26 September 2020: Clinical Research
Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19
Cuixing Zhou1BCDE, Yimeng Chen1ABC, Yun Ji2BC, Xiaozhou He1AF*, Dong Xue1AFGDOI: 10.12659/MSM.926178
Med Sci Monit 2020; 26:e926178
Abstract
BACKGROUND: The aim of this study was to assess the diagnostic utility of iron homeostasis determinations for prediction of severity of COVID-19.
MATERIAL AND METHODS: This was a retrospective study enrolling a total of 50 patients diagnosed with the novel coronavirus disease-19 (COVID-19) from February 27, 2020 to March 30, 2020, including a severe group (12 patients) and a mild group (38 patients). For the control group, 50 healthy people were examined during the same period. We compared clinical laboratory data and iron homeostasis biomarkers among the 3 groups. ROC curve analysis was used to assess diagnoses.
RESULTS: Patients diagnosed with severe COVID-19 had higher hepcidin and serum ferritin levels than in other groups (p<0.001). A combination test of hepcidin and serum ferritin provided the best specificity and sensitivity in the prognosis of COVID-19 severity. Logistic regression analysis showed hepcidin and serum ferritin independently contributed to the severity of COVID-19. Hepcidin and serum ferritin tandem testing predicted COVID-19 severity with 94.6% specificity, while hepcidin and serum ferritin parallel testing had a sensitivity of 95.7%.
CONCLUSIONS: Iron homeostasis had a robust association with the occurrence of severe COVID-19. Iron homeostasis determinations were specific and sensitive for the early prediction of disease severity in COVID-19 patients and thus have clinical utility.
Keywords: COVID-19, Hepcidins, Iron Metabolism Disorders, Area Under Curve, Betacoronavirus, COVID-19, Coronavirus Infections, Ferritins, Iron, Logistic Models, Pandemics, Pneumonia, Viral, ROC Curve, SARS-CoV-2, Sensitivity and Specificity
Background
Coronavirus disease 19 (COVID-19) is an infectious disease caused by SARS-CoV-2 infection and can be accompanied by multiple organ dysfunction, and, in severe cases, it can lead to death [1]. Since the first report of COVID-19 in Wuhan in December 2019, the epidemic has progressed rapidly [2]. As of May 4, 2020, more than 3.5 million confirmed cases of infection have been reported worldwide, with more than 250 000 deaths. The WHO declared SARS CoV2 infections a pandemic, and the epidemic situation is still very serious and deserves urgent attention [3].
As a new respiratory disease, COVID-19 has been found not only to cause lung damage, but also to damage other organs. Patients with severe disease often have multiple organ dysfunction syndrome (MODS) [4]. Thus, early diagnosis and timely treatment of severe COVID-9 are crucial. In this study, we analyzed iron homeostasis in COVID-19 patients and its correlation with the severity of COVID-19 disease. We also examined the clinical utility of iron homeostasis in predicting the severity of COVID-19 disease.
Material and Methods
PATIENTS:
This was a retrospective study. Analysis of the clinical data collected from patients diagnosed with COVID-9 was conducted from February 27, 2020 to March 30, 2020 in the Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, China. Diagnosis of the patients was carried out based on the interim guidance for COVID-19 issued by the WHO. All COVID-19 diagnoses were confirmed using fluorescence RT-PCR. We recruited 50 patients age (45.9±19.8) years, consisting of 25 males and 25 females. Patients with COVID-19 were subsequently subcategorized into 2 groups according to their disease severity: the severe group (12 patients, 7 males and 5 females) and the mild group (38 patients, 16 males and 22 females). Assignment to the mild group was based on fever, respiratory tract symptoms, and pneumonia imaging. Patients with the following symptoms were assigned to the severe group: respiratory distress (RR ≥30 times/min); insufficient oxygen saturation (≤93%) at rest; and low partial oxygen pressure (PaO2 ≤60 mmHg) and oxygen absorption concentration (FiO2) ratio (≤300 mmHg). Data from 50 healthy controls (25 males and 25 females) were collected and assessed in the control group. Blood samples were collected and used for iron homeostasis tests. All tests were approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University. All patients who participated in this study provided written informed consent.
CLINICAL LABORATORY DATA:
Routine blood tests performed included: white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HB), platelet count (PLT), eosinophil (EO), lymphocytes (LY), and monocytes (MO). Iron homeostasis test results (hepcidin, serum ferritin [SF]) were quantified using enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA). A parallel chest CT scan was performed for all cases. Data from mild patients were collected from the initial laboratory tests at admission, and data from severe patients were collected from the latest laboratory tests prior to physicians making the clinical diagnosis of severe disease. In this study, for severe cases, the disease usually progressed from mild to severe stage at about 3–7 days after admission. All procedures in this study were carried out by specially assigned personnel and were carried out strictly according to the instructions.
STATISTICAL ANALYSIS:
Data are expressed as mean±SD. All results were obtained from 3 or more independent experiments. All statistical analyses were carried out using SPSS 26.0 and GraphPad Prism 5.0. Data were assessed by
Results
BASELINE DATA OF THE 3 GROUPS:
There were 100 participants enrolled in this study. The average age of the 50 patients (25 males and 25 females) diagnosed with COVID-19 was (45.9 ± 19.8) years, including 12 patients (7 males and 5 females) categorized as severe cases that had an average age of (48.2±18.3) years; and 38 patients (22 males and 16 females) categorized as mild cases that had an average age of (41.7±17.6) years. No significant differences in sex or age were detected between the severe and mild group. The mean age of the 50 healthy controls (25 males and 25 females) was (46.5±15.7) years. Baseline characteristics of all participants are shown Table 1.
CLINICAL LABORATORY DATA AND IRON HOMEOSTASIS BIOMARKERS IN THE 3 GROUPS:
The hematological characteristics of all participants are shown in Table 2 and Figure 1. The levels of RBC, HB, PLT, and MO were not significantly different among the 3 groups (p>0.05). The expression of WBC, LY, and EO in patients with COVID-19 was significantly lower than in healthy controls (p<0.05). The hepcidin and serum ferritin levels were higher in patients with COVID-19 than in healthy controls (p<0.001), and the levels were also higher in the severe group than in the mild group (p<0.001), suggesting that they could be used as biomarkers for predicting the severity of COVID-19 disease.
ANALYSIS BY RECEIVER OPERATING CHARACTERISTIC CURVE (ROC):
ROC curves were used in the analysis of prediction efficacy and the optimal prediction threshold for worsening of condition in COVID-19 patients. As shown in Table 3 and Figure 2, the AUC of hepcidin was 0.795 (p<0.0001), indicating that it better predicts the development of severe pneumonia in COVID-19. The optimum critical point of hepcidin was 32.7 ng/ml, which defined the highest limit of the cases without severe pneumonia. The serum ferritin AUC for the prediction of the pneumonia severity was 0.767 (p=0.0005), with an optimum critical point of 162 ng/mL, which defined the highest limit of the cases without severe pneumonia. For the combined detection factor, we first performed binary logistic analysis by setting hepcidin and serum ferritin as covariates and disease diagnosis as the dependent. The B values of hepcidin (BHep) and serum ferritin (BSF) were calculated. The variate of combined detection factor was determined according to the formula: combined detection factor=Hepcidin+serum ferritin*BSF/BHep. In the combined detection of both hepcidin and serum ferritin, the AUC was 0.814 (p<0.0001).
EFFECTS OF HEPCIDIN AND SERUM FERRITIN ON THE SEVERITY COVID-19:
In logistic regression analysis, diagnosis of COVID-19 (yes=1, no=0) was a dependent variable, while hepcidin (>32.7 ng/ml=1, ≤32.7 ng/ml=0) and serum ferritin (>162 ng/ml=1, ≤162 ng/ml=0) were set as independent variables. As a result, hepcidin (OR=10.85 [95% CI 2.322, 50.688], p=0.002) and serum ferritin (OR=10.01 [95% CI 2.306, 43.362], p=0.002) had independent influences on the prediction of COVID-19 severity. The regression equation was Logit (P)=−2.561+2.384 (hepcidin)+2.303 (SF), and the difference was statistically significant (p=0.001). As shown in Table 4, the prediction accuracy was 75%.
EFFECTIVENESS OF INDICATORS (HEPCIDIN AND SERUM FERRITIN) IN THE PREDICTION OF SEVERITY OF COVID-19:
With hepcidin over 32.7 ng/mL, the COVID-19 severity was predicted (sensitivity 56.5%, specificity 97.3%). The COVID-19 severity was predicted when serum ferritin was higher than 162 ng/mL (sensitivity 86.9%, specificity 70.3%). When hepcidin was higher than 32.7 ng/mL or serum ferritin was higher than 162 ng/mL, the sensitivity and the specificity were 95.7% and 72.2%, respectively, while the corresponding AUC was 0.815. The combination of hepcidin and serum ferritin in tandem testing displayed sensitivity and specificity of 56.5% and 94.6%, respectively, with a corresponding AUC of 0.690. The specificity was at the highest point (94.6%) in tandem testing combining hepcidin and serum ferritin. The sensitivity was 95.7% in parallel testing combining hepcidin and serum ferritin, as shown in Figure 3 and Table 5. The results indicated that there may be additional ways to combine these 2 parameters in different regions.
Discussion
Since its initial occurrence, COVID-19 has spread worldwide. Human susceptibility, high infectivity, and mortality are significantly higher than those of influenza, and it has received worldwide attention [5]. Most patients show mild symptoms and have a good prognosis. In contrast, some patients develop severe symptoms, including severe pneumonia, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome (MODS). Currently, the occurrence, progression, and immune conditions of COVID-19 patients remain unclear. In critically ill patients, new coronavirus pneumonia (SARS-CoV-2) infection is also associated with inflammatory cytokine storm [6]. The inflammatory response changes iron homeostasis [7]. In the present study, we analyzed the features of iron homeostasis in patients with COVID-19.
In this study, we analyzed that the blood routine biomarkers from COVID-19 patients, showing that WBC, LY, and EO in COVID-19 patients were significantly lower than those in healthy controls. Zhong [8] found that patients with severe COVID-19 had significantly more leukopenia, thrombocytopenia, and high C-reactive protein (p<0.05). Leukopenia occurs when a large number of cytokines are secreted after viral pneumonia infection, resulting in an increase in the marginal granulocytes in white blood cells and a decrease in circulating pools. After activation, cytokines tend to reach the lungs and other target organs, releasing inflammatory factors, and an “inflammatory storm” occurs; this process causes tissue and organ damage [6]. It was found that eosinophils decreased significantly or even disappeared, and eosinophils may indicate a poor prognosis [9,10]. In the present study, no significant changes in WBC, LY, and EO counts were detected between the severe group and the mild group. The results showed that routine blood test results were changed in COVID-19 patients but could not identify patients with severe conditions.
The inflammatory response in COVID-19 is involved in the progression from mild cases to severe cases [11]. Inflammation regulates iron homeostasis. Hepcidin is a newly discovered marker that regulates iron absorption through regulation of the gastrointestinal tract and iron release, which are both important pathways involved in regulation of iron availability for incorporation. Hepcidin levels are upregulated in inflammations commonly induced by infections [12]. The levels of hepcidin and serum ferritin in patients in the severe group were significantly higher than those in the mild group. In joint prediction using both hepcidin and serum ferritin, the ROC curve integral of severe COVID-19 was 0.814 (p<0.01), indicating that they are promising markers in the prediction of severe COVID-19, and that the combined detection produces better outcomes. Logistic regression analysis demonstrated that hepcidin and serum ferritin can predict severe COVID-19. The combination for severe COVID-19 prediction can be significantly improved by using different combinations of tests dependent on different disease situations. Also, early prediction is critical in the clinical treatment of COVID-19.
Conclusions
In summary, our findings suggest that hepcidin and serum ferritin levels are appropriate for use in the prediction of COVID-19 severity. Iron homeostasis appears to have diagnostic utility for use in patients with severe COVID-19. When appropriate, the levels of hepcidin and serum ferritin can be measured and used as clinical markers for predicting disease severity in patients diagnosed with COVID-19.
Figures
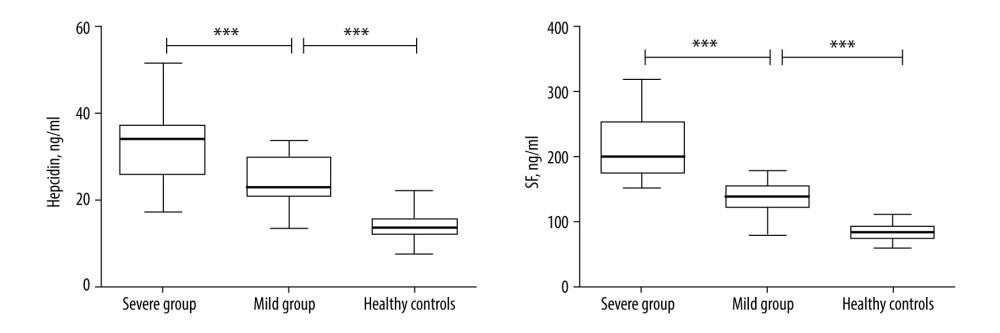 Figure 1. Changes in hepcidin and serum ferritin (SF) among severe group, mild group, and healthy controls. * p<0.05; ** p<0.01; *** p<0.001.
Figure 1. Changes in hepcidin and serum ferritin (SF) among severe group, mild group, and healthy controls. * p<0.05; ** p<0.01; *** p<0.001. 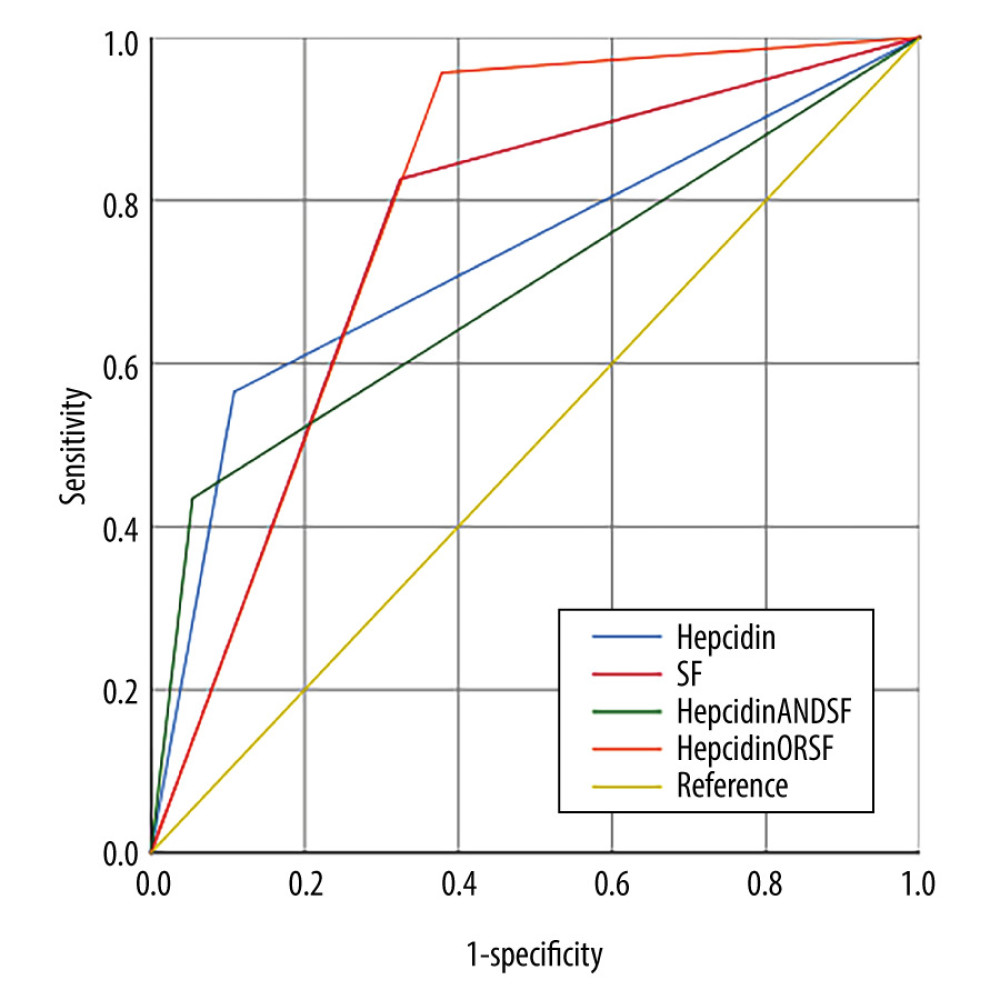 Figure 2. ROC curves comparing the prediction of severe COVID-19 variables for hepcidin combined with serum ferritin.
Figure 2. ROC curves comparing the prediction of severe COVID-19 variables for hepcidin combined with serum ferritin. 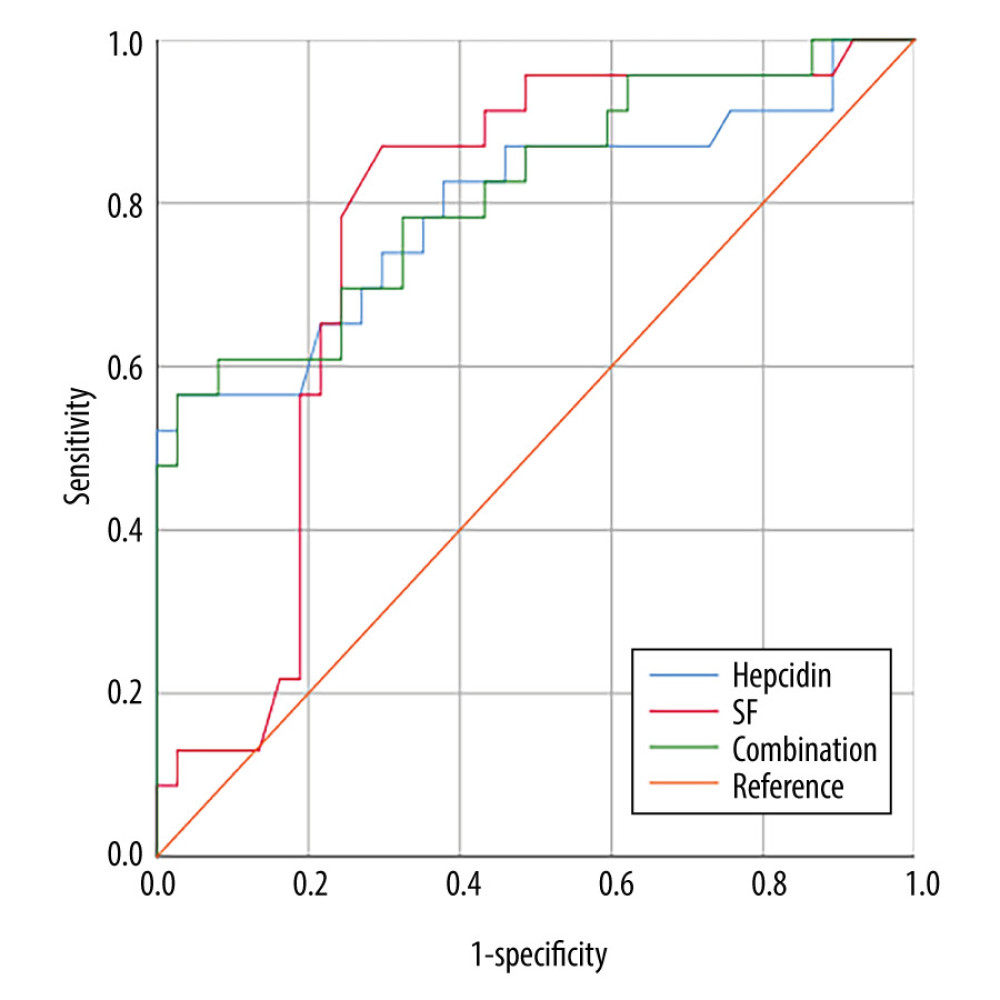 Figure 3. ROC curves of independent and joint detection were obtained when hepcidin and serum ferritin both took the best critical values. Hepcidin or serum ferritin represented serial detection. Hepcidin and serum ferritin represented parallel detection.
Figure 3. ROC curves of independent and joint detection were obtained when hepcidin and serum ferritin both took the best critical values. Hepcidin or serum ferritin represented serial detection. Hepcidin and serum ferritin represented parallel detection. Tables
Table 1. Baseline characteristics of all participants. Table 2. Clinical laboratory data of participants.
Table 2. Clinical laboratory data of participants.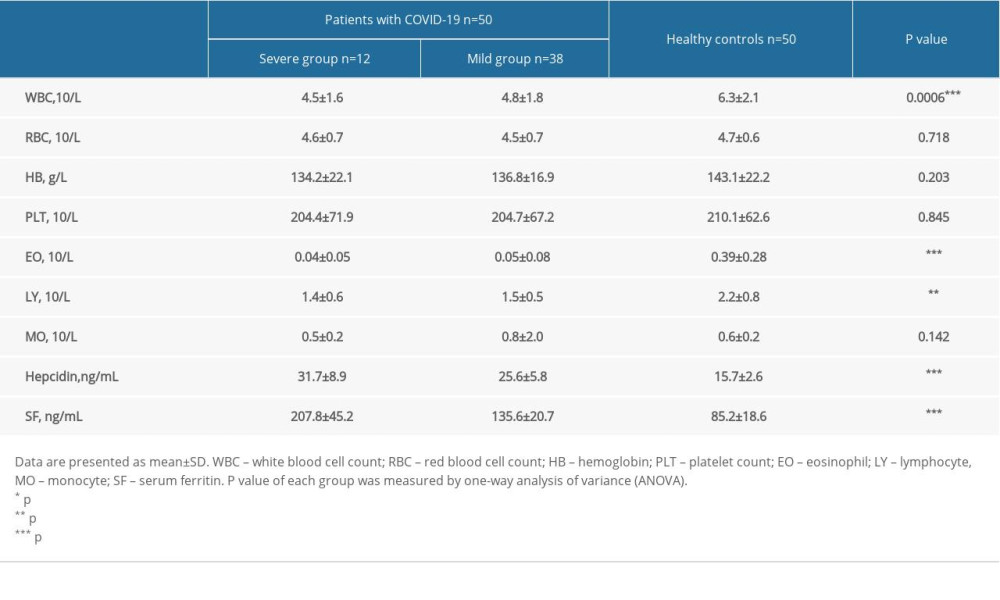 Table 3. ROC curve analysis of iron homeostasis biomarkers.
Table 3. ROC curve analysis of iron homeostasis biomarkers.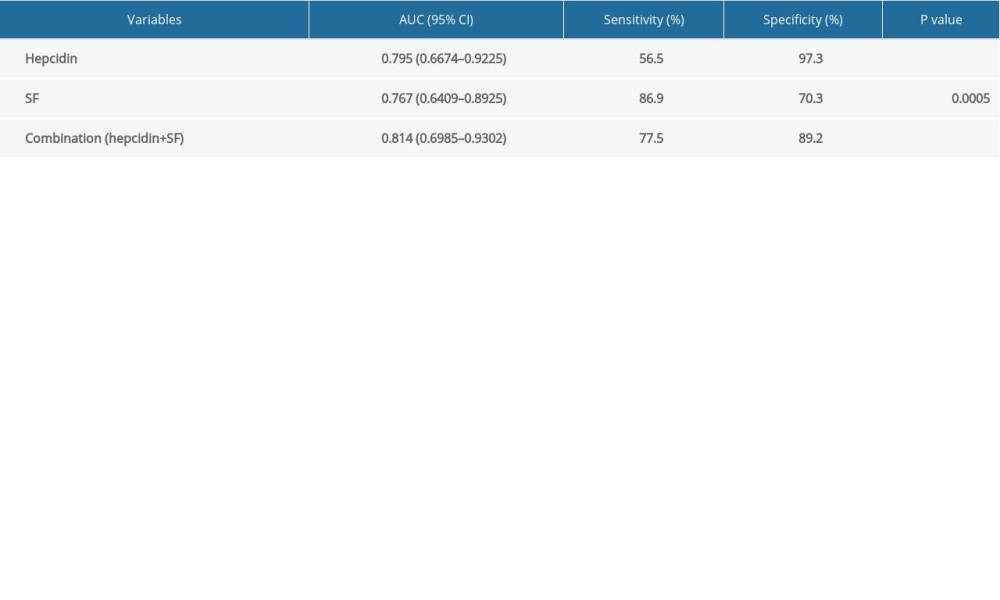 Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers.
Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers.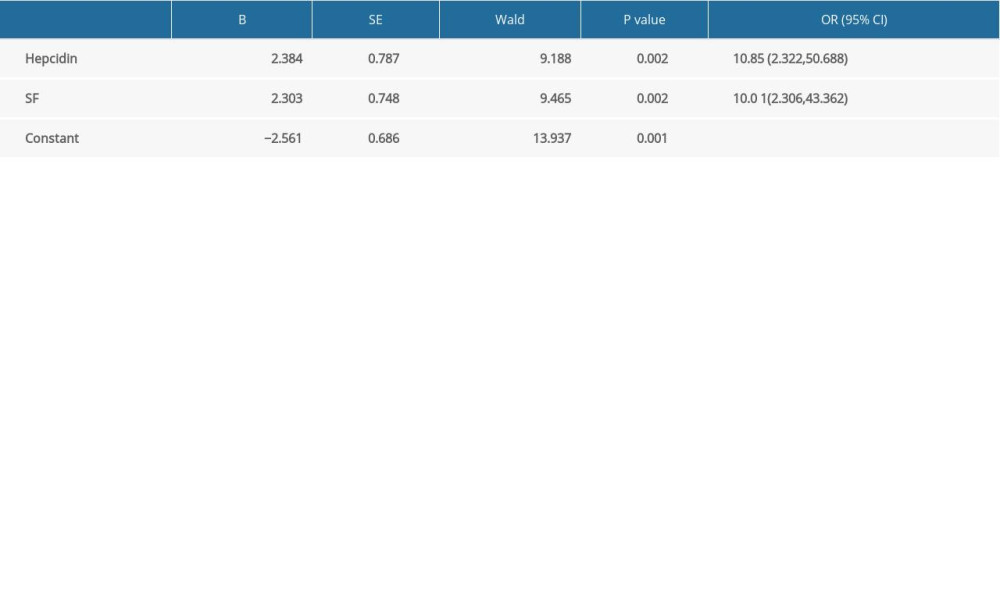 Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19.
Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19.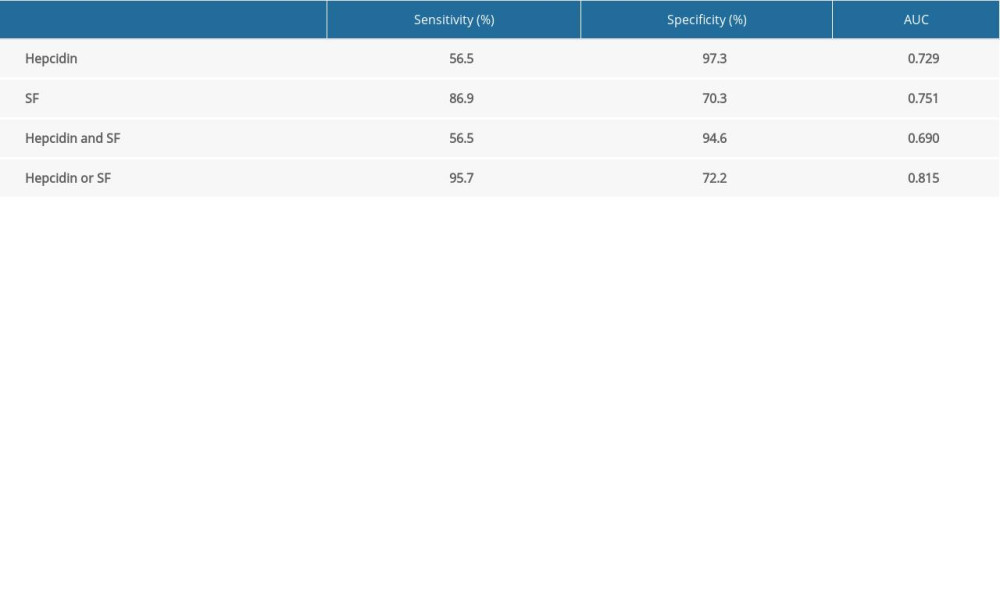
References
1. Mahase E, COVID-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction: BMJ, 2020; 368; m1036
2. Wu JT, Leung K, Leung GM, Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study: Lancet, 2020; 395(10225); 689-97
3. Zhang X, Epidemiology of COVID-19: N Engl J Med, 2020; 382; 1869-70
4. Chen N, Zhou M, Dong X, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study: Lancet, 2020; 395(10223); 507-13
5. Thompson R, Pandemic potential of 2019-nCoV: Lancet Infect Dis, 2020; 20(3); 280
6. Mehta P, McAuley DF, Brown M, COVID-19: Consider cytokine storm syndromes and immunosuppression: Lancet, 2020; 395(10229); 1033-34
7. Weiss G, Ganz T, Goodnough LT, Anemia of inflammation: Blood, 2019; 133(1); 40-50
8. Guan WJ, Ni ZY, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
9. Du Y, Tu L, Zhu P, Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study: Am J Respir Crit Care Med, 2020; 201(11); 1372-79
10. Zhu L, Xu X, Ma K, Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression: Am J Transplant, 2020; 20(7); 1859-63
11. Stebbing J, Phelan A, Griffin I, COVID-19: combining antiviral and anti-inflammatory treatments: Lancet Infect Dis, 2020; 20(4); 400-2
12. Fillebeen C, Wilkinson N, Charlebois E, Hepcidin-mediated hypoferremic response to acute inflammation requires a threshold of Bmp6/Hjv/Smad signaling: Blood, 2018; 132(17); 1829-41
Figures
 Figure 1. Changes in hepcidin and serum ferritin (SF) among severe group, mild group, and healthy controls. * p<0.05; ** p<0.01; *** p<0.001.
Figure 1. Changes in hepcidin and serum ferritin (SF) among severe group, mild group, and healthy controls. * p<0.05; ** p<0.01; *** p<0.001. Figure 2. ROC curves comparing the prediction of severe COVID-19 variables for hepcidin combined with serum ferritin.
Figure 2. ROC curves comparing the prediction of severe COVID-19 variables for hepcidin combined with serum ferritin. Figure 3. ROC curves of independent and joint detection were obtained when hepcidin and serum ferritin both took the best critical values. Hepcidin or serum ferritin represented serial detection. Hepcidin and serum ferritin represented parallel detection.
Figure 3. ROC curves of independent and joint detection were obtained when hepcidin and serum ferritin both took the best critical values. Hepcidin or serum ferritin represented serial detection. Hepcidin and serum ferritin represented parallel detection. Tables
 Table 1. Baseline characteristics of all participants.
Table 1. Baseline characteristics of all participants. Table 2. Clinical laboratory data of participants.
Table 2. Clinical laboratory data of participants. Table 3. ROC curve analysis of iron homeostasis biomarkers.
Table 3. ROC curve analysis of iron homeostasis biomarkers. Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers.
Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers. Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19.
Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19. Table 1. Baseline characteristics of all participants.
Table 1. Baseline characteristics of all participants. Table 2. Clinical laboratory data of participants.
Table 2. Clinical laboratory data of participants. Table 3. ROC curve analysis of iron homeostasis biomarkers.
Table 3. ROC curve analysis of iron homeostasis biomarkers. Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers.
Table 4. Relationship between occurrence of COVID-19 and iron homeostasis biomarkers. Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19.
Table 5. Analysis of the effectiveness of individual and joint indicators (hepcidin and serum ferritin) for predicting the occurrence of severe COVID-19. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








