15 February 2021: Clinical Research
Polymorphisms in and Genes Are Associated with Rheumatoid Arthritis in a Chinese Han Population
Yuqin Peng1BC, Bingqian Chen1BCD, Xiaowen Sheng1DE, Yufeng Qian1AEG*DOI: 10.12659/MSM.928455
Med Sci Monit 2021; 27:e928455
Abstract
BACKGROUND: The IRF5 and TYK2 gene polymorphisms are associated with autoimmune diseases. However, the relationship between the IRF5 and TYK2 gene polymorphisms and RA risk in the Chinese Han population was inconsistent.
MATERIAL AND METHODS: A total of 578 RA patients (case group) and 578 healthy controls (control group) were assessed in a case-control study. Genotyping of IRF5 (Exon 6 insertion/deletion (in/de), rs2004640, rs2070197, rs10954213) and TYK2 (rs280500, rs280519, rs280521, rs8108236, rs12720253) was performed by direct sequencing method. Data analysis was performed by SHEsis.
RESULTS: The rs2004640T allele (P=0.0003) and the dominant (P=0.001) and recessive (P=0.01) models of rs2004640 were associated with RA risk after stringent Bonferroni correction (0.05/4). The IRF5 exon 6 (in), rs2070197 and rs10954213 were not associated with RA (P>0.05). Two haplotypes of IRF5 (DTAT and DTGG) were associated with RA susceptibility (P<0.05). In addition, the frequencies of TYK2 rs280500A, rs280521A, and rs8108236A were significantly higher in the RA group compared with the control group (P<0.05). TYK2 rs280500, rs280521, and rs8108236 were associated with RA susceptibility in the dominant model, but the same was not observed for rs280519 and rs12720253 (P<0.05). Furthermore, 3 risk haplotypes (AAAGT, AGGAT, and GAAAT) and a protective haplotype (GAGGT) of TYK2 gene were associated with RA susceptibility (P<0.05).
CONCLUSIONS: Our results suggest that IRF5 rs2004640, TYK2 rs280500, rs280521, rs8108236, and haplotypes IRF5 (DTAT and DTGG) and TYK2 (AAAGT, AGGAT, GAAAT, and GAGGT) are susceptible factors for RA in a Chinese Han population.
Keywords: Arthritis, Juvenile, Genetic Association Studies, Polymorphism, Genetic, Alleles, Arthritis, Rheumatoid, Asians, Case-Control Studies, ethnicity, Genetic Predisposition to Disease, Genotype, Haplotypes, Interferon Regulatory Factors, Polymorphism, Single Nucleotide, TYK2 Kinase
Background
Rheumatoid arthritis (RA) is a kind of multisystem inflammatory disease that mainly affects synovium and surrounding tissues [1,2]. The prevalence rate of RA is 0.8–1.0% in Europeans and Americans, and around 0.5% in Chinese [3]. The incidence is highest in adults aged 20–50 years, and the incidence rate in women is 2–3 times higher than that in men [4]. The etiology of RA is unknown and is thought to be related to genetic factors and environmental factors such as infection, smoking, and pregnancy [5–7].
Recent studies have confirmed that the interferon family and its immunomodulatory pathways, especially type I interferon (IFNs) pathway, play an important role in the pathogenesis of RA [8]. IFNs are cytokines produced, among others, by plasmacytoid dendritic cells during infection [9]. Studies have found that IFNs gene expression is abnormal in whole blood of patients with autoimmune diseases, especially systemic lupus erythematosus (SLE) [10] and RA [11]. Genetic association analysis has confirmed that interferon regulator 5 (
IRF-5 is a member of the IRF family of transcription factors and plays an important role in inflammation and autoimmune response [13]. The gene encoding IRF5 maps to chromosome 7q32 [14]. Overexpression of
TYK2 is a member of the non-receptor tyrosine kinase-linked Janus kinase (JAK) family and is part of the Janus kinase/signal transduction and transcriptional activator 4(JAK-STAT) pathway [21]. Several studies have confirmed the important role of TYK2 in the type I interferon signaling pathway [22,23], and in SLE [12], systemic sclerosis (SS) [24], multiple sclerosis [25], and other immunological diseases. Zheng et al reported that expression of the
Therefore, the present study sought to investigate the genetic association between
Material and Methods
SAMPLES:
The Ethics Committee of Changshu Hospital Affiliated to Soochow University (CSHEC-190922) approved the study protocol. Written informed consent for genetics analysis was obtained from all subjects. The case group was composed of 578 unrelated patients (51 men and 527 women) from Jiangsu province who fulfilled the American College of Rheumatology 1982 criteria for RA [29]. The control group was composed of 578 healthy controls (55 men and 523 women) matched for sex, ethnicity, and age. Clinical features of RA patients and controls are shown in Table 1. All the individuals were of Chinese Han ethnicity.
SINGLE-NUCLEOTIDE POLYMORPHISMS (SNPS) SELECTION AND GENOTYPING:
Genomic DNA was extracted from peripheral leukocytes using the standard phenol-chloroform method. The SNP selection of the IRF5 gene was performed using the methods described by Tang et al [12]. Four SNPs in the IRF5 gene were selected: exon 6 (de/in), rs2004640 (G/T), rs2070197 (C/T), and rs10954213 (G/A). The SNP selection of the TYK2 gene was performed using Haploview Software with minor allele frequency (MAF) higher than 0.05 and r2 ≥0.8 based on the HapMap database (CHB, Chinese Han population) (http://www.hapmap.org/index.-html.ja). After screening, 5 tag SNPs (rs280500, rs280519, rs280521, rs8108236, and rs12720253) were selected. All the SNPs were genotyped by direct sequencing with the ABI 3730XL DNA Sequencer.
STATISTICAL ANALYSIS:
SHEsis software was used in statistical analysis (
Results
ASSOCIATION OF IRF5 POLYMORPHISMS AND RA:
The frequency of rs2004640T was significantly higher in cases than in controls (P=0.0003) after stringent Bonferroni correction (0.05/4) (Table 3). Genotype analysis ascertained that rs2004640 was associated with RA according to the dominant and recessive models (dominant model: P=0.001; recessive model: P=0.01) after stringent Bonferroni correction (0.05/4) (Table 3). No significant difference in the other 3 variants – exon 6 (de/in), rs2070197, and rs10954213 – were detected between the cases and healthy controls (P>0.05) (Tables 2, 3).
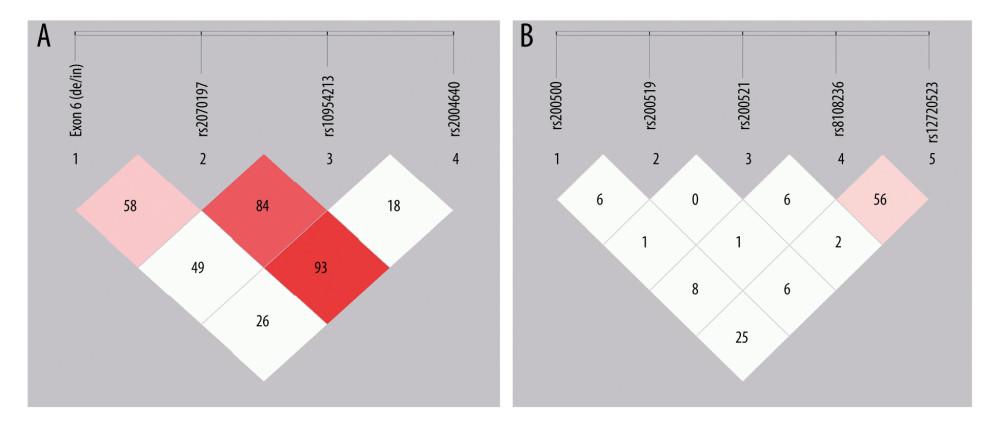
As shown in Table 4, 8 major haplotypes were identified by 3 SNPs and the exon 6 insertion/deletion with the lowest frequency threshold (LFT)>0.01). Significant associations were observed between the haplotype (IH1) involving exon 6 (de), rs2070197T, rs10954213A, and rs2004640T and RA (P=0.0009, OR (95%CI): 1.54 [1.191~1.976]) after stringent Bonferroni correction (0.05/8) (Table 2). Additionally, a protective haplotype (IH4) carrying the exon 6 deletion, rs2070197T, rs10954213G, and rs2004640G, was identified (P=4.45e-005, OR (95%CI): 0.48 [0.337~0.688]) after stringent Bonferroni correction (0.05/8) (Table 2).
ASSOCIATION OF TYK2 POLYMORPHISMS AND RA:
Significant association between rs280500A, rs280521A, and rs8108236A and RA were observed after the stringent Bonferroni correction (0.05/5) (rs280500: P=0.0001; rs280521: P=1.06e-008; rs8108236: P=0.0002) (Table 3). Similar results were obtained for the association between the dominant model of rs280500, rs280521, and rs8108236 and RA (rs280500: P=0.0001; rs280521: P=1.35e-008; rs8108236: P=0.0003) after stringent Bonferroni correction (0.05/5) (Table 2). No correlation was detected between the TYK2 rs280519 and rs12720253 polymorphisms and RA risk (P>0.05) (Tables 2, 3).
As shown in Table 4, 15 major haplotypes containing rs280500, rs280519, rs280521, rs8108236, and rs12720253 alleles with the lowest frequency threshold (LFT)>0.01) were identified. Haplotypes containing rs280500A- rs280519A- rs280521A- rs8108236G- rs12720253T(TH1) (P=9.88e-005, OR (95% CI): 3.18 [1.723~5.878]), rs280500A- rs280519G- rs280521G- rs8108236A- rs12720253T(TH6) (P=9.82e-005, OR (95% CI): 2.35 [1.513~3.667]), and rs280500G- rs280519A- rs280521A- rs8108236A- rs12720253T (TH8) (P=0.001, OR (95% CI): 2.52 [1.427~4.460]) were shown to be significantly associated with RA after stringent Bonferroni correction (0.05/15). A protective haplotype – rs280500G- rs280519A-rs280521G- rs8108236G- rs12720253T (TH11) – was also identified in our study (P=2.60e-005, OR (95% CI): 0.65 [0.538~0.799]) after stringent Bonferroni correction (0.05/15).
Discussion
Our results confirmed significant associations between rs2004640 and RA in a Chinese Han population according to allele, dominant, and recessive models. The frequencies of rs2004640 T allele and TT genotype were lower in controls than in the RA cases, which is consistent with previous studies conducted in Chinese Han populations with RA [30,31]. Kozyrev et al found that
No0, no association was found between the
Significant associations were detected between the rs280500A, rs280521A, and rs8108236A alleles in
Few studies have been conducted on the association between
Furthermore, research in a white population confirmed a significant association between
Haplotype analysis has shown that the haplotype (IH1) involving exon 6 (de), rs2070197T, rs10954213A, and rs2004640T and haplotype (IH4) carrying the exon 6 deletion, rs2070197T, rs10954213G, and rs2004640G were associated with the RA susceptibility. This is the first time that haplotypes defined by exon 6, rs2070197, rs10954213, and rs2004640 polymorphisms were found to be associated with RA in a Chinese Han population. However, comparison of
There are limitations in the present study. First, although the calculation power indicated that the sample size is large enough to detect an association at an odds ratio of 1.5, the sample size included in the study was relatively small. Our results need to be confirmed in studies with larger sample sizes. Second, both genetic and environmental factors were determined to play a role in the development of RA, but we did not assess the influence of environmental factors and RA risk due to insufficient data.
Conclusions
Our results suggest that
Tables
Table 1. Clinical Characteristics of patients with rheumatoid arthritis and healthy controls (n=578).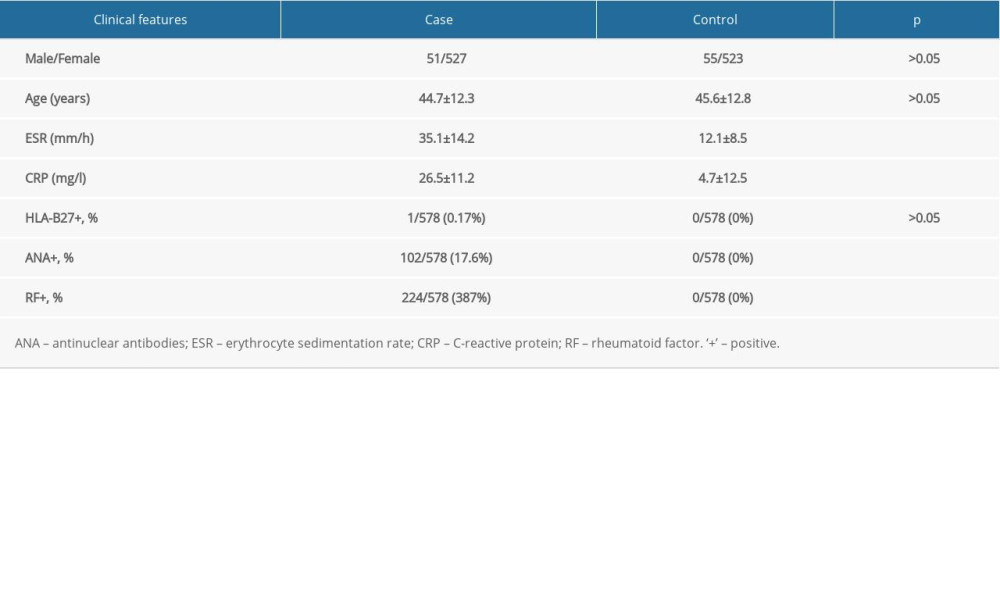 Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls.
Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls.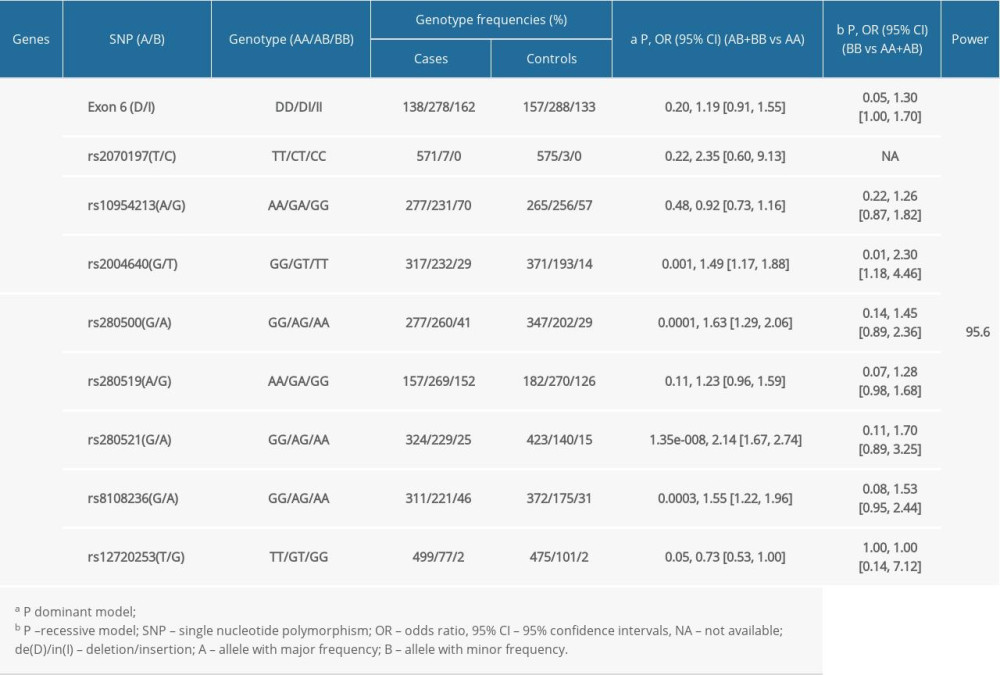 Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk.
Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk.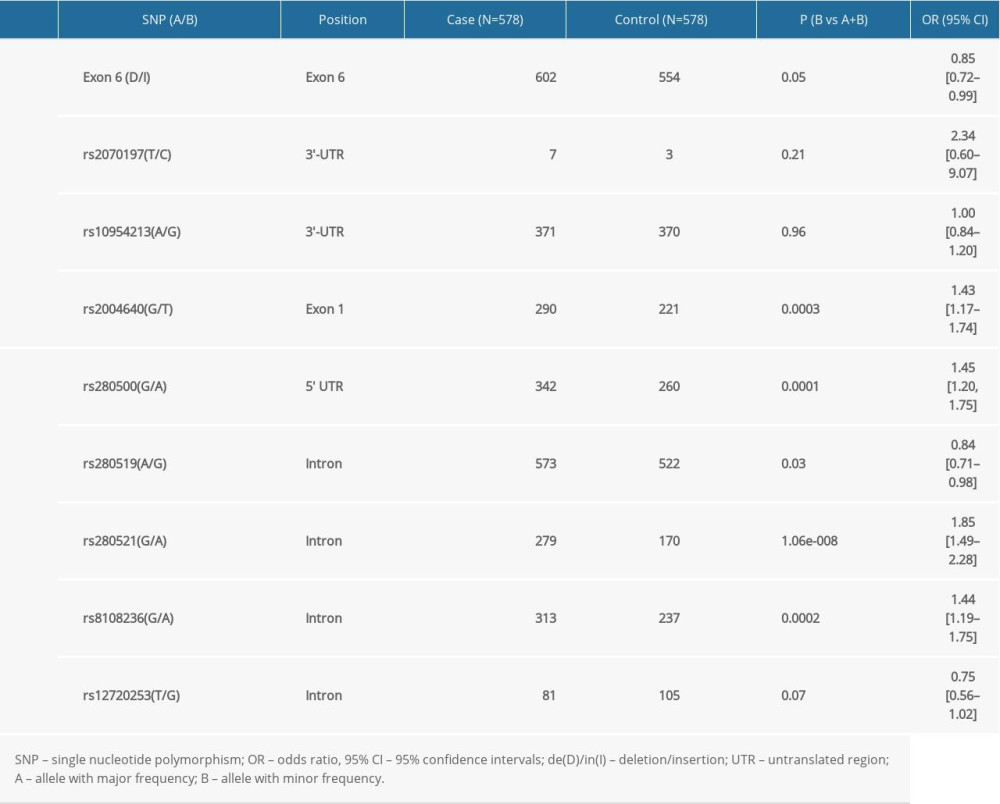 Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism.
Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism.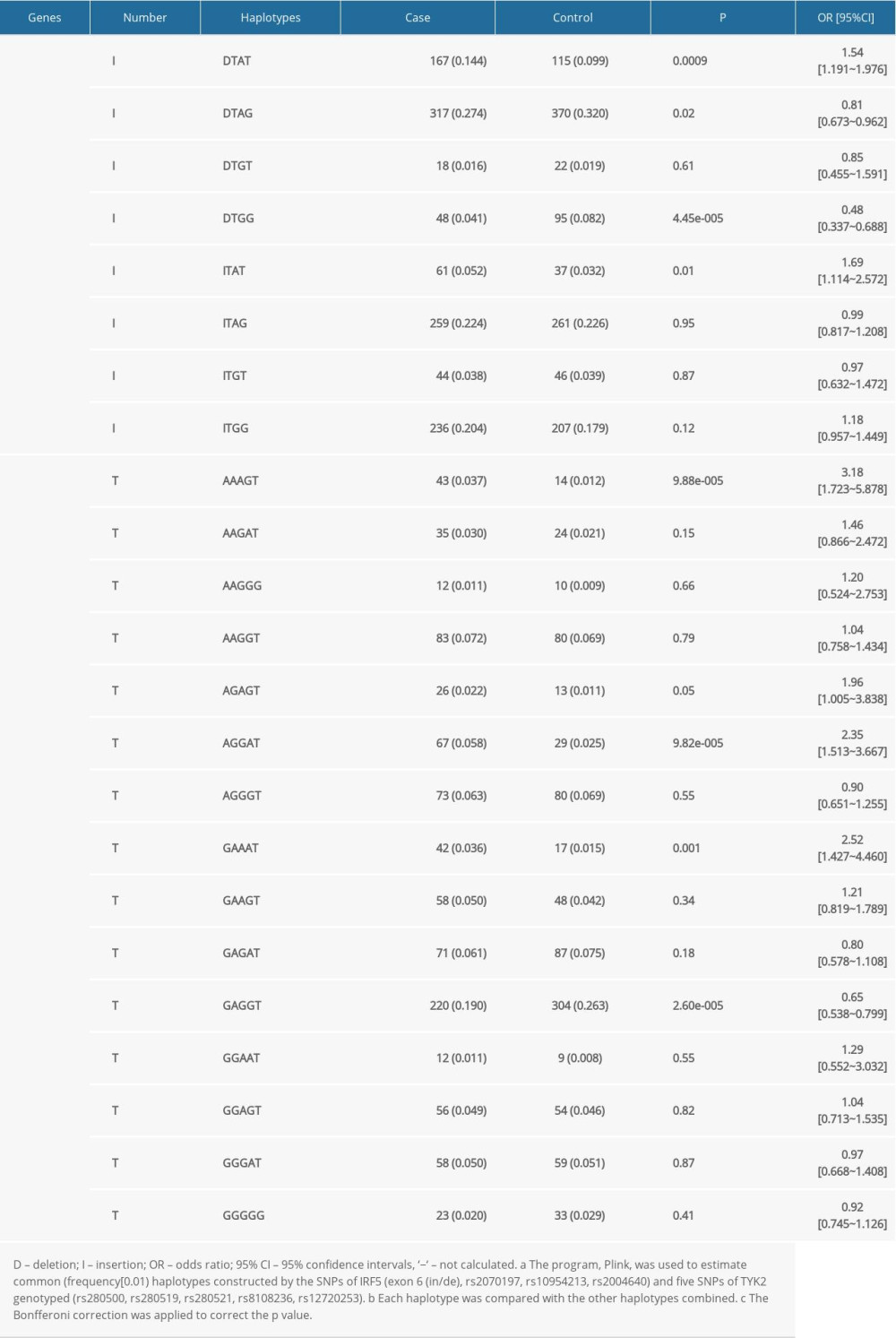 Supplementary Table 1. The Hardy-Weinberg equilibrium of IRF5 and TYK2 gene polymorphisms in case and control groups.
Supplementary Table 1. The Hardy-Weinberg equilibrium of IRF5 and TYK2 gene polymorphisms in case and control groups.
References
1. Wells PM, Adebayo AS, Bowyer RCE, Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: A cross-sectional study: Lancet Rheumatology, 2020; 2(7); e418-27
2. Mehta N, Schneider LK, McCardell E, Rheumatoid arthritis: Selecting monotherapy versus combination therapy: J Clin Rheumatol, 2017 [Online ahead of print]
3. Gulati M, Farah Z, Mouyis M, Clinical features of rheumatoid arthritis: Medicine, 2018; 211-15
4. Kawahito YGuidelines for the management of rheumatoid arthritis: Nippon Rinsho, 2016; 74(6); 939-43 [in Japanese]
5. Kim H, Sookgyung Cho, Lee J, Increased risk of opportunistic infection in early rheumatoid arthritis: Int J Rheum Dis, 2019; 22(1); 355-60
6. Ummarino D, Smoking influences autoimmunity to vimentin: Nat Rev Rheumatol, 2016; 12; 624
7. Smith WD, West HF, Pregnancy and rheumatoid arthritis: Acta Rheumatologica Scandinavica, 2006; 6(1–4); 189-201
8. Huang JJ, Feng G, Li Z, Clinical implications of type I interferon signature gene expression in patients with rheumatoid arthritis: Acta Academiae Medicinae Wannan, 2018; 3
9. Uddin S, Platanias LC, Mechanisms of type-I interferon signal transduction: J Biochem Mol Biol, 2004; 37(6); 635-41
10. Rönnblom L, Eloranta ML, Alm GV, The type I interferon system in systemic lupus erythematosus: Arthritis Rheum, 2010; 54(2); 408-20
11. Higgs BW, Liu Z, Zhu W, Type I interferon in rheumatoid arthritis, systemic lupus erythematosus, myositis and systemic scleroderma – a review of transcript profiling studies in the blood: European Musculoskeletal Review, 2012; 7(1); 22-28
12. Tang L, Wan PC, Wang Y, Genetic association and interaction between the IRF5 and TYK2 genes and systemic lupus erythematosus in the Han Chinese population: Inflamm Res, 2015; 64(10); 817-24
13. Hammami A, Charpentier T, Smans M, IRF-5-mediated inflammation limits CD8+ T cell expansion by inducing HIF-1α and impairing dendritic cell functions during leishmania infection: PLoS Pathog, 2015; 11(6); e1004938
14. Reykande SE, Rezaei A, Sadr M, Association of interferon regulatory factor 5 (IRF5) gene polymorphisms with juvenile idiopathic arthritis: Clin Rheumatol, 2018; 37(10); 2661-65
15. Weiss M, Byrne AJ, Blazek K, IRF5 controls both acute and chronic inflammation: Proc Natl Acad Sci, 2015; 112(35); 11001-6
16. van der Helm-van Mil, Toes REM, Huizinga TWJ, Genetic variants in the prediction of rheumatoid arthritis: Ann Rheum Dis, 2010; 69(9); 1694-96
17. Graham RR, Kozyrev SV, Baechler EC, A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus: Nat Genet, 2006; 38(5); 550-55
18. Rueda B, Reddy MVPL, González-Gay MA, Analysis of IRF5 gene functional polymorphisms in rheumatoid arthritis: Arthritis Rheum, 2014; 54(12); 3815-19
19. Maalej A, Hamad MB, Reba A, Association of IRF5 gene polymorphisms with rheumatoid arthritis in a Tunisian population: Scand J Rheumatol, 2008; 37(6); 414-18
20. Shimane K, Kochi Y, Yamada R, A single nucleotide polymorphism in the IRF5 promoter region is associated with susceptibility to rheumatoid arthritis in the Japanese patients: Ann Rheum Dis, 2008; 68(3); 377-83
21. Yin Q, Wu LC, Zheng L, Comprehensive assessment of the association between genes on JAK-STAT pathway (IFIH1, TYK2, IL-10) and systemic lupus erythematosus. A meta-analysis: Arch Dermatol Res, 2018; 310; 711-28
22. Yokota SI, Yokosawa N, Kubota T, Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and Janus kinases during an early infection stage: Virology, 2001; 286(1); 119-24
23. Gongora C, Mechti NInterferon signaling pathways: Bull Cancer, 1999; 86(11); 911-19 [in French]
24. Johnson BA, Wang J, Taylor EM, Multiple sclerosis susceptibility alleles in African Americans: Genes Immun, 2010; 11(4); 343-50
25. Dyment DA, Cader MZ, Chao MJ, Exome sequencing identifies a novel multiple sclerosis susceptibility variant in the TYK2 gene: Neurology, 2012; 79(5); 406-11
26. Zheng XL, Yao ZJStudy on expression and role of TYK2 gene in rheumatoid arthritis: Chinese Journal of Immunology, 2020; 36; 1868-73 [in Chinese]
27. Mori Y, Hirose K, Suzuki K, Tyk2 is essential for IFN-alpha-induced gene expression in mast cells: Int Arch Allergy Immunol, 2004; 134(Suppl 1); 25-29
28. Graham RR, Kyogoku C, Sigurdsson S, Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus: Proc Natl Acad Sci USA, 2007; 104; 6758-63
29. Arnett FC, Edworthy SM, Bloch DA, The American Rheumatism Association 1987 revised criteria for the Classification of rheumatoid arthritis: Arthritis Rheum, 1988; 31; 315-24
30. Li XB, Li T, Yan MFAssociation of interferon regulatory factor 5 gene polymorphisms with rheumatoid arthritis in Shanxi Han Chinese population: Chin J Rheumatol, 2015; 19(7); 440-46 [in Chinese]
31. Shen GY, Shu R, Cui LFAssociation of interferon regulatory factor 5 gene polymorphism with rheumatoid arthritis: Suzhou University Journal of Medical Science, 2010; 30(6); 1246-48 [in Chinese]
32. Kozyrev SV, Lewe’n S, Reddy PM, Structural insertion/deletion variation in IRF5 is associated with a risk haplotype and defines the precise isoforms expressed in SLE: Arthritis Rheum, 2007; 56; 1234-41
33. Sigurdsson S, Nordmark G, Göring HH, Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus: Am J Hum Genet, 2005; 76; 528-37
34. Li P, Chang YK, Shek KW, Lau YL, Lack of association of TYK2 gene polymorphisms in Chinese patients with systemic lupus erythematosus: J Rheumatol, 2011; 38; 177-78
35. Cunninghame Graham DS, Morris DL, Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus: PLoS Genet, 2011; 7; e1002341
36. Kyogoku C, Morinobu A, Nishimura K, Lack of association between tyrosine kinase 2 (TYK2) gene polymorphisms and susceptibility to SLE in a Japanese population: Mod Rheumatol, 2009; 19; 401-6
37. Kawasaki A, Kyogoku C, Ohashi J, Association of IRF5 polymorphisms with systemic lupus erythematosus in a Japanese population: Support for a crucial role of intron 1 polymorphisms: Arthritis Rheum, 2008; 58; 826-34
Tables
 Table 1. Clinical Characteristics of patients with rheumatoid arthritis and healthy controls (n=578).
Table 1. Clinical Characteristics of patients with rheumatoid arthritis and healthy controls (n=578). Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls.
Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls. Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk.
Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk. Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism.
Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism. Table 1. Clinical Characteristics of patients with rheumatoid arthritis and healthy controls (n=578).
Table 1. Clinical Characteristics of patients with rheumatoid arthritis and healthy controls (n=578). Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls.
Table 2. Distributions of the genotypes of IRF5 and TYK2 polymorphisms in cases and controls. Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk.
Table 3. The association between the alleles of IRF5 and TYK2 gene polymorphisms and RA risk. Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism.
Table 4. Haplotypes structure and frequencies of IRF5 and TYK2 gene polymorphism. Supplementary Table 1. The Hardy-Weinberg equilibrium of IRF5 and TYK2 gene polymorphisms in case and control groups.
Supplementary Table 1. The Hardy-Weinberg equilibrium of IRF5 and TYK2 gene polymorphisms in case and control groups. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952