspase-3), with their respective loading controls (GAPDH). (D) Relative density of immunoblot of BAX. (E) Relative density of immunoblot of bcl-2. (F) Relative density of immunoblot of cleaved-Caspase-3. Data are presented as percentage of control (or pro-Caspase-3) and values are presented as mean±SD, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ## P<.01, ### P<.001: DM+Z-LIG group vs. DM group.
Figure 6. Z-LIG attenuates retinal cell apoptosis after 12 weeks of treatment. (A) Representative TUNEL images. (B) Expression of TUNEL (CON%). (C) Representative Western blots (BAX, bcl-2, procaspase and cleaved-Caspase-3), with their respective loading controls (GAPDH). (D) Relative density of immunoblot of BAX. (E) Relative density of immunoblot of bcl-2. (F) Relative density of immunoblot of cleaved-Caspase-3. Data are presented as percentage of control (or pro-Caspase-3) and values are presented as mean±SD, n=4. * P<.05, *** P<.001: DM group, DM+Z-LIG group vs. control group; ## P<.01, ### P<.001: DM+Z-LIG group vs. DM group. In Press
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








