19 September 2021: Clinical Research
Risk Factors and Prognosis of New-Onset Chronic Kidney Disease Following Orthotopic Liver Transplantation: A Retrospective Case-Control Study
Yi DuanDOI: 10.12659/MSM.931834
Med Sci Monit 2021; 27:e931834
Abstract
BACKGROUND: We have undertaken this investigation to explore the perioperative risk factors of new-onset chronic kidney disease (NOCKD) after orthotopic liver transplantation (OLT), and to provide an early prediction model for the screening of NOCKD high-risk populations.
MATERIAL AND METHODS: A retrospective case-control study was performed in adult recipients who received OLT in our center between January 2018 and January 2020. Perioperative data were collected using the center’s electronic medical record system. Logistics regression analysis was used to determine risk factors for NOCKD within 1 year following OLT. Kaplan-Meier and log-rank tests were used to evaluate the 1-year survival of recipients with NOCKD or without NOCKD.
RESULTS: A total of 174 patients were included in this study, and 29 patients developed NOCKD after OLT. Logistic multivariate regression analysis showed that preoperative diabetes, high model for end-stage liver disease (MELD) score, postoperative acute kidney injury (AKI), and postoperative renal replacement therapy (RRT) were independent risk factors for NOCKD 1 year after OLT. The 1-year survival rate of NOCKD recipients waas significantly lower than that of patients who did not receive NOCKD.
CONCLUSIONS: Diabetes mellitus, MELD score, postoperative AKI, and requirement for postoperative RRT are independent risk factors for NOCKD after OLT, which may have great potential for personalized decision making and predicting the 1-year postoperative mortality of the recipient.
Keywords: Diabetes Mellitus, Liver Transplantation, Renal Insufficiency, Chronic, Acute Kidney Injury, Case-Control Studies, Female, Humans, Postoperative Complications, Renal Replacement Therapy, Risk Assessment, Risk Factors, Severity of Illness Index
Background
Chronic kidney disease (CKD) is a global public health issue, which also widely occurs among transplant recipients. Convincing evidence suggests that a large number of recipients experience renal function decline after organ transplantation [1], and a considerable number of them will progress to CKD [2]. The incidence of CKD within 1 year after orthotopic liver transplantation (OLT) is 4.0% to 27.5% [3], second only to the rates in heart and lung transplantation recipients. CKD may further develop into end-stage renal disease (ESRD), requiring several years of renal replacement therapy (RRT) or even kidney transplantation, which causes a heavy social and economic burden [4] and adversely impacts quality of life. In addition, CKD is associated with an increased incidence of cardiovascular events following transplantation and leads to increased readmission and mortality risk [5]. Thus, there is an urgent need for analysis and insight into the causes of and a potential prediction model for CKD after OLT [6–8].
Previous studies suggested that age, female, hypertension, diabetes, model for end-stage liver disease (MELD) score, preoperative glomerular filtration rate, hemoglobin levels, and duration of renal impairment are correlated with the onset and progression of CKD in patients with cirrhosis [9–12]. However, the risk factors and their effects on CKD remain controversial. Moreover, most previous studies focused on transplant recipients with preoperative renal dysfunction. Postoperative CKD may be attributable to preoperative renal parenchymal injury. Nevertheless, little is known about perioperative risk factors for NOCKD in patients without CKD prior to OLT. Defining the risk factors for NOCKD after OLT may facilitate early identification of potential high-risk groups, help personalize perioperative anesthesia management, and avoid the use of nephrotoxic drugs such as calcineurin inhibitors (CNI) in postoperative treatment [8,13], so as to improve prognosis and short- and long-term survival rates. Moreover, in Asian countries there are no available prediction models that can identify patients at increased risk of NOCKD and related poor prognosis after OLT based only on their perioperative characteristics. This study aimed to retrospectively analyze the perioperative risk factors of NOCKD after OLT in adults with preoperative estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73 m2, and to provide an early prediction model for the screening of NOCKD high-risk groups.
Material and Methods
STUDY DESIGN AND POPULATION:
This single-center, retrospective study included all consecutive adult patients who underwent LT between 1 January 2018 and 1 February 2020. All recipients were tested for serum creatinine levels and assessed for eGFR prior to transplantation, 1 to 7 days after transplantation, and 1, 3, 6, 9, and 12 months after transplantation, according to the conventional treatment strategy. Patients who underwent the following procedures were excluded: (1) Patients under 18 years of age, (2) Renal replacement treatment before OLT or baseline eGFR <60 ml/(min·1.73·m2), (3) Survival less than 3 months, (4) Lack of important data, (5) Surgery cancelled after anesthesia. The requirement for informed consent was waived for this study.
DIAGNOSTIC CRITERIA FOR ACUTE KIDNEY INJURY (AKI) AND NOCKD:
According to the revised definition of AKI in patients with cirrhosis by the Kidney Disease Improvement Global Outcomes Organization (KDIGO) [14] and the recommendation of the International Club of ASCITES (ICA) [15], AKI is defined as increase in serum creatinine (sCr) by ≥26.4 μmol/L (0.3 mg/dL) in <48 h or 50% increase in sCr from baseline, which is known or presumed to have occurred within the prior 7 days (the baseline value refers to the stable value within 3 months before surgery. If not available, the measured value at admission was used. Stage 1 refers to sCr increase 1.5–1.9 times baseline, or Cr increase ≥0.3 mg/dl (26.5 μmol/L); stage 2 refers to sCr increase 2.0–2.9 times baseline; and stage 3 refers to sCr increase 3.0 times baseline, or sCr increase to ≥4.0 mg/dl (353.6 μmol/L), or initiation of renal replacement therapy.
According to the 2012 KDIGO [16] guideline for the diagnosis and management of adults and children with CKD, the diagnostic criteria for CKD is eGFR <60 ml/(min·1.73·m2) for >3 months. In this study, NOCKD was defined as the diagnosis of CKD after OLT in recipients with preoperative eGFR ≥60 mL/(min·1.73·m2).
IMMUNOSUPPRESSIVE MANAGEMENT:
Methylprednisolone was administered in a 500-mg intravenous bolus 30 min before graft reperfusion. Following liver transplantation, patients were maintained on MMF-, tacrolimus-, and prednisone-based immunosuppressive therapy. The tacrolimus concentration was maintained at 8–10 ng/ml for 3 months after the operation. MMF was administrated at 0.5 g b.i.d. in the first 3 months and then reduced to 0.25 g. MMF was slowly discontinued at 6 months after transplantation. Methylprednisolone was administered orally at 240 mg, reduced by 40 mg daily until reduced to 40 mg daily. Then, methylprednisolone was changed to oral prednisolone 20 mg daily, reduced by 2.5 mg weekly until reduced to 5 mg and maintained for 1 month, and stopped thereafter.
In patients with hepatitis C or malignant tumors, tacrolimus trough levels were maintained at 6~8 ng/ml within 3 months after the operation. MMF was administered at 0.5 g b.i.d. until the end of the third month. Prednisone was reduced by 5 mg weekly for 1 month, and stopped thereafter.
For patients with renal insufficiency after transplantation, 20 mg Basiliximab was administered on post-transplant day 1 and day 4, without CNI administration. If the arterial condition was good, rapamycin (a mTOR inhibitor) was given orally at the dose of 2 mg per day 1 week after surgery. If the arterial condition was poor, MMF was used alone and increased to 1 g every 12 h, and then gradually changed to rapamycin or rapamycin combined with MMF 1 month after liver transplantation.
DATA COLLECTION:
The following data were collected through the outpatient or inpatient electronic medical records of the Hospital Information System (HIS), the Docare Anesthesia Clinical Information System (V5.0) and follow-up visits:
Preoperative basic information: recipient age, sex, body mass index (BMI), MELD score, complications including acute liver failure, ascites, diabetes mellitus, hypertension, and coronary heart disease, as well as type of the primary disease (eg, post-hepatitis B cirrhosis, post-hepatitis C cirrhosis, alcoholic cirrhosis and non-alcoholic fatty liver disease, liver hydatid disease, liver cancer), preoperative hemoglobin level, preoperative albumin level, preoperative total bilirubin level, preoperative glutamic-pyruvic transaminase level, preoperative sCr, preoperative international normalized ratio (INR), preoperative serum sodium levels, donor age, and warm and cold ischemia time.
Intraoperative and anesthetic information included operation duration, portal vein occlusion time, amount of crystal fluid infusion, 5% albumin infusion, red blood cell infusion, fresh frozen plasma infusion, blood loss, urine volume, norepinephrine dosage, and duration of intraoperative minimum blood pressure.
Postoperative outcome information included postoperative anti-rejection treatment, incidence and severity of AKI, rate of postoperative RRT, length of postoperative ICU stay, postoperative hospital stay, postoperative incidence of CKD within 1 year, and postoperative cause of death and mortality within 1 year.
FOLLOW-UP:
Patients were followed and events recorded until 31 January 2021 and/or death.
STATISTICAL ANALYSIS:
Data were analyzed using SPSS 26.0 statistical software. Normally distributed data were expressed as mean±standard deviation (SD) and compared by independent-sample
ETHICS:
The study was approved by the Beijing Tsinghua Changgung Hospital Ethics Committee (approval number: 21017-6-01) and the requirement the informed consent was waived by Beijing Tsinghua Changgung Hospital Ethics Committee. Our study was carried out in accordance with the Declaration of Helsinki (2013) of the World Medical Association and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for human observational studies.
Results
A total of 235 patients who underwent orthotopic allogeneic OLT in our center were included in the study. Among them, 3 recipients were younger than 18 years old, 38 recipients received preoperative renal replacement therapy or preoperative eGFR <60 mL/(min·1.73·m2), 16 recipients died within 3 months after surgery, 3 recipients had incomplete case information, and 1 recipient cancelled the OLT. A total of 174 patients were included in the study for data analysis (Figure 1). We identified 54 patients (31.0%) who developed AKI within 1 week after surgery, with stage 1 in 19 patients, stage 2 in 17 patients, and stage 3 in 18 patients. In this study, a total of 29 (16.7%) recipients developed NOCKD after OLT.
Recipients were divided into a NOCKD group and a non-CKD group according to whether they were diagnosed with NOCKD after surgery. Univariate analysis showed that the preoperative prevalence of diabetes was higher in the NOCKD group (55.2% vs 15.2%,
No immunosuppressant toxicity-related AKI was observed in NOCKD group and non-CKD group. The incidence of AKI in the NOCKD group was higher than that in the non-CKD group within 1 week after OLT (75.9% vs 22.1%,
The results of regression analyses are shown in Table 3. Preoperative diabetes, MELD score, postoperative AKI, and postoperative RRT were independent risk factors for NOCKD. A prediction model containing the 4 variables was developed using multivariable logistic regression analyses. The formula of the original model was: R=−3.616+1.188× (diabetes)+0.084× (preoperative MELD score)+1.269×(postoperative AKI)+2.524× (postoperative RRT).
R was divided by 0.084 to obtain the predictive factor R1[R1=(preoperative MELD score) −43+14 (if preoperative diabetes)+15 (if postoperative AKI)+30 (if postoperative RRT required)]. The ROC curve was further obtained (Figure 3A, AUC: 0.879, 95% CI: 0.802~0.957). The lower the value of R1, the lower the risk of NOCKD within 1 year after surgery. The cutoff value was −17 (Figure 3B).
There were 174 recipients followed up for 6–34 months, and the median follow-up duration was 21 months. A total of 150 patients were followed up for 1 year, and the 1-year survival rate was 96.0%. During the follow-up, 6 patients died, among which 3 patients had multiple organ failure, 2 patients had respiratory complications, and 1 patient had cerebral hemorrhage. Log-rank survival statistics showed that the 1-year survival rate in the NOCKD group was significantly lower than that in the non-CKD group (88.9% vs 97.6%,
Discussion
LIMITATIONS:
There are several limitations to the present study. First, this was a single-center study, so bias may exist due to lack of data from other centers. Second, the sample size of this study was relatively small. With the development of the transplantation center in our hospital, more patients can be included in future studies. Third, the median length of follow-up was 21 months, which was relatively short. Our further studies will extend the follow-up period and continue to observe the incidence of CKD at 3 and 5 years postoperatively, as well as the outcomes of CKD. Finally, the prediction model in this study should be validated in future prospective studies.
Conclusions
In conclusion, diabetes mellitus, MELD score, postoperative AKI, and requirement for postoperative RRT are independent risk factors for NOCKD in OLT recipients, which may be clinically significant to predict 1-year postoperative mortality. The model established in this study may facilitate early identification of the high-risk population of NOCKD after OLT, establishment of perioperative renal protection strategy, evidence for early reduction or cessation of immunosuppressive agents with nephrotoxicity, and improvement of prognosis.
Figures
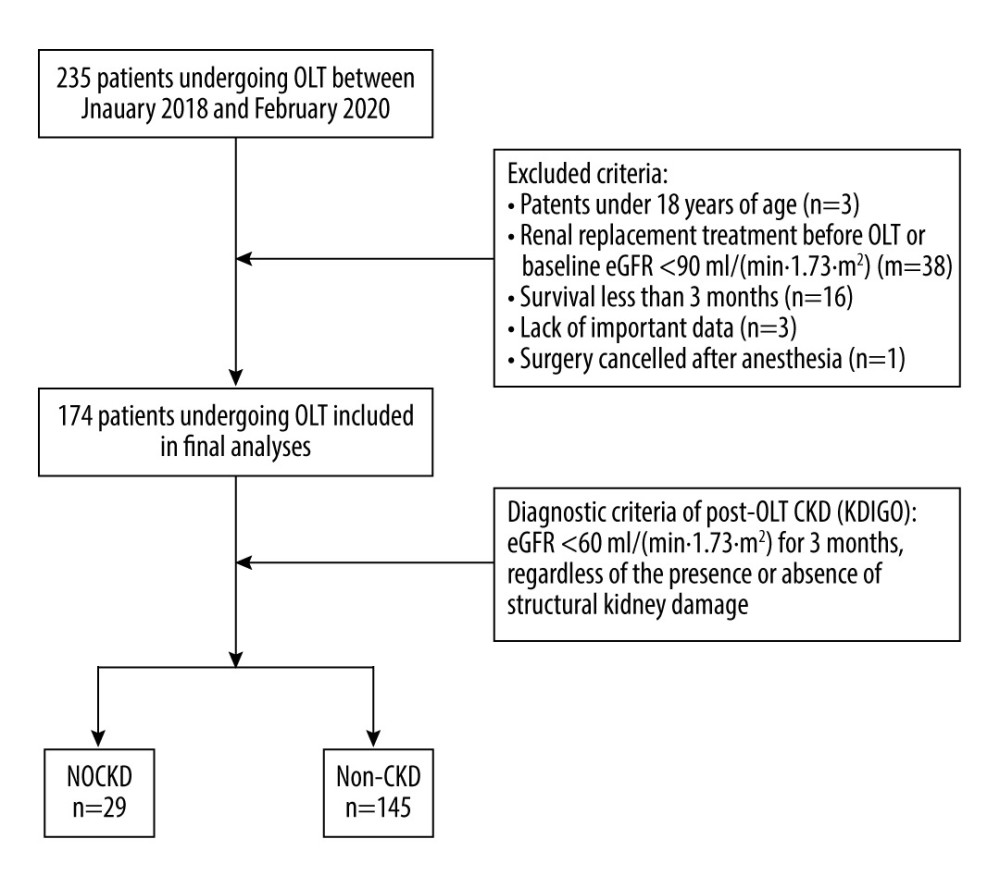 Figure 1. Flow chart of patient selection. OLT – orthotopic liver transplantation; eGFR – estimated glomerular filtration rate; KDIGO – Kidney Disease of Improving Global Outcomes; NOCKD – new-onset chronic kidney disease; CKD – chronic kidney disease.
Figure 1. Flow chart of patient selection. OLT – orthotopic liver transplantation; eGFR – estimated glomerular filtration rate; KDIGO – Kidney Disease of Improving Global Outcomes; NOCKD – new-onset chronic kidney disease; CKD – chronic kidney disease. 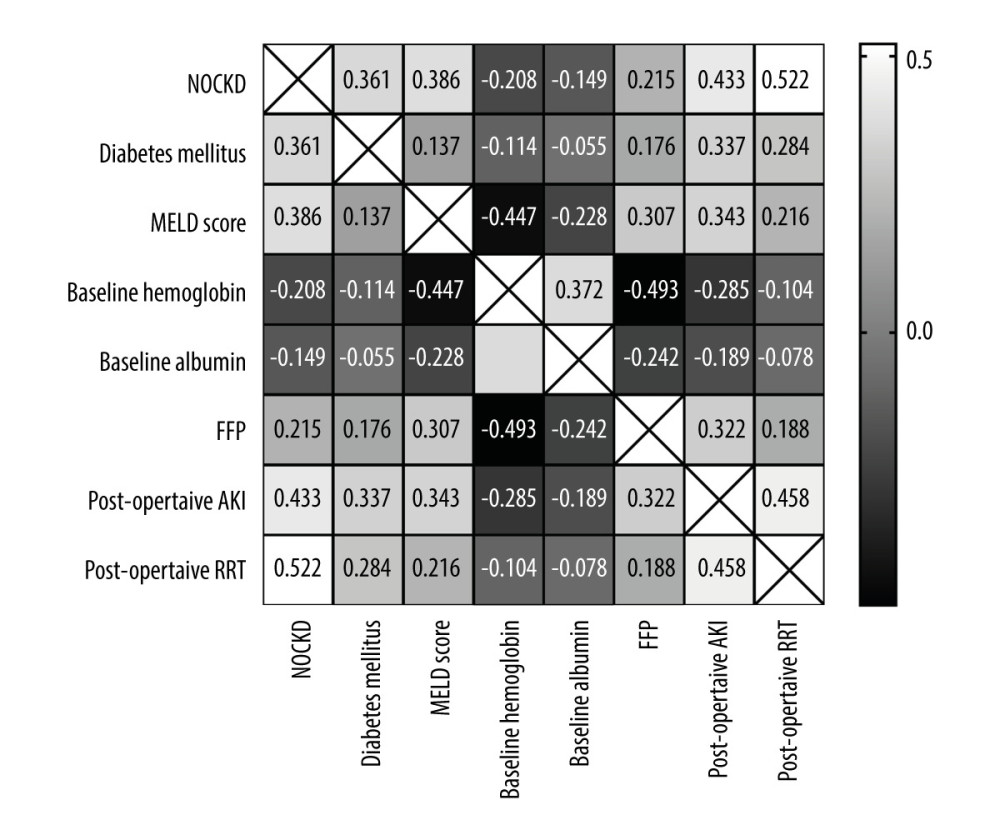 Figure 2. The heatmap of correlations between NOCKD and correlated factors. Correlation coefficients are shown. Diabetes, MELD score, baseline hemoglobin, baseline albumin, fresh frozen plasma (FFP), postoperative AKI, and postoperative RRT were factors correlated with NOCKD (P all <0.05).
Figure 2. The heatmap of correlations between NOCKD and correlated factors. Correlation coefficients are shown. Diabetes, MELD score, baseline hemoglobin, baseline albumin, fresh frozen plasma (FFP), postoperative AKI, and postoperative RRT were factors correlated with NOCKD (P all <0.05). 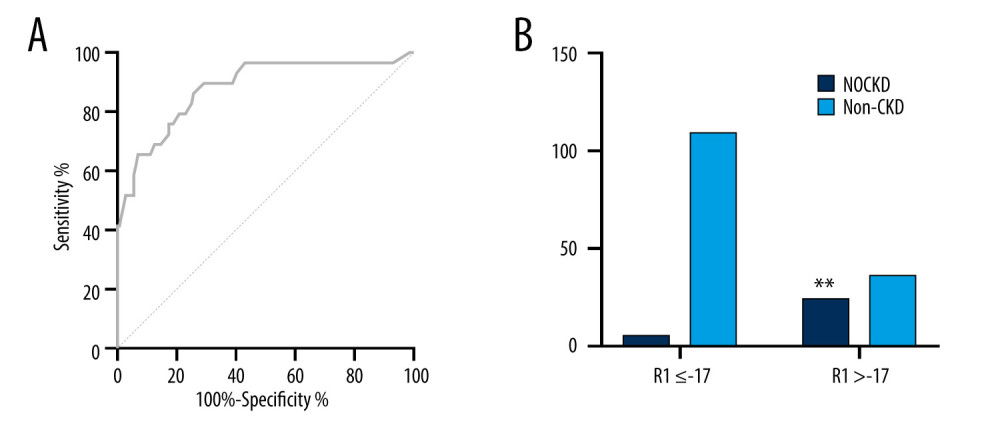 Figure 3. Verification for the risk prediction model. (A) ROC curve of the prediction model for patients with high risk of NOCKD. ROC – receiver operating characteristic. (B) R1 >-17 indicated a lower incidence of NOCKD. ** P<0.05.
Figure 3. Verification for the risk prediction model. (A) ROC curve of the prediction model for patients with high risk of NOCKD. ROC – receiver operating characteristic. (B) R1 >-17 indicated a lower incidence of NOCKD. ** P<0.05. 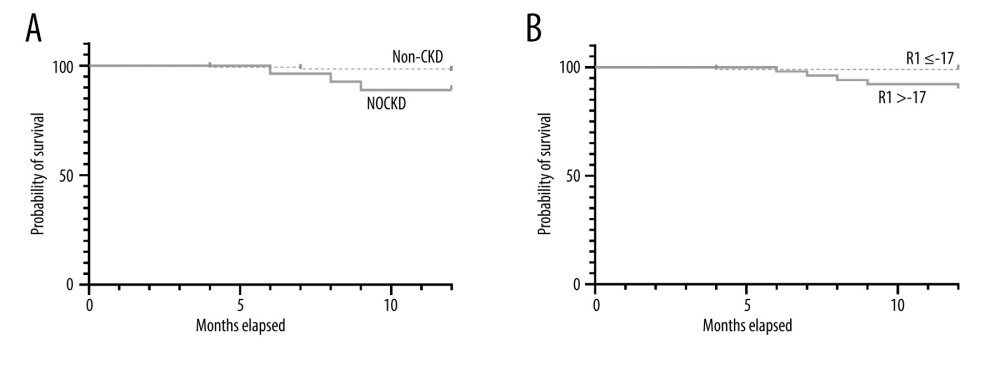 Figure 4. One-year survival in patients with or without postoperative NOCKD. (A) Kaplan-Meier plot showed that recipient survival for patients who developed CKD (dotted line) was significantly worse compared with those who did not develop CKD (solid line) (88.9% vs 97.6%, P=0.036). (B) Recipients survival for patients whose R1 >-17 (dotted line) was significantly worse compared with those whose R1 ≤-17 (solid line) (90.2% vs 99.0%, P=0.01)
Figure 4. One-year survival in patients with or without postoperative NOCKD. (A) Kaplan-Meier plot showed that recipient survival for patients who developed CKD (dotted line) was significantly worse compared with those who did not develop CKD (solid line) (88.9% vs 97.6%, P=0.036). (B) Recipients survival for patients whose R1 >-17 (dotted line) was significantly worse compared with those whose R1 ≤-17 (solid line) (90.2% vs 99.0%, P=0.01) References
1. Herrero JI, Cuervas-Mons V, Gómez-Bravo MÁ, Prevalence and progression of chronic kidney disease after liver transplant: A prospective, real-life, observational, two-year multicenter study: Rev Esp Enferm Dig, 2018; 110(9); 538-43
2. Bassegoda O, Huelin P, Ariza X, Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes: J Hepatol, 2020; 72(6); 1132-39
3. Weber ML, Ibrahim HN, Lake JR, Renal dysfunction in liver transplant recipients: Evaluation of the critical issues: Liver Transpl, 2012; 18(11); 1290-301
4. von Zur-Mühlen B, Wintzell V, Levine A, Healthcare resource use, cost, and sick leave following kidney transplantation in Sweden: A population-based, 5-year, retrospective study of outcomes: COIN: Ann Transplant, 2018; 23; 852-66
5. Erratum: Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group, KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017; 7:1–59: Kidney Int Suppl (2011), 2017; 7(3); e1
6. Allen AM, Kim WR, Therneau TM, Chronic kidney disease and associated mortality after liver transplantation – a time-dependent analysis using measured glomerular filtration rate: J Hepatol, 2014; 61(2); 286-92
7. Longenecker JC, Estrella MM, Segev DL, Atta MG, Patterns of kidney function before and after orthotopic liver transplant: Associations with length of hospital stay, progression to end-stage renal disease, and mortality: Transplantation, 2015; 99(12); 2556-64
8. Ojo AO, Held PJ, Port FK, Chronic renal failure after transplantation of a nonrenal organ: N Engl J Med, 2003; 349(10); 931-40
9. Ruebner R, Goldberg D, Abt PL, Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction: Am J Transplant, 2012; 12(11); 2958-65
10. Israni AK, Xiong H, Liu J, Predicting end-stage renal disease after liver transplant: Am J Transplant, 2013; 13(7); 1782-92
11. Sharma P, Goodrich NP, Schaubel DE, Patient-specific prediction of ESRD after liver transplantation: J Am Soc Nephrol, 2013; 24(12); 2045-52
12. Maiwall R, Pasupuleti SSR, Bihari C, Incidence, risk factors, and outcomes of transition of acute kidney injury to chronic kidney disease in cirrhosis: A prospective cohort study: Hepatology, 2020; 71(3); 1009-22
13. O’Leary JG, Levitsky J, Wong F, Protecting the kidney in liver transplant candidates: Practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice: Am J Transplant, 2016; 16(9); 2516-31
14. Jagarlamudi N, Wong F, Acute kidney injury: Prediction, prognostication and optimisation for liver transplant: Hepatol Int, 2020; 14(2); 167-79
15. Angeli P, Ginès P, Wong F, Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites: J Hepatol, 2015; 62(4); 968-74
16. Stevens PE, Levin AKidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members, Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline: Ann Intern Med, 2013; 158(11); 825-30
17. Peng JC, Li YJ, Wang J, Incidence of chronic kidney disease after orthotopic liver transplantation in a Chinese cohort: Clin Exp Nephrol, 2020; 24(9); 806-12
18. Gojowy D, Kubis P, Gorecka M, Chronic kidney disease in patients after liver transplantation: A long-term retrospective analysis from 1 transplantation center: Transplant Proc, 2020; 52(8); 2492-96
19. Li Y, Li B, Wang W, Lv J, Risk factors for new-onset chronic kidney disease in patients who have received a liver transplant: Exp Ther Med, 2018; 15(4); 3589-95
20. Menon S, Pollack AH, Sullivan E, Acute kidney injury and chronic kidney disease after non-kidney solid organ transplantation: Pediatr Transplant, 2020; 24(6); e13753
21. Gonwa TA, Mai ML, Melton LB, End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: Risk of development and treatment: Transplantation, 2001; 72(12); 1934-39
22. Ye L, Zhang Y, Tang H, Prediction of chronic kidney disease progression used by calcineurin inhibitor concentration and estimated glomerular filtration rate early after liver transplantation: Niger J Clin Pract, 2020; 23(10); 1387-94
23. Maurel P, Prémaud A, Carrier P, Evaluation of longitudinal exposure to tacrolimus as a risk factor of chronic kidney disease occurrence within the first year post-liver transplantation: Transplantation, 2020 [Online ahead of print]
24. Zitta S, Schaffellner S, Gutschi J, The effect of mammalian target of rapamycin versus calcineurin inhibitor-based immunosuppression on measured versus estimated glomerular filtration rate after orthotopic liver transplantation: Transplantation, 2015; 99(6); 1250-56
25. Zhang W, Fung J, Limitations of current liver transplant immunosuppressive regimens: Renal considerations: Hepatobiliary Pancreat Dis Int, 2017; 16(1); 27-32
26. Saliba F, Duvoux C, Dharancy S, Early switch from tacrolimus to everolimus after liver transplantation: Outcomes at 2 years: Liver Transpl, 2019; 25(12); 1822-32
27. Calne RY, Rolles K, White DJ, Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers: Lancet, 1979; 2(8151); 1033-36
28. Lederer AK, Haffa D, Felgendreff P, The impact of immunosuppression on postoperative graft function after graft-unrelated surgery: A retrospective controlled cohort study: BMC Nephrol, 2019; 20(1); 170
29. Aguiar D, Martínez-Urbistondo D, Baroja-Mazo A, Real-world multicenter experience of immunosuppression minimization among 661 liver transplant recipients: Ann Transplant, 2017; 22; 265-75
30. Buchholz BM, Ferguson JW, Schnitzbauer AA, Randomized sirolimus-based early calcineurin inhibitor reduction in liver transplantation: Impact on renal function: Transplantation, 2020; 104(5); 1003-18
31. Guilianelli C, Baeza-Squiban A, Boisvieux-Ulrich E, Effect of mineral particles containing iron on primary cultures of rabbit tracheal epithelial cells: Ppossible implication of oxidative stress: Environ Health Perspect, 1993; 101(5); 436-42
32. Jha V, Garcia-Garcia G, Iseki K, Chronic kidney disease: Global dimension and perspectives: Lancet, 2013; 382(9888); 260-72
33. Lee K, Jeon J, Kim JM, Perioperative risk factors of progressive chronic kidney disease following liver transplantation: Analyses of a 10-year follow-up single-center cohort: Ann Surg Treat Res, 2020; 99(1); 52-62
34. Guo M, Gao Y, Wang L, Early acute kidney injury associated with liver transplantation: A retrospective case-control study: Med Sci Monit, 2020; 26; e923864
35. Trinh E, Alam A, Tchervenkov J, Cantarovich M, Impact of acute kidney injury following liver transplantation on long-term outcomes: Clin Transplant, 2017; 31(1); ctr.12863
36. Karkar A, Ronco C, Prescription of CRRT: A pathway to optimize therapy: Ann Intensive Care, 2020; 10(1); 32
37. Zou H, Hong Q, Xu G, Early versus late initiation of renal replacement therapy impacts mortality in patients with acute kidney injury post cardiac surgery: A meta-analysis: Crit Care, 2017; 21(1); 150
38. Zarbock A, Kellum JA, Schmidt C, Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN Randomized Clinical Trial: JAMA, 2016; 315(20); 2190-99
39. Gaudry S, Hajage D, Benichou N, Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: A systematic review and individual patient data meta-analysis of randomised clinical trials: Lancet, 2020; 395(10235); 1506-15
40. Safwan M, Gosnell J, Collins K, Effects of intraoperative continuous renal replacement therapy on outcomes in liver transplantation: Transplant Proc, 2020; 52(1); 265-70
Figures
 Figure 1. Flow chart of patient selection. OLT – orthotopic liver transplantation; eGFR – estimated glomerular filtration rate; KDIGO – Kidney Disease of Improving Global Outcomes; NOCKD – new-onset chronic kidney disease; CKD – chronic kidney disease.
Figure 1. Flow chart of patient selection. OLT – orthotopic liver transplantation; eGFR – estimated glomerular filtration rate; KDIGO – Kidney Disease of Improving Global Outcomes; NOCKD – new-onset chronic kidney disease; CKD – chronic kidney disease. Figure 2. The heatmap of correlations between NOCKD and correlated factors. Correlation coefficients are shown. Diabetes, MELD score, baseline hemoglobin, baseline albumin, fresh frozen plasma (FFP), postoperative AKI, and postoperative RRT were factors correlated with NOCKD (P all <0.05).
Figure 2. The heatmap of correlations between NOCKD and correlated factors. Correlation coefficients are shown. Diabetes, MELD score, baseline hemoglobin, baseline albumin, fresh frozen plasma (FFP), postoperative AKI, and postoperative RRT were factors correlated with NOCKD (P all <0.05). Figure 3. Verification for the risk prediction model. (A) ROC curve of the prediction model for patients with high risk of NOCKD. ROC – receiver operating characteristic. (B) R1 >-17 indicated a lower incidence of NOCKD. ** P<0.05.
Figure 3. Verification for the risk prediction model. (A) ROC curve of the prediction model for patients with high risk of NOCKD. ROC – receiver operating characteristic. (B) R1 >-17 indicated a lower incidence of NOCKD. ** P<0.05. Figure 4. One-year survival in patients with or without postoperative NOCKD. (A) Kaplan-Meier plot showed that recipient survival for patients who developed CKD (dotted line) was significantly worse compared with those who did not develop CKD (solid line) (88.9% vs 97.6%, P=0.036). (B) Recipients survival for patients whose R1 >-17 (dotted line) was significantly worse compared with those whose R1 ≤-17 (solid line) (90.2% vs 99.0%, P=0.01)
Figure 4. One-year survival in patients with or without postoperative NOCKD. (A) Kaplan-Meier plot showed that recipient survival for patients who developed CKD (dotted line) was significantly worse compared with those who did not develop CKD (solid line) (88.9% vs 97.6%, P=0.036). (B) Recipients survival for patients whose R1 >-17 (dotted line) was significantly worse compared with those whose R1 ≤-17 (solid line) (90.2% vs 99.0%, P=0.01) Tables
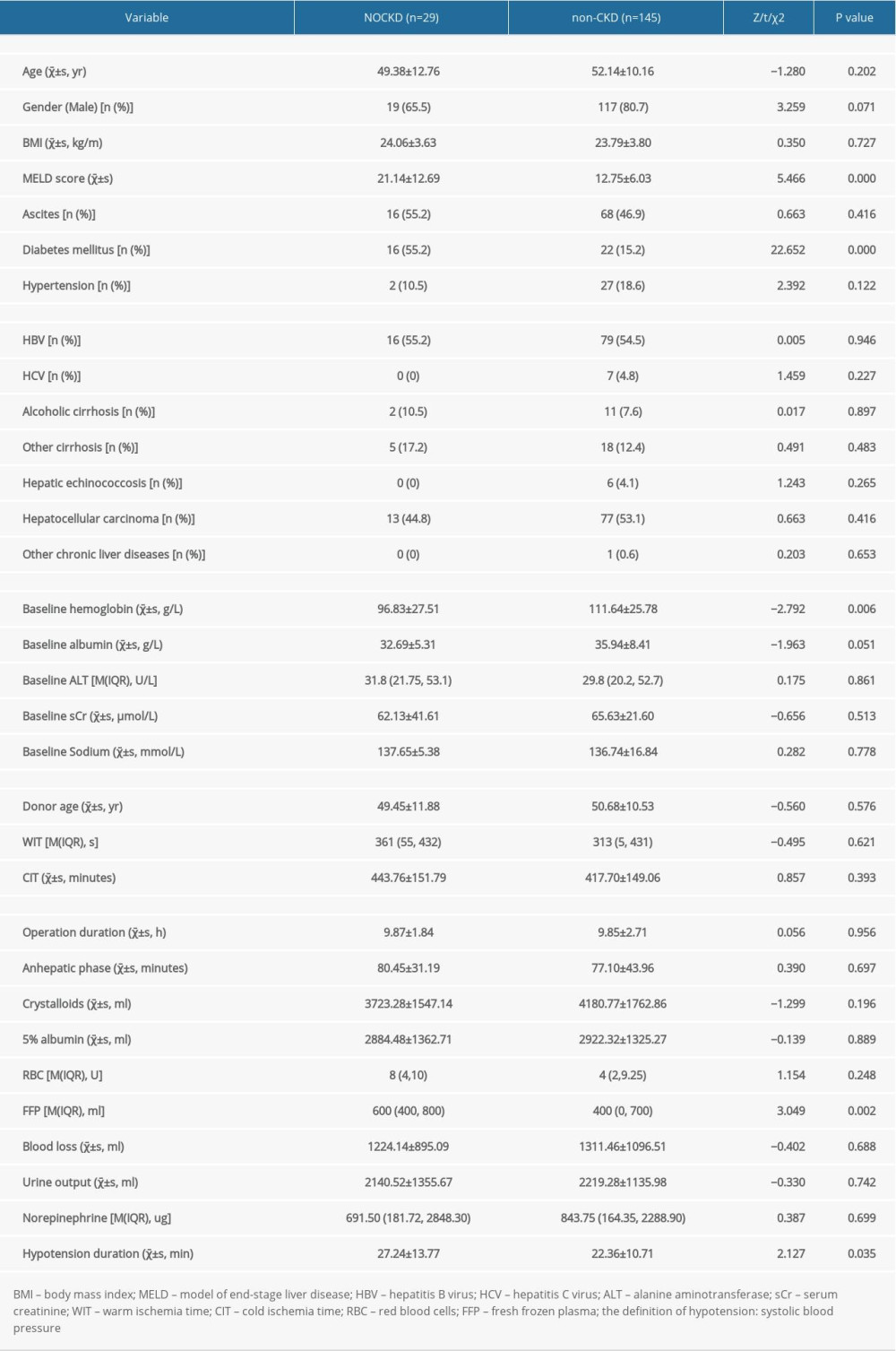 Table 1. Demographic and characteristics of the 174 patients, stratified by NOCKD.
Table 1. Demographic and characteristics of the 174 patients, stratified by NOCKD.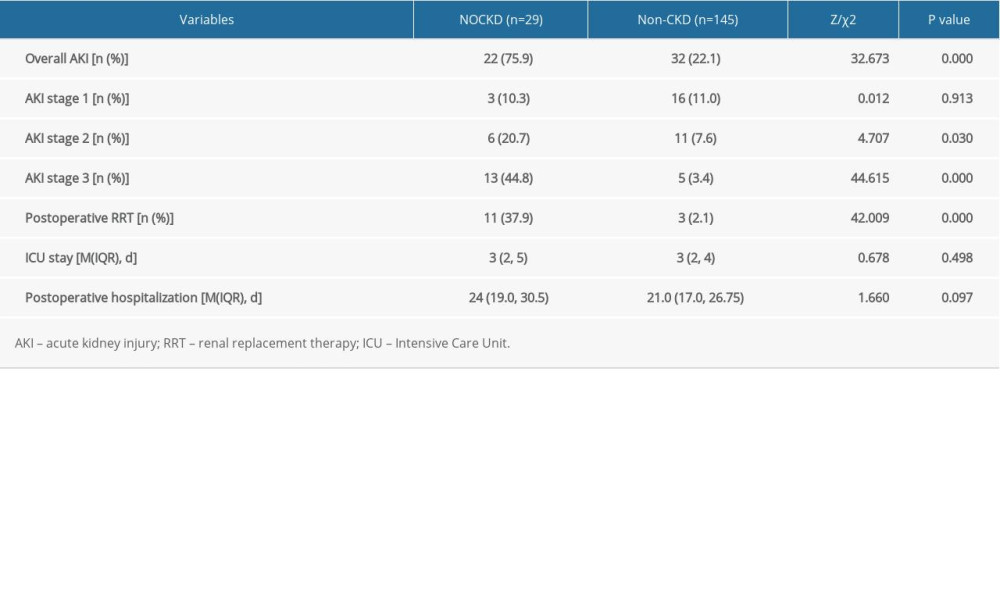 Table 2. Postoperative outcomes of patient with or without NOCKD.
Table 2. Postoperative outcomes of patient with or without NOCKD.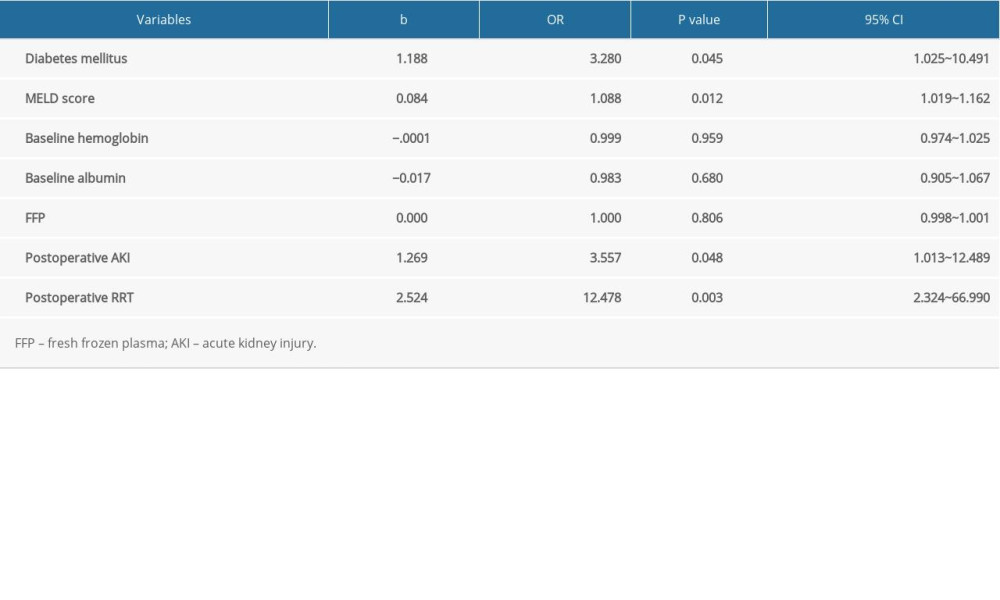 Table 3. Logistic regression analysis of patients with or without NOCKD.
Table 3. Logistic regression analysis of patients with or without NOCKD. Table 1. Demographic and characteristics of the 174 patients, stratified by NOCKD.
Table 1. Demographic and characteristics of the 174 patients, stratified by NOCKD. Table 2. Postoperative outcomes of patient with or without NOCKD.
Table 2. Postoperative outcomes of patient with or without NOCKD. Table 3. Logistic regression analysis of patients with or without NOCKD.
Table 3. Logistic regression analysis of patients with or without NOCKD. In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








