13 August 2021: Clinical Research
Percutaneous Argon-Helium Cryoablation for Small Hepatocellular Carcinoma Located Adjacent to a Major Organ or Viscus: A Retrospective Study of 92 Patients at a Single Center
Wei Zhang1BCE, Xudong Gao1DF, Jie Sun1EF, Jiamin Cheng1B, Yanli Hu2BE, Zheng Dong1B, Huifang Kong1BF, Huixin Zhang1BF, Chunping Wang1BG, Yongping Yang1AG*DOI: 10.12659/MSM.931473
Med Sci Monit 2021; 27:e931473
Abstract
BACKGROUND: Cryoablation of hepatocellular carcinoma (HCC) close to major organs or viscus is challenging because it can cause complications. This retrospective study aimed to investigate the safety and efficacy of percutaneous argon-helium cryoablation of small HCC located adjacent to major organs or viscus.
MATERIAL AND METHODS: Ninety-two patients who underwent percutaneous argon-helium cryoablation between February 2012 and December 2018 at the Fifth Medical Center of the Chinese People’s Liberation Army General Hospital were included. Treatment efficacy was evaluated by magnetic resonance imaging or triphasic computed tomography scan within 1 week after each cryoablation procedure. Local tumor progression, distant recurrence, and overall survival were analyzed using the Kaplan-Meier method and log-rank test.
RESULTS: A total of 92 patients with small HCC located adjacent to major organs or viscus who underwent cryoablation were retrospectively reviewed. The number of patients with tumors adjacent to the gallbladder, portal or hepatic vein, diaphragm, stomach, heart, and intestine was 22, 1, 39, 6, 8, and 16, respectively. Cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. Cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively. Major complications were observed in 5 (5.4%) patients. Minor complications were observed in 85 (92.4%) patients.
CONCLUSIONS: This experience from a single center showed that percutaneous argon-helium cryoablation was safe and effective in the management of small HCC that is located adjacent to major organs or viscus.
Keywords: Carcinoma, Hepatocellular, Cryosurgery, Tomography Scanners, X-Ray Computed, Argon, Helium, Liver, Liver Neoplasms, Magnetic Resonance Imaging, Organs at Risk, Postoperative Complications
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies and causes approximately 1.25 million deaths each year [1,2]. With the development of diagnostic imaging technology and the wide application of screening methods in high-risk groups, the early diagnosis rate of small HCC is increasing yearly [3]. For many years, surgical treatment (liver resection and transplantation) for small HCC has been used as a first-line therapeutic option; however, its development is still limited by donor availability and is accompanied by many complications [4,5].
Recently, with the development of percutaneous local ablative therapy, percutaneous argon-helium cryoablation has been regarded as a safe and effective method for the treatment of very early or early-stage HCC [1,4,6,7]. Compared with open surgery, percutaneous cryoablation has the advantages of safety, cost-effectiveness, minimal invasiveness, and faster postoperative recovery [8]. However, complete cryoablation is still a challenge when the tumor is located in certain locations such as the gallbladder, portal or hepatic vein, diaphragm, and heart [9,10]. Previous studies have reported catastrophic consequences of radiofrequency ablation of HCC at these locations [11–13]. In the report by Moumouh et al [13], hemorrhagic cardiac tamponade occurred during surgery on HCC located in the second segment of the liver, near the diaphragm.
Cryoablation of HCC close to major organs or viscus is challenging because it can cause complications. At present, few studies have evaluated the safety and efficacy of cryoablation for small HCC that is located adjacent to major organ or viscus. Therefore, this retrospective study aimed to investigate the safety and efficacy of percutaneous argon-helium cryoablation of small HCC located adjacent to major organ or viscus.
Material and Methods
PATIENTS:
This retrospective study was approved by the Ethics Committee of the Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, Beijing, China. Informed consent was waived. Ninety-two patients underwent cryoablation for the treatment of small HCC between February 2012 and December 2018 at the Fifth Medical Center of the Chinese People’s Liberation Army General Hospital. The diagnostic criteria for HCC were based on current clinical guidelines [14–17]. Patients were enrolled in this retrospective study based on the following inclusion criteria: (1) presence of a single nodule ≤5 cm or 3 or fewer nodules with ≤3 cm [18]; (2) absence of vascular invasion or distant metastasis; (3) Child-Pugh class A or B; (4) the HCC was located in a high-risk location; and (5) the patient had not received any previous surgical treatment. The exclusion criteria were as follows: (1) recurrent HCC; (2) serious comorbidities such as cardiopulmonary and renal insufficiency; (3) severe coagulation disturbance (prothrombin time >25 s, prothrombin activity <40%, and platelet count <50×109/L); and (4) severe infection. All cases of HCC were discussed in a multidisciplinary meeting consisting of surgeons, medical oncologists, and radiologists. Treatment with cryoablation or radiofrequency ablation/microwave coagulation therapy or resection or liver transplant was determined using the following criteria: (1) cryoablation for tumors in close vicinity to major hepatic veins, hepatic hilum, secondary branches of the portal pedicles, or other organs; (2) microwave coagulation therapy for tumors of ≤1 cm; and (3) patient preference. All patients gave their written consent after being fully informed of each treatment procedure.
DEFINITION OF HCC LOCATED ADJACENT TO MAJOR ORGANS OR VISCUS:
Cryoablation of HCC located adjacent to major organs or viscus is challenging because it can cause some complications. The HCC located adjacent to major organs or viscus was defined as the tumor in close vicinity to organs, such as the stomach, gallbladder, portal or hepatic vein, heart, intestine, and diaphragm, with a maximum distance of 1.0 cm [9,19]. The distance from the edge of the tumor to neighboring organs was independently analyzed by 2 radiologists on sagittal and coronal reconstruction computed tomography (CT) images or magnetic resonance imaging (MRI) of axial and coronal/sagittal scans before the procedure.
PERCUTANEOUS ARGON-HELIUM CRYOABLATION:
Cryoablation was performed with an argon-helium-based EndoCare system (EndoCare, Irvine, CA, USA) as previously described [4,20]. Cryoprobes with different diameters (2 mm or 3 mm) were applied. The frozen area covered the whole tumor and at least 5 mm to 10 mm of paraneoplastic liver tissue. Following the administration of local anesthesia, the cryoprobes were inserted percutaneously into the tumor site under CT guidance. The cryoprobe advanced until it reached the distal edge of the target lesion. The double freeze-thaw cycle included freezing for 20 min, thawing for 10 min, and further freezing for 15 min. After removing the probes, all tracts were packed with Surgicel (Johnson & Johnson, Arlington, TX, USA) through the sheath introducer to control bleeding, and the sheath introducer was removed.
FOLLOW-UP PROTOCOL AFTER TREATMENT:
Treatment efficacy was assessed by MRI or triphasic CT scan within 1 week after cryoablation. Serum α-fetoprotein (AFP) was also evaluated 2 weeks after cryoablation and was rechecked every month thereafter. All patients were followed up every 3 months in the first year after cryoablation and twice a year thereafter to observe tumor recurrence. The follow-up visits included routine physical examination, laboratory tests (such as total bilirubin, serum albumin, prothrombin time, and tumor marker levels), and contrast-enhanced imaging including CT or MRI.
CLINICAL OUTCOME ASSESSMENT:
The treatment was considered a technical success when the cryoablation area was shown to completely cover the tumor on the CT that was performed immediately after the procedure, and when the procedure was without acute complications. Complete ablation was defined as a lack of enhancement within the cryoablation area on the initial 1-month follow-up CT scan or MRI [21]. Local recurrence was defined as tumor recurrence within or at the periphery of the area that was previously considered to be completely ablated. Distant intrahepatic recurrence was defined as a new lesion that appeared at least 1 cm from an area previously considered to be completely ablated. Extrahepatic recurrence referred to the recurrence of any tumor outside the liver. Patients who developed local recurrence with or without distant intrahepatic recurrence or extrahepatic recurrence were considered to have local tumor progression, while patients with distant intrahepatic recurrence or extrahepatic recurrence or both were considered to have distant recurrence. Overall survival was calculated as the time from the first cryoablation treatment to death or the final follow-up [4].
STATISTICAL ANALYSIS:
SPSS version 21.0 software (SPSS, Chicago, IL) was used to perform statistical analyses. The quantitative variables are expressed as the mean and standard deviation, while the qualitative variables are expressed as the frequency or percentage. The rates of local tumor progression, distant recurrence, and overall survival were calculated using the Kaplan-Meier method. A 2-sided
Results
BASELINE CHARACTERISTICS:
A total of 92 consecutive patients with small HCC located adjacent to major organs or viscus who underwent cryoablation were retrospectively reviewed. Among the 92 patients, 90 patients had a single tumor and 2 patients had 2 tumors. The numbers of patients with Child-Pugh grades of A and B were 83 and 9, respectively. The range of tumor size was 2.1±0.6 cm in diameter (from 0.6 to 4.0 cm). The number of patients with tumors adjacent to the gallbladder, portal or hepatic vein, diaphragm, stomach, heart, and intestine was 22, 1, 39, 6, 8, and 16, respectively (Figures 1, 2). Depending on the size and location of the tumors, 1 to 2 cryoprobes were used (Table 1).
TECHNICAL EFFECTIVENESS AND CLINICAL OUTCOMES:
The technical success rate and the complete ablation rate were 100%. The median follow-up time was 20 months (range, 5–63 months). During the follow-up period, local tumor progression and distant recurrence were found in 4 (4.3%) and 13 (14.1%) patients, respectively (Table 2). Among them, 8 patients underwent cryoablation again, 2 patients were treated with transarterial chemoembolization, 3 patients underwent cryoablation in combination with ethanol injection, and the remaining patients were not treated. The median duration from cryoablation to the first recurrence was 11.5±6.2 months. The cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. The cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively (Figure 3).
COMPLICATIONS:
Cryoablation of HCC located adjacent to major organs or viscus is challenging because it can cause some complications. In this study, no treatment-related deaths occurred. Major complications were observed in 5 (5.4%) patients, including spontaneous bacterial peritonitis in 2 patients (2.2%) and pleural effusion in 3 patients (3.1%) (Table 2). The patients with spontaneous bacterial peritonitis were treated with antibiotic therapy and recovered within 1 week. The cases of pleural effusion were usually self-limited to within 2 weeks. Minor complications were observed in 85 (92.4%) patients, including 15 patients (16.3%) with postoperative surgical-related pain, 19 patients (20.7%) with postoperative fever, 77 patients (83.7%) with transient elevation of aminotransferase, and 2 patients (2.2%) with bleeding at the probe insertion point. Consistent with previous studies [20], postoperative surgical-related pain, fever, and transient elevation of aminotransferase were the most common minor complications of cryoablation, and these complications in patients were usually self-limited, with patients recovering within 2 weeks without treatment.
Discussion
This study aimed to evaluate the experience from our center of treating patients with small HCC located adjacent to major organs or viscus. Our results showed that percutaneous argon-helium cryoablation is feasible for the treatment of small HCC located adjacent to major organs or viscus, with acceptable clinical outcomes and rare postoperative complications. For small HCC, percutaneous argon-helium cryoablation should be considered as one of the standard local ablation modalities in these patients.
With the significant development of cryotherapy technology, minimally invasive percutaneous cryoablation is considered as a promising option for the treatment of early-stage HCC. According to guidelines of the American Association for the Study of Liver Diseases and European Association for the Study of the Liver, local ablation is the best option for patients with early-stage HCC who are not suitable for liver resection or transplantation [6,22]. Compared with other local ablation techniques, including microwave ablation and radiofrequency ablation, cryoablation has a more obvious ablative periphery and larger ablative area and causes less pain for the patient [23]. Also, the safety and efficacy of cryoablation have been confirmed by previous studies. In a large cohort of cirrhosis-based HCC, Rong et al found that the overall survival rates at 1, 3, 5, and 10 years were 85.7%, 44.6%, 25.7%, and 9.2%, respectively. The rates of local recurrence at 1, 3, 5, and 10 years were 14.8%, 23.5%, 32.7%, and 39.3%, respectively [4]. In a multicenter randomized controlled study comparing percutaneous cryoablation and radiofrequency ablation, Wang et al found that the incidence of local tumor progression in patients undergoing cryoablation treatment was significantly reduced. However, for tumors that are larger than 2.5 cm to 3 cm, multifocal, or near high-risk locations, such as major vascular or biliary structures, ablation techniques often fail to achieve complete ablation coverage of the tumor [22]. In addition, it can cause many complications. Ma et al [9] reported that the incidences of biloma, capsular injury, subcapsular planting metastasis, and pneumothorax after cryoablation treatment of liver cancer in special sites were 1.4%, 18.8%, 1.4%, and 2.8%, respectively. Some previous studies have reported that fatal complications such as cardiac perforation, tamponade, and arrhythmia can occur when tumors are adjacent to the heart [11–13]. Therefore, tumors located adjacent to major organs or viscus are still a challenge for the cryoablation procedure.
To date, few studies have focused on the safety and efficacy of cryoablation treatment in small HCC. However, there have been several reports showing evidence that cryoablation is a safe and effective option for small HCC treatment. Orlacchio et al [24] conducted a case series of 4 patients and reported the safety and effectiveness of percutaneous cryoablation with ultrasound guidance and CT monitoring for the treatment of small HCC. After a 6-month follow-up, all 4 patients survived without short-term or long-term complications. Shimizu et al [25] reported the outcomes of MRI-guided percutaneous cryoablation in 15 patients with 16 small HCCs. The results showed that 1-year and 3-year overall survival rates were 93.8% and 79.3%, respectively. The complete ablation rate was 80.8% at 3 years. In our cohort, the technical success rate and the complete ablation rate was 100%. Major complications occurred in 5.4% of the 92 patients, with no procedure-related mortality. Our results are consistent with previous studies [9,26], and provide a further clinical reference for the application of percutaneous ablation in the treatment of HCC located adjacent to major organs or viscus.
The heat-sink effect is a common phenomenon that is characterized by the production of warm blood flow to protect the heart and peritumoral hepatic vessels from ablation damage [27]. The convective influx of warm blood weakens the effect of ablation and makes the ablation coverage of a tumor insufficient. Periportal tumors are difficult to treat owing to their complex anatomical location and are accompanied by a higher recurrence rate. Kang et al [28] found that the periportal location has a significant association with the development of aggressive intrasegmental recurrence after radiofrequency ablation. Some authors considered that the high recurrence rate of perivascular tumors is due not only to the heat-sink effect, but also to the sudden release of internal pressure of the tumor during ablation, which causes tumor cells to spread through the hepatic portal vein [10]. Therefore, compared with radiofrequency and other types of thermal ablation, cryoablation has certain advantages in treating perivascular HCC. In our study, there were only 2 patients (2 of 9) that had distant intrahepatic recurrence with HCC adjacent to the portal vein and heart. We consider that the use of cryoablation may prevent the spread of tumor cells and is more suitable for the treatment of perivascular HCC than is radiofrequency ablation.
The tumors adjacent to the diaphragm can easily cause damage to the diaphragm, pleural, and lungs. A previous study of 3670 patients who underwent radiofrequency ablation for liver tumors adjacent to the diaphragm showed that the incidence of diaphragm damage was 0.16% (6 of 3670 patients) [29]. Kang et al [30] reported a higher rate of diaphragmatic and lung injuries of 25% following radiofrequency ablation. Yang et al found that 24 patients (39.3%) undergoing cryoablation for HCC adjacent to the diaphragm experienced intrathoracic adverse events. In our present study, there were no diaphragmatic injuries, perforations, or other serious complications in patients with small HCC adjacent to the diaphragm. Three patients (3 of 39) developed pleural effusion after surgery, and they recovered within 2 weeks, without further treatment. It is worth noting that all cases of pleural effusion occurred in patients with tumors adjacent to the diaphragm. The probes were inserted through the transthoracic transpulmonary approach when tumors were adjacent to the diaphragm, so the pleural effusion was likely caused by the stimulation of the probe puncture. Consistent with our results, Wang et al [31] found that post-treatment asymptomatic pleural effusion is related to the treatment of tumors located in the diaphragm dome. Yang et al also found that the incidence of intrathoracic complications of tumors adjacent to the diaphragm is higher than that of other tumors.
The present study has certain limitations. First, the retrospective nature of the study may lead to selection bias. Second, although the current literature on the efficacy of liver tumors located adjacent to major organs or viscus is limited, the sample size of this study is still small, so multicenter studies and studies with larger sample sizes are needed. Third, our team has carried out percutaneous argon-helium cryoablation technology since the 2000s. All patients included in this study were treated by the same surgeon who had nearly 30 years of surgical experience. Therefore, the differences in surgical experience may lead to inconsistent results of efficacy. Finally, the median follow-up time was only 20 months. Therefore, some late complications such as tumor seeding may not be well evaluated.
Conclusions
This experience from a single center has shown that percutaneous argon-helium cryoablation was safe and effective in the management of small HCC located adjacent to a major organ or viscus.
Figures
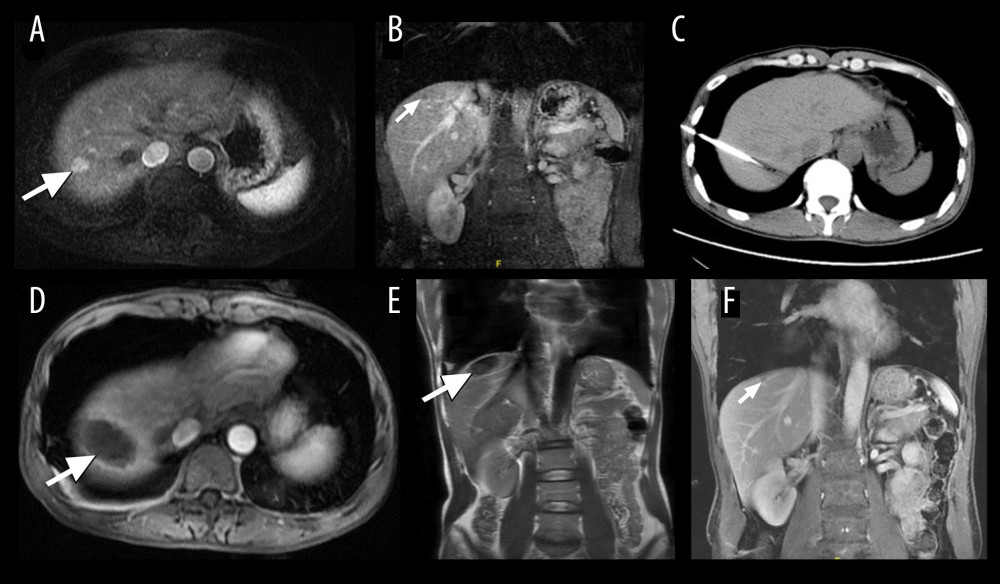 Figure 1. A 45-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC). (A, B) Axial and coronal magnetic resonance imaging (MRI) obtained during the arterial phase shows a nodule of approximately 1.1×1.5 cm (white arrow), located in liver segment VIII, abutting the diaphragm. (C) Cryoprobes were inserted into the tumor under computed tomography guidance. (D, E) MRI scanning 4 days after cryoablation of HCC. The HCC lesion was completely ablated, showing a hypovascular zone with a hypervascular inflammatory rim around the ablation zone (white arrow). (F) MRI scanning at 12 months after treatment shows that the ablated area had shrunk significantly (white arrow).
Figure 1. A 45-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC). (A, B) Axial and coronal magnetic resonance imaging (MRI) obtained during the arterial phase shows a nodule of approximately 1.1×1.5 cm (white arrow), located in liver segment VIII, abutting the diaphragm. (C) Cryoprobes were inserted into the tumor under computed tomography guidance. (D, E) MRI scanning 4 days after cryoablation of HCC. The HCC lesion was completely ablated, showing a hypovascular zone with a hypervascular inflammatory rim around the ablation zone (white arrow). (F) MRI scanning at 12 months after treatment shows that the ablated area had shrunk significantly (white arrow). 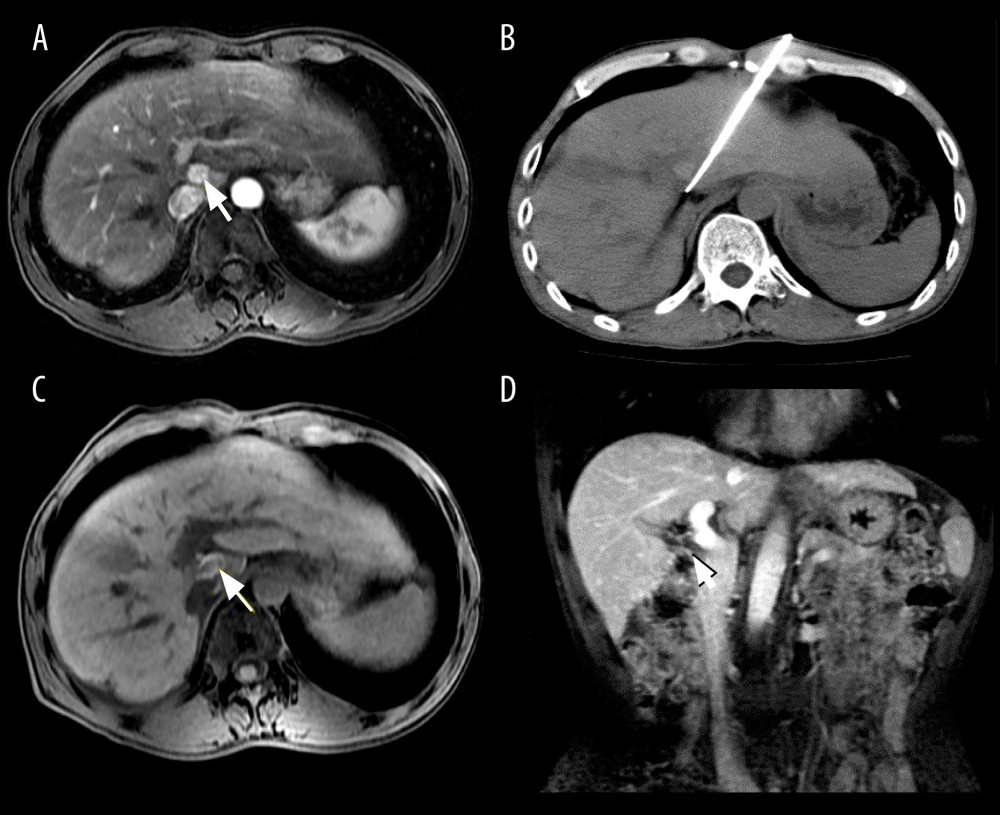 Figure 2. Images from a 51-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC) located in liver segment I. (A) Magnetic resonance imaging (MRI) axial scan in the arterial phase before cryoablation; 1 nodule located in liver segment I (white arrow). (B) Cryoprobes were inserted into the tumor under CT guidance. (C) MRI scan preformed 3 days after cryoablation indicated technical success. (D) MRI scanning shows that local tumor recurrence (white arrow) was not found at the 18-month follow-up.
Figure 2. Images from a 51-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC) located in liver segment I. (A) Magnetic resonance imaging (MRI) axial scan in the arterial phase before cryoablation; 1 nodule located in liver segment I (white arrow). (B) Cryoprobes were inserted into the tumor under CT guidance. (C) MRI scan preformed 3 days after cryoablation indicated technical success. (D) MRI scanning shows that local tumor recurrence (white arrow) was not found at the 18-month follow-up. 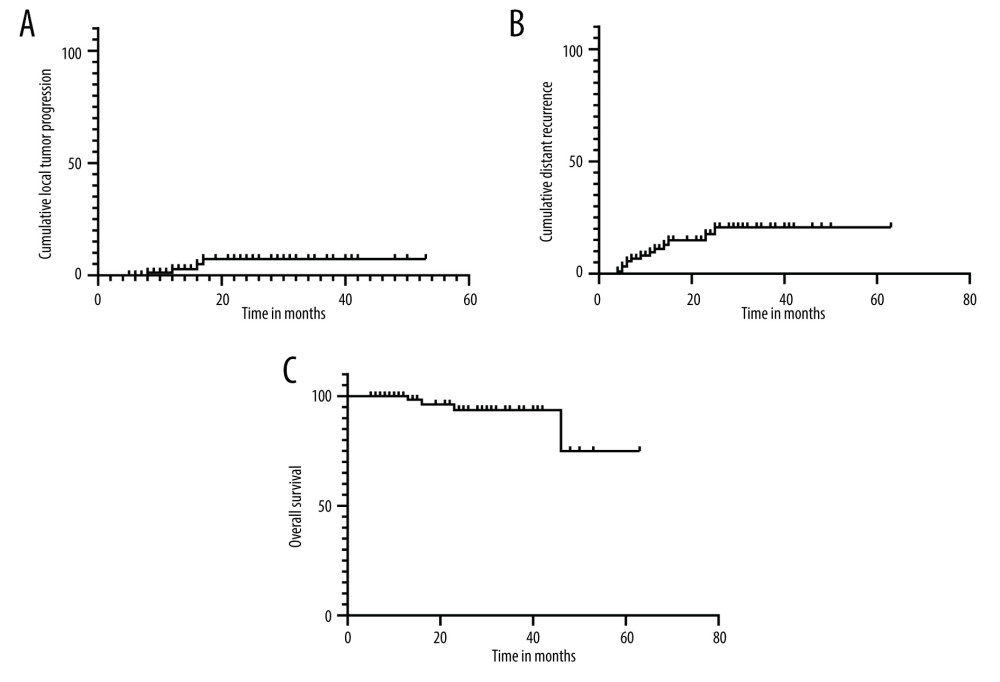 Figure 3. Kaplan-Meier curves show the cumulative local tumor progression rates, cumulative distant recurrence rates, and cumulative overall survival rates. (A) The cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. (B) The cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. (C) The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively.
Figure 3. Kaplan-Meier curves show the cumulative local tumor progression rates, cumulative distant recurrence rates, and cumulative overall survival rates. (A) The cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. (B) The cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. (C) The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively. References
1. Asham EH, Kaseb A, Ghobrial RM, Management of hepatocellular carcinoma: Surg Clin North Am, 2013; 93; 1423-50
2. Croasdell G, The Digital International Liver Congress 2020 – European Association for the Study of the Liver (EASL). Virtual Conference – August 27–29, 2020: Drug Future, 2020; 45; 775
3. Zhou Y, Zhao Y, Li B, Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: BMC Gastroenterol, 2010; 10; 78
4. Rong G, Bai W, Dong Z, Cryotherapy for cirrhosis-based hepatocellular carcinoma: A single center experience from 1595 treated cases: Front Med, 2015; 9; 63-71
5. Bruix J, Sherman M, Management of hepatocellular carcinoma: An update: Hepatology (Baltimore, Md), 2011; 53; 1020-22
6. Llovet JP, Ducreux M, Lencioni REuropean Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer: EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma, 2012
7. Lencioni R, Loco-regional treatment of hepatocellular carcinoma: Hepatology (Baltimore, Md), 2010; 52; 762-73
8. Hirokawa F, Hayashi M, Miyamoto Y, Short- and long-term outcomes of laparoscopic versus open hepatectomy for small malignant liver tumors: A single-center experience: Surg Endosc, 2015; 29; 458-65
9. Ma J, Wang F, Zhang W, Percutaneous cryoablation for the treatment of liver cancer at special sites: An assessment of efficacy and safety: Quant Imaging Med Surg, 2019; 9; 1948-57
10. Kim R, Kang T, Cha D, Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications: Eur Radiol, 2019; 29; 654-62
11. Carberry GA, Smolock A, Christescu M, Safety and efficacy of percutaneous microwave hepatic ablation near the heart: J Vasc Interv Radiol, 2017; 4; 490-97
12. Beng LK, Ismail BS, Jeet ABJ, Hemorrhagic cardiac tamponade: Rare complication of radiofrequency ablation of hepatocellular carcinoma: Korean J Radiol, 2012; 13; 643-47
13. Moumouh A, Hannequin JM, Chagneau C, A tamponade leading to death after radiofrequency ablation of hepatocellular carcinoma: Eur Radiol, 2005; 15; 234-37
14. Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC), 2014 Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guideline for the Management of Hepatocellular Carcinoma: Korean J Radiol, 2015; 16; 465-522
15. Vogel A, Martinelli EESMO Guidelines Committee, Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines: Ann Oncol, 2021 [Online ahead of print]
16. Bruix J, Sherman M, Management of hepatocellular carcinoma: Hepatology, 2005; 42; 1208-36
17. , EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma: J Hepatol, 2018; 69; 182-236
18. Mazzaferro V, Regalia E, Doci R, Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis: N Engl J Med, 1996; 334; 693-99
19. Qi C, Gao H, Zhao Q, Zhang L, Computed tomography-guided percutaneous cryoablation for subcardiac hepatocellular carcinoma: Safety, efficacy, therapeutic results and risk factors for survival outcomes: Cancer Manag Res, 2020; 12; 3333-42
20. Wang C, Lu Y, Chen Y, Prognostic factors and recurrence of hepatitis B-related hepatocellular carcinoma after argon-helium cryoablation: A prospective study: Clin Exp Metastasis, 2009; 26; 839-48
21. Kwon JH, Won JY, Han K, Safety and efficacy of percutaneous cryoablation for small hepatocellular carcinomas adjacent to the heart: J Vasc Interv Radiol, 2019; 8; 1223-28
22. Heimbach JK, Kulik LM, Finn RS, AASLD guidelines for the treatment of hepatocellular carcinoma: Hepatology, 2018; 67; 358-80
23. Littrup PJ, Aoun HD, Adam B, Krycia M, Percutaneous cryoablation of hepatic tumors: Long-term experience of a large U.S. series: Abdom Radiol, 2016; 41; 767-80
24. Orlacchio A, Bazzocchi G, Pastorelli D, Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: Initial experience: Cardiovasc Intervent Radiol, 2008; 31; 587-94
25. Shimizu T, Sakuhara Y, Abo D, Outcome of MR-guided percutaneous cryoablation for hepatocellular carcinoma: J Hepatobiliary Pancreat Surg, 2009; 16(6); 816-23
26. Hu J, Chen S, Wang X, Image-guided percutaneous microwave ablation versus cryoablation for hepatocellular carcinoma in high-risk locations: intermediate-term results: Cancer Manag Res, 2019; 11; 9801-11
27. Lu D, Raman S, Vodopich D, Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: Assessment of the “heat sink” effect: Am J Roentgenol, 2002; 178; 47-51
28. Kang T, Lim H, Lee M, Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: Risk factors and clinical significance: Radiology, 2015; 276; 274-85
29. Mulier S, Mulier P, Ni Y, Complications of radiofrequency coagulation of liver tumours: Br J Surg, 2002; 89(10); 1206-22
30. Kang TW, Rhim H, Kim EY, Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: Assessment of safety and therapeutic efficacy: Korean J Radiol, 2009; 10(1); 34-42
31. Wang C, Wang H, Yang W, Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma: Hepatology (Baltimore, Md), 2015; 61; 1579-90
Figures
 Figure 1. A 45-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC). (A, B) Axial and coronal magnetic resonance imaging (MRI) obtained during the arterial phase shows a nodule of approximately 1.1×1.5 cm (white arrow), located in liver segment VIII, abutting the diaphragm. (C) Cryoprobes were inserted into the tumor under computed tomography guidance. (D, E) MRI scanning 4 days after cryoablation of HCC. The HCC lesion was completely ablated, showing a hypovascular zone with a hypervascular inflammatory rim around the ablation zone (white arrow). (F) MRI scanning at 12 months after treatment shows that the ablated area had shrunk significantly (white arrow).
Figure 1. A 45-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC). (A, B) Axial and coronal magnetic resonance imaging (MRI) obtained during the arterial phase shows a nodule of approximately 1.1×1.5 cm (white arrow), located in liver segment VIII, abutting the diaphragm. (C) Cryoprobes were inserted into the tumor under computed tomography guidance. (D, E) MRI scanning 4 days after cryoablation of HCC. The HCC lesion was completely ablated, showing a hypovascular zone with a hypervascular inflammatory rim around the ablation zone (white arrow). (F) MRI scanning at 12 months after treatment shows that the ablated area had shrunk significantly (white arrow). Figure 2. Images from a 51-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC) located in liver segment I. (A) Magnetic resonance imaging (MRI) axial scan in the arterial phase before cryoablation; 1 nodule located in liver segment I (white arrow). (B) Cryoprobes were inserted into the tumor under CT guidance. (C) MRI scan preformed 3 days after cryoablation indicated technical success. (D) MRI scanning shows that local tumor recurrence (white arrow) was not found at the 18-month follow-up.
Figure 2. Images from a 51-year-old man who underwent cryoablation for small hepatocellular carcinoma (HCC) located in liver segment I. (A) Magnetic resonance imaging (MRI) axial scan in the arterial phase before cryoablation; 1 nodule located in liver segment I (white arrow). (B) Cryoprobes were inserted into the tumor under CT guidance. (C) MRI scan preformed 3 days after cryoablation indicated technical success. (D) MRI scanning shows that local tumor recurrence (white arrow) was not found at the 18-month follow-up. Figure 3. Kaplan-Meier curves show the cumulative local tumor progression rates, cumulative distant recurrence rates, and cumulative overall survival rates. (A) The cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. (B) The cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. (C) The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively.
Figure 3. Kaplan-Meier curves show the cumulative local tumor progression rates, cumulative distant recurrence rates, and cumulative overall survival rates. (A) The cumulative local tumor progression rates at 1 and 2 years were 2.8% and 7.3%, respectively. (B) The cumulative distant recurrence rates at 1, 2, and 3 years were 11.1%, 17.6%, and 20.7%, respectively. (C) The overall survival rates at 1, 2, and 4 years were 100%, 93.6%, and 74.9%, respectively. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952