07 December 2021: Animal Study
Tissue-Protective Effect of Erdosteine on Multiple-Organ Injuries Induced by Fine Particulate Matter
Lei Cao1ACDE, Fen Ping2AG*, Fengrui Zhang2BCF, Haixiang Gao3CDF, Ping Li2BCF, Xiaohui Ning2BCF, Guohuan Cui2DF, Zheng Ma4BC, Xin Jiang5CD, Suyan Li6CD, Shuzhi Han2ACDGDOI: 10.12659/MSM.930909
Med Sci Monit 2021; 27:e930909
Abstract
BACKGROUND: Fine particulate matter (PM2.5) is the air pollutant that most threatens global public health. The purpose of this study was to observe the inflammatory and oxidative stress injury of multiple organs induced by PM2.5 in rats and to explore the tissue-protective effect of erdosteine.
MATERIAL AND METHODS: We randomly divided 40 male Wistar rats into a blank control group, a saline group, a PM2.5 exposure group, and an erdosteine intervention group. We assessed changes in organs tissue homogenate and biomarkers of inflammation and oxidative stress in serum and bronchoalveolar lavage fluid (BALF).
RESULTS: (1) The expressions of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in serum and BALF of the PM2.5 exposure group increased, but decreased after treatment with erdosteine, suggesting that erdosteine treatment attenuates inflammatory and oxidative stress injury. (2) The expression of γ-GCS in serum and lungs in the PM2.5 exposure group increased, but did not change significantly after treatment with erdosteine. This suggests that PM2.5 upregulates the level of γ-GCS, while erdosteine does not affect this protective response. (3) The expression of T-AOC in serum, lungs, spleens, and kidneys of the PM2.5 exposure group decreased, but increased after treatment with erdosteine. Our results suggest that PM2.5 can cause imbalance of oxidation/anti-oxidation in multiple organs, and erdosteine can alleviate this imbalance.
CONCLUSIONS: PM2.5 exposure can lead to inflammatory and oxidative stress damage in serum and organ tissues of rats. Erdosteine may be an effective anti-inflammatory and antioxidant that can reduce this injury.
Keywords: Inflammation, Particulate Matter, Protein Carbonylation, Animals, Bronchoalveolar Lavage Fluid, Disease Models, Animal, Expectorants, Kidney, Lung Injury, Male, Rats, Rats, Wistar, Spleen, Thioglycolates, Thiophenes
Background
On 26 May 2015, the World Health Organization for the first time clearly defined air pollution as the world’s largest single environmental health risk factor. Particulate matter (PM), especially fine particulate matter (PM2.5) in inhalable particles, has been recognized as the most harmful and representative air pollutant affecting human health among the recognized atmospheric pollutants [1]. Many studies have reported the correlation between long-term exposure to fine particulate matter and mortality. For example, an extended follow-up of the Harvard Six-Cities Study from 1974 to 2009 [2] and a study on 73 711 subjects living in California conducted by the American Cancer Society [3] showed that particulate matter was associated with all-cause mortality. In 2017, a 13-year follow-up study published in the USA, involving nearly 61 million people with health insurance (including those from small cities and rural areas), showed that long-term exposure to PM2.5 increased the risk of death, and when PM2.5 increased by 10 μg per cubic meter, the all-cause mortality increased by 7.3% [4].
The primary causes of PM2.5 pollution are energy sector coal combustion, motor vehicle exhaust emissions, large-scale infrastructure work, and residential burning. Particulate matters consist of harmful components such as polycyclic aromatic hydrocarbons (PAHs) and heavy metals, which cause damage to multiple organs of the body [5]. Some scholars showed that heavy metals, such as lead, manganese, aluminum, and copper, carried by PM2.5, can enter the circulation after respiratory exposure and selectively accumulate in the target organs, including blood, brain, liver, and lungs, with a significant dose-effect relationship [6]. Other scholars exposed mice to PM2.5 for 3 months and found that increased levels of DNA lesions were correlated with the occurrence of oxidative stress in the lungs, liver, and kidneys, in parallel with decreased global levels of 5-hmC (the currently best-characterized epigenetic marker) in lung and liver DNA [7]. In 2019, scholars studied the changes in multiple-organ DNA methylation (5-MC) caused by inhalation of PM2.5 in animal experiments. They demonstrated that overall hypomethylation of DNA was observed in the lungs and heart after acute PM2.5 exposure. After chronic PM2.5 exposure, global DNA methylation levels were decreased in most organs, including lungs, testis, thymus, spleen, epididymal fat, hippocampus, and blood. Decreased DNA methylation levels can activate the expression of some deleterious genes, such as oncogenes and cytokines, leading to multi-organ damage in the organism [8]. In 2018, Chinese scholars showed that PM2.5 exposure through long-term intratracheal instillation in Sprague-Dawley rats induced spleen damage. PM2.5 triggered oxidative stress and activated ERS (endoplasmic reticulum stress) and the autophagy signaling pathway, which facilitated the spleen injury process and toxic effects in a concentration-dependent manner [9]. Other studies have confirmed that sub-chronic exposure to PM2.5 can lead to histological alterations in renal tissue, such as fibrosis, mesangial expansion, and decreased glomerular and tubular lumen volumes, and found that this was associated with early-stage renal dysfunction [10]. Human experiments have come to the same conclusion. Epidemiological studies have documented that human exposure to PM2.5 is positively associated with cardiovascular disease morbidity and mortality (eg, myocardial infarction, heart failure, and rhythm disturbances), and can lead to a decline in renal function [11–14]. There are other studies on PM2.5 and the occurrence and development of lung diseases [15–20]. Based on the great threat to human health caused by PM2.5, in addition to preventive measures such as controlling the environment and reducing pollution, it is urgent to find safe and effective drugs that can reduce this damage.
Erdosteine was originally developed as a mucolytic for treatment of chronic bronchitis and chronic obstructive pulmonary disease. It thins the viscosity of mucus and dissolves mucus, promotes mucociliary transport, and reduces the adhesion between mucus and the airway wall. Erdosteine plays an antioxidant role by eliminating free radicals, and also has anti-inflammatory and antibacterial effects by inhibiting the release of inflammatory mediators and reducing bacterial adhesion [21,22]. Erdosteine shows antioxidant properties and anti-inflammatory effects in many diseases. Its broad-spectrum dose (LD50: 3500–5000 mg/kg) has protective effects on many diseases [23]. Mutneja et al showed that erdosteine attenuated myocardial necrosis induced by isoproterenol (ISO) by inhibiting the MAPK and Nrf-2/HO-1 pathways [24]. In another study, Ugurel et al reported that erdosteine can protect the ovaries from torsional/resetting damage by avoiding scavenging free radicals, releasing oxidative free radicals, and by blocking pro-inflammatory processes [25]. A previous study showed that erdosteine treatment significantly decreased serum liver function. The study also found that erdosteine can improve the antioxidant capacity and stress parameters of the liver [26]. It has been recently suggested that erdosteine treatment increases serum antioxidant enzymes, thereby reducing the level of oxidative stress after ischemia-reperfusion injury in the kidneys [27]. Erdosteine also has beneficial effects on ischemia-reperfusion injury in various organs, including the lungs, brain, kidneys, intestines, ovaries, and spinal cord [28–31]. Given that inflammation and oxidative stress damage are the most important damage mechanisms of PM2.5, we speculated that erdosteine, which has anti-inflammatory and antioxidant effects, can alleviate PM2.5-induced damage to the body.
The purpose of this study was to explore the multiple-organ inflammatory and oxidative stress injury caused by sub-acute exposure to PM2.5, and to assess the effects of erdosteine treatment on levels of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat serum and BALF, and to measure the changes in T-AOC and γ-GCS in serum and in lung, heart, liver, kidney, and spleen tissue homogenate in rats. We also explored whether there is an oxidation/anti-oxidation imbalance in rats who underwent PM2.5 tracheal instillation, and studies the protective effect of erdosteine on the rat organ injury induced by PM2.5.
Material and Methods
MAIN INSTRUMENTS AND REAGENTS:
Automatic microplate reader (Thermo Scientific Co., USA, Multiskan MK3), tissue homogenizer (IKN Co., Germany, CMD2000/4), desktop cryogenic high-speed centrifuge (Thermo Co., USA, Micro CL21R),optical microscope (Olympus Co., Japan, CX33), electric calorstat (Shanghai Senxin Experimental Instruments, Ltd, DGG-9070B), γ-GCS test kit (Nanjing Jiancheng Bioengineering Institute, A091), T-AOC test kit (Nanjing Jiancheng Bioengineering Institute, A015), rat IL-6 ELISA kit (Wuhan Liuhe Biotechnology, LH-E10103RA), rat IL-1β ELISA kit (Wuhan Liuhe Biotechnology, LH-E1099RA), rat TNF-α ELISA kit (Wuhan Liuhe Biotechnology, LH-E10183RA), rat 4-HNE ELISA kit (Shanghai Blue Gene Biotechnology, JL19014), rat 8-OHdG ELISA kit (Shanghai Blue Gene Biotechnology, XL02323) PCC test kit (Nanjing Jiancheng Bioengineering Institute, A087), and erdosteine capsules (Tantong, Xi’an Haixin Pharmaceutical).
PREPARATION OF PM2.5 SUSPENSION:
PM2.5 was provided by the Environmental Monitoring Center of Hebei Province. Before use, it was autoclaved, and prepared at 7.5 mg/ml concentration with sterilized water for injection, then mixed by ultrasound oscillation.
GROUPING OF EXPERIMENTAL ANIMALS AND METHODS OF ADMINISTRATION:
Forty male Wistar rats (provided by Experimental Animal Center of Hebei Medical University) were selected from March to September in 2017, weighing 190 to 220 g, and routinely raised to 250 g to start the experiment. They were divided into 4 groups, with 10 rats in each group, by random number table: the 2 control groups were the blank control group and the saline group, and the 2 experimental groups were the PM2.5 exposure group and the erdosteine intervention group. The blank control group received no intervention. The saline group was given tracheal instillation of normal saline (1 ml/kg) once a week for 4 weeks. Rats in the PM2.5 exposure group were given tracheal instillation of PM2.5 suspension 1 ml/kg (mixed as 7.5 mg/ml with the sterilized water for injection in advance) once a week for 4 weeks. The PM suspension was prepared immediately before instillation). The erdosteine intervention group was given intragastric administration of erdosteine (500 mg/kg/day, dissolved in water for injection ahead of time) for 30 days in total; tracheal instillation was started 7 days after the administration of erdosteine, and tracheal instillation of PM2.5 suspension was given once a week (7.5 mg/ml, dissolved into 1 ml/kg of water droplets) for 4 times. During the experiment, the general state of the rats was observed and the rats were killed 24 h after the last tracheal instillation. All animals were managed and treated in accordance with the laboratory animal guidelines of Hebei Medical University. All animal experiments complied with the guidelines of the Ethics Committee of Hebei General Hospital, China.
As the exposure levels of PM2.5 determine the toxicological effects, the dosages in the study were carefully selected. Several animal publications have adopted the method of tracheal PM2.5 drip, with some scholars applied 0.2–2.7 mg/kg for tracheal drip every 3 days for 2 months, with a maximum cumulative dose of 54 mg/kg. Some scholars applied 1.8–16.2 mg/kg concentration every 3 days for 1 month, and others applied 10–40 mg/kg once a week for up to 12 times, and all of them proved that the toxic effects of PM2.5 were concentration-dependent [32,33]. Based on daily respiratory volume of rats, which is about 0.288 m3 and the average concentration of PM2.5 in our city (as high as hundreds or even thousands ug/m3 in winter, the average is about 600 ug/m3), the total amount of PM2.5 inhaled by rats per week was calculated. When the body weight of an adult rat is considered as 250 g, the PM2.5 exposure concentration was approximately 5 mg/kg once every 7 days. Therefore, we used 3 doses of 5 mg/kg, 7.5 mg/kg, and 10 mg/kg for the pre-experiment, and found that an intermediate dose produced significant toxic effects; no significant behavioral abnormalities were found in the animals, no significant changes in body weight were observed, and no animal deaths occurred. Therefore, we selected an intermediate dose (7.5 mg/kg) for the experiment.
METHOD OF TRACHEAL INSTILLATION OF PM2.5:
After weighing, the Wistar rats were anesthetized with pentobarbital sodium (40 mg/kg) intraperitoneal injection, it was laid on their backs on a rat operating table with a 30°~40° slope, teeth and limbs were restrained, and a lamp vertically illuminated its neck. With the tongue sticking out of the mouth and pressing the tongue, we could clearly see the rat’s glottis with the aid a light. We inserted a no. 12 lavage needle into the trachea, gently dripped a certain volume of PM2.5 suspension until we could hear moist rales and see bubbles coming out of the windpipe, which proves the success of tracheal drip. The lavage needle was taken out. Rats were removed from the operating table after 10 min and were kept in supine position until waking.
SPECIMEN COLLECTION:
The rats were killed by abdominal aorta bleeding 24 h after the last tracheal instillation (the blank control group rats were killed 4 weeks after the start of the experiment) using intraperitoneal injection of pentobarbital sodium (50 mg/kg) to anesthetize them. Blood samples were collected from the abdominal aorta, centrifuged at 3000 r/min (1509.3×g) for 10 min, and collected the serum and stored it in a freezer at -80°C for later testing.
We separated the trachea and main bronchus, clamped the right main bronchus with a hemostatic forceps, cut a small oblique opening on the trachea, then inserted a No. 16 gavage feeding needle, and fixed it with a suture. We performed bronchoalveolar lavage with 5 ml pre-cool phosphate buffer (PBS) 3 times, then BALF was obtained, centrifuged at 1500r/min (377.3×g) for 10 min at 4°C, and the supernatant was collected and stored in a low-temperature freezer at −80°C before testing.
The lung tissue of the right lower lobe of the rats without lavage was fixed with formaldehyde solution, embedded in conventional paraffin, sectioned, and stained with hematoxylin-eosin (HE). Pathological changes were observed under a light microscope, and the rest of the right lung, heart, liver, and spleen tissues were filled with liquid nitrogen and transferred to a freezer at −80°C after being completely frozen for preparing tissue homogenate.
PREPARATION OF TISSUE HOMOGENATE:
The lung, heart, liver, kidney, and spleen tissues were accurately weighed, and the ratio of homogenization was selected as 10% (ie, homogenization was performed by adding 9-fold volume) of normal saline according to weight (g): volume (ml), and homogenized on an ice bath using a tissue homogenizer, centrifuged at 5000r/min (4193.3×g) for 15 min, and we collected the supernatant as the sample to be tested immediately.
INDEX TEST:
IL-6, IL-1β, TNF-α, 8-OHdG, and 4-HNE in serum and BALF of rats were tested by ELISA (enzyme-linked immunosorbent assays) using plate-based assays for identification and quantification a specific protein in complex biological samples. Briefly, the capture antibody was used to target specific protein and was coated on the microplate at 2–8°C overnight. The coated microplate was washed with blocking buffer for 1 h at room temperature. Then, the standards and biological samples were injected to designated wells to bind to the capture antibody with gentle shaking at room temperature for 1 h. After that, the excess substances were removed using PBS buffer solution to wash excess unbound compounds. The detection antibody was added to combine with the immobilized proteins, followed by washing with washing buffer. Finally, the HRP and TMB substrate solution was added to each well and the stop was injected. The obtained solution was measured, and the results were calculated using the curve-fit method. PCC was tested by using ultraviolet colorimetry and spectrophotometer according to the test kit instructions (Nanjing Institute of Bioengineering).
The contents of γ-GCS and T-AOC in serum and tissue homogenate of rats were measured, and γ-GCS and T-AOC were tested by using a chemical spectrophotometer according to the test kit instructions.
STATISTICAL ANALYSIS:
All data were in accordance with normal distribution (Shapiro-Wilk test of normality). The experiment results are presented as the mean±standard deviation (mean±SEM). All statistical analyses were conducted using SPSS 21.0 Statistics Software. Means among multiple groups were compared by one-way ANOVA followed by Student-Newman-Keuls (SNK) post hoc tests for different pairwise comparisons. A difference was considered statistically significant if
Results
HISTOPATHOLOGY OF LUNG IN RATS:
There were no obvious abnormalities in pathological sections of rat lung tissues in the blank control group and the saline group under light microscopy; there were injuries in the rat lung tissues of the PM2.5 exposure group and the erdosteine intervention group with varying degrees. Infiltration of bronchiole and peripheral inflammatory cells could be seen in the PM2.5 exposure group under light microscopy, the inflammatory cells in the lumen increased, the lumen became smaller and narrower, the alveolar septa thickened, the alveolar cavity shrank, inflammatory cells exuded, peripheral inflammatory cells infiltrated, interstitial became edematous, capillary congested, small vessel wall thickened, smooth muscle became hyperplastic, and the lumen became smaller. Pathological sections of rat lung tissues in the erdosteine intervention group showed the degree of destruction was reduced (Figure 1).
:
There were statistically significant differences in IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in serum among the blank control group, the saline group, the PM2.5 exposure group, and the erdosteine intervention group (
:
There was statistically significant difference in IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in BALF among the blank control group, the saline group, the PM2.5 exposure group, and the erdosteine intervention group (
:
There was a statistically significant difference in γ-GCS in serum and lung tissues among the blank control group, the saline group, the PM2.5 exposure group, and the erdosteine intervention group (
:
There was a statistically significant difference in T-AOC expression in serum and lung, spleen, kidney, and liver tissues among the blank control group, the saline group, the PM2.5 exposure group, and the erdosteine intervention group (
Discussion
Currently, environmental pollution is the leading cause of premature death and disability worldwide [34,35]. PM2.5 is the air pollutant that poses the greatest threat to global public health [36]. It is estimated that environmental exposure to PM2.5 results in 4.2 million deaths and 103.1 million days of labor loss according to GBD 2015, which accounts for 7.6% of global mortality and 4.2% of total labor losses [37]. The premature death due to air pollution is caused mainly by ischemic heart disease and chronic obstructive pulmonary disease (COPD) [38], which indicates that there is a close correlation between the fine particle matters and cardiovascular and respiratory diseases. The short-term exposure to fine particle matter weakens the beneficial cardiopulmonary effects of walking in patients with chronic obstructive pulmonary disease or ischemic heart disease [39]. Air pollution exposure is closely related to deterioration of respiratory function and symptoms in patients with COPD, and even low concentrations of PM2.5 can lead to decreased lung function and increase the risks of COPD [40]. The acute effects of air pollution are reflected in more respiratory symptoms, as well as increased cardiovascular events, hospitalization rates. and mortality. The long-term effects include lung growth retardation in children and adolescents, decreased lung function in adults, cardiovascular disease and lung cancer [41]. PM2.5 exposure is associated with liver diseases, stroke, diabetes, myocardial infarction, hypertension, heart failure, anxiety, lung cancer, and other system diseases [42–47].
Particulate matters consist of harmful components such as polycyclic aromatic hydrocarbons (PAHs) and heavy metals, which mainly come from industrial emissions and traffic exhaust. It has been reported that PAHs can produce reactive oxygen species (ROS) through the redox cycle, which leads to oxidative alteration of DNA and lipids in the body. Some heavy metals, such as arsenic, copper, cobalt, and chromium, can generate ROS through Fenton reaction. The generation of ROS can cause oxidative damage to biological macromolecules such as lipids, DNA, and proteins in the body [48–53]. Many studies have suggested that the pathological mechanism of the increase of mortality caused by PM2.5 is mainly reflected in the following aspects: (1) PM2.5 and oxidative stress; (2) PM2.5 and inflammatory injury; (3) PM2.5 and nucleic acid repair; and (4) PM2.5 and immune reaction [54]. Oxidative stress is a normal oxidation/anti-oxidation imbalance caused by oxidant excess and/or antioxidant reduction in the body, which is the common basis for multi-system and multi-organ oxidative injury, and the key to the occurrence, development, and deterioration of disease triggered by PM2.5. Recently, many studies have confirmed that the inflammation and oxidative stress injury caused by acute PM2.5 exposure can affect multiple organs throughout the body [55–57]. The antagonists against this mechanism may provide protection for the prevention and treatment of the health effects of PM2.5 air pollution.
TNF-α is a potent mediator of immune and inflammatory responses. IL-6 and IL-1β are acute cytokines that induce other cells to produce cytokines and produce-acute phase reactions. The activated macrophages produce pro-inflammatory mediators, such as IL-1β and TNF-α, which are involved in a variety of respiratory symptoms. Airway hyper-responsiveness and asthma are associated with the increase of IL-1β and TNF-α level [58–60]. PM2.5 enters the alveoli and is deposited on the alveolar surface and is phagocytosed by macrophages on the alveolar surface, which can lead to the expression and release of cytokines such as TNF-α and IL-6 in macrophages [61]. After PM2.5 treatment of human bronchial epithelial cells (BEAS-2B), with increasing exposure time and exposure dose, the genes expression of TNF-α, IL-1β, IL-6, IL-8, and other inflammatory factors was enhanced [62]. In 2015, animal experiments showed that PM2.5 caused lung congestion and inflammatory cells infiltration in rats, and increases inflammatory cell numbers in BALF. With the increase of PM2.5 concentration, the concentration of pro-inflammatory mediators, such as TNF-α, IL-6, IL-β, in lung tissue, increased too, and accompanied by changes in oxidative stress, which may have a critical role in the susceptibility to respiratory diseases caused by PM2.5 [63]. PCC is the most common and most commonly used biomarker for evaluating oxidative injury in protein. Human diseases associated with PCC include Alzheimer’s disease, chronic lung disease, chronic renal failure, diabetes, and sepsis [64–66]. 8-OHdG is a specific biomarker of DNA oxidative injury caused by a variety of endogenous and exogenous factors [67]. It is one of the main forms of oxidative damage induced by free radicals and has been widely used as a biomarker of oxidative stress and carcinogenesis [68]. Studies have shown that PM2.5 can increase the level of 8-OHdG in human urine [69]. 4-HNE is the product of oxidative injury and a marker of lipid peroxidation. PM2.5 exposure has been reported to increase 4-HNE levels in the blood of mice [70]. All of these are classical oxidative stress biomarkers. Because inflammatory and oxidative stress injury is an important mechanism of the respiratory damage caused by PM2.5, we examined the trends of the above classical inflammatory and oxidative stress markers in serum and BALF. We observed that HE staining of rat lung tissues after PM2.5 exposure showed significant inflammatory manifestations, the inflammatory factors such as IL-6, IL-1β, and TNF-α, and the above-mentioned classical oxidative stress markers were elevated compared to normal, again demonstrating the mechanism of lung injury caused by PM2.5.
γ-GCS is the rate-limiting enzyme in the synthesis of GSH; its activity directly affects GSH levels [71]; the expression of γ-GCS is increased during the oxidative stress process induced by cigarettes, and the expression levels of γ-GCS mRNA and its protein expression in bronchopulmonary tissues gradually increased with the prolongation of smoking. At present, there is no relevant research on whether PM2.5 affects the level of γ-GCS. T-AOC is the total antioxidant capacity, and its decrease means that the oxidative stress defense system of humans and animals will be compromised [73]. Therefore, we observed the changes of γ-GCS and T-AOC in serum and various organs after PM2.5 exposure, and found the γ-GCS level in serum and lung was increased, but other organs did not change much, suggesting that the lung is the primary organ in which γ-GCS is expressed. PM2.5 exposure upregulates GSH synthesis by upregulating γ-GCS level, which is a protective response against oxidative stress in the body. However, we also observed the decrease of T-AOC in serum and lung, liver, kidney, and spleen, which indicates that there is an oxidation/anti-oxidation imbalance in multiple organs, and the upregulated GSH did not affect the additional injuries of pro-inflammatory mediators and oxides, which ultimately leads to a decrease in total antioxidant capacity.
Chinese scholars have confirmed that a Chinese herbal decoction reduces oxidative stress injury in COPD rats by lowering the levels of 8-OHdG, 4-HNE, and γ-GCS [72]. Other studies on the toxicity of PM2.5 explored the effects after intervention. Pretreatment with selenium before tracheal instillation to PM2.5-exposed rats can decrease indicators (TNF-α, IL-1β, soluble intercellular adhesion molecule sICAM-1) of inflammatory response in rat BALF, increase the indicators of antioxidant capacity among related oxidative stress indicators, such as T-AOC, total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), decrease malondialdehyde (MDA), a lipid peroxidation product, and decrease the cell injury biomarkers lactate dehydrogenase (LDH), total protein (TP), and alkaline phosphatase (AKP). Selenium has a protective effect on the inflammatory oxidative stress injury in lung tissue caused by PM2.5 [73]. Other scholars have found that early administration of curcumin can reduce the expression of TNF-α and IL-6, increase T-AOC, and alleviate lung injury induced by PM2.5 in mice [74]. Therefore, we speculated that pretreatment with drugs with anti-inflammatory and antioxidant mechanisms can reduce the organ damage caused by PM2.5.
Erdosteine contains a closed sulfhydryl group in its molecular structure, which exerts mucolytic effects through hepatic biotransformation into active metabolites containing free sulfhydryl groups. Recent studies have shown that the metabolites of hepatic biotransformation also have free radical scavenging and anti-oxidative stress damage functions. Erdosteine prevents the accumulation of accumulated oxygen radicals and increases the antioxidant capacity of cells. The final result is a protective effect on tissues, reducing lipid peroxidation, neutrophil infiltration, and cellular noxious agent-mediated apoptosis [75]. In recent years, several studies on erdosteine supported the protective role of erdosteine in various tissue injuries mediated by oxidative stress products and inflammatory factors [76–79]. Currently, there are no international studies related to PM2.5 damage and treatment using erdosteine. We provided early treatment with erdosteine in animal experiments to observe whether it could reduce the damage caused by PM2.5 exposure, and we found that the IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC levels in the erdosteine intervention group were lower than those of the PM2.5 exposure group, and increased the total antioxidant capacity of serum, lung, spleen, and kidney. Erdosteine can alleviate the inflammatory and oxidative stress damage caused by PM2.5 exposure, and may be an effective anti-inflammatory and antioxidant to reduce the damage caused by PM2.5 exposure. Meanwhile, we observed that the γ-GCS level did not change much in serum and lung tissue after erdosteine intervention, which suggested that erdosteine does not affect this protective response. Of course, in our experiment, we also found that there was little change in the expression of T-AOC in liver tissue after the intervention of erdosteine, and there was no significant difference in cardiac T-AOC among the 4 groups. We suspect that this may be related to the different expression level of T-AOC in different organs and the different concentration of erdosteine in organs, and we do not rule out the loss of some effects in the process of tissue sampling and production. We hope that this can be further assessed in future experiments.
This study provides a theoretical basis for seeking the effective clinical therapeutic drugs against oxidative stress injury caused by PM2.5. However, in this experiment, we did not further explore the mechanism of the increase of γ-GCS caused by PM2.5 and whether the exposure time of PM2.5 will lead to dynamic change of γ-GCS. Further in-depth research on erdosteine is needed, the safety and related adverse effects at different doses have not been discussed, and there is a lack of relevant data from human experiments.
Conclusions
PM2.5 exposure can cause inflammatory and oxidative stress damage in serum and organ tissues of rats. Animal experiments have shown that early erdosteine intervention has a certain protective effect on this injury, which may be due to the downregulation of the levels of inflammatory and oxidative stress factors caused by PM2.5 exposure and the increase of T-AOC. Our results suggest that erdosteine may be an effective anti-inflammatory and antioxidant to reduce the damage caused by PM2.5.
Figures
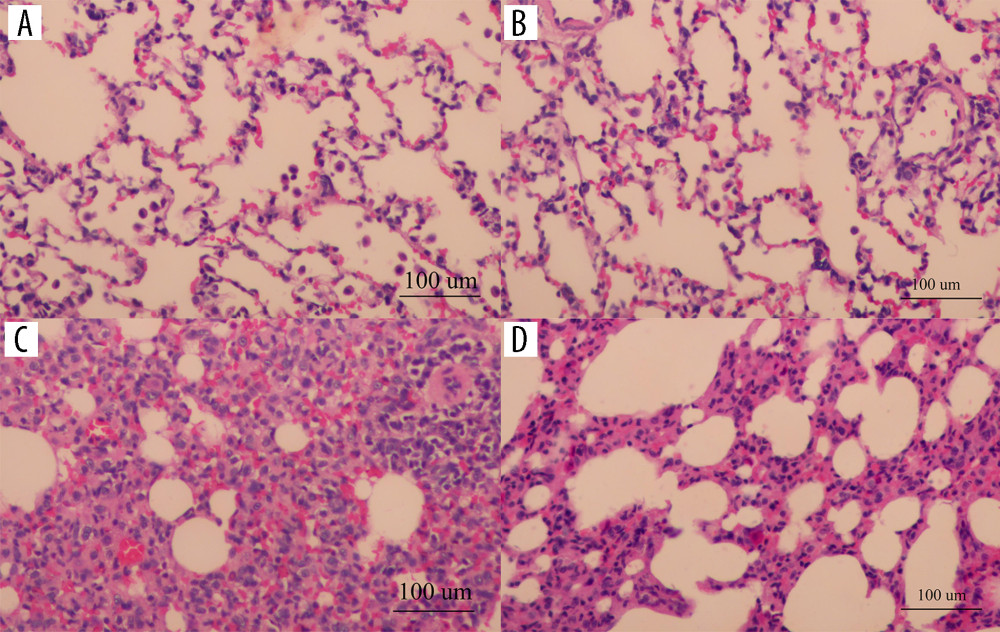 Figure 1. The pathological sections of rat lung tissues under light microscopic in each group (HE staining, 200×): the blank control group (A), the saline group (B), the PM2.5 exposure group (C), the erdosteine intervention group (D) (Adobe Illustrator CC 2019, Adobe Systems Incorporated).
Figure 1. The pathological sections of rat lung tissues under light microscopic in each group (HE staining, 200×): the blank control group (A), the saline group (B), the PM2.5 exposure group (C), the erdosteine intervention group (D) (Adobe Illustrator CC 2019, Adobe Systems Incorporated). 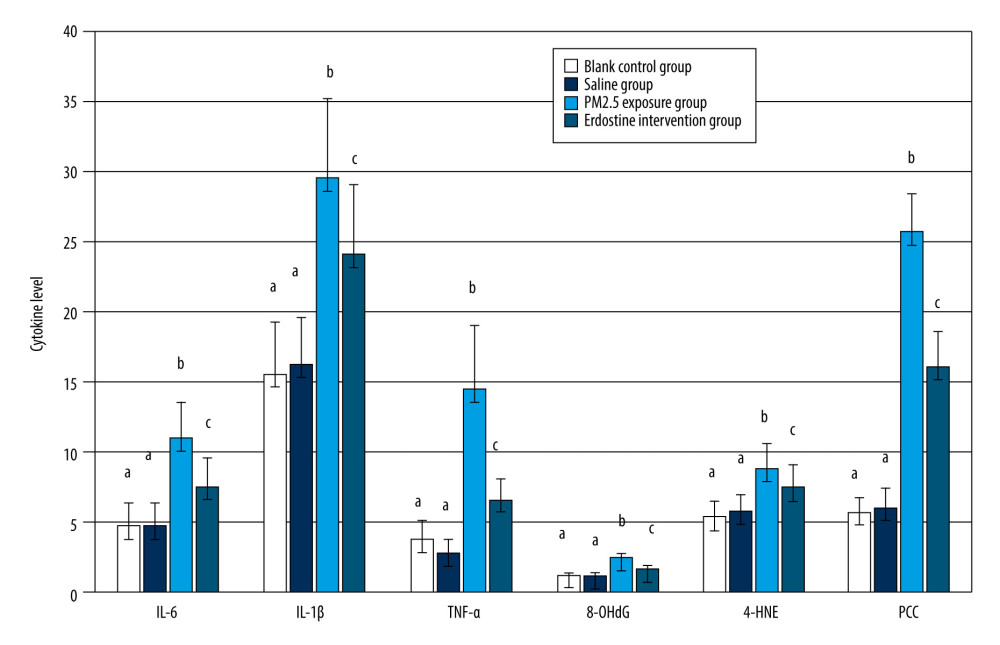 Figure 2. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat serum of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 2. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat serum of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). 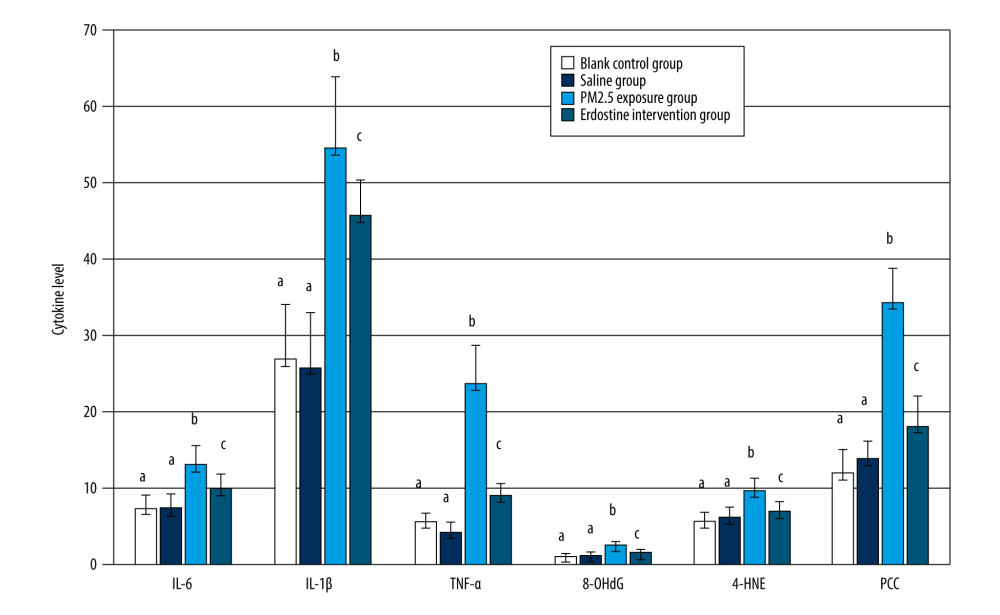 Figure 3. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat BALF of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 3. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat BALF of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). 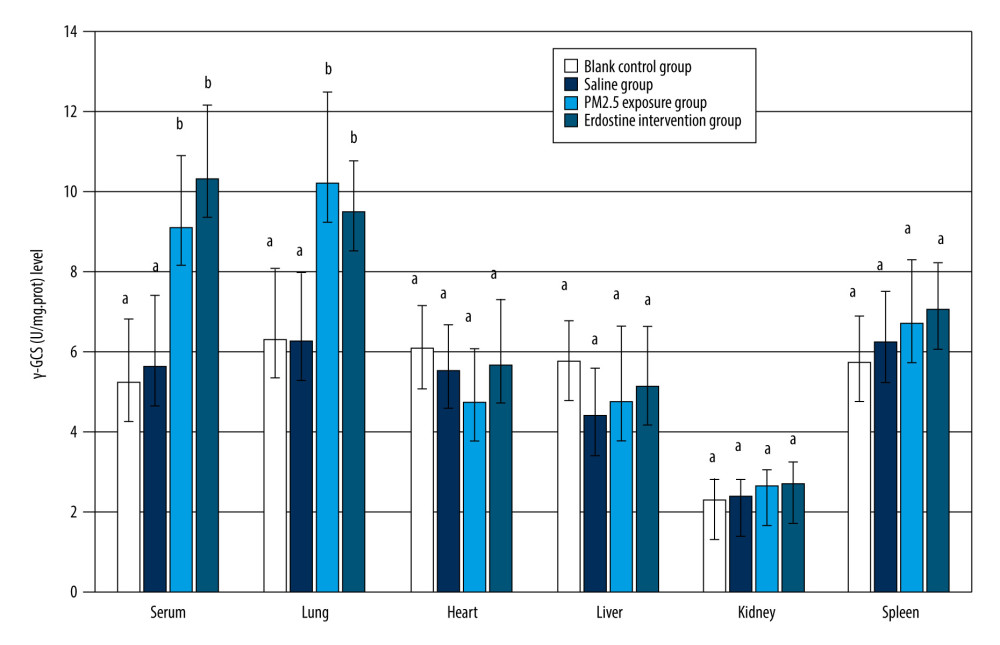 Figure 4. Comparisons of γ-GCS(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate non statistical differences, p>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 4. Comparisons of γ-GCS(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate non statistical differences, p>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). 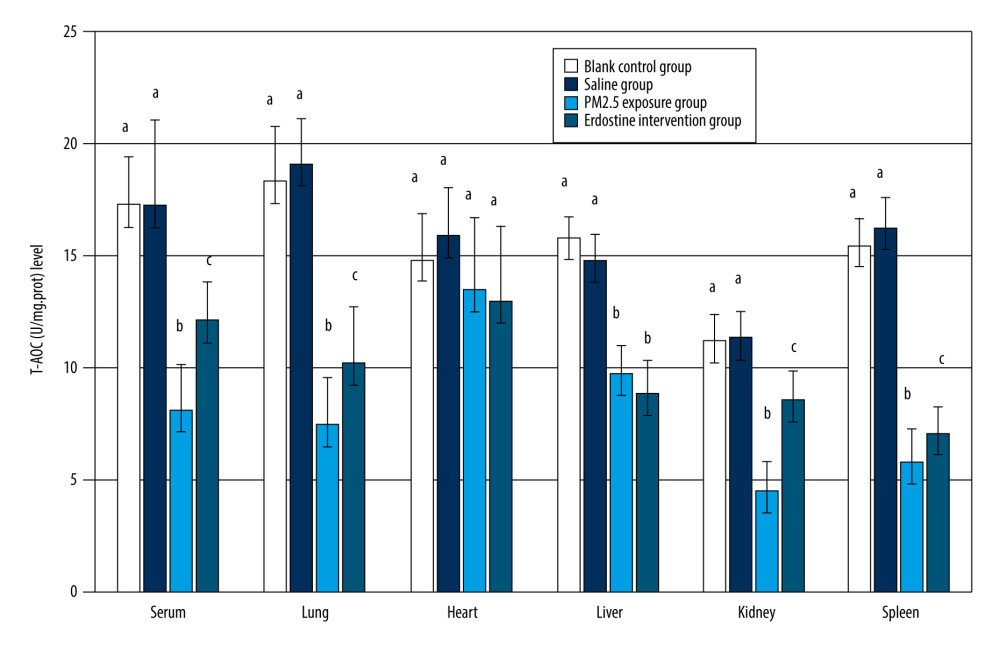 Figure 5. Comparisons of T-AOC(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 5. Comparisons of T-AOC(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). Tables
Supplementary Table 1. Comparisons of cytokine level in rat serum of each group (n=10).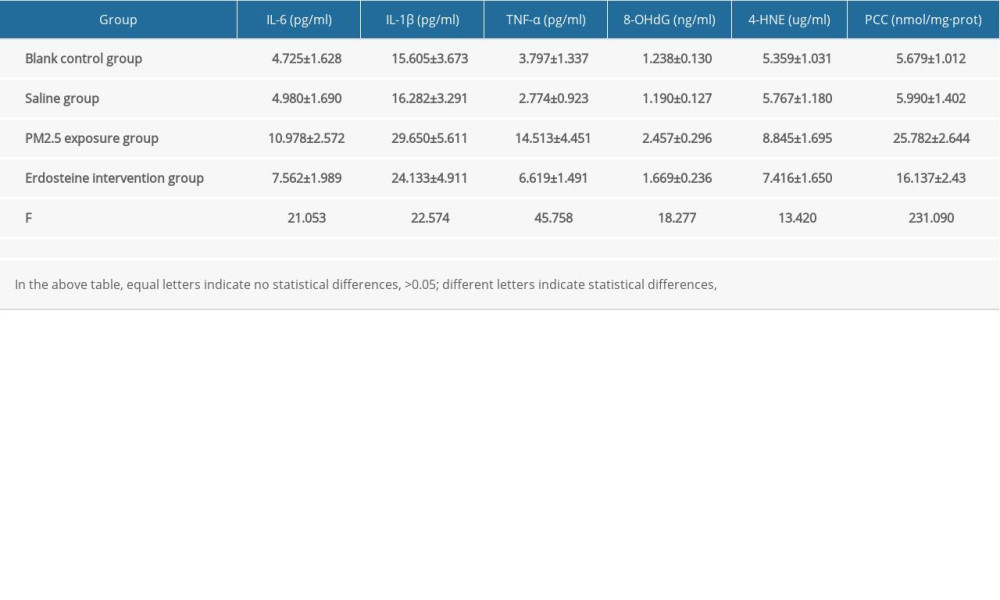 Supplementary Table 2. Comparisons of cytokine level in rat BALF of each group (n=10).
Supplementary Table 2. Comparisons of cytokine level in rat BALF of each group (n=10).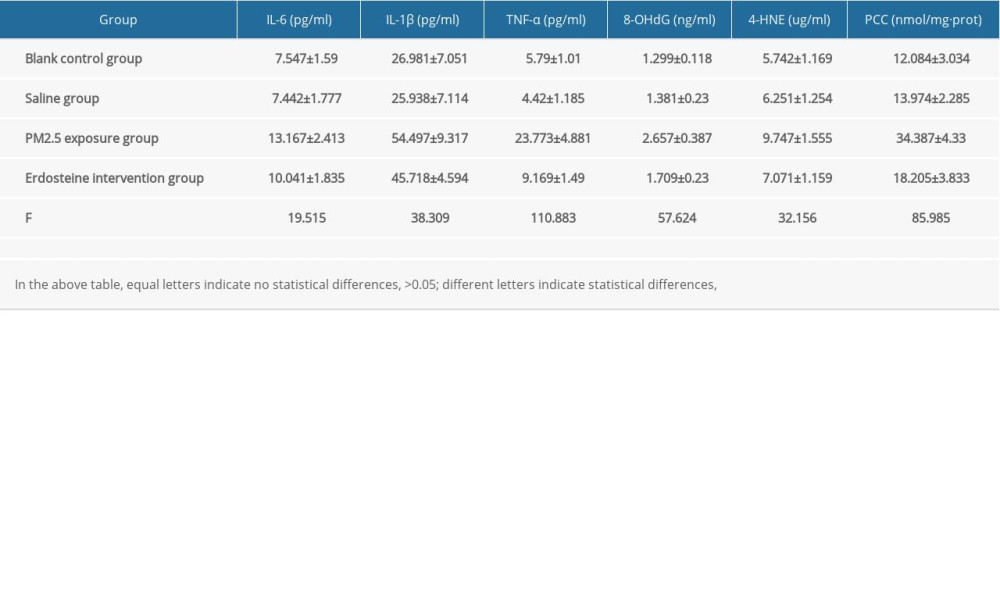 Supplementary Table 3. Comparisons of γ-GCS (U/mg·prot) level in rat organs of each group (n=10).
Supplementary Table 3. Comparisons of γ-GCS (U/mg·prot) level in rat organs of each group (n=10).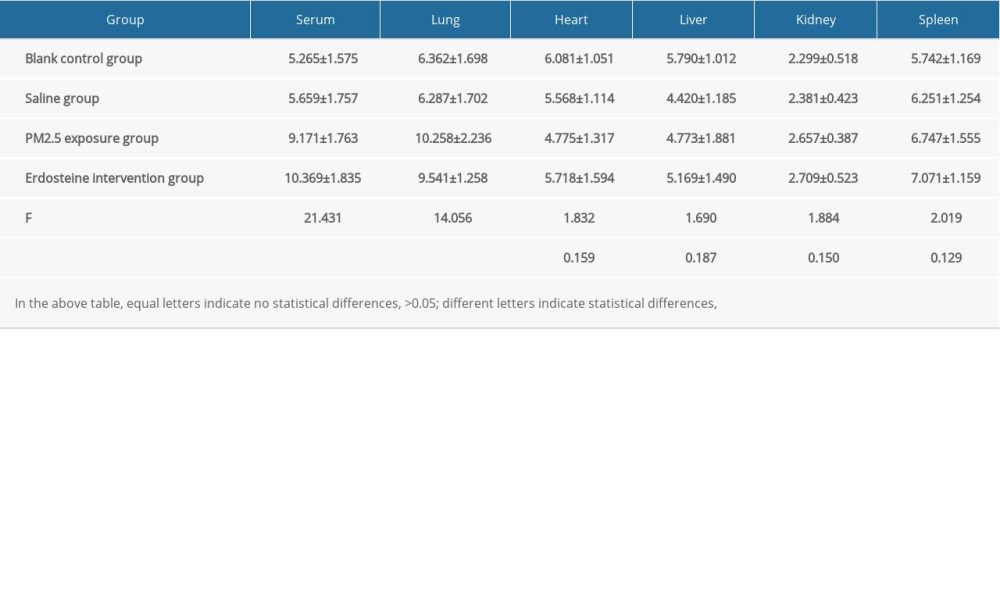 Supplementary Table 4. Comparisons of T-AOC (U/mg·prot) level in rat organs of each group (n=10).
Supplementary Table 4. Comparisons of T-AOC (U/mg·prot) level in rat organs of each group (n=10).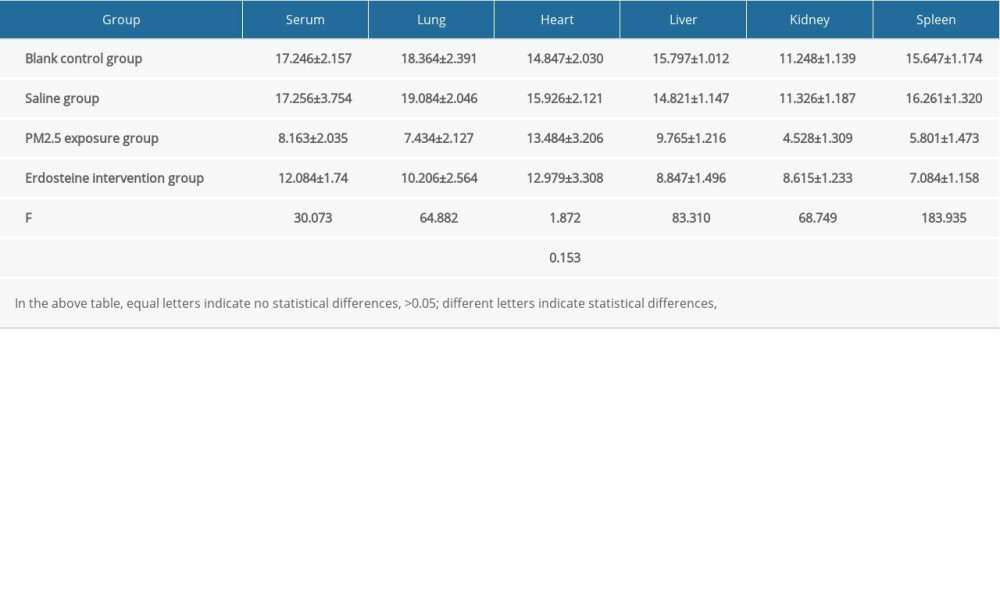
References
1. Iriti M, Piscitelli P, Missoni E, Miani A, Air pollution and health: The need for a medical reading of environmental monitoring data: Int J Environ Res Public Health, 2020; 17(7); 2174
2. Lepeule J, Laden F, Dockery D, Schwartz J, Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009: Environ Health Perspect, 2012; 120(7); 965-70
3. Jerrett M, Burnett RT, Beckerman BS, Spatial analysis of air pollution and mortality in California: Am J Respir Crit Care Med, 2013; 188(5); 593-99
4. Di Q, Wang Y, Zanobetti A, Air pollution and mortality in the medicare population: N Engl J Med, 2017; 376(26); 2513-22
5. Chung Y, Dominici F, Wang Y, Associations between long-term exposure to chemical constituents of fine particulate matter (PM2.5) and mortality in Medicare enrollees in the eastern United States: Environ Health Perspect, 2015; 123(5); 467-74
6. Li Q, Liu H, Alattar M, The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats: Sci Rep, 2015; 5; 16936
7. de Oliveira AAF, de Oliveira TF, Dias MF, Genotoxic and epigenotoxic effects in mice exposed to concentrated ambient fine particulate matter (PM2.5) from São Paulo city, Brazil: Part Fibre Toxicol, 2018; 15(1); 40
8. Li Z, Li N, Guo C, Genomic DNA methylation signatures in different tissues after ambient air particulate matter exposure: Ecotoxicol Environ Saf, 2019; 179; 175-81
9. Su R, Jin X, Lyu L, The potential immunotoxicity of fine particulate matter based on SD rat spleen: Environ Sci Pollut Res Int, 2019; 26(23); 23958-66
10. Tavera Busso I, Mateos AC, Juncos LI, Kidney damage induced by sub-chronic fine particulate matter exposure: Environ Int, 2018; 121(Pt 1); 635-42
11. Zhang LW, Chen X, Xue XD, Long-term exposure to high particulate matter pollution and cardiovascular mortality: A 12-year cohort study in four cities in northern China: Environ Int, 2014; 62; 41-47
12. Lee BJ, Kim B, Lee K, Air pollution exposure and cardiovascular disease: Toxicol Res, 2014; 30; 71-75
13. Li J, Liu F, Liang F, Long-term effects of high exposure to ambient fine particulate matter on coronary heart disease incidence: A population-based Chinese cohort study: Environ Sci Technol, 2020; 54(11); 6812-21
14. Mehta AJ, Zanobetti A, Bind MA, Long-term exposure to ambient fine particulate matter and renal function in older men: The veterans administration normative aging study: Environ Health Perspect, 2016; 124(9); 1353-60
15. Huang SK, Zhang Q, Qiu Z, Chung KF, Mechanistic impact of outdoor air pollution on asthma and allergic diseases: J Thorac Dis, 2015; 7(1); 23-33 [Erratum in: J Thorac Dis. 2015;7(10):E521]
16. Garcia E, Berhane KT, Islam T, Association of changes in air quality with incident asthma in children in California, 1993–2014: JAMA, 2019; 321(19); 1906-15
17. Hamra GB, Guha N, Cohen A, Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis: Environ Health Perspect, 2014; 122(9); 906-11 [Erratum in: Environ Health Perspect. 2014;122(9):A236]
18. Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH, Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults: Am J Epidemiol, 2017; 186(8); 961-69
19. Hertz-Picciotto I, Baker RJ, Yap PS, Early childhood lower respiratory illness and air pollution: Environ Health Perspect, 2007; 115(10); 1510-18
20. Sint T, Donohue JF, Ghio AJ, Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease: Inhal Toxicol, 2008; 20(1); 25-29
21. Park JS, Park MY, Cho YJ, Anti-inflammatory effect of erdosteine in lipopolysaccharide-stimulated RAW 264.7 cells: Inflammation, 2016; 39(4); 1573-81
22. Dal Negro RW, Visconti M, Erdosteine reduces the exercise-induced oxidative stress in patients with severe COPD: Results of a placebo-controlled trial: Pulm Pharmacol Ther, 2016; 41; 48-51
23. Dobrakowski M, Machoń-Grecka A, Nowak P, The influence of erdosteine administration on lead-induced oxidative stress in rat muscle: Drug Chem Toxicol, 2019 [Online ahead of print]
24. Mutneja E, Verma VK, Malik S, Erdosteine salvages cardiac necrosis: Novel effect through modulation of MAPK and Nrf-2/HO-1 pathway: J Biochem Mol Toxicol, 2020; 34(12); e22590
25. Ugurel V, Cicek AC, Cemek M, Antioxidant and antiapoptotic effects of erdosteine in a rat model of ovarian ischemia-reperfusion injury: Iran J Basic Med Sci, 2017; 20(1); 53-58
26. Barlas AM, Kismet K, Erel S, Erdosteine ameliorates the harmful effects of ischemia-reperfusion injury on the liver of rats: Acta Cir Bras, 2017; 32(10); 796-806
27. Sirmali R, Armağan A, Öktem F, Protective effects of erdosteine, vitamin E, and vitamin C on renal injury induced by the ischemia-reperfusion of the hind limbs in rats: Turk J Med Sci, 2015; 45(1); 33-37
28. Kurtoglu T, Sacar M, Inan BK, Erdosteine ameliorates lung injury induced by transient aortic occlusion in rats: Cardiovasc J Afr, 2007; 18(6); 367-70
29. Gurel A, Armutcu F, Cihan A, Erdosteine improves oxidative damage in a rat model of renal ischemia-reperfusion injury: Eur Surg Res, 2004; 36(4); 206-9
30. Ugurel V, Cicek AC, Cemek M, Antioxidant and antiapoptotic effects of erdosteine in a rat model of ovarian ischemia-reperfusion injury: Iran J Basic Med Sci, 2017; 20(1); 53-58
31. Ege E, Ilhan A, Gurel A, Erdosteine ameliorates neurological outcome and oxidative stress due to ischemia/reperfusion injury in rabbit spinal cord: Eur J Vasc Endovasc Surg, 2004; 28(4); 379-86
32. Zhang J, Zhou Q, Su R, Cardiac dysfunction and metabolic remodeling due to seasonally ambient fine particles exposure: Sci Total Environ, 2020; 721; 137792
33. Feng L, Wei J, Liang S, miR-205/IRAK2 signaling pathway is associated with urban airborne PM2.5-induced myocardial toxicity: Nanotoxicology, 2020; 14(9); 1198-212
34. Viegi G, Baldacci S, Maio S, Health effects of air pollution: A Southern European perspective: Chin Med J (Engl), 2020; 133(13); 1568-74
35. Beelen R, Raaschou-Nielsen O, Stafoggia M, Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project: Lancet, 2014; 383(9919); 785-95
36. Landrigan PJ, Fuller R, Hu H, Pollution and global health – an agenda for prevention: Environ Health Perspect, 2018; 126(8); 084501
37. Landrigan PJ, Fuller R, Acosta NJR, The Lancet Commission on pollution and health: Lancet, 2018; 391(10119); 462-512 [Erratum in: Lancet. 2018;391(10119):430]
38. Lelieveld J, Evans JS, Fnais M, The contribution of outdoor air pollution sources to premature mortality on a global scale: Nature, 2015; 525(7569); 367-71
39. Sinharay R, Gong J, Barratt B, Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study: Lancet, 2018; 391(10118); 339-49 [Erratum in: Lancet. 2018;391(10118):308]
40. Kariisa M, Foraker R, Pennell M, Short- and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects: Arch Environ Occup Health, 2015; 70(1); 56-62
41. Berend N, Contribution of air pollution to COPD and small airway dysfunction: Respirology, 2016; 21(2); 237-44
42. Shah AS, Lee KK, McAllister DA, Short term exposure to air pollution and stroke: Systematic review and meta-analysis: BMJ, 2015; 350; h1295 [Erratum in: BMJ. 2016;354:i4851]
43. Eze IC, Hemkens LG, Bucher HC, Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis: Environ Health Perspect, 2015; 123(5); 381-89
44. Héritier H, Vienneau D, Foraster M, A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: A nationwide cohort study in Switzerland: Eur Heart J, 2019; 40(7); 598-603
45. Fuks KB, Weinmayr G, Basagaña X, Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE): Eur Heart J, 2017; 38(13); 983-90
46. Power MC, Kioumourtzoglou MA, Hart JE, The relation between past exposure to fine particulate air pollution and prevalent anxiety: Observational cohort study: BMJ, 2015; 350; h1111
47. Hamra GB, Guha N, Cohen A, Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis: Environ Health Perspect, 2014; 122(9); 906-11 [Erratum in: Environ Health Perspect. 2014;122(9):A236]
48. Hu W, Wang Y, Wang T, Ambient particulate matter compositions and increased oxidative stress: Exposure-response analysis among high-level exposed population: Environ Int, 2021; 147; 106341
49. Contini D, Cesari D, Conte M, Donateo A, Application of PMF and CMB receptor models for the evaluation of the contribution of a large coal-fired power plant to PM10 concentrations: Sci Total Environ, 2016; 560–561; 131-40
50. Tan C, Lu S, Wang Y, Long-term exposure to high air pollution induces cumulative DNA damages in traffic policemen: Sci Total Environ, 2017; 593–594; 330-36
51. Zhang X, Staimer N, Gillen DL, Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort: Environ Res, 2016; 150; 306-19
52. Xuan Y, Gào X, Holleczek B, Prediction of myocardial infarction, stroke and cardiovascular mortality with urinary biomarkers of oxidative stress: Results from a large cohort study: Int J Cardiol, 2018; 273; 223-29
53. Zhang Y, Chu M, Zhang J, Urine metabolites associated with cardiovascular effects from exposure of size-fractioned particulate matter in a subway environment: A randomized crossover study: Environ Int, 2019; 130; 104920
54. Yue W, Tong L, Liu X, Short term Pm2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy: Redox Biol, 2019; 22; 101161
55. Bekki K, Ito T, Yoshida Y, PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways: Environ Toxicol Pharmacol, 2016; 45; 362-69
56. Li B, Guo L, Ku T, PM2.5 exposure stimulates COX-2-mediated excitatory synaptic transmission via ROS-NF-κB pathway: Chemosphere, 2018; 190; 124-34
57. Li X, Zheng M, Pu J, Identification of abnormally expressed lncRNAs induced by PM2.5 in human bronchial epithelial cells: Biosci Rep, 2018; 38(5); BSR20171577
58. Berry MA, Hargadon B, Shelley M, Evidence of a role of tumor necrosis factor alpha in refractory asthma: N Engl J Med, 2006; 354(7); 697-708
59. Pauwels NS, Bracke KR, Dupont LL, Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD: Eur Respir J, 2011; 38(5); 1019-28
60. Huang K, Li W, Chen Y, Zhu J, Effect of PM2.5 on invasion and proliferation of HeLa cells and the expression of inflammatory cytokines IL-1 and IL-6: Oncol Lett, 2018; 16(6); 7068-73
61. Mitkus RJ, Powell JL, Zeisler R, Squibb KS, Comparative physicochemical and biological characterization of NIST Interim Reference Material PM2.5 and SRM 1648 in human A549 and mouse RAW264.7 cells: Toxicol In Vitro, 2013; 27(8); 2289-98
62. Dieme D, Cabral-Ndior M, Garçon G, Relationship between physicochemical characterization and toxicity of fine particulate matter (PM2.5) collected in Dakar city (Senegal): Environ Res, 2012; 113; 1-13
63. Li R, Kou X, Xie L, Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats: Environ Sci Pollut Res Int, 2015; 22(24); 20167-76
64. Piao MJ, Ahn MJ, Kang KA, Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis: Arch Toxicol, 2018; 92(6); 2077-91
65. Al Housseiny H, Singh M, Emile S, Identification of toxicity parameters associated with combustion produced soot surface chemistry and particle structure by in vitro assays: Biomedicines, 2020; 8(9); 345
66. Piao MJ, Kang KA, Zhen AX, Particulate Matter 2.5 mediates cutaneous cellular injury by inducing mitochondria-associated endoplasmic reticulum stress: Protective effects of ginsenoside Rb1: Antioxidants (Basel), 2019; 8(9); 383
67. Ndong Ba A, Cazier F, Verdin A, Physico-chemical characterization and in vitro inflammatory and oxidative potency of atmospheric particles collected in Dakar city’s (Senegal): Environ Pollut, 2019; 245; 568-81
68. Liu Y, Li X, Zhang B, CYP1A1 methylation mediates the effect of smoking and occupational polycyclic aromatic hydrocarbons co-exposure on oxidative DNA damage among Chinese coke-oven workers: Environ Health, 2019; 18(1); 69
69. Mu G, Zhou M, Wang B, Personal PM2.5 exposure and lung function: Potential mediating role of systematic inflammation and oxidative damage in urban adults from the general population: Sci Total Environ, 2021; 755(Pt 1); 142522
70. Wang N, Ma Y, Liu ZL, Hydroxytyrosol prevents PM2.5-induced adiposity and insulin resistance by restraining oxidative stress related NF-κB pathway and modulation of gut microbiota in a murine model: Free Radic Biol Med, 2019; 141; 393-407
71. Han ZH, Jiang Y, Duan YY, Oxidative stress in a rat model of cotton smoke inhalation-induced pulmonary injury: Afr J Tradit Complement Altern Med, 2016; 13(5); 132-38
72. Li C, Shi Q, Yan Y, Kong Y, Recuperating lung decoction attenuates the oxidative stress state of chronic obstructive pulmonary disease by inhibiting the MAPK/AP-1 signal pathway and regulating γ-GCS: Evid Based Complement Alternat Med, 2017; 2017; 9264914
73. Liu J, Yang Y, Zeng X, Investigation of selenium pretreatment in the attenuation of lung injury in rats induced by fine particulate matters: Environ Sci Pollut Res Int, 2017; 24(4); 4008-17
74. Huang K, Shi C, Min J, Study on the mechanism of curcumin regulating lung injury induced by outdoor fine Particulate Matter (PM2.5): Mediators Inflamm, 2019; 2019; 8613523
75. Moretti M, Erdosteine: its relevance in COPD treatment: Expert Opin Drug Metab Toxicol, 2009; 5(3); 333-43
76. Kim SJ, Park C, Lee JN, Erdosteine protects HEI-OC1 auditory cells from cisplatin toxicity through suppression of inflammatory cytokines and induction of Nrf2 target proteins: Toxicol Appl Pharmacol, 2015; 288(2); 192-202
77. Erdem A, Gedikli E, Yersal N, Protective role of erdosteine pretreatment on oleic acid-induced acute lung injury: J Surg Res, 2017; 213; 234-42
78. Waissbluth S, Garnier D, Akinpelu OV, The impact of erdosteine on cisplatin-induced ototoxicity: A proteomics approach: Eur Arch Otorhinolaryngol, 2017; 274(3); 1365-74
79. Dobrakowski M, Machoń-Grecka A, Nowak P, The influence of erdosteine administration on lead-induced oxidative stress in rat muscle: Drug Chem Toxicol, 2019 [Online ahead of print]
Figures
 Figure 1. The pathological sections of rat lung tissues under light microscopic in each group (HE staining, 200×): the blank control group (A), the saline group (B), the PM2.5 exposure group (C), the erdosteine intervention group (D) (Adobe Illustrator CC 2019, Adobe Systems Incorporated).
Figure 1. The pathological sections of rat lung tissues under light microscopic in each group (HE staining, 200×): the blank control group (A), the saline group (B), the PM2.5 exposure group (C), the erdosteine intervention group (D) (Adobe Illustrator CC 2019, Adobe Systems Incorporated). Figure 2. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat serum of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 2. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat serum of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). Figure 3. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat BALF of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 3. Comparisons of IL-6, IL-1β, TNF-α, 8-OHdG, 4-HNE, and PCC in rat BALF of each group, as well as cytokine levels, were described by mean±standard deviation (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). Figure 4. Comparisons of γ-GCS(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate non statistical differences, p>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 4. Comparisons of γ-GCS(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate non statistical differences, p>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). Figure 5. Comparisons of T-AOC(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation).
Figure 5. Comparisons of T-AOC(U/mg.prot) level in rat organs of each group (n=10). Equal letters indicate no statistically significant differences, P>0.05; different letters indicate statistical differences, P<0.05 (Microsoft Office 2010, Microsoft Corporation). Tables
 Supplementary Table 1. Comparisons of cytokine level in rat serum of each group (n=10).
Supplementary Table 1. Comparisons of cytokine level in rat serum of each group (n=10). Supplementary Table 2. Comparisons of cytokine level in rat BALF of each group (n=10).
Supplementary Table 2. Comparisons of cytokine level in rat BALF of each group (n=10). Supplementary Table 3. Comparisons of γ-GCS (U/mg·prot) level in rat organs of each group (n=10).
Supplementary Table 3. Comparisons of γ-GCS (U/mg·prot) level in rat organs of each group (n=10). Supplementary Table 4. Comparisons of T-AOC (U/mg·prot) level in rat organs of each group (n=10).
Supplementary Table 4. Comparisons of T-AOC (U/mg·prot) level in rat organs of each group (n=10). In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








