10 September 2021: Clinical Research
Epidemiology and Mortality of Sepsis in Intensive Care Units in Prefecture-Level Cities in Sichuan, China: A Prospective Multicenter Study
Lianghai Cao1ABE, Min Xiao2ABE, Yong Wan3AE*, Chaogui Zhang1B, Xiaofeng Gao4C, Xuemei Chen5B, Xiangde Zheng6B, Xianhua Xiao7B, Mingquan Yang8B, Yuanhua Zhang9BDOI: 10.12659/MSM.932227
Med Sci Monit 2021; 27:e932227
Abstract
BACKGROUND: Studies on the epidemiology of sepsis in intensive care units (ICUs) of prefecture-level hospitals in China are rare. This study aimed to investigate the epidemiological characteristics and mortality risk factors of sepsis in ICUs of tertiary hospitals in Sichuan, China.
MATERIAL AND METHODS: In this prospective, multicenter, observational study, patients admitted to the ICU of 7 tertiary hospitals in Sichuan (China) between October 10, 2017 and January 9, 2018 were screened for sepsis using the Sepsis-3 criteria. Patients with sepsis were included.
RESULTS: Of the 1604 patients screened for sepsis, 294 (18.3%) had sepsis, and 140 (47.6%) had septic shock. Of these, 169 (57.5%) died. Multivariable analysis showed that central nervous system dysfunction (odds ratio [OR]=2.59, 95% confidence interval [CI]: 1.15-5.84, P=0.022), lowest blood phosphorus level (OR=2.56, 95% CI: 1.21-5.44, P=0.014), highest lactate level (OR=1.20, 95% CI: 1.10-1.32, P<0.001), 24-h Acute Physiologic Assessment and Chronic Health Evaluation-II (APACHE-II) score (OR=1.08, 95% CI: 1.03-1.13, P=0.002), and lung infection (OR=2.57, 95% CI: 1.30-5.09, P=0.007) were independently associated with mortality in patients with sepsis.
CONCLUSIONS: The prevalence and mortality rates of sepsis are high in tertiary hospital ICUs in Sichuan, China. The APACHE-II score on day 1 after diagnosis, acute central nervous system dysfunction, lowest blood phosphorus, high serum lactate, and lung infection were independent risk factors of mortality in patients with sepsis.
Keywords: Epidemiology, Mortality, Risk Factors, Sepsis, Shock, Septic, Cities, Female, Humans, Intensive Care Units, Prospective Studies, Tertiary Care Centers
Background
The Third International Consensus Definition of Sepsis (Sepsis-3) defines sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Sepsis occurs when the host response to an infectious pathogen causes life-threatening organ dysfunction, manifested as an increase in the Sequential Organ Failure Assessment (SOFA) score of ≥2 [1]. The SOFA score is an objective assessment of 6 body functions: pulmonary (oxygen requirements), renal (creatinine), neurologic status (Glasgow Coma Score), coagulation (platelet counts), liver function (bilirubin), and cardiovascular (systolic blood pressure) [2]. Septic shock is the ultimate complication of sepsis and leads to death in 39% of inpatients [3]. A meta-analysis of studies from developed countries revealed global annual estimates of about 31.5 million sepsis cases and 19.4 million severe sepsis cases, with potentially 5.3 million deaths in the hospital setting [4]. Mortality may increase dramatically with the aging of the population [5]. Most patients with sepsis require treatment in an Intensive Care Unit (ICU), which puts tremendous emotional and financial pressure on patients, their families, and society.
A good deal of research goes into the study of sepsis, but most of these studies are focused in developed areas such as Europe and America, whereas other regions are severely lacking in research work, especially Asia and mainland China [6]. Among the few multicenter studies conducted in mainland China, Cheng et al [7] showed that the prevalence of sepsis in surgical ICUs in 10 university-affiliated hospitals in mainland China was 8.7%, and the in-hospital mortality rate was 48.7%. A 2-month prospective observational cohort study of 22 ICUs in China conducted by the Clinical Research Group of Critical Care Medicine found that the prevalence of sepsis in the ICU was 37.3% and that the ICU mortality and hospital mortality rates were 28.7% and 33.5%, respectively [8]. These 2 studies were carried out in top hospitals in economically and medically developed areas of mainland China. As China is still a developing country, the regional economic development and the medical resources and conditions may not be the same across rural and urban areas, so those 2 studies may not reflect the true situation of the sepsis epidemic in the underdeveloped areas of mainland China. Because primary hospitals generally do not have the potential to treat patients with sepsis, and because it is impossible for all patients with sepsis to go to the top hospitals in the provincial capital cities for treatment, the vast majority of patients are found in the ICU of tertiary hospitals.
Sichuan Province is in the western part of China, which comprises less developed areas in mainland China. This study aimed to investigate the epidemiology of sepsis in ICU patients in the prefecture-level cities of Sichuan Province. The results reflect the sepsis epidemiology in areas of China with an underdeveloped economy and scarce medical resources.
Material and Methods
PATIENTS:
This was a multicenter prospective study of patients who were admitted to the comprehensive ICU of 7 tertiary hospitals in Sichuan from October 10, 2017 to January 9, 2018. Patients who were expected to stay in the ICU <24 h or who were <18 years of age were excluded. The patients diagnosed with sepsis according to the criteria of Sepsis-3 [1] were included in the mortality analysis. For patients with repeated infections during ICU admission, only the data related to the first sepsis event were collected [8]. The patients diagnosed with sepsis were divided into the septic shock and no septic shock groups.
The study was approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College (No. 2017ER (R) 022). The committee waived the requirement of obtaining written informed consent because this study was non-interventional, only observational in nature, and because of the difficulties in communicating with the patients. The study was approved by the ethics committees of all other participating hospitals under the same conditions. This study was registered in the China Clinical Trial Center (#ChiCTR-EOC-17012679).
STUDY PROCEDURE:
The patients were screened for sepsis every day in the ICU according to the Sepsis-3 criteria [1]. Hospital-acquired infections refer to infections acquired within 48 h of admission or discharge. Acquired infections in the ICU are defined as infections occurring within 48 h after admission to ICU. Multidrug-resistant bacteria (MDRB) refer to those bacteria that are usually sensitive to 3 or more commonly used antibiotics and show resistance at the same time. Mixed infection refers to an infection involving 2 or more different pathogenic microorganisms at the same time. Multisite infection refers to an infection occurring in 2 or more parts of a patient. When the same pathogen is detected in different parts of a patient, only 1 infection of this pathogen is recorded.
DATA COLLECTION AND DEFINITIONS:
Data were collected from patient records and hospital electronic databases. Each ICU provided a person in charge and at least 1 researcher. The researchers identified the patients and collected data on paper forms during the study period. The paper forms were collected by the Affiliated Hospital of North Sichuan Medical College after study completion. The integrity and accuracy of data collection were audited by specialists appointed by the main research institution. After eliminating the omissions and logical errors, the specialists double-input the data into the pre-designed Epidata 3.0 database and checked the correctness of the final data.
The data included age, sex, place of living, patient sources, major diseases for ICU admission, comorbidities, infection type, etiology, Acute Physiologic Assessment and Chronic Health Evaluation-II (APACHE-II) score within 24 h after diagnosis, the components of the SOFA score, the highest SOFA score within 24 h after diagnosis, the minimum and maximum biochemical indicators during ICU stay and after diagnosis, duration of ventilation, ICU hospitalization time, total hospitalization time, ICU intervention measures, and mortality during the hospital stay. The mortality included deaths during hospitalization and the deaths that were confirmed by phone after discharge.
STATISTICAL ANALYSIS:
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The data related to age, the APACHE-II score on the first day after diagnosis, SOFA score on the first day, ICU hospitalization time, total hospitalization time, mechanical ventilation time, and laboratory indices of patients with sepsis were not normally distributed according to the Shapiro-Wilk test, so the median (Q1, Q3) was used to describe them, and the group design rank-sum test was used for analysis. Categorical data are presented as n (%) and analyzed using Fisher’s exact test. Age and APACHE-II scores in the different prognosis groups were compared. Multiple comparisons were adjusted using the Bonferroni method. The variables with P<0.05 were analyzed by multivariable logistic regression using the forward method. P<0.05 was considered statistically significant.
Results
PARTICIPANTS:
A total of 2018 patients were admitted to the 7 ICUs. The characteristics of the ICUs are shown in Table 1. After excluding 52 re-transfers and 362 patients who did not meet the eligibility criteria, 1604 patients were screened for sepsis, of which 294 were diagnosed with sepsis. Thus, the prevalence of sepsis in ICU patients was 18.3% (294/1604), and 140/294 (47.6%) of these sepsis patients had septic shock.
CHARACTERISTICS OF THE PATIENTS:
Table 2 presents the characteristics of the patients. Men accounted for 62.9% (n=185) of the subjects. The median age was 67 (IQR, 55–76) years. Rural household registration accounted for 61.2% (n=180) of all registrations. More than half of the patients (51.4%) were transferred from general wards/ICUs in other hospitals. Respiratory diseases (38.4%) and digestive system diseases (31.3%) were the major diseases leading to ICU admission of patients with sepsis. More than two-thirds of the patients (69.4%) had comorbidities or limited ambulation. Patients with 4 or more dysfunctional organs accounted for 66.2% (n=194) of all registrations, and the respiratory system (96.2%) was found to be most affected. Of the 2 infection types, community-acquired infections accounted for most patients (84.0%).
The most common sites of infection were the lungs (n=191, 65%) and abdomen (n=104, 35.4%). Urinary and hematogenous infections accounted for 8.8% and 6.1% of cases, respectively. Multiple-site infections (>2 site infections) accounted for 24% of cases. The mortality rates of patients with lung infection and abdomen infection in the septic shock group were both higher than those in the non-septic shock group (lung: 74.4% vs 57.5%, P=0.017; abdomen: 57.1% vs 36.6%, P=0.04) (Table 3).
Table 4 presents the median biochemical and hematological scores, and hospitalization characteristics of the patients. The APACHE-II and SOFA scores on the first day were 26 (21–32) and 10 (7–12), duration of ventilation was 48 (11–130) h, ICU stay was 2 (2–8) days, and hospitalization duration was 8 (2–19) days.
The APACHE-II score within 24 h after diagnosis of sepsis was higher in the death group than in the survival group (29 [IQR, 24–36] vs 23 [IQR, 17.5–26], P<0.001). The SOFA score was higher in the death group than in the survival group (11 [IQR, 8–14] vs 8 [IQR, 6–10], P<0.001). The mechanical ventilation time after diagnosis of sepsis (including invasive and non-invasive) was longer in the death group than in the survival group (55 [IQR, 17–140] vs 37 [IQR, 0–116], P=0.049). ICU hospitalization and total hospitalization time were shorter in the death group than in the survival group (4.00 [IQR, 2–8] vs 6 [IQR, 4–10], P<0.001), (3 [IQR, 1–8] vs 18 [IQR, 11–26.5], P<0.001). The lowest level of serum phosphorus was higher in the death group than in the survival group (0.90 [0.58–1.36] vs 0.69 [0.49–0.90], P<0.001). The highest level of serum lactate was higher in the death group than in the survival group (6.5 [3.2–10.1] vs 3.3 [2.1–4.7], P<0.001).
MICROORGANISMS:
Of the 294 patients, 25 were not tested for pathogenic microorganisms (Table 5). A total of 139 pathogenic microorganisms were cultured from 136 specimens obtained from 117 patients within 48 h after the diagnosis of sepsis. Of them, 70 (50.4%) were gram-negative bacteria, 48 (34.5%) were gram-positive bacteria, and 19 (13.7%) were fungi. Of those bacteria, 48 (34.5%) were multidrug-resistant. The most common bacteria were Klebsiella pneumoniae and Escherichia coli (Table 5).
RISK FACTORS FOR MORTALITY:
A total of 37 patients with sepsis died during hospitalization, and 132 patients who gave up treatment and then were discharged died either due to severe illnesses or due to other reasons, which was confirmed over the telephone. One patient was still hospitalized. The mortality rates due to sepsis and septic shock were 51.9% and 63.6%, respectively (Table 2), and the overall mortality due to sepsis and septic shock in the ICU during the observation period was 57.5%.
Univariable analyses were performed using death as the dependent variable, and acute dysfunction of the central nervous system, lowest blood phosphorus, highest lactate, 24-h APACHE-II score, and lung infection were selected as variables for the multivariable analysis. The results of the multivariable analysis showed that central nervous system dysfunction (odds ratio [OR]=2.59, 95% confidence interval [CI]: 1.15–5.84, P=0.022), lowest blood phosphorus (OR=2.56, 95% CI: 1.21–5.44 P=0.014), highest lactate (OR=1.20, 95% CI: 1.10–1.32, P<0.001), 24-h APACHE-II score (1.08, 95% CI: 1.03–1.13, P=0.002), and lung infection (OR=2.57, 95% CI: 1.30–5.09, P=0.007) were independently associated with mortality in patients with sepsis (Table 6).
Discussion
In this study, the prevalence of sepsis in ICU patients was 18.3%. The most common sites of infection are the lungs and abdomen, and half of the pathogens were gram-negative bacteria. The mortality rate of sepsis in the ICU was 57.5%. The mortality rates of sepsis without shock and with septic shock were 51.9% and 63.6%, respectively. Acute central nervous system dysfunction, lowest blood phosphorus, highest lactate, 24-h APACHE-II, and lung infection were independently associated with mortality of patients with sepsis. Septic encephalopathy and pulmonary infection are high-risk factors for death from sepsis. Many similar studies have been carried out, but there are few studies on blood phosphorus as a high-risk factor, which is a promising topic for future discussion.
Compared with previous studies, the prevalence of sepsis among ICU patients in this study was similar to that in ICUs in tertiary hospitals in Thailand [9], higher than that in ICUs in Italy [10], Germany [11], and France [12], but lower than that in 28 ICUs in Europe [13], adult ICUs in Brazil [14], and 22 ICUs in mainland China [8]. The prevalence of sepsis in ICU patients in different countries and regions is different due to various factors. First, sepsis is defined differently in different studies, and different definitions of sepsis may lead to different outcomes. Most studies screened patients based on the definition of severe sepsis from Sepsis-1 [15] or Sepsis-2 [16], and the prevalence of severe sepsis fluctuates between 6.0% and 18.9%, according to the definitions [9,11–13,17–19]. In this study, sepsis was defined according to Sepsis-3, which is more accurate than Sepsis-1 and Sepsis-2 [1]. Second, different inclusion criteria may lead to different results, for example, different age ranges. The 2-month prospective observational cohort study of 22 ICUs in China by the Chinese Critical Care Medical Clinical Research Group included patients >15 years of age, compared with >18 years in this study [8]. Third, different subjects may lead to different results. The prevalence of sepsis in the surgical ICU of 10 affiliated hospitals of universities in mainland China (8.7%) [7] was slightly more than the prevalence of severe sepsis in the surgical ICU of northern Taiwan (6.9%) [20]. The Clinical Research Group of Critical Disease Medicine in China studied the prevalence of sepsis in 22 ICUs in mainland China and found the prevalence to be 37.3%, and of those 22 ICUs, 18 were comprehensive ICUs, 3 were surgical ICUs, and 1 was an internal ICU. The subjects were mostly medical patients [8]. Finally, the season of study [12,13,21] and age composition [5,22,23] may also have an impact on the degree of prevalence of sepsis in ICUs.
Compared with previous studies, the mortality rate of sepsis patients in this study was similar to that of severe sepsis patients in Indian ICUs [24] and Turkish ICUs [25], but higher than that of most previous studies [8,18,26–29]. There are many reasons for the high mortality rate. Differences in the APACHE-II and SOFA scores, 2 different scoring systems used to evaluate patients with sepsis, can affect the final outcomes of sepsis. Nevertheless, consistent with previous studies [7,9,25,28], this study found that a high APACHE-II score on the first day after diagnosis was an independent risk factor for sepsis mortality. Second, economic and medical conditions also lead to a high mortality rate. Studies have shown that the mortality rate of sepsis patients is related to the availability of adequate resources and appropriate treatment [13,14,25]. The fatality rate of sepsis in developed areas is lower than that in underdeveloped areas. A meta-analysis of studies conducted over the past 10 years showed that the in-hospital mortality rate of sepsis patients and that of severe sepsis patients was [4] very similar to that revealed by another study of 28 ICUs in developed European countries [13]. On the other hand, a multicenter study in Asia showed that the in-hospital mortality rate of sepsis was high [27]. The in-hospital mortality rate of severe sepsis patients in Australia and New Zealand is also high (38.0%) [18]. The mortality rate of severe sepsis and septic shock patients in Turkish ICUs was found to be even higher (55.7% and 70.4%, respectively) [25]. In India, the situation is no better: ICU mortality rate, in-hospital mortality rate, and 28-day mortality rate are high (56%, 63.6%, and 62.8%, respectively) [24]. The in-hospital mortality rate of severe sepsis patients in China is 33.5–48.7% [7,8]. This difference may be due to poor fluid resuscitation and cluster management of sepsis patients in most parts of Asia [27]. This study was conducted in prefecture-level cities in Sichuan Province, where the economy and medical treatment facilities are underdeveloped, so the mortality rate was relatively high. The season of study is also a factor affecting the mortality rate of sepsis patients. This study was conducted in autumn and winter, so the mortality rate was relatively high.
The reasons for the high mortality rate in less developed areas are as follows. First, the doctors’ lack of awareness of sepsis can play a major role [30,31], and medical staff in underdeveloped areas may have few opportunities to receive critical medical education and professional training. Second, medical resources are insufficient in less developed countries or regions compared with developed countries in Europe and North America [32,33]. Providing adequate medical resources and training and education on sepsis management to clinicians will help reduce the mortality rate of sepsis patients. The training of clinical doctors and nurses should include not only ICU doctors but also all clinical doctors and nurses attending sepsis patients in areas such as emergency, anesthesia, and surgery.
The results of this study are consistent with the results of Chinese and foreign studies in that across all studies, the most common sites of infection were found to be the lungs and abdomen [7,8,13,18, 34,35]. More than half of the pathogenic bacteria were gram-negative bacteria, a finding similar to the Chinese research results [7,8]. In thisstudy,
This study has certain limitations. Because some blood indicators were not tested, the results may be biased. Most people in China want to visit their relatives before their relatives die. Since ICU wards do not allow visitors, the families often choose to take the patients home before they die. In China, it is considered impolite for a doctor to follow up on a patient’s condition after the patient’s death. Therefore, some bias may arise due to non-confirmed data.
Conclusions
This study showed that the prevalence of sepsis and mortality of sepsis patients in ICUs of Grade A level III hospitals in prefecture-level cities in Sichuan were higher than those in developed countries and in other regions of China. The APACHE-II score on the first day after diagnosis, acute organ dysfunction involving the central nervous system, lowest blood P, high serum lactate, and lung infection were independent risk factors associated with death of patients with sepsis.
Tables
Table 1. Characteristics of the participating ICUs.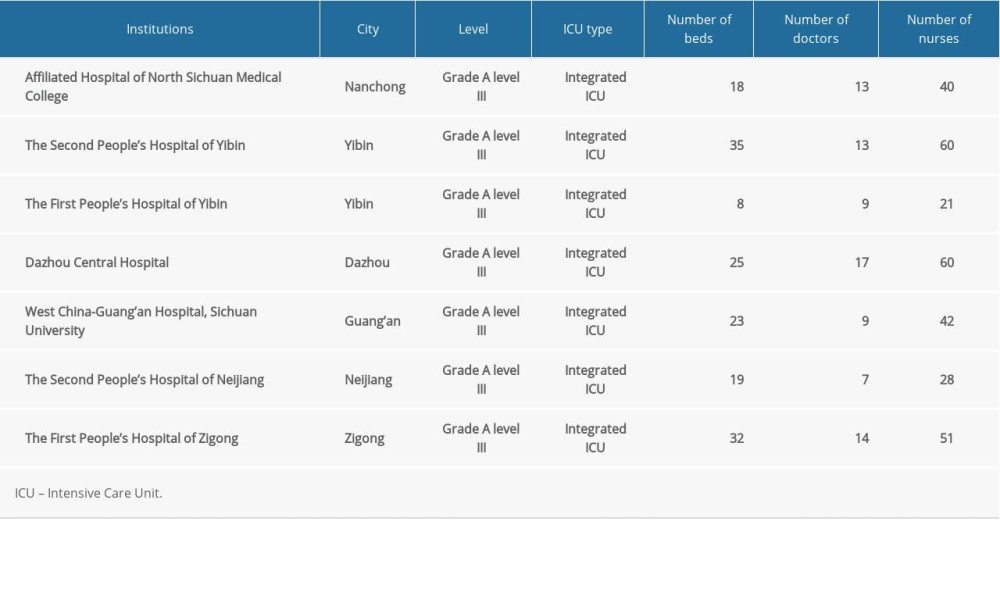 Table 2. Characteristics of patients with sepsis.
Table 2. Characteristics of patients with sepsis.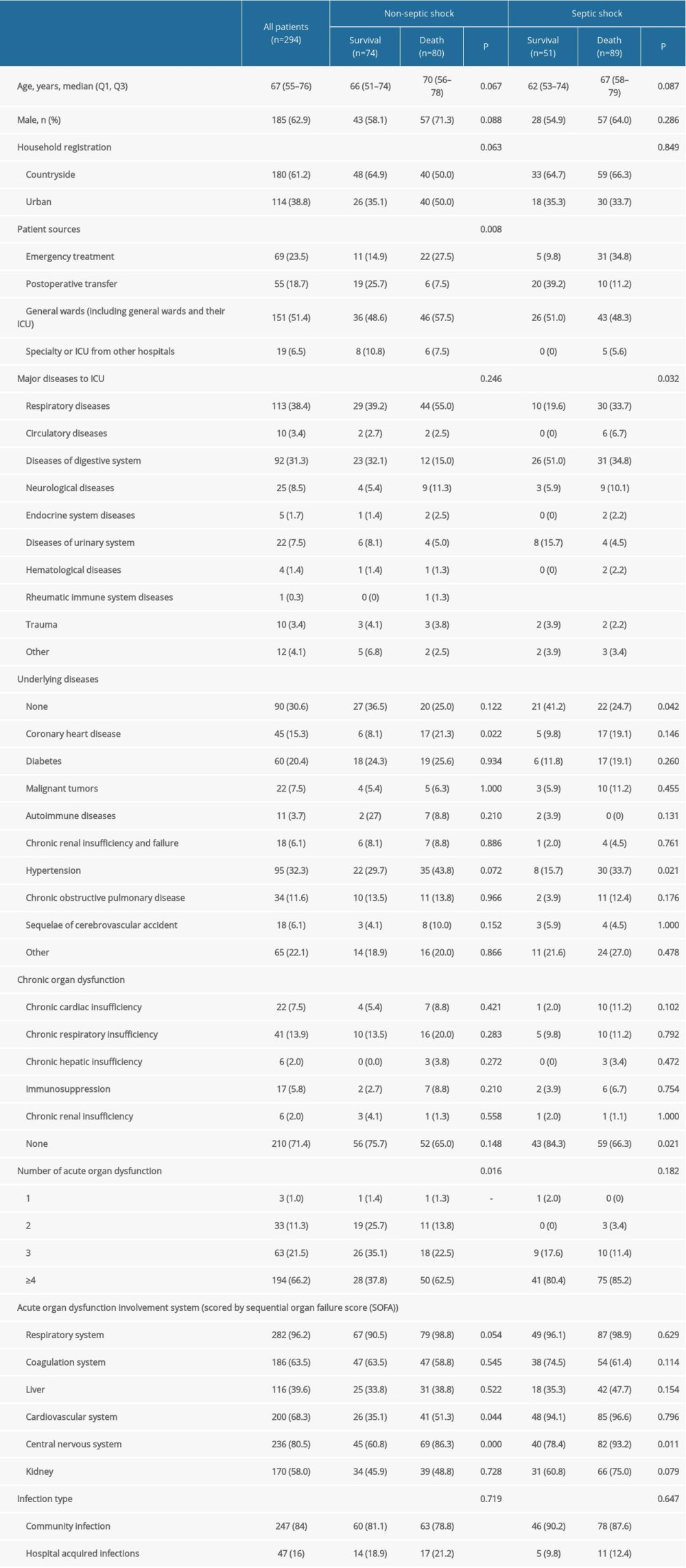 Table 3. Comparison of mortality rate in patients with different infection sites between non-septic shock and septic shock groups.
Table 3. Comparison of mortality rate in patients with different infection sites between non-septic shock and septic shock groups.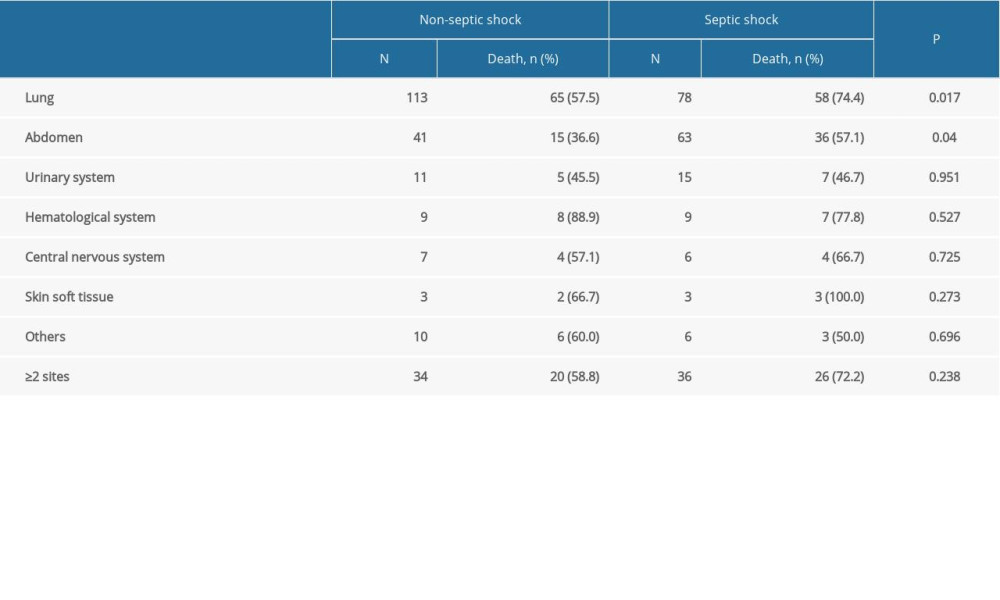 Table 4. Laboratory indices, severity scores, and hospitalization characteristics of sepsis patients during ICU admission.
Table 4. Laboratory indices, severity scores, and hospitalization characteristics of sepsis patients during ICU admission.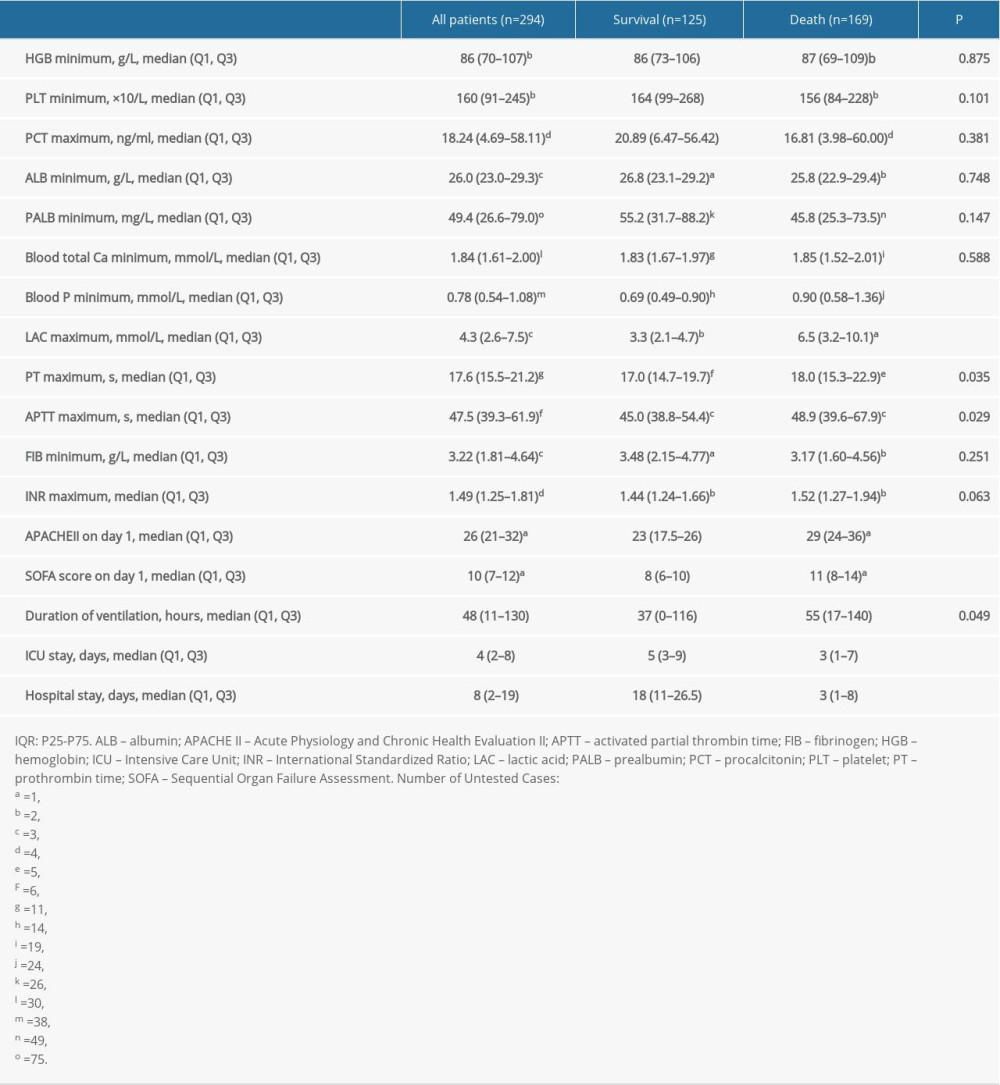 Table 5. Pathogenic microorganisms of sepsis (%).
Table 5. Pathogenic microorganisms of sepsis (%).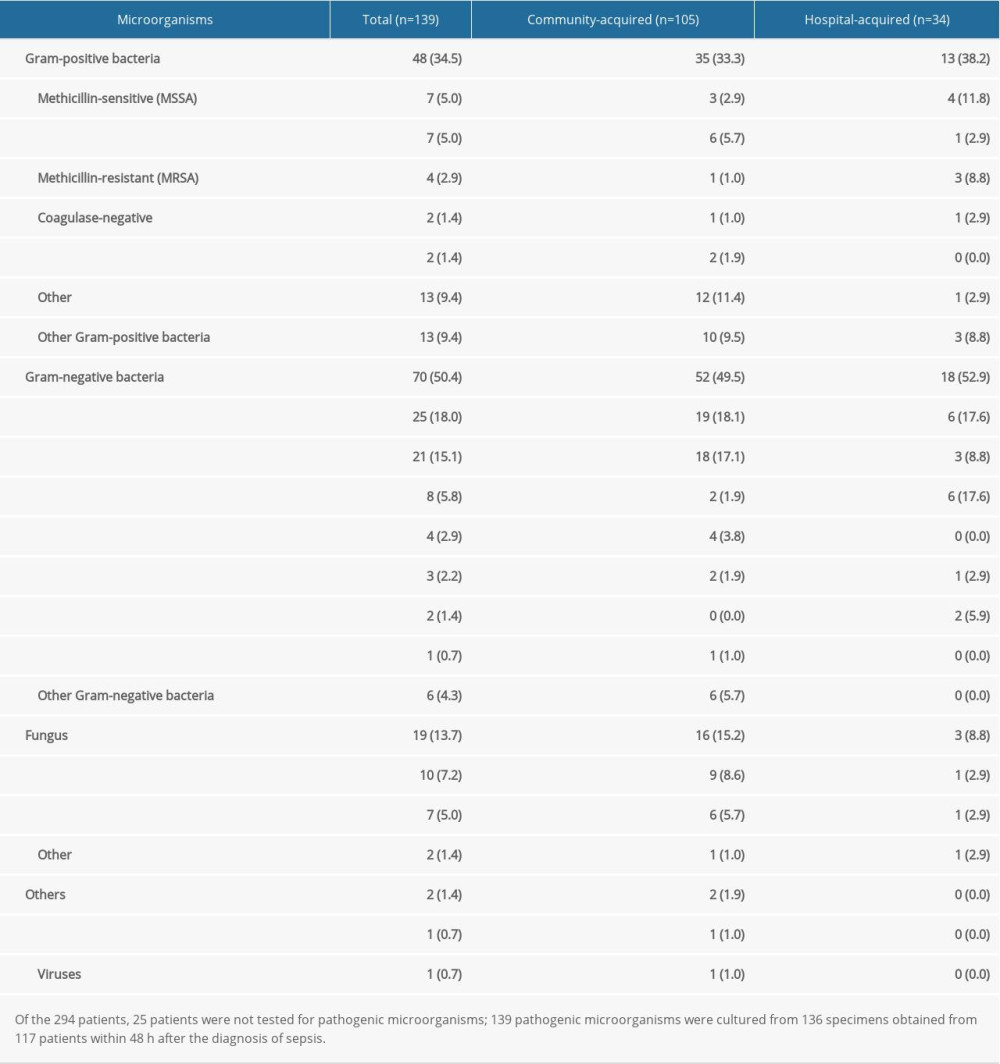 Table 6. Univariable and multivariable logistic analysis for mortality in sepsis patients.
Table 6. Univariable and multivariable logistic analysis for mortality in sepsis patients.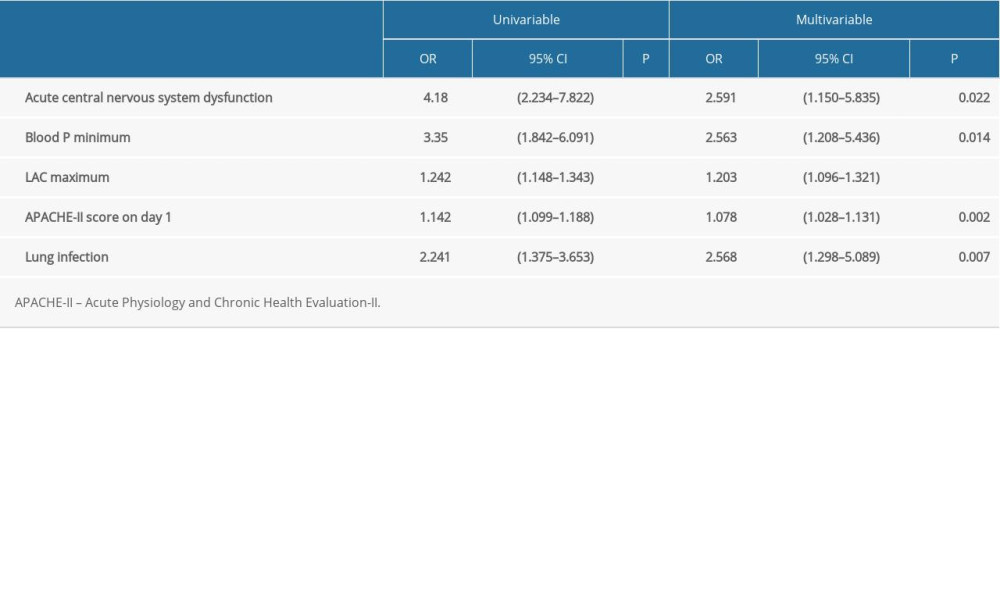
References
1. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315; 801-10
2. Lambden S, Laterre PF, Levy MM, Francois B, The SOFA score-development, utility and challenges of accurate assessment in clinical trials: Crit Care, 2019; 23; 374
3. Vincent JL, Jones G, David S, Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis: Crit Care, 2019; 23; 196
4. Fleischmann C, Scherag A, Adhikari NK, Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations: Am J Respir Crit Care Med, 2016; 193; 259-72
5. Angus DC, Linde-Zwirble WT, Lidicker J, Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care: Crit Care Med, 2001; 29; 1303-10
6. Liao X, Du B, Lu M, Current epidemiology of sepsis in mainland China: Ann Transl Med, 2016; 4; 324
7. Cheng B, Xie G, Yao S, Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China: Crit Care Med, 2007; 35; 2538-46
8. Zhou J, Qian C, Zhao M, Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China: PLoS One, 2014; 9; e107181
9. Khwannimit B, Bhurayanontachai R, The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting: Epidemiol Infect, 2009; 137; 1333-41
10. Salvo I, de Cian W, Musicco M, The Italian SEPSIS study: Preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock: Intensive Care Med, 1995; 21(Suppl 2); S244-49
11. Engel C, Brunkhorst FM, Bone HG, Epidemiology of sepsis in Germany: Results from a national prospective multicenter study: Intensive Care Med, 2007; 33; 606-18
12. Brun-Buisson C, Meshaka P, Pinton P, Vallet B, EPISEPSIS: A reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units: Intensive Care Med, 2004; 30; 580-88
13. Vincent JL, Sakr Y, Sprung CL, Sepsis in European intensive care units: Results of the SOAP study: Crit Care Med, 2006; 34; 344-53
14. Machado FR, Cavalcanti AB, Bozza FA, The epidemiology of sepsis in Brazilian Intensive Care Units (the Sepsis PREvalence Assessment Database, SPREAD): An observational study: Lancet Infect Dis, 2017; 17; 1180-89
15. Bone RC, Balk RA, Cerra FB, Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine: Chest, 1992; 101; 1644-55
16. Levy MM, Fink MP, Marshall JC, 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference: Intensive Care Med, 2003; 29; 530-38
17. Brun-Buisson C, Doyon F, Carlet J, Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis: JAMA, 1995; 274; 968-74
18. Finfer S, Bellomo R, Lipman J, Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units: Intensive Care Med, 2004; 30; 589-96
19. Karlsson S, Varpula M, Ruokonen E, Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: The Finnsepsis study: Intensive Care Med, 2007; 33; 435-43
20. Huang CT, Tsai YJ, Tsai PR, Epidemiology and outcome of severe sepsis and septic shock in surgical Intensive Care Units in Northern Taiwan: Medicine (Baltimore), 2015; 94; e2136
21. Danai PA, Sinha S, Moss M, Haber MJ, Martin GS, Seasonal variation in the epidemiology of sepsis: Crit Care Med, 2007; 35; 410-15
22. Martin GS, Mannino DM, Eaton S, Moss M, The epidemiology of sepsis in the United States from 1979 through 2000: N Engl J Med, 2003; 348; 1546-54
23. Kaukonen KM, Bailey M, Suzuki S, Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand. 2000–2012: JAMA, 2014; 311; 1308-16
24. Chatterjee S, Bhattacharya M, Todi SK, Epidemiology of adult-population sepsis in India: A single center 5 year experience: Indian J Crit Care Med, 2017; 21; 573-77
25. Baykara N, Akalın H, Arslantaş MK, Epidemiology of sepsis in Intensive Care Units in Turkey: A multicenter, point-prevalence study: Crit Care, 2018; 22; 93
26. Silva E, de Pedro MA, Sogayar AC, Brazilian Sepsis Epidemiological Study (BASES study): Crit Care, 2004; 8; R251-60
27. Phua J, Koh Y, Du B, Management of severe sepsis in patients admitted to Asian Intensive Care Units: Prospective cohort study: BMJ, 2011; 342; d3245
28. Herrán-Monge R, Muriel-Bombín A, García-García MM, Epidemiology and changes in mortality of sepsis after the implementation of surviving sepsis campaign guidelines: J Intensive Care Med, 2019; 34; 740-50
29. Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K, Epidemiology of sepsis and septic shock in Critical Care Units: Comparison between sepsis-2 and sepsis-3 populations using a national critical care database: Br J Anaesth, 2017; 119; 626-36
30. Tang H, Liu D, Zhang H-Y, Epidemiology of sepsis in ICUs of Western China: J Acute Dis, 2016; 5; 210-15
31. Tufan ZK, Eser FC, Vudali E, The knowledge of the physicians about sepsis bundles is suboptimal: A multicenter survey: J Clin Diagn Res, 2015; 9; Oc13-16
32. Schultz MJ, Dunser MW, Dondorp AM, Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future: Intensive Care Med, 2017; 43; 612-24
33. Baelani I, Jochberger S, Laimer T, Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: A self-reported, continent-wide survey of anaesthesia providers: Crit Care, 2011; 15; R10
34. Záhorec R, Firment J, Straková J, Epidemiology of severe sepsis in Intensive Care Units in the Slovak Republic: Infection, 2005; 33; 122-28
35. Sakr Y, Elia C, Mascia L, Epidemiology and outcome of sepsis syndromes in Italian ICUs: A muticentre, observational cohort study in the region of Piedmont: Minerva Anestesiol, 2013; 79; 993-1002
Tables
 Table 1. Characteristics of the participating ICUs.
Table 1. Characteristics of the participating ICUs. Table 2. Characteristics of patients with sepsis.
Table 2. Characteristics of patients with sepsis. Table 3. Comparison of mortality rate in patients with different infection sites between non-septic shock and septic shock groups.
Table 3. Comparison of mortality rate in patients with different infection sites between non-septic shock and septic shock groups. Table 4. Laboratory indices, severity scores, and hospitalization characteristics of sepsis patients during ICU admission.
Table 4. Laboratory indices, severity scores, and hospitalization characteristics of sepsis patients during ICU admission. Table 5. Pathogenic microorganisms of sepsis (%).
Table 5. Pathogenic microorganisms of sepsis (%). Table 6. Univariable and multivariable logistic analysis for mortality in sepsis patients.
Table 6. Univariable and multivariable logistic analysis for mortality in sepsis patients. In Press
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








