07 November 2020: Clinical Research
High-Resolution Vessel Wall Magnetic Resonance Imaging of the Middle Cerebral Artery: Comparison of 3D CUBE T1-Weighted Sequence with and without Fat Suppression
Yejun Wu1ABCDE, Fangbing Li1CDF, Yilin Wang2CDF, Tianxiang Hu2BC, Liang Xiao2G*DOI: 10.12659/MSM.928931
Med Sci Monit 2020; 26:e928931
Abstract
BACKGROUND: Fat suppression is an important technique in magnetic resonance imaging (MRI). Comprehensive and quantitative assessment of the influence of fat suppression (FS) on T1-weighted imaging of intracranial vessel wall imaging is needed. In this study, we compared the three-dimensional (3D) variable-flip-angle turbo-spin-echo (CUBE) T1-weighted sequence with and without FS to investigate the differences between the 2 sequences in imaging of the middle cerebral artery (MCA) vessel walls.
MATERIAL AND METHODS: A 3D CUBE T1-weighted sequence with and without FS by 3.0T MRI was used to obtain intracranial vessel wall images of 105 MCA stenosis patients. The image signal intensity, signal-to-noise ratio, and contrast-to-noise ratio were calculated and compared. Two observers evaluated the image quality of the 2 sequences twice, and interobserver and intraobserver consistency were determined. Differences between the 2 sequences in the area of lumen and plaque were compared.
RESULTS: The signal intensity, signal-to-noise ratio, and contrast-to-noise ratio of the 3D CUBE T1-weighted sequence without FS were higher, whereas the noise level was lower. In terms of subjective scores, the 3D CUBE T1-weighted sequence without FS performed better. No significant difference was observed in the measurement of the vascular lumen area between the 2 sequences, although there were statistically significant differences in the measurement of plaque area (i.e., the measurement obtained with 3D CUBE T1-weighted sequence without FS was larger).
CONCLUSIONS: 3D CUBE T1-weighted sequence without FS performed better for MCA vessel walls imaging than 3D CUBE T1-weighted sequence with FS.
Keywords: Magnetic Resonance Imaging, Middle Cerebral Artery, Plaque, Atherosclerotic, Adiposity, Constriction, Pathologic, Imaging, Three-Dimensional, Reproducibility of Results, Signal Processing, Computer-Assisted
Background
Intracranial atherosclerotic disease (ICAD) is one of the most common causes of intracranial arterial stenosis [1,2]. The middle cerebral artery (MCA) is an important branch of the intracranial carotid artery and is susceptible to ICAD, leading to lumen stenosis of the MCA and corresponding clinical symptoms. Therefore, it is clinically important to evaluate MCA stenosis caused by ICAD.
With the development of imaging technology, an increasing number of diagnostic imaging methods have been used to evaluate vascular stenosis caused by atherosclerotic diseases, and high-resolution vessel wall magnetic resonance imaging (HR VW-MRI) is considered one of the most reliable methods for detecting atherosclerotic plaques
Currently, T1-weighted, T2-weighted, and proton density-weighted sequences are commonly used as scanning sequences for HR VW-MRI. The T1-weighted sequence is one of the most commonly used sequences for assessing intracranial arterial vessel walls. The 3D T1-weighted sequence can fully display the location, spatial distribution, size, shape, vascular remodeling, and signal characteristics of intracranial arterial plaques. Importantly, 3D imaging can cover a larger imaging area and even the whole brain in a similar scanning time as 2D imaging. Furthermore, 3D imaging can achieve isotropic resolution and visualize the morphology and distribution characteristics of intracranial arterial lesions from multiple perspectives through multi-planner reformation or curved planner reformation [4].
The generally accepted scanning standard is high-resolution cerebrospinal fluid suppression and blood suppression [4,5]. Some previous studies have applied the T1-fat suppression (FS) sequence in intracranial vascular wall imaging [6–9], whereas others have not [10–13], and it remains unclear whether the use of FS has an influence on HR VW-MRI of MCA [14]. Although it is generally known that FS reduces the signal-to-noise ratio (SNR) of MRI, there is no evidence that clearly demonstrates it. Therefore, it is necessary to perform a comprehensive and quantitative assessment of the influence of FS on theT1-weighted sequence of MCA vessel wall imaging. We hypothesized that the 3D variable-flip-angle turbo-spin-echo (CUBE) T1-weighted sequence without FS would perform better in terms of MCA imaging. In the current study, we compared image quality and relevant scanning results of 3D CUBE T1-weighted sequence with and without FS using subjective evaluation and objective indexes. The overall aim of this study was to compare the performances of these 2 sequences of MCA vessel wall imaging.
Material and Methods
PATIENTS:
The study procedures and informed consent were reviewed by the Ethics Committee of our college. All patients who participated in this study provided written informed consent before undergoing non-invasive HR VW-MRI examination.
A prospective study conducted in this study from March 2019 to July 2019, in which 129 MCA stenosis patients were enrolled. Clinical data (age, sex, neurological clinical symptoms, and clinical history) were recorded for all patients. Patients were diagnosed with MCA stenosis by neurologists according to the atherosclerotic intracranial arterial stenosis diagnostic criteria [15].
The exclusion criteria were: (1) contraindications to MRI, (2) MCA dissection or Moyamoya disease, (3) generation of motion artifacts during MRI examination that affected the diagnosis, and (4) implantation of a stent in the head or carotid artery.
SCANNING PROTOCOL:
A 3.0-T MRI scanner (GE Discovery MR750; GE Healthcare, Global Diagnostic Imaging, WI, USA) performed HR VW-MRI of the MCA, with an 8-channel head coil. All subjects were scanned using the same parameters (Table 1). We used the FS technique of frequency-selective fat saturation (FatSat).
A 3D time-of-flight (TOF) MRA image from each patient was used as the positioning image for scanning the 3D CUBE T1-weighted sequence with and without FS. The imaging range included the MCA M1 segment. In patients with MCA stenosis, MRA results were used to assess MCA stenosis and the select responsible vessels to be scanned. All source data obtained after scanning were uploaded to a GE workstation (Advantage Workstation 4.6, GE Healthcare), and 2 observers conducted further image analysis and comparisons.
IMAGING PROCESSING AND OBJECTIVE EVALUATION:
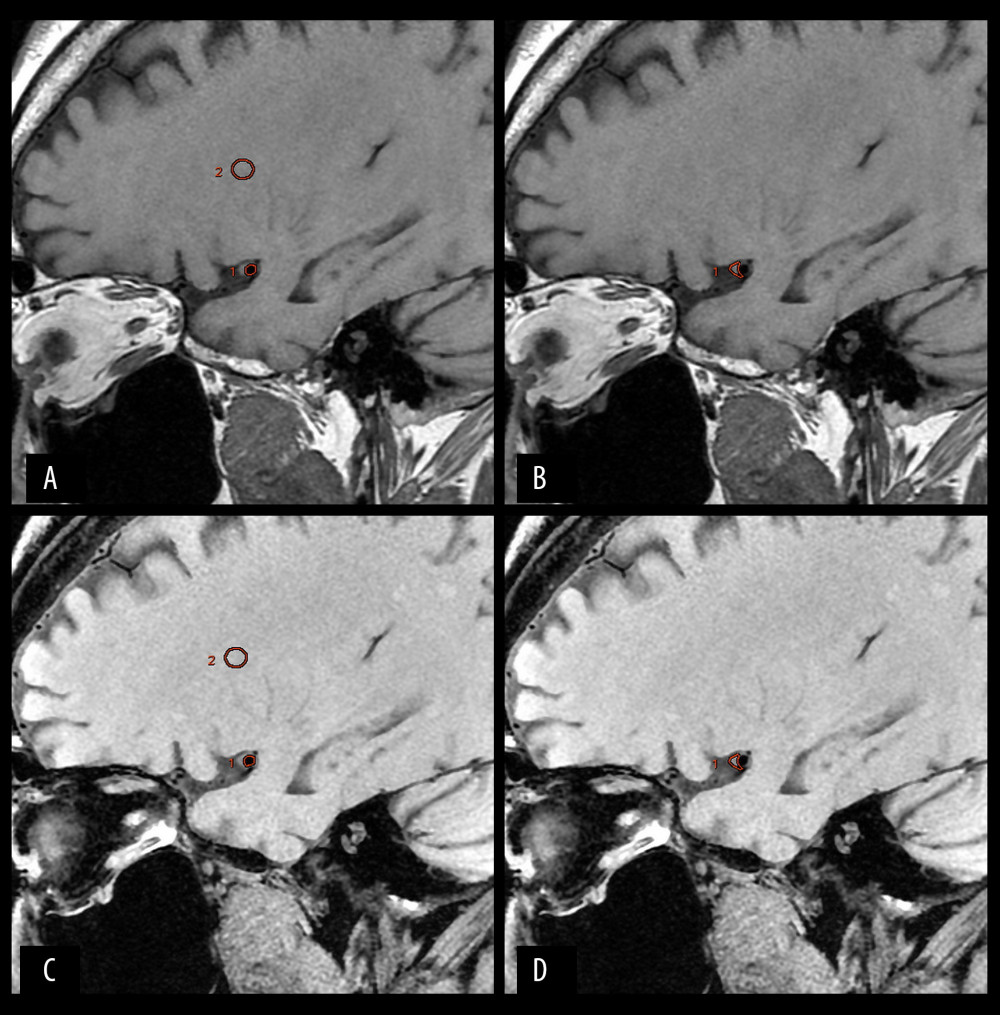
To select the regions of interest (ROI; mm2): we imported the source images of the 3D CUBE T1-weighted sequence with and without FS into the workstation in “comparison mode” and ensured that the ROIs of the 2 sequences were at the same level. To more accurately evaluate image noise, we drew an area equal to 250 mm2 within the brain white matter (ROIB) and obtained its standard deviation as the noise of the image [16]. In patients with MCA stenosis, the vessel wall at the narrowest vascular cross-section of MCA was used to draw the ROIV, and the lumen and plaque of interest were drawn separately (ROIL and ROIPQ, mm2) (Figure 1). All cases were reviewed in random order. The GE workstation automatically calculated the signal intensity (including the vascular cross-section SV, brain white matter SB, vessel lumen SL, plaque SPQ) and noise standard deviation (σN) of each ROI. SNR=S/σN, contrast-to-noise-ratio (CNR)=(SNRB–SNRV or L or PQ)/σN.
SUBJECTIVE IMAGING ASSESSMENT:
Subjective evaluation of the images was performed on the GE workstation. First, the 2 observers evaluated all images independently according to predetermined scoring criteria. The 2 observers were blinded to the other sequencing images when analyzing the images. After 4 weeks, the 2 observers assessed the image quality again. Parameter information of the images and clinical information were removed before the assessment, and the assessment order was randomized. The image quality score was divided into the following 5 components: noise, lumen visualization, vascular wall visualization, contrast resolution between the vessel wall and surrounding structures, plaque visualization, and contrast resolution between the plaque and vessel wall. A 5-point scale was defined as 1 (very poor), 2 (poor), 3 (adequate), 4 (good), and 5 (excellent).
STATISTICAL ANALYSIS:
All statistical analyses were performed using MedCalc 19.0.7 (MedCalc Software bvba, Ostend, Belgium). Continuous variables are described as mean±standard deviation, and classified variables are expressed as count (percentage). The Kolmogorov-Smirnov test was used to determine whether the continuous variables were normally distributed. The intraclass correlation coefficient (ICC) was used to quantify the intraobserver and interobserver reproducibility. An ICC value >0.75 was used to indicate a high level of reproducibility, 0.40≤ ICC ≤0.75 denoted moderate reproducibility, and ICC <0.4 denoted low reproducibility. The Wilcoxon signed-rank test was used to verify the differences in measurement and enumeration data. All statistical methods used
Results
RESULTS OF OBJECTIVE MEASUREMENT OF IMAGES:
Compared with 3D CUBE T1-weighted sequence with FS, 3D CUBE T1-weighted sequence without FS had higher signal intensity and a higher SNR of brain white matter, vascular cross-section, lumen, and plaque, and they had a higher CNR of vascular cross-section, lumen, and plaque. In addition, the noise level of the 3D CUBE T1-weighted sequence without FS was lower. The differences were statistically significant (Table 2).
There was no significant difference in the vascular cross-section (T1
RESULTS OF SUBJECTIVE EVALUATION OF IMAGES:
The subjective scores indicated no significant difference between the 2 sequences in the display scores of the vascular lumen. However, the noise score was significantly higher in the 3D CUBE T1-weighted sequence without FS. For other scores, the 3D CUBE T1-weighted sequence without FS scores were slightly higher than the 3D CUBE T1-weighted sequence with FS scores (Table 3). There was a high level of consistency between the 2 observers in subjective scores (Table 4).
Discussion
To the best of our knowledge, no previous studies have reported the effect of FS on 3D CUBE T1 sequence in vessel wall imaging in detail. The present study assessed the influence of the use of fat suppression on the image quality of 3D CUBE T1 sequences in depicting the MCA. Although expert consensus has recommended that fat suppression is not necessary for intracranial vessel wall imaging [14], it is still important to perform a comprehensive and quantitative assessment of the influence of fat suppression on the image. In the present study, we compared a 3D CUBE T1-weighted sequence with and without FS for imaging the MCA vessel walls. Based on the signal intensity measurements, as well as qualitative evaluation by 2 observers, our results indicate that the 3D CUBE T1-weighted sequence without FS visualizes the intracranial vessel walls better than when using FS.
HR VW-MRI of the carotid artery requires the use of FS technique because the adjacent tissues of the carotid arteries contain fat, which displays a high signal on the T1-weighted sequence. When the high signal of fat is suppressed, the carotid artery wall can be displayed more clearly [17]. Some previous studies have used FS technique in HR VW MRI of intracranial arterial; those experiments might have referred to the techniques of carotid vessel wall imaging. However, the intracranial arterial blood vessel has no fat content and is very different from the cervical internal carotid artery and other large arteries [18,19]. For intracranial scanning, MCA is not affected by clivus bone marrow [20].
Common FS techniques consist of the following various modalities: short tau inversion recovery, spectral attenuated inversion recovery, FatSat, water-selective excitation, and Dixon technique [21], and each is based on a different physical phenomenon. In this study, we used FatSat technique to suppress the fat signal. This technique takes advantage of the difference in the precession frequency of water and fat to selectively apply presaturation pulses to fat, with the goal of fat suppression. It is widely used in clinical practice and is one of the most commonly used methods of fat inhibition by high-field magnetic resonance [22]. We selected the FatSat technique for FS mainly because it is suitable for almost all MRI sequences and only slightly increases the scanning time.
Both the SNR and CNR of the source images of the 3D CUBE T1-weighted sequence with FS are lower because the application of FS technology increases imaging noise. The M1 segment of MCA is 3.0–5.0 mm in diameter on average, and is located along the cerebral sulcus, traveling tortuously [23]. To accurately evaluate the MCA vessel wall, plaque characteristics, and the signal characteristics of both, higher resolution and the relative SNR are required [24,25]. The increase in noise and decrease in signal intensity, SNR, and CNR affected the evaluation of image quality and vascular wall details, leading to a difference in scores between the 2 observers for the 2 sequence images, except for the visualization of the vascular lumen.
From the subjective evaluation, the 2 sequences showed no significant difference in the inhibition of blood flow, and the boundary between the vascular lumen and vessel wall could be distinguished. Therefore, there was no statistically significant difference in the measurement of vascular lumen area between the 2 sequences. However, from the perspective of plaque and vessel wall visualization, the increase in noise and the decrease in signal intensity affected the detailed observation of MCA vessel wall imaging, including the resolution of the plaque and vessel wall and the resolution of the vessel wall and surrounding tissues. Because the 3D CUBE T1-weighted sequence without FS has a high SNR, high CNR, and low noise, it can better display the plaque and distinguish the boundary between the plaque and the vessel wall. Accordingly, the differences were noted in the measurements of plaque area by the 2 observers.
In this study, although the subjective score of blood vessel wall and the surrounding tissue comparison of the 3D CUBE T1-FS sequence was slightly lower, there was no significant difference in the cross-sectional area between the 2 objectively measured blood vessels. This may be because vascular sections are relatively easy to measure as compared with plaques, which offsets the disadvantage of local vascular walls being indistinguishable from surrounding tissues.
There are some limitations to our study. Currently, the most widely accepted scanning scheme for HR VW-MRI is multi-sequence scanning using the T1-weighted sequence, T2-weighted sequence, and proton density-weighted sequence, as well as the T1-enhanced sequence. In the current study, only the T1 sequence was selected for study. The T1-enhanced sequence may provide a more accurate display of the vascular lumen and plaque. However, injection of contrast medium is invasive and there is a risk of gadolinium deposition in the brain. Most patients enrolled in this study refused the minimally invasive examination, and the application of the contrast agent was beyond our scope and budget. Fat suppression technology leads to decreased SNR and CNR of images. This phenomenon may exist in the T1-enhanced sequence. Therefore, further studies on the evaluation of T1-enhanced sequence with and without FS will be necessary. In addition, other vascular stenoses caused by intracranial atherosclerotic diseases, including basilar artery stenosis, should be evaluated in future studies.
Conclusions
When using high-resolution vessel wall MRI of the MCA, the 3D CUBE T1-weighted sequence without FS performed better than the 3D CUBE T1-weighted sequence with FS.
Tables
Table 1. Vessel wall imaging protocol with a GE Discovery 3.0T MRI scanner. Table 2. Comparison of signal characteristics between the two sequences.
Table 2. Comparison of signal characteristics between the two sequences.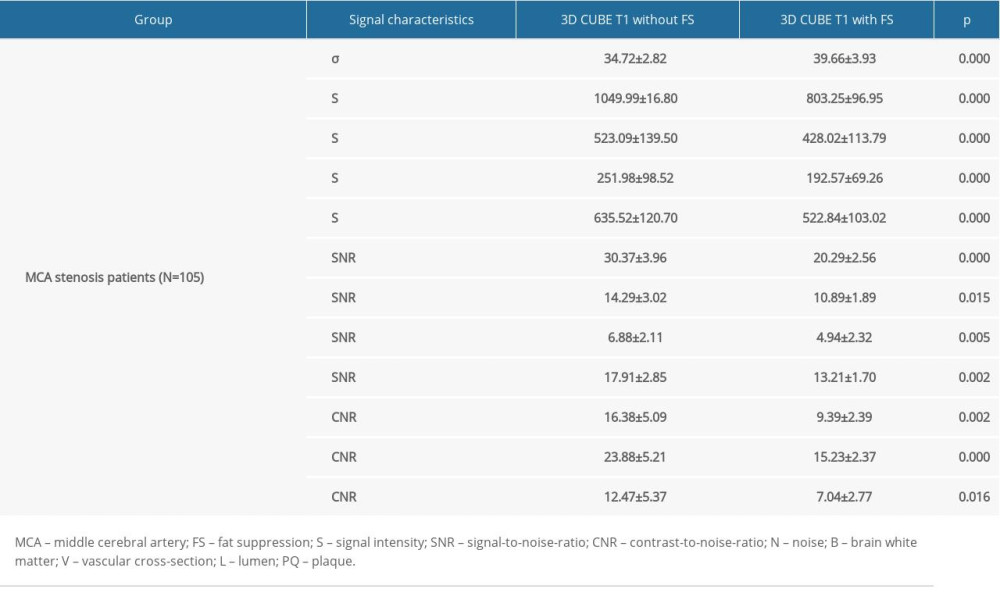 Table 3. Comparison of image quality between two sequences datasets as scored by two readers.
Table 3. Comparison of image quality between two sequences datasets as scored by two readers.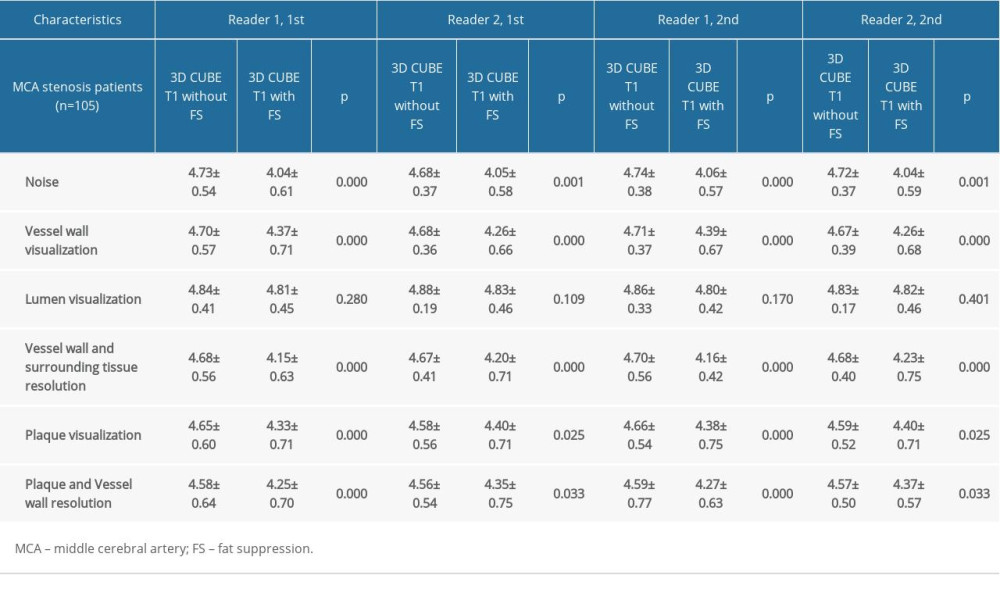 Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences.
Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences.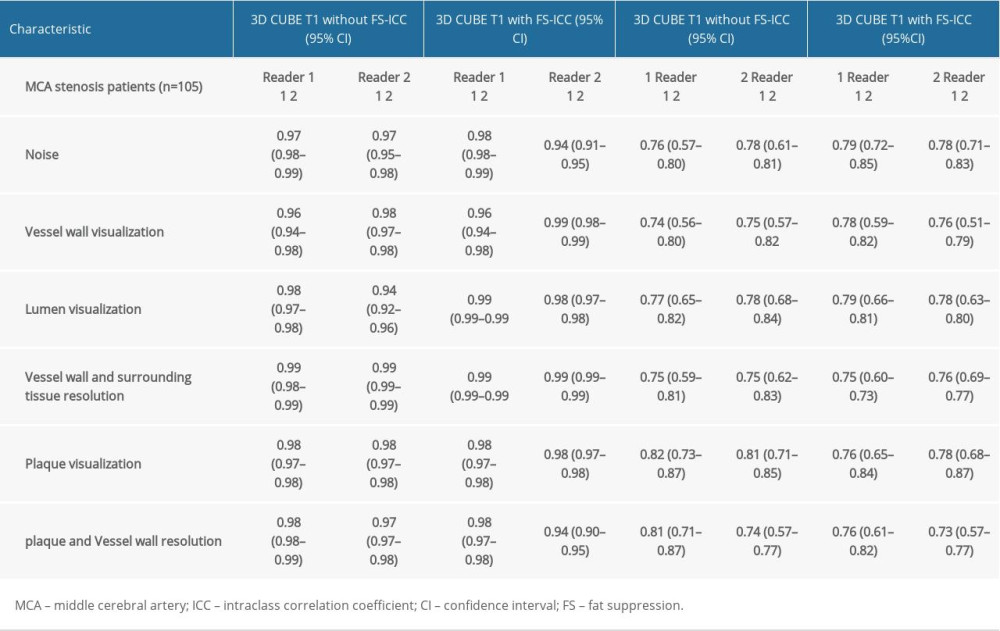
References
1. Gorelick PB, Wong KS, Bae HJ, Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier: Stroke, 2008; 39; 2396-99
2. Shi MC, Wang SC, Zhou HW, Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: A high-resolution MRI and microemboli monitoring study: Neurol Res, 2012; 34; 153-58
3. Tan HW, Chen X, Maingard J, Intracranial vessel wall imaging with magnetic resonance imaging: Current techniques and applications: World Neurosurg, 2018; 112; 186-98
4. Alexander MD, de Havenon A, Kim SE, Assessment of quantitative methods for enhancement measurement on vessel wall magnetic resonance imaging evaluation of intracranial atherosclerosis: Neuroradiology, 2019; 61; 643-50
5. Wu F, Ma Q, Song H, Differential features of culprit intracranial atherosclerotic lesions: A whole-brain vessel wall imaging study in patients with acute ischemic stroke: J Am Heart Assoc, 2018; 7; e009705
6. Wong SK, Mobolaji-Iawal M, Arama L: World J Radiol, 2014; 6; 192-202
7. de Havenon A, Chung L, Park M, Intracranial vessel wall MRI: A review of current indications and future applications: Neurovasc Imaging, 2016; 2; 10
8. Bhogal P, Navaei E, Makalanda HL, Intracranial vessel wall MRI: Clin Radiol, 2016; 71; 293-303
9. Huang J, Jiao S, Zhao X, Characteristics of patients with enhancing intracranial atherosclerosis and association between plaque enhancement and recent cerebrovascular ischemic events: A high-resolution magnetic resonance imaging study: Acta Radiologica, 2019; 60; 1301-7
10. Schaafsma JD, Rawal S, Coutinho JM, Diagnostic impact of intracranial vessel wall MRI in 205 patients with ischemic stroke or tia: Am J Neuroradiol, 2019; 40; 1701-6
11. Qiao Y, Zeiler SR, Mirbagheri S, Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images: Radiology, 2014; 271; 534-42
12. Dieleman N, Yang W, Abrigo JM, Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis: Stroke, 2016; 47; 1797-802
13. Alexander MD, Yuan C, Rutman A, High-resolution intracranial vessel wall imaging: Imaging beyond the lumen: J Neurol Neurosurg Psychiatry, 2016; 87; 589-97
14. Mandell DM, Mossa-Basha M, Qiao Y, Intracranial vessel wall MRI: Principles and expert consensus recommendations of the American society of neuroradiology: Am J Neuroradiol, 2017; 38; 218-29
15. Samuels OB, Joseph GJ, Lynn MJ, A standardized method for measuring intracranial arterial stenosis: Am J Neuroradiol, 2000; 21; 643-46
16. Zhang Z, Fan Z, Carroll TJ, Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla: Invest Radiol, 2009; 44; 619-26
17. Adhithyan R, Kesav P, Thomas B, High-resolution magnetic resonance vessel wall imaging in cerebrovascular diseases: Neurol India, 2018; 66; 1124-32
18. Li ML, Xu YY, Hou B, High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence: Eur J Radiol, 2016; 85; 803-7
19. Saba L, Yuan C, Hatsukami TS, Carotid artery wall imaging: Perspective and guidelines from the ASNR vessel wall imaging study group and expert consensus recommendations of the American society of neuroradiology: Am J Neuroradiol, 2018; 39; E9-31
20. Zhang N, Zhang F, Deng Z, 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: A study on the reliability in the quantification of intracranial vessel dimensions: J Cardiovasc Magn Reson, 2018; 20; 39
21. Partridge SC, Singer L, Sun R, Diffusion-weighted MRI: Influence of intravoxel fat signal and breast density on breast tumor conspicuity and apparent diffusion coefficient measurements: J Magn Reson Imaging, 2011; 29; 1215-21
22. Bley TA, Wieben O, Francois CJ, Fat and water magnetic resonance imaging: J Magn Reson Imaging, 2010; 31; 4-18
23. Chung GH, Kwak HS, Hwang SB, High-resolution MR imaging in patients with symptomatic middle cerebral artery stenosis: Eur J Radiol, 2012; 81; 4069-74
24. Harteveld AA, van der Kolk AG, van der Worp HB, High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: Comparison of 3T and 7T: Eur Radiol, 2017; 27; 1585-95
25. de Havenon A, Mossa-Basha M, Shah L, High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease: Neuroradiology, 2017; 59; 1193-202
Tables
 Table 1. Vessel wall imaging protocol with a GE Discovery 3.0T MRI scanner.
Table 1. Vessel wall imaging protocol with a GE Discovery 3.0T MRI scanner. Table 2. Comparison of signal characteristics between the two sequences.
Table 2. Comparison of signal characteristics between the two sequences. Table 3. Comparison of image quality between two sequences datasets as scored by two readers.
Table 3. Comparison of image quality between two sequences datasets as scored by two readers. Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences.
Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences. Table 1. Vessel wall imaging protocol with a GE Discovery 3.0T MRI scanner.
Table 1. Vessel wall imaging protocol with a GE Discovery 3.0T MRI scanner. Table 2. Comparison of signal characteristics between the two sequences.
Table 2. Comparison of signal characteristics between the two sequences. Table 3. Comparison of image quality between two sequences datasets as scored by two readers.
Table 3. Comparison of image quality between two sequences datasets as scored by two readers. Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences.
Table 4. ICC values of intraobserver, and interobserver reproducibility for two sequences. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952