12 May 2021: Molecular Biology
Decreased HLF Expression Predicts Poor Survival in Lung Adenocarcinoma
Zaiyan Wang1BE, Xiaoning Li1BE, Hao Chen1CF, Li Han1CD, Xiaobin Ji1BD, Qiubo Wang1DF, Li Wei1BF, Yafang Miao1CF, Jing Wang1F, Jianfeng Mao1F, Zeming Zhang1AG*DOI: 10.12659/MSM.929333
Med Sci Monit 2021; 27:e929333
Abstract
BACKGROUND: Lung adenocarcinoma (LUAD) is a type of non-small cell carcinoma. Its pathogenesis is being explored and there is no cure for the disease.
MATERIAL AND METHODS: The Gene Expression Omnibus (GEO) was searched to obtain data on expression of messenger RNA. GEO2R, an interactive web tool, was used to calculate the differentially expressed genes (DEGs) in LUAD. All the DEGs from different datasets were imported into VENNY 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) to identify the intersection of the DEGs. An online analysis tool, the Database for Annotation, Visualization, and Integrated Discovery (DAVID), was used to help understand the biological meaning of DEG enrichment in LUAD. Cytoscape 3.7.2 was used to perform centrality analysis and visualize hub genes and related networks. Furthermore, the prognostic value of the hub genes was evaluated with the Kaplan-Meier plotter survival analysis tool.
RESULTS: The GEO database was used to obtain RNA sequencing information for LUAD and normal tissue from the GSE118370, GSE136043, and GSE140797 datasets. A total of 376 DEGs were identified from GSE118370, 248 were identified from GSE136403, and 718 DEGs were identified from GSE140797. The 10 genes with the highest degrees of expression – the hub genes – were CAV1, TEK, SLIT2, RHOJ, DGSX, HLF, MEIS1, PTPRD, FOXF1, and ADRB2. In addition, Kaplan-Meier survival evaluation showed that CAV1, TEK, SLIT2, HLF, MEIS1, PTPRD, FOXF1, and ADRB2 were associated with favorable outcomes for LUAD.
CONCLUSIONS: CAV1, TEK, SLIT2, HLF, MEIS1, PTPRD, FOXF1, and ADRB2 are hub genes in the DEG interaction network for LUAD and are involved in the development of and prognosis for the disease. The mechanisms underlying these genes should be the subject of further studies.
Keywords: Biological Markers, A549 cells, Adenocarcinoma of Lung, Basic-Leucine Zipper Transcription Factors, Biomarkers, Tumor, Computational Biology, Databases, Genetic, Gene Expression Profiling, gene ontology, Gene Regulatory Networks, Protein Interaction Maps
Background
Non-small cell lung carcinoma (NSCLC) includes large cell carcinoma, squamous cell carcinoma (SCC), and adenocarcinoma [1]. Unlike squamous cell lung cancer, lung adenocarcinoma (LUAD) is more common in non-smokers and women [2]. The incidence of LUAD is lower than for SCC and undifferentiated cancer and patients diagnosed with it are younger [2]. In general, there are no obvious clinical symptoms in the early stage of the disease and it often is diagnosed on a chest X-ray. LUAD tends to spread through the bloodstream in early stages, whereas metastasis through the lymphatic system occurs much later [3].
The pathogenesis of lung cancer is complex and involves multiple causes and genes [4]. Many studies are focusing on the pathological mechanism of LUAD [1,4]. However, the cause of lung cancer is still unclear, and there is no cure for it [2]. Exploring the process of LUAD at the gene and protein level can provide detailed and useful information for a comprehensive understanding of the disease.
Based on big data, bioinformatics methods are an increasingly popular way of exploring the role of differentially expressed genes (DEGs) in disease [5]. Hub genes can be identified by analyzing the DEG interaction network. Bioinformatics has been used to identify some diseases, especially in oncology [6]. However, few studies have combined assessment of DEG interaction networks and survival analysis for LUAD. Therefore, the aim of the present study was to use bioinformatics methods to retrieve the DEGs for LUAD, analyze the interaction network of the DEGs, identify hub genes in LUAD, and perform a Kaplan-Meier survival analysis.
Material and Methods
SEARCH OF GENE CHIP DATA:
The Gene Expression Omnibus (GEO) is a gene expression database created and maintained by the National Center for Biotechnology Information of the National Library of Medicine in the United States. We searched this database for gene chip data that compared tissue from LUAD with normal tissue. The keywords used were “lung adenocarcinoma.” Matrix data on messenger RNA (mRNA) expression were downloaded for analysis.
IDENTIFICATION OF DIFFERENTIALLY EXPRESSED GENES:
GEO2R is online software used to analyze data from GEO. We imported the data into the tool and analyzed it with the default settings. The DEG information was provided by GEP2R, which sorted the DEGs with LogFC.
GENE ONTOLOGY AND KYOTO ENCYCLOPEDIA OF GENES AND GENOMES PATHWAY ENRICHMENT ANALYSIS OF GENES:
We used the Database for Annotation, Visualization and Integrated Discovery (DAVID) 6.8 to explore the biological roles of involved genes [7,8]. Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed with DAVID. DEGs were imported into DAVID with the setting “gene list” and “gene symbol,” and the associated GO and KEGG terms were obtained. The top 3 terms for biological process, cellular component, and molecular function, and the KEGG pathway with the highest P value were selected.
PROTEIN–PROTEIN INTERACTION NETWORK ANALYSIS:
The Protein–Protein Interaction (PPI) network can be used to show the functional relationship between common DEGs. Upregulated and downregulated genes were imported into the Search Tool for the Retrieval of Interacting Genes (STRING) to establish the network. The file “string_interactions.tsv” was downloaded. Then the PPI network “string_interactions.tsv” was imported into Cytoscape software (version 3.7.2) and visualized [9]. Using the software Centiscape 2.2 in Cytoscape, the DEGs were ranked by degree centrality. That information for the top 10 DEGs in the network was visualized with ggplot2 [10]. The enrichment results for the top 10 DEGs and the GO and KEGG terms were visualized with GOplot [11].
KAPLAN-MEIER SURVIVAL ANALYSIS OF HUB GENES:
All the hub genes were imported into the Kaplan-Meier plotter web tool (http://kmplot.com/analysis/) [12]. The Kaplan-Meier plotter can assess the effect of 54 000 genes on survival in 21 cancer types, helping us discover and validate survival biomarkers [13]. Kaplan-Meier survival analysis results for each hub gene were obtained with default settings: “lung adenocarcinoma,” “auto select best cutoff,” and “all the follow-up threshold.” P<0.01 was defined as a statistically significant difference.
CELL CULTURE AND TRANSFECTION:
The LUAD cell line A549 was purchased from the Shanghai Cell Bank. Dulbecco’s modified Eagle’s medium and 10% fetal bovine serum were used to culture all the cells at 37°C in 5% CO2. For transfection, the negative control small interfering RNA (siRNA) and HLF siRNA were designed by Suzhou GenePharma Biotechnology Co., Ltd. A549 cells were transfected with 100 nM NC siRNA or HLA siRNA using Lipofectamine® 3000.
QUANTITATIVE REVERSE TRANSCRIPTION POLYMERASE CHAIN REACTION:
Invitrogen TRIzol was used to extract the total RNA from the cells and tissues. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was performed to determine the HLF mRNA level. The primers were designed by Shanghai Bio Tech, as follows:
The 2−ΔΔCq approach was used to quantify the HLF mRNA level.
Results
IDENTIFICATION OF DEGS:
Gene expression profiles from the GSE118370, GSE136043, and GSE140797 datasets were obtained from the GEO database. GSE118370 is a LUAD dataset; GSE136043 is an mRNA microarray from lung cancer not otherwise specified, and GSE140797 is 7 pairs of LUAD tissue and normal tissue. There were 6 LUAD and 6 normal specimens in GSE118370, 5 LUAD and 5 normal in GSE136043, and 7 LUAD and 7 normal specimens in GSE140797 (Table 1). Next, 245 upregulated and 686 downregulated DEGs were identified in GSE118370; 617 upregulated and 687 downregulated DEGs were identified in GSE136043; and 1073 upregulated and 1281 downregulated DEGs were identified in GSE140797. Finally, 35 common upregulated and 142 common downregulated DEGs were obtained from the 3 datasets (Figure 1).
GENE ONTOLOGY AND KYOTO ENCYCLOPEDIA OF GENES AND GENOMES ENRICHMENT ANALYSIS OF DEGS:
For all the common DEGs, GO and KEGG analysis results showed that during biological processes, common DEGs were significantly enriched in cell adhesion, morphogenesis of a branching structure, and angiogenesis. In molecular function, common DEGs were significantly enriched in protein binding, lipid binding, transcriptional activator activity, and RNA polymerase II core promoter proximal region sequence-specific binding. In the cellular component, common DEGs were significantly enriched as an integral component of the plasma membrane, the membrane raft, and the plasma membrane. In the KEGG pathway, common DEGs were significantly enriched in extracellular matrix-receptor interaction, protein digestion and absorption, and axon guidance (Table 2).
PPI NETWORK CONSTRUCTION AND MODULE ANALYSIS:
The network of protein interactions was obtained using STRING and then the top 10 hub genes with high degrees of expression were identified using Cytoscape (Figure 2). Those hub genes were CAV1, TEK, SLIT2, RHOJ, DGSX, FOXF1, HLF, MEIS1, PTPRD, and ADRB2, all of which were downregulated in LUAD. The degree centrality information for the hub genes is shown in Figure 3 and Table 3. Results of GO and KEGG enrichment analysis of the hub genes are shown in Figure 4. GO and KEGG analysis is one of the most important parts of bioinformatics. It can help us systematically understand the ways in which DEGs, including hub genes, affect biological functions. In the present study, the results of GO and KEGG analysis revealed the function of DEGs in LUAD, as shown in Table 2.
KAPLAN-MEIER SURVIVAL ANALYSIS OF HUB GENES:
The relationship between hub genes and prognostic conditions was evaluated with the Kaplan-Meier plotter online analysis tool. CAV1, TEK, SLIT2, FOXF1, HLF, MEIS1, PTPRD, and ADRB2 were found to be associated with favorable overall survival (OS) in patients with LUAD (Figure 5). The probes for hub genes that we used are shown in Table 4. The schema for this study is shown in Figure 6.
INHIBITION OF HLF PROMOTES A549 CELL PROLIFERATION:
To determine the expression of HLF with the lowest log rank P value, qRT-PCR analysis was used to compare the HLF expression between LUAD and adjacent normal tissue. A significantly lower level of HLF mRNA was found in the LUAD (Supplementary Figure 1A). Next, to study the function of HLF in LUAD, HLF siRNA was used to knock down its expression in A549 cells. The qRT-PCR result indicated an acceptable knockdown efficiency (Supplementary Figure 1B). In addition, to explore the impact of HLF on the proliferation of A549 cells, a qRT-PCR assay was performed to determine the expression of Cyclin D1 and Cyclin D3. Compared with the siRNA-NC group, the expression of Cyclin D1/3 was significantly increased (Supplementary Figure 1C).
Discussion
LUAD is a malignant tumor that originates from bronchial mucosal glandular epithelium and which accounts for about 33% of all lung carcinomas [14]. Lung cancer may not cause any symptoms, especially in the early stages of the disease. Patients with LUAD may develop a cough and chest symptoms. In the late stage, the tumor can block off an airway and interfere with breathing. Current treatments for LUAD include targeted therapy, radiotherapy, immunotherapy, chemotherapy, and surgery, but there is no criterion standard for the disease. The mechanism of LUAD is still unclear and there is controversy about what causes the disease. DEG analysis based on bioinformatic methods can help us explore the pathogenesis of LUAD and find novel targeted treatments for it.
As reported in some studies,
The
The literature regarding the relationship between
In the present study,
There are some limitations of the present study. First, there was no in vitro or in vivo validation. Second, only 3 datasets were used. More data could provide more convincing evidence about our results. Nevertheless, we believe that this bioinformatics-based study provides some useful information for further research on LUAD.
Conclusions
In this bioinformatics-based study,
Figures
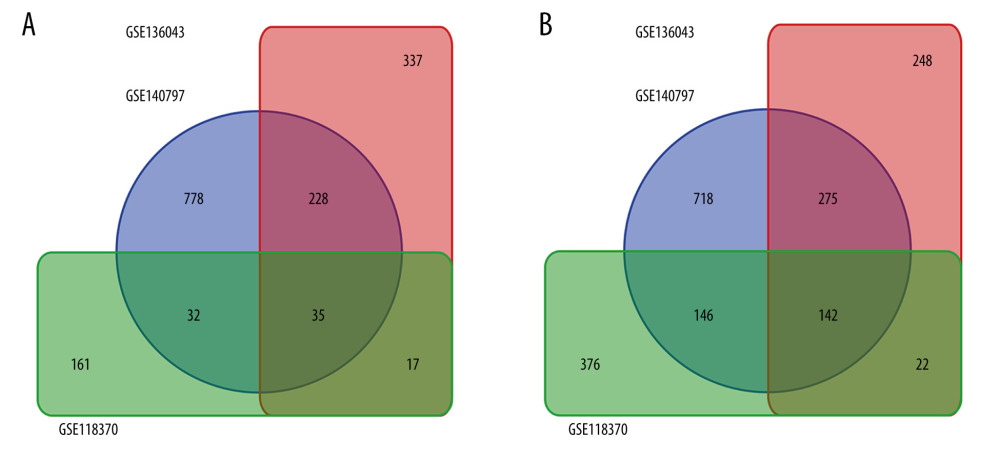 Figure 1. Common differentially expressed genes (DEGs) in GSE118370, GSE136043, and GSE140797. (A) There were 35 upregulated DEGs common to the 3 datasets. (B) There were 142 downregulated DEGs common to the 3 datasets.
Figure 1. Common differentially expressed genes (DEGs) in GSE118370, GSE136043, and GSE140797. (A) There were 35 upregulated DEGs common to the 3 datasets. (B) There were 142 downregulated DEGs common to the 3 datasets. 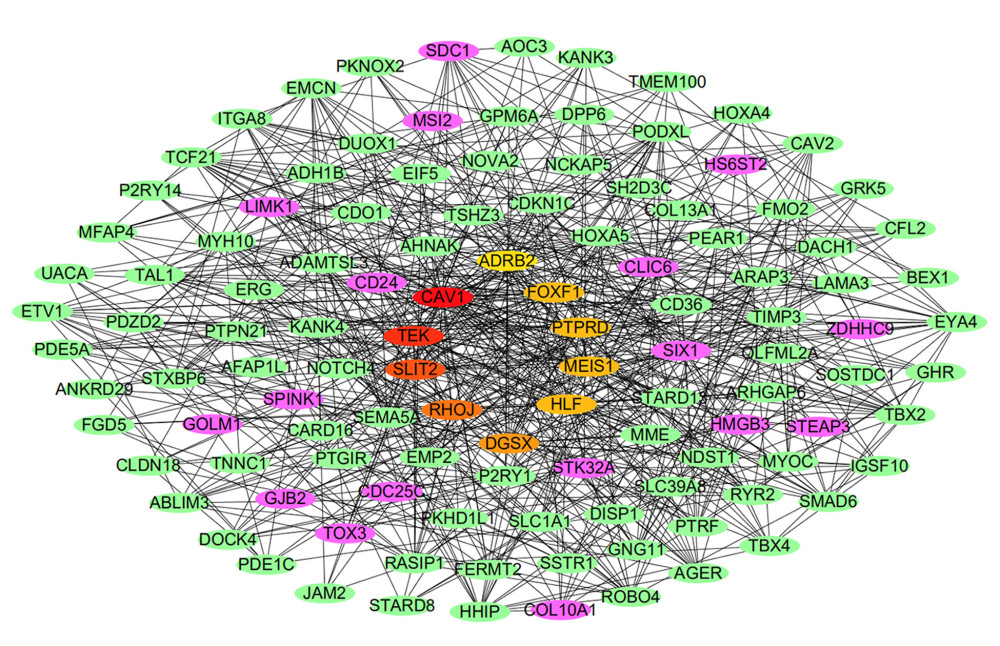 Figure 2. Protein-protein interaction network of the differentially expressed genes (DEGs) constructed with Cytoscape 3.7.2. DEGs shown in purple are upregulated and those in green are downregulated. The 10 hub genes ranked by Degree Centrality, which are located in the middle, are CAV1, TEK, SLIT2, RHOJ, DGSX, FOXF1, HLF, MEIS1, PTPRD, and ADRB2.
Figure 2. Protein-protein interaction network of the differentially expressed genes (DEGs) constructed with Cytoscape 3.7.2. DEGs shown in purple are upregulated and those in green are downregulated. The 10 hub genes ranked by Degree Centrality, which are located in the middle, are CAV1, TEK, SLIT2, RHOJ, DGSX, FOXF1, HLF, MEIS1, PTPRD, and ADRB2. 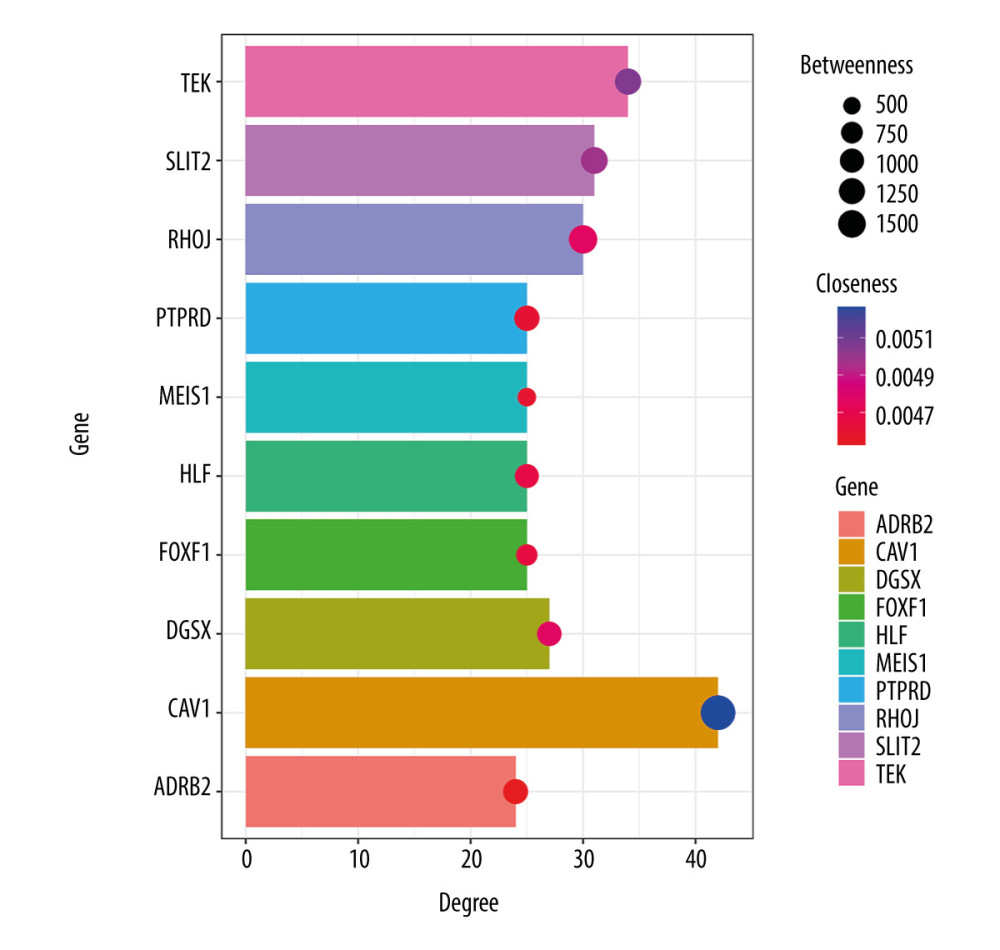 Figure 3. Information about the degree, betweenness, and closeness of hub genes. The size of the bubbles represents betweenness. The depth of color indicates closeness. The length of the column represents degree. As depicted in the figure, CAV1 has the greatest degree of expression, betweenness, and closeness.
Figure 3. Information about the degree, betweenness, and closeness of hub genes. The size of the bubbles represents betweenness. The depth of color indicates closeness. The length of the column represents degree. As depicted in the figure, CAV1 has the greatest degree of expression, betweenness, and closeness. 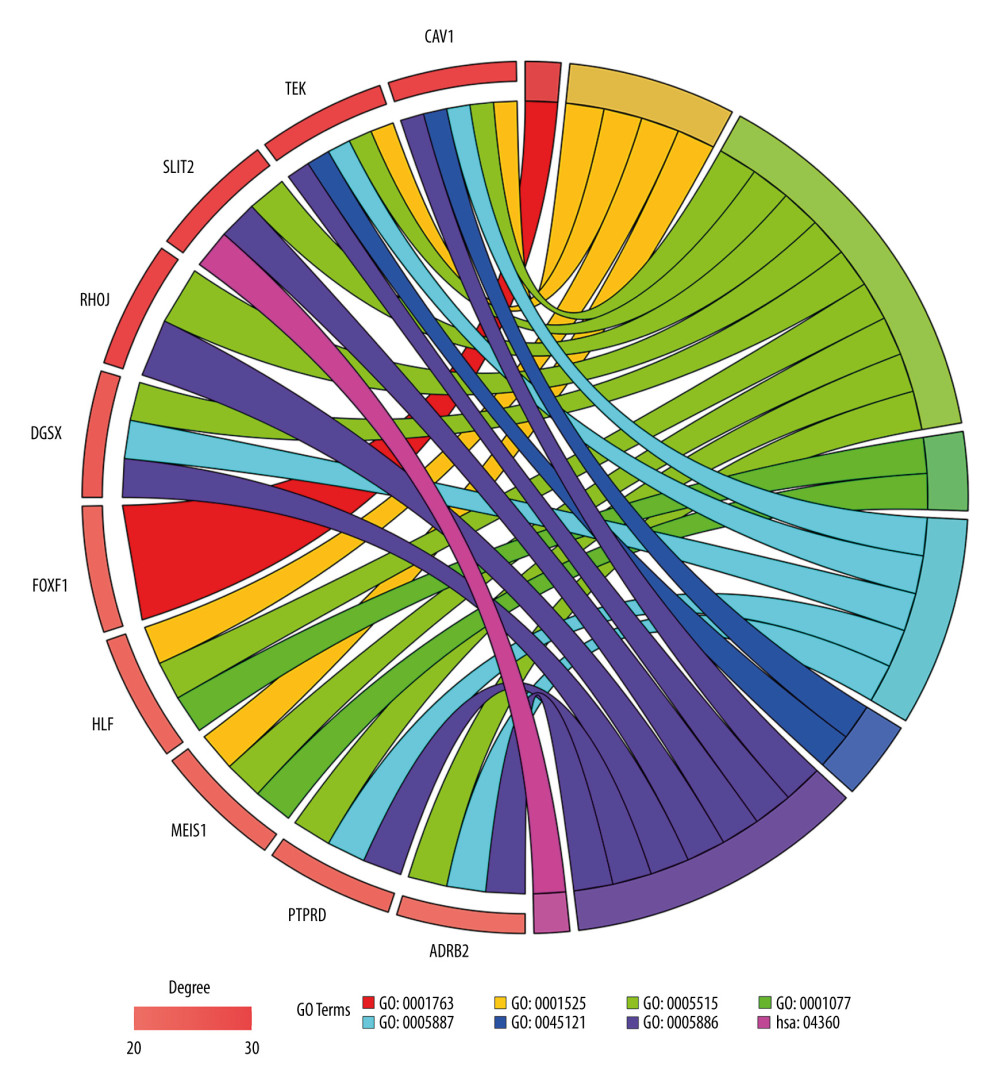 Figure 4. Enrichment results for the hub genes based on assessment of the top Gene Ontology and Kyoto Encyclopedia of Genes and Genomes terms. GO: 0001763, morphogenesis of a branching structure; GO: 0001525, angiogenesis; GO: 0005515, protein binding; GO: 0001077, transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding; GO: 0005887, integral component of plasma membrane; GO: 45121, membrane raft; GO: 0005886, plasma membrane; hsa: 04360, axon guidance.
Figure 4. Enrichment results for the hub genes based on assessment of the top Gene Ontology and Kyoto Encyclopedia of Genes and Genomes terms. GO: 0001763, morphogenesis of a branching structure; GO: 0001525, angiogenesis; GO: 0005515, protein binding; GO: 0001077, transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding; GO: 0005887, integral component of plasma membrane; GO: 45121, membrane raft; GO: 0005886, plasma membrane; hsa: 04360, axon guidance. 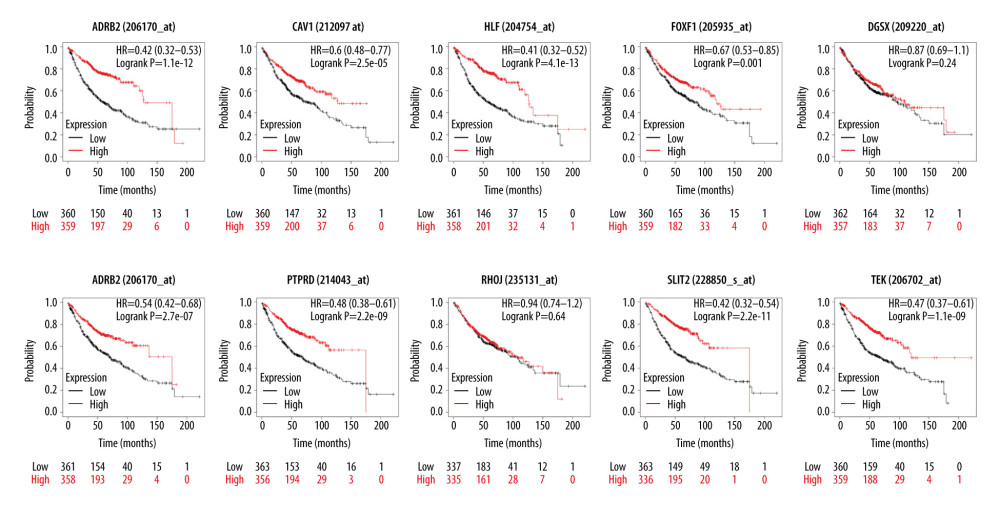 Figure 5. Results of Kaplan-Meier overall analyses for hub genes identified in the study. CAV1, TEK, SLIT2, FOXF1, HLF, MEIS1, PTPRD, and ADRB2 were found to be associated with favorable overall survival in patients with LUAD.
Figure 5. Results of Kaplan-Meier overall analyses for hub genes identified in the study. CAV1, TEK, SLIT2, FOXF1, HLF, MEIS1, PTPRD, and ADRB2 were found to be associated with favorable overall survival in patients with LUAD.  Figure 6. Schema of the study.
Figure 6. Schema of the study. References
1. Zheng M, Classification and pathology of lung cancer: Surg Oncol Clin N Am, 2016; 25(3); 447-68
2. Zappa C, Mousa SA, Non-small cell lung cancer: Current treatment and future advances: Transl Lung Cancer Res, 2016; 5(3); 288-300
3. Abid W, Seguin-Givelet A, Brian E, Second pulmonary resection for a second primary lung cancer: Analysis of morbidity and survival: Eur J Cardiothorac Surg, 2020 [Online ahead of print]
4. Aftabi Y, Ansarin K, Shanehbandi D, Long non-coding RNAs as potential biomarkers in the prognosis and diagnosis of lung cancer: A review and target analysis: IUBMB Life, 2020; 73(2); 307-27
5. Yu T, You X, Zhou H, P53 plays a central role in the development of osteoporosis: Aging (Albany NY), 2020; 12(11); 10473-87
6. Liang R, Chen W, Chen XY, Dihydroartemisinin inhibits the tumorigenesis and invasion of gastric cancer by regulating STAT1/KDR/MMP9 and P53/BCL2L1/CASP3/7 pathways: Pathol Res Pract, 2020; 218; 153318
7. Huang DW, Sherman BT, Lempicki RA, Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists: Nucleic Acids Res, 2009; 37(1); 1-13
8. Huang DW, Sherman BT, Lempicki RA, Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources: Nat Protoc, 2009; 4(1); 44-57
9. Shannon P, Markiel A, Ozier O, Cytoscape: A software environment for integrated models of biomolecular interaction networks: Genome Res, 2003; 13(11); 2498-504
10. Wickham H: ggplot2: Elegant graphics for data analysis; 2016, New York, Springer-Verlag
11. Walter W, Sánchez-Cabo F, Ricote M, GOplot: An R package for visually combining expression data with functional analysis: Bioinformatics, 2015; 31(17); 2912-14
12. Győrffy B, Surowiak P, Budczies J, Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer: PLoS One, 2013; 8(12); e82241
13. Li MX, Jin LT, Wang TJ, Identification of potential core genes in triple negative breast cancer using bioinformatics analysis: Onco Targets Ther, 2018; 11; 4105-12
14. Ridge CA, McErlean AM, Ginsberg MS, Epidemiology of lung cancer: Semin Intervent Radiol, 2013; 30(2); 93-98
15. Shi YB, Li J, Lai XN, Multifaceted roles of Caveolin-1 in lung cancer: A new investigation focused on tumor occurrence, development and therapy: Cancers (Basel), 2020; 12(2); 291
16. Goetz JG, Lajoie P, Wiseman SM, Caveolin-1 in tumor progression: The good, the bad and the ugly: Cancer Metastasis Rev, 2008; 27(4); 715-35
17. Yang G, Truong LD, Timme TL, Elevated expression of caveolin is associated with prostate and breast cancer: Clin Cancer Res, 1998; 4(8); 1873-80
18. Demirci NS, Dogan M, Erdem GU, Is plasma caveolin-1 level a prognostic biomarker in metastatic pancreatic cancer: Saudi J Gastroenterol, 2017; 23(3); 183-89
19. Torrejón B, Cristóbal I, Rojo F, García-Foncillas J, Caveolin-1 is markedly downregulated in patients with early-stage colorectal cancer: World J Surg, 2017; 41(10); 2625-30
20. Wiechen K, Diatchenko L, Agoulnik A, Caveolin-1 is downregulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene: Am J Pathol, 2001; 159(5); 1635-43
21. Butkiewicz D, Gdowicz-Kłosok A, Krześniak M, Association of genetic variants in ANGPT/TEK and VEGF/VEGFR with progression and survival in head and neck squamous cell carcinoma treated with radiotherapy or radiochemotherapy: Cancers (Basel), 2020; 12(6); 1506
22. Liu D, Martin V, Fueyo J, Tie2/TEK modulates the interaction of glioma and brain tumor stem cells with endothelial cells and promotes an invasive phenotype: Oncotarget, 2010; 1(8); 700-9
23. Makhoul I, Todorova VK, Siegel ER, Germline genetic variants in TEK, ANGPT1, ANGPT2, MMP9, FGF2 and VEGFA are associated with pathologic complete response to bevacizumab in breast cancer patients: PLoS One, 2017; 12(1); e0168550
24. Peng T, Yang F, Sun Z, TEK suppresses lung adenocarcinoma cell phenotypes by interacting with miR-19a-3: Research Square, 2020 https://www.researchsquare.com/article/rs-38816/v1
25. Tseng RC, Lee SH, Hsu HS, SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis: Cancer Res, 2010; 70(2); 543-51
26. Dallol A, Da SNF, Viacava P, SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers: Cancer Res, 2002; 62(20); 5874-80
27. Tamura M, Sasaki Y, Koyama R, Forkhead transcription factor FOXF1 is a novel target gene of the p53 family and regulates cancer cell migration and invasiveness: Oncogene, 2014; 33(40); 4837-46
28. Wu CY, Chan CH, Dubey NK, Highly expressed FOXF1 inhibit non-small-cell lung cancer growth via inducing tumor suppressor and G1-phase cell-cycle arrest: Int J Mol Sci, 2020; 21(9); 3227
29. Herrera-Merchan A, Cuadros M, Rodriguez MI, The value of lncRNA FENDRR and FOXF1 as a prognostic factor for survival of lung adenocarcinoma: Oncotarget, 2020; 11(13); 1172-85
30. Wang Z, Wei Y, Zhang R, Multi-omics analysis reveals a HIF network and hub gene EPAS1 associated with lung adenocarcinoma: EBioMedicine, 2018; 32; 93-101
31. Zhen Q, Zhang Y, Gao L, EPAS1 promotes peritoneal carcinomatosis of non-small-cell lung cancer by enhancing mesothelial-mesenchymal transition: Strahlenther Onkol, 2020; 197(2); 141-49
32. Li W, Huang K, Guo H, Meis1 regulates proliferation of non-small-cell lung cancer cells: J Thorac Dis, 2014; 6(6); 850-55
33. VanOpstall C, Perike S, Brechka H, MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans: Elife, 2020; 9; e53600
34. Kohno T, Otsuka A, Girard L, A catalog of genes homozygously deleted in human lung cancer and the candidacy of PTPRD as a tumor suppressor gene: Genes Chromosomes Cancer, 2010; 49(4); 342-52
35. Veeriah S, Brennan C, Meng S, The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers: Proc Natl Acad Sci USA, 2009; 106(23); 9435-40
36. Bae WJ, Ahn JM, Byeon HE, PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin: J Exp Clin Cancer Res, 2019; 38(1); 484
37. Ortiz B, White JR, Wu WH, Deletion of PTPRD and CDKN2A cooperate to accelerate tumorigenesis: Oncotarget, 2014; 5(16); 6976-82
38. Zhang X, Zhang Y, He Z, Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2: Cell Death Dis, 2019; 10(11); 788
39. Wang H, Hao B, Chen X, Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: A case-control study in a Chinese population: Cancer Lett, 2006; 240(2); 297-305
Figures
 Figure 1. Common differentially expressed genes (DEGs) in GSE118370, GSE136043, and GSE140797. (A) There were 35 upregulated DEGs common to the 3 datasets. (B) There were 142 downregulated DEGs common to the 3 datasets.
Figure 1. Common differentially expressed genes (DEGs) in GSE118370, GSE136043, and GSE140797. (A) There were 35 upregulated DEGs common to the 3 datasets. (B) There were 142 downregulated DEGs common to the 3 datasets. Figure 2. Protein-protein interaction network of the differentially expressed genes (DEGs) constructed with Cytoscape 3.7.2. DEGs shown in purple are upregulated and those in green are downregulated. The 10 hub genes ranked by Degree Centrality, which are located in the middle, are CAV1, TEK, SLIT2, RHOJ, DGSX, FOXF1, HLF, MEIS1, PTPRD, and ADRB2.
Figure 2. Protein-protein interaction network of the differentially expressed genes (DEGs) constructed with Cytoscape 3.7.2. DEGs shown in purple are upregulated and those in green are downregulated. The 10 hub genes ranked by Degree Centrality, which are located in the middle, are CAV1, TEK, SLIT2, RHOJ, DGSX, FOXF1, HLF, MEIS1, PTPRD, and ADRB2. Figure 3. Information about the degree, betweenness, and closeness of hub genes. The size of the bubbles represents betweenness. The depth of color indicates closeness. The length of the column represents degree. As depicted in the figure, CAV1 has the greatest degree of expression, betweenness, and closeness.
Figure 3. Information about the degree, betweenness, and closeness of hub genes. The size of the bubbles represents betweenness. The depth of color indicates closeness. The length of the column represents degree. As depicted in the figure, CAV1 has the greatest degree of expression, betweenness, and closeness. Figure 4. Enrichment results for the hub genes based on assessment of the top Gene Ontology and Kyoto Encyclopedia of Genes and Genomes terms. GO: 0001763, morphogenesis of a branching structure; GO: 0001525, angiogenesis; GO: 0005515, protein binding; GO: 0001077, transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding; GO: 0005887, integral component of plasma membrane; GO: 45121, membrane raft; GO: 0005886, plasma membrane; hsa: 04360, axon guidance.
Figure 4. Enrichment results for the hub genes based on assessment of the top Gene Ontology and Kyoto Encyclopedia of Genes and Genomes terms. GO: 0001763, morphogenesis of a branching structure; GO: 0001525, angiogenesis; GO: 0005515, protein binding; GO: 0001077, transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding; GO: 0005887, integral component of plasma membrane; GO: 45121, membrane raft; GO: 0005886, plasma membrane; hsa: 04360, axon guidance. Figure 5. Results of Kaplan-Meier overall analyses for hub genes identified in the study. CAV1, TEK, SLIT2, FOXF1, HLF, MEIS1, PTPRD, and ADRB2 were found to be associated with favorable overall survival in patients with LUAD.
Figure 5. Results of Kaplan-Meier overall analyses for hub genes identified in the study. CAV1, TEK, SLIT2, FOXF1, HLF, MEIS1, PTPRD, and ADRB2 were found to be associated with favorable overall survival in patients with LUAD. Figure 6. Schema of the study.
Figure 6. Schema of the study. Tables
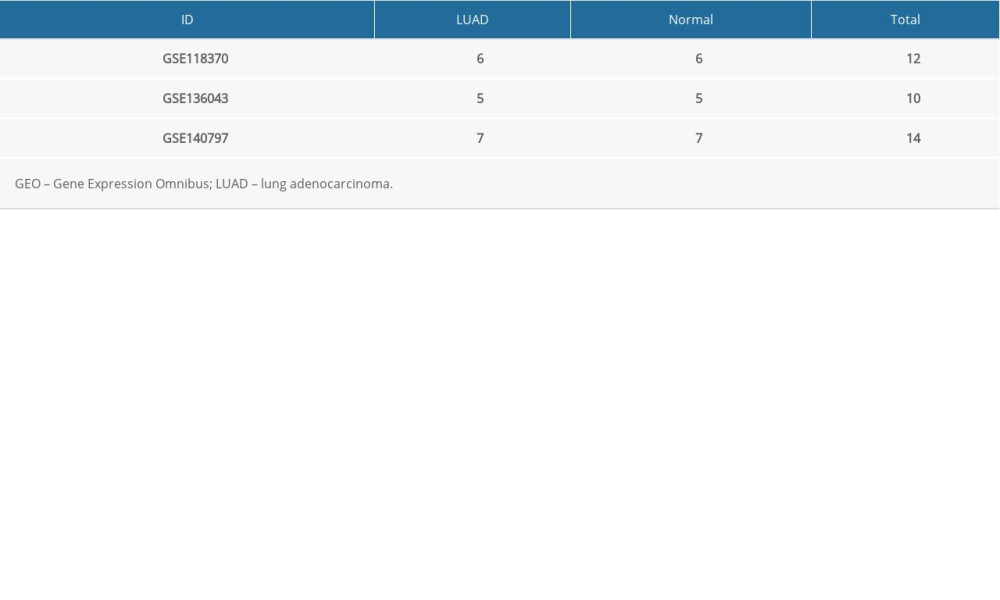 Table 1. Information about the 3 datasets obtained from GEO.
Table 1. Information about the 3 datasets obtained from GEO.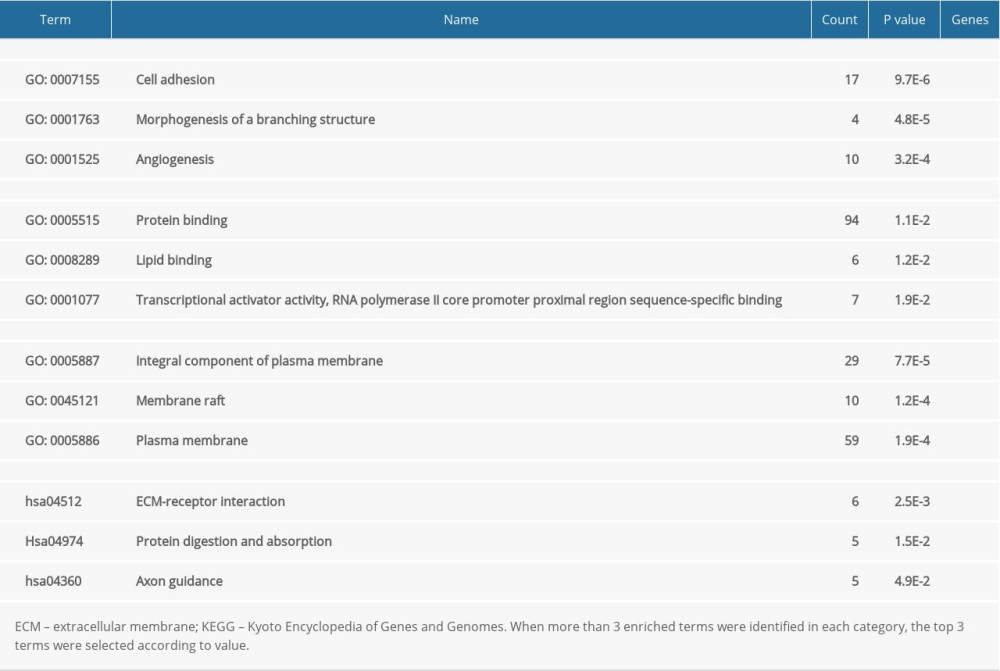 Table 2. Functional and pathway enrichment analysis of the DEGs.
Table 2. Functional and pathway enrichment analysis of the DEGs.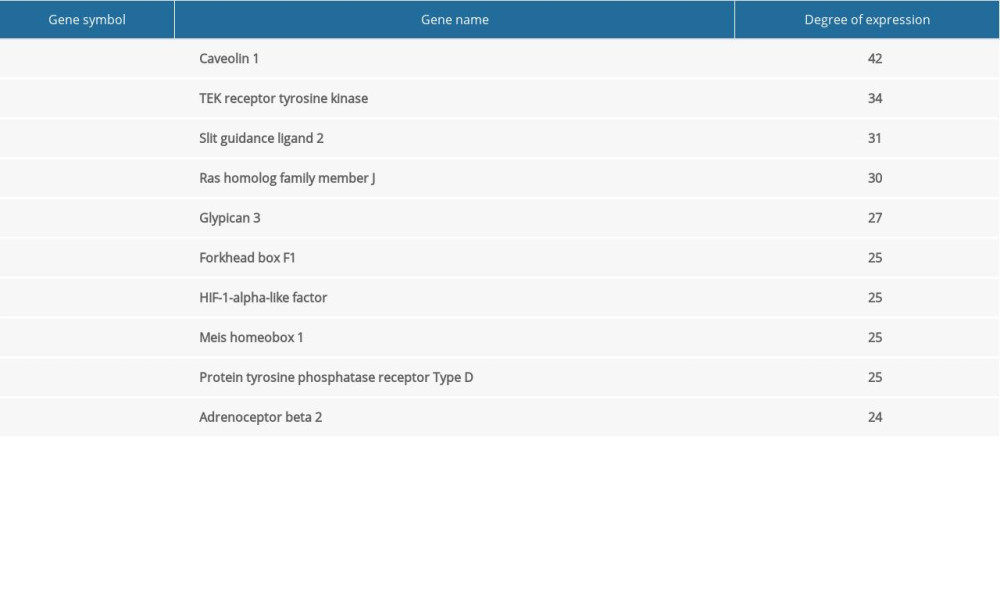 Table 3. Information for the top 10 genes with high degrees of expression.
Table 3. Information for the top 10 genes with high degrees of expression.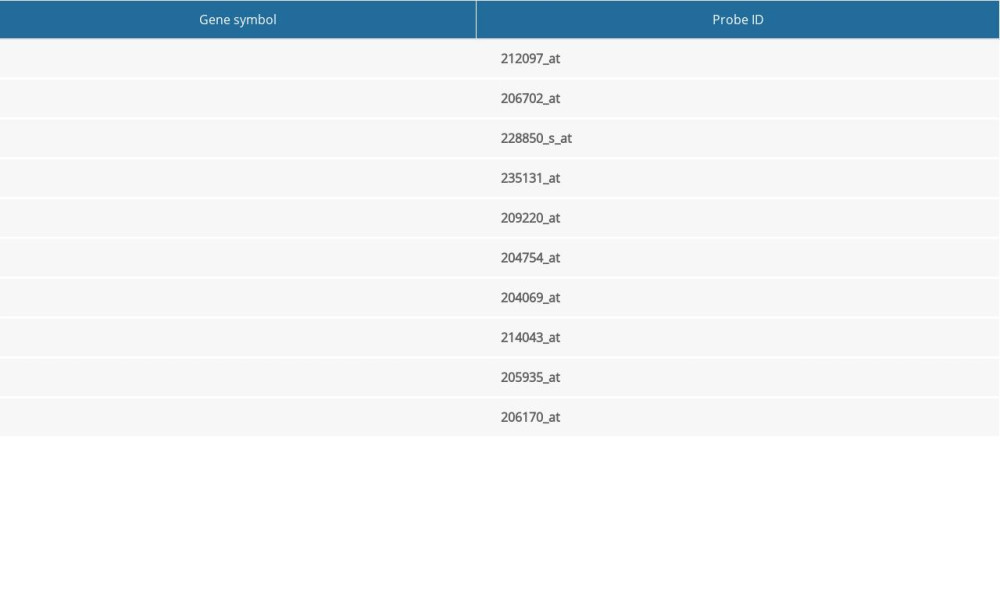 Table 4. Hub genes in the Kaplan-Meier plotter database and corresponding probes.
Table 4. Hub genes in the Kaplan-Meier plotter database and corresponding probes. Table 1. Information about the 3 datasets obtained from GEO.
Table 1. Information about the 3 datasets obtained from GEO. Table 2. Functional and pathway enrichment analysis of the DEGs.
Table 2. Functional and pathway enrichment analysis of the DEGs. Table 3. Information for the top 10 genes with high degrees of expression.
Table 3. Information for the top 10 genes with high degrees of expression. Table 4. Hub genes in the Kaplan-Meier plotter database and corresponding probes.
Table 4. Hub genes in the Kaplan-Meier plotter database and corresponding probes. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








