16 September 2021: Clinical Research
Contrast-Enhanced Ultrasound Imaging Features of Focal Splenic Tuberculosis
Ying Zhang1BCE, Tianzhuo Yu1CF, Wenzhi Zhang1BD, Gaoyi Yang1AG*DOI: 10.12659/MSM.932654
Med Sci Monit 2021; 27:e932654
Abstract
BACKGROUND: The aim of this study was to characterize the contrast-enhanced ultrasound imaging features of focal splenic tuberculosis.
MATERIAL AND METHODS: We retrospectively analyzed the conventional ultrasound (US) and contrast-enhanced ultrasound (CEUS) imaging features of 22 patients with splenic TB confirmed by surgical histopathology or biopsy.
RESULTS: Conventional US demonstrated that 15 of the 22 patients had a single lesion, while 7 had multiple lesions. The maximum diameter of the lesions ranged from 1.0 to 3.7 cm. Of the 22, 17 were detected with hypoecho and 5 were detected with complex echo by conventional US. Seven (7/22) were detected with blood flow signals by color Doppler flow image (CDFI). CUES demonstrated that 18 cases (81.8%, 18/22) began to enhance in the arterial phase, mostly followed by slow wash-out in the intermediate or late parenchymal phase, and 4 (18.2%, 4/22) presented with non-enhancement during all phases. The enhancement patterns were categorized into 4 types: Type I, homogeneous enhancement (2/22); Type II, rim-like enhancement (12/22); Type III, septation-like enhancement (4/22); and Type IV, non-enhancement (4/22).
CONCLUSIONS: CEUS showed that splenic TB lesions were enhanced in the arterial phase, followed by slow washed out or persistent enhancement in the intermediate and late parenchymal phases. The rim- or septation-like enhancement may be helpful for diagnosing splenic TB. The splenic lesions presenting round hypoecho by conventional US and complete non-enhancement by CEUS are highly suspicious of splenic TB.
Keywords: Hospitals, Chronic Disease, Splenic Diseases, Ultrasonography, Doppler, Adolescent, Contrast Media, Diagnosis, Differential, Female, Humans, Image Enhancement, Spleen, Tuberculosis, Splenic, Ultrasonography, young adult
Background
Splenic tuberculosis (TB) is a rare disorder that generally results from the dissemination of pulmonary or biliary TB and was first reported by Coley in 1846 [1,2]. The most common manifestations of splenic TB are splenomegaly (13.2–100%), fever (82.3%), and weight loss and fatigue (44.12%) [3]. It was reported that splenic tuberculosis accounted for nearly 10% of abdominal TB [1] and the spleen is the third most common organ involved in miliary TB, with incidence rates up to 75% [4,5]. Moreover, splenic TB was found in up to 80–100% of autopsied patients with disseminated tuberculosis [6,7]. With the increasing number of drug abusers, HIV carriers, immunosuppressor use, and drug-resistant-mycobacterium tuberculosis, the incidence of splenic TB is estimated to be increasing [7,8].
Previous studies used contrast-enhanced ultrasound (CEUS) to identify lesions in the spleen and found that CEUS could reflect the blood flow of the normal and abnormal tissues, improve the diagnostic sensitivity, and contribute to the differential diagnosis between the benign and malignant lesions involved in the spleen [9–11]. However, there are few CEUS studies characterizing splenic TB [9–11]. The present study retrospectively analyzed the CEUS imaging features of 22 patients with splenic TB, aiming to provide more information for clinical diagnosis.
Material and Methods
PATIENTS:
We retrospectively reviewed 22 patients who were diagnosed with splenic tuberculosis confirmed by biopsy (n=18) or surgical histopathology (n=4) and who underwent both conventional US and CEUS examinations prior to biopsy or surgery from January 2010 to September 2018. All the splenic tuberculosis was caused by
The criterion standard method of splenic tuberculosis diagnosis involves performing a splenectomy and taking biopsies [3,12]. Considering the present multidrug-resistant TB surge and extensive drug-resistant tuberculosis cases, invasive procedures such as splenic biopsy/resection are encouraged for further confirmation [3]. Splenic trauma and due to radical surgical oncological clearance of adjacent tumor is an indication for splenectomy [13]. In this study, 1 patient underwent radical surgery for pancreatic carcinoma, and incidental detection of splenic TB was seen in 3 patients who underwent splenectomy for trauma. Informed consent was obtained from all enrolled patients before CEUS examinations and biopsy. The inclusion criteria of patients were: 1) presence of at least 1 splenic nodule; 2) written informed consent; and 3) absence of CEUS-related contraindications and no allergy to the contrast agent; 4) preoperative routine examinations of coagulation function were normal; 5) all cases had histopathological results. This was a retrospective study and was approved by the Hospital Ethics Committee to use the patient database.
US EXAMINATION:
We used an iU22 Ultrasound instrument (Philips, Amsterdam, The Netherlands) with C5–1, L12–5, and L9–3 probes, and the corresponding frequency was 1–5 MHz, 5–12 MHz, and 3–9 MHz, respectively. All of the patients underwent conventional US examinations in supine or right lateral position. The number, diameter, shape, border, echoic pattern, liquid, calcification, and blood distribution of the splenic nodules were detected. Since lesions with diameter less than 1.0 cm are easily missed by the low spatial resolution of conventional US, they were not included in this study. For cases with multiple splenic nodules, the largest nodule was chosen for further characterization.
CEUS EXAMINATION:
For CEUS examination, SonoVue (Bracco, Milan, Italy) diluted with 5 mL saline was applied as a contrast agent. Following intravenous injection of 2.4 mL contrast agent, the perfusion status and the echoic pattern of the lesions were observed in real time for up to 5 min. All of the images were recorded and stored in a computer. Digital cine clips of splenic lesions were registered during arterial phase (5–30 s after injection), intermediate parenchymal phase (60–90 s after injection), and late parenchymal phase (180–300 s after injection) [14]. Compared with the surrounding splenic parenchyma, the enhancement pattern of the lesions was classified into hyper-enhancement, iso-enhancement, hypo-enhancement, and non-enhancement; the enhancement distribution pattern was classified into homogeneous enhancement, rim-like enhancement, and septation-like enhancement.
Conventional US and CEUS were both conducted by radiologists with over 5 years’ experience. The images were analyzed by 2 experienced radiologists and discordant findings were discussed by both experts to reach a consensus.
STATISTICAL ANALYSIS:
Values are presented as mean±SD and were analyzed with SPSS software (version 23.0; IBM SPSS Statistics).
Results
PATIENT CHARACTERISTICS:
The male-to-female ratio was 13: 9 and the mean age of the 22 patients was 30.7 years (range 18–50 years). Of the 22 cases, 8 were accompanied with pulmonary TB, 6 were with peritonitis TB, 3 were with cervical tuberculous lymphadenitis, and 2 were with multiple organs TB. In addition, 10 of the 22 patients had immunodeficiency, including 1 patient with acquired immune deficiency syndrome (AIDS), while the other 12 patients were immunocompetent. The common clinical manifestations of the 22 included patients were fatigue complicated with weight loss (n=6) or with fever (n=2).
CONVENTIONAL US:
Findings from conventional US examinations showed that 15 patients had a single splenic lesion and 7 patients had multiple splenic lesions, the number of which ranged from 2 to 9. Of the 22 cases, 2 lesions were located at the splenic capsule with a fusiform shape and the others were all located in the spleen parenchyma with a round shape. The mean diameter of the lesions was 2.3±0.6 cm (range 1.0–3.7 cm). Of all 22 lesions, 17 presented with hypoecho and 5 were with complex echo. Color Doppler flow imaging (CDFI) demonstrated that 7 of the 22 cases were detected with punctate blood flow signal and others were with no blood flow signal.
CEUS:
Of the 22 lesions, 18 lesions began enhancement in arterial phase. Of these 18 lesions, 1 exhibited a “rapid wash-out” enhancement manner, starting to wash out in the arterial phase and presenting hypo-enhancement in the intermediate and late parenchymal phases; 2 started to wash out in the intermediate parenchymal phase and presented iso- or hypo-enhancement compared to the peripheral splenic parenchyma in the late parenchymal phase; and the remaining 15 exhibited a “slow wash-out” manner, starting to wash out in the late parenchymal phase and presented iso- or hypo-enhancement. Among the 22 lesions, 4 were observed with non-enhancement during the whole arterial, intermediate parenchymal, and late parenchymal phases.
According to the peak enhancement conditions of the lesions, they were categorized into 4 types. Type I was homogeneous enhancement (n=2, 9.1%), in which the lesions were homogeneously enhanced with hyper-enhancement compared to the peripheral splenic parenchyma, followed by wash-out with hypo-enhancement (Figure 1); Type II was rim-like enhancement (n=12, 54.5%), in which the lesion was enhanced in its periphery in a rim-like shape and non-enhancement in its interior, it could be washed out, and it presented iso- or hypo-enhancement (Figure 2); Type III was septation-like enhancement (n=4, 18.2%), in which the interior of the lesion showed septation-like hyper-enhancement followed by almost complete wash-out (Figures 3, 4); and Type IV was non-enhancement (n=4, 18.2%), in which the lesion was non-enhanced during the whole arterial, intermediate parenchymal, and late parenchymal phases and presented a well-defined border (Figure 5). In this study, there were no significant differences in CEUS between the 10 immunocompromised and 12 immunocompetent patients.
Discussion
Primary and isolated splenic TB is an extremely rare disease. However, it is a common finding in patients with disseminated or miliary tuberculosis [5,15]. Its clinical manifestation can be only the splenic enlargement complicated with several tiny splenic lesions that cannot be visualized by the naked eye during surgery or by imaging examinations [1,6,16]. It is commonly characterized by a focal nodule in the splenic parenchyma as detected by imaging, and can be divided into micronodules with diameter less than 1.0 cm and macronodules with diameter no less than 1.0 cm [12,17]. However, since the assessment of micronodular lesions is limited by the low spatial resolution of conventional US, in the present study we only enrolled the macronodular splenic lesions. As shown by gray-scale US, the diameter of the enrolled lesions ranged from 1.0 to 3.7 cm, and 20 of the 22 lesions were round nodules, with 17 (77.2%) presenting hypoecho and 5 (22.7%) presenting complex echo.
Previous studies reported that CDFI had limited value in evaluating the blood flow of the splenic lesions, and could not provide much valuable information in differentiation between benign and malignant splenic lesions, as most splenic lesions are avascular or hypovascular except for intra-splenic pseudo-aneurysms [18]. In our study, 15 cases were not detected with any blood flow signal by CDFI, and 11 of these 15 cases presented with contrast enhancement as detected by CEUS. This indicated that compared with conventional US and CDFI, CUES can more accurately and sensitively reflect the blood flow of the lesions. CEUS is a rapidly growing ultrasound technology that can visualize the low-speed blood flow and detect the perfusion characteristics in real time, thereby improving the diagnosis of splenic lesions [9–11,19]. Compared with contrast-enhanced computer tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI), CEUS is a relatively safe, cost-effective, and time-saving technique. The sonographic contrast agent SonoVue has been proven to have high spleen-specific enhancement since it can be specifically detained by the splenic reticuloendothelial system or sinusoid endotheliocyte, allowing for more time to visualize the enhancement pattern of the splenic lesions [9–11,20]. Therefore, its unique spleen specificity would greatly contribute to the identification and characterization of splenic lesions.
Studies demonstrated that CEUS can differentiate between benign and malignant splenic lesions and reported that the benign splenic lesions typically showed iso-enhancement or hyper-enhancement in the arterial phase followed by slow and incomplete wash-out, while malignant lesions initially showed slight hypo-enhancement followed by rapid and complete wash-out [10,20]. The iso-enhancement or hyper-enhancement of benign splenic lesions can reflect their rich vascular structures and enhanced arterial supply. The slow wash-out can result from the presence of sinusoidal epithelium [20]. Consistent with this, in our study, 15 of the 22 lesions showed hyper-enhancement during the atrial phase and hyper- or iso-enhancement during the intermediate parenchymal phase, followed by slow wash-out during the late parenchymal phase. However, 1 of the 22 cases was initially mistaken for a malignant lesion due to its rapid hyper-enhancement in the arterial phase followed by rapid wash-out, and ultimately was proven to be splenic TB by surgical histopathology. This enhancement pattern was inconsistent with the results from a previous study, which demonstrated that splenic TB lesions generally presented persistent enhancement during the intermediate parenchymal phase, and the rapid wash-out pattern was common in malignant splenic lesions but was rare in benign lesions [10,21]. We assumed that this unusual enhancement pattern is be likely associated with the neovascularization and the formation of arterio-venous fistula involved in splenic TB [20].
According to the peak contrast enhancement distribution, the included lesions can be categorized into 4 types: homogeneous enhancement (Type I), rim-like enhancement (Type II), septation-like enhancement (Type III), and non-enhancement (Type IV). Of the 22 lesions, 12 were classified as Type II, presenting a rim-like enhancement with their peripheral tissues rapidly enhanced during the arterial phase while their internal tissues were non-enhanced during all the phases. It was reported that the lesions of splenic TB could be observed with peripheral rim-like enhancement as detected by contrast-enhanced MRI [6,22], and this is in line with the results from our CEUS imaging. The rim-like enhancement was considered to be the result of the hyperperfusion of the granuloma surrounding the lesions or the inflammatory hyperemia of the tissues surrounding the lesions [6,22,23]. In combination with their pathological results, we found that the rim-like enhanced areas were observed with tuberculous granuloma, and the peripheral splenic parenchyma was observed with telangiectasis and inflammatory cells infiltration. It was speculated that during the formation of splenic TB lesions, the tuberculous granuloma was formed and enlarged, stimulating the immune response and subsequent inflammatory responses of the peripheral splenic parenchyma. The granulomas and rich capillaries in the peripheral lesions lead to hyperperfusion, while caseous necroses in the internal lesions with no blood flow present non-enhancement.
Four cases (18.2%) were classified as Type III, presenting thin septation-like hyper-enhancement or iso-enhancement in the arterial phase and hypo-enhancement or complete wash-out in the late parenchymal phase. Considering the incomplete caseous necrosis of the lesions, the septation-like hyperenhanced tissues may be the residual normal splenic tissues, and since the vessels of the lesions were dysfunctional or impaired, the contrast agent had a brief retention and rapid wash-out. Nevertheless, this septation-like enhancement has not been reported in the MRI studies [6,22]. There may be 2 reasons for this: 1) CEUS can reflect the blood perfusion in real time, while MRI was scanned in different time phases and would not detect the transient perfusion of contrast agent; and 2) SonoVue would not diffuse into the interstitial space, unlike the MRI imaging contrast [6,9].
In our study, another 4 lesions (18.2%) with well-defined borders were classified as Type IV, showing non-enhancement during all phases, including the arterial, the intermediate parenchymal, and late parenchymal phases. These non-enhanced lesions were confirmed by surgical histopathology or biopsy to be caseous necrosis and liquefaction necrosis. The non-enhanced splenic TB needs to be differentiated from the splenic infarct and splenic cystic lesions. It was reported that the splenic infarct generally showed a wedge-shape non-enhancement, and the splenic cystic lesions showed anechoic and thin cystic wall-like enhancement as detected by conventional US and CEUS [10,11]. Therefore, hypoechoic round splenic lesions with non-enhancement during all phases of CEUS examination are highly indicative of splenic TB.
Two of the 22 splenic TB patients were shown by conventional US to have multiple hypoechoic nodular lesions. Of these 2 patients, one had a history of intestinal tumor surgery and the splenic lesions were thus initially considered as metastases, while the other one had a history of hepatic lymphoma, and the splenic lesions were thus considered to be splenic lymphomas. However, CEUS demonstrated that the former patient presented with rim-like enhancement that was maintained during the intermediate parenchymal phase, followed by slow wash-out in the late parenchymal phase; the latter patient showed non-enhancement in all phases. These were inconsistent with the findings from the study by Ioanitescu et al, which showed the malignant splenic lesions like splenic metastases and splenic lymphoma had rapid wash-out [24]. Therefore, malignant lesions were not considered for these 2 cases, and the 2 cases were finally confirmed by biopsy to be splenic TB.
In addition, CEUS can also be valuable for identifying the position and blood supply of the lesions. In this study, 2 were located in the diaphragmatic surface of the splenic envelope and grew in parallel to the splenic envelope. They presented fusiform shapes (Figure 4), likely due to the limited growth space between the spleen and diaphragm. Furthermore, CEUS found that the blood supply of the lesions originated from the splenic envelope, making it clear that the lesions were located at the spleen rather than in the parietal peritoneum. CEUS can not only accurately detect the blood perfusion of the lesions, but can also increase the contrast between the lesions and the peripheral normal spleen tissues, making the margin of the lesions more explicit and the number of the lesions more precise. In our study, conventional US showed 1 patient with only 3 lesions, whereas 5 lesions with clearer borders were detected by CEUS. Thus, CEUS can be helpful for increasing the lesion detection rate, which is in agreement with the results of Ioanitescu et al [24].
Conclusions
In conclusion, the rim-like or septation-like enhancement pattern may provide some valuable information for the diagnosis of splenic TB. Round hypoechoic lesions as detected by conventional US and complete non-enhancement detected by CEUS are highly suspicious for splenic TB. CEUS is a mini-invasive, safe, and convenient approach that can rapidly reflect the blood perfusion of lesions in real time. It can provide substantial valuable information for the diagnosis and differentiation of splenic TB.
Figures
 Figure 1. A splenic TB lesion with hyper-enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a relatively well-defined border (arrows); (B–D), CEUS demonstrates that the lesion become diffuse enhancement at 8 s after injection of SonoVue (B), followed by homogeneous enhancement at 10 s after injection (C, arrows) and hypo-enhancement in the late parenchymal phase (D, arrows).
Figure 1. A splenic TB lesion with hyper-enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a relatively well-defined border (arrows); (B–D), CEUS demonstrates that the lesion become diffuse enhancement at 8 s after injection of SonoVue (B), followed by homogeneous enhancement at 10 s after injection (C, arrows) and hypo-enhancement in the late parenchymal phase (D, arrows). 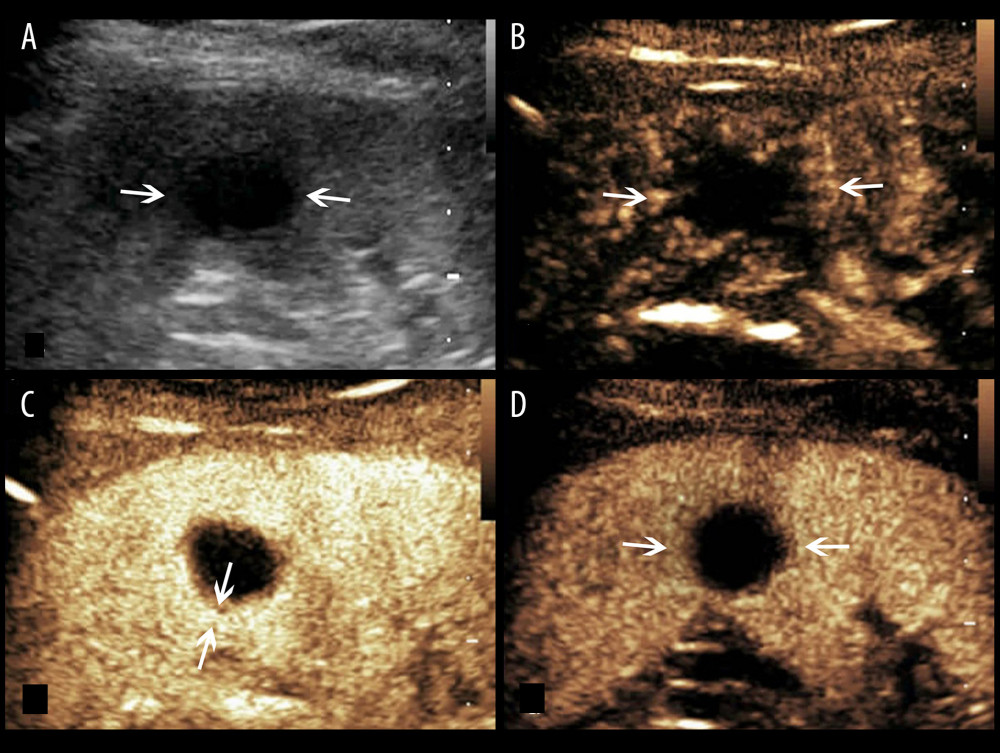 Figure 2. A splenic TB lesion with rim-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with relatively well-defined boundary (arrows); (B–D), CEUS demonstrates that the lesion began to enhance from the margin at 12 s after injection of the SonoVue (B, arrows) and presents rim-like enhancement at 25 s (C, arrows), followed by hypo-enhancement compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows).
Figure 2. A splenic TB lesion with rim-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with relatively well-defined boundary (arrows); (B–D), CEUS demonstrates that the lesion began to enhance from the margin at 12 s after injection of the SonoVue (B, arrows) and presents rim-like enhancement at 25 s (C, arrows), followed by hypo-enhancement compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows). 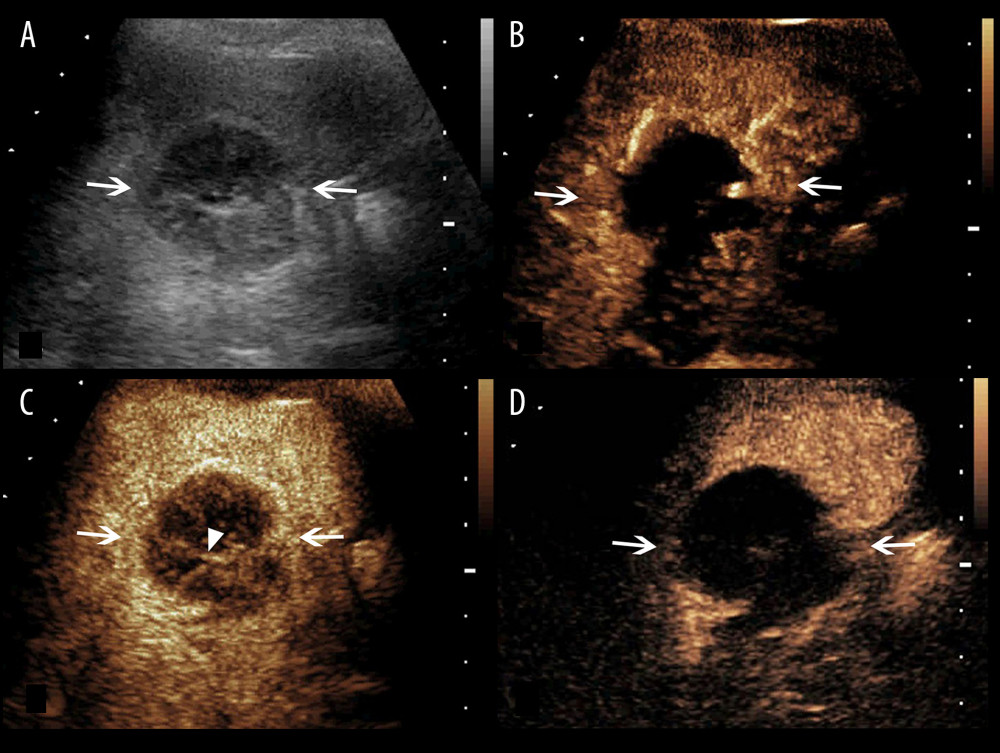 Figure 3. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a well-defined border and heterogeneous(arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the margin at 13 s after injection of SonoVue (B, arrows) to the internal in a septation-like manner at 30 s (C, head arrows). The internal septation-like enhancement is washed out and the marginal tissues become iso-enhanced compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows).
Figure 3. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a well-defined border and heterogeneous(arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the margin at 13 s after injection of SonoVue (B, arrows) to the internal in a septation-like manner at 30 s (C, head arrows). The internal septation-like enhancement is washed out and the marginal tissues become iso-enhanced compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows). 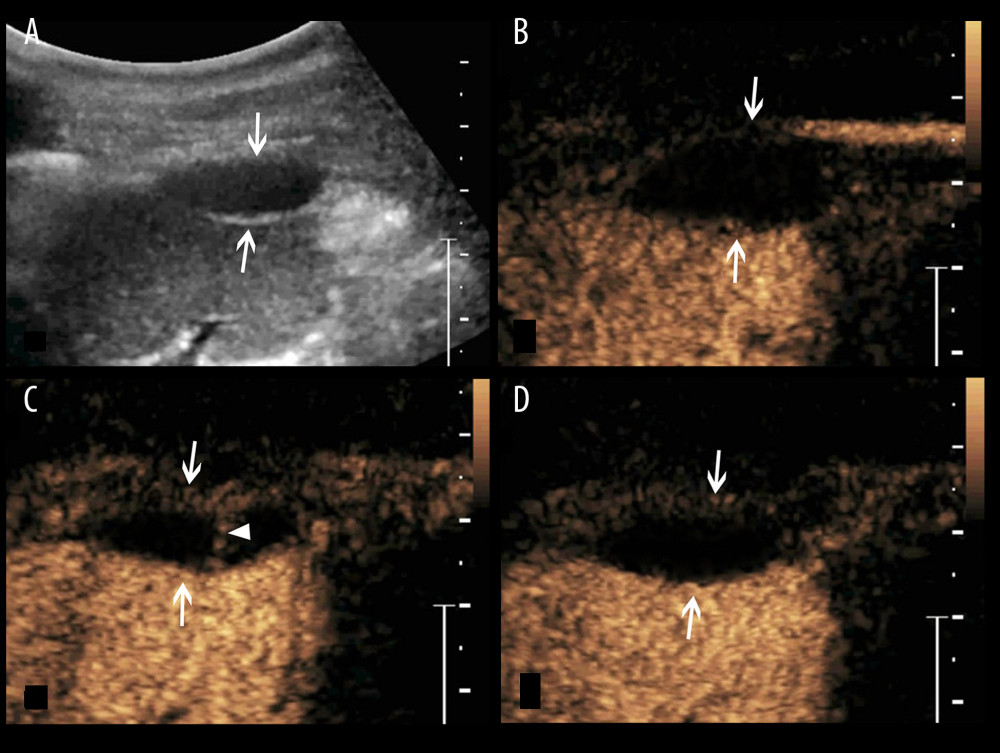 Figure 4. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoehoic lesion in the splenic envelope with a well-defined border (arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the splenic envelope at 30 s after injection of SonoVue (B, arrow) toward the peritoneum. Its internal area is presented with septation-like enhancement at 32 s (C, head arrows), which is completely washed out in the late parenchymal phase. The marginal area is iso-enhanced compared with the peripheral splenic parenchyma (D, arrows).
Figure 4. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoehoic lesion in the splenic envelope with a well-defined border (arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the splenic envelope at 30 s after injection of SonoVue (B, arrow) toward the peritoneum. Its internal area is presented with septation-like enhancement at 32 s (C, head arrows), which is completely washed out in the late parenchymal phase. The marginal area is iso-enhanced compared with the peripheral splenic parenchyma (D, arrows). 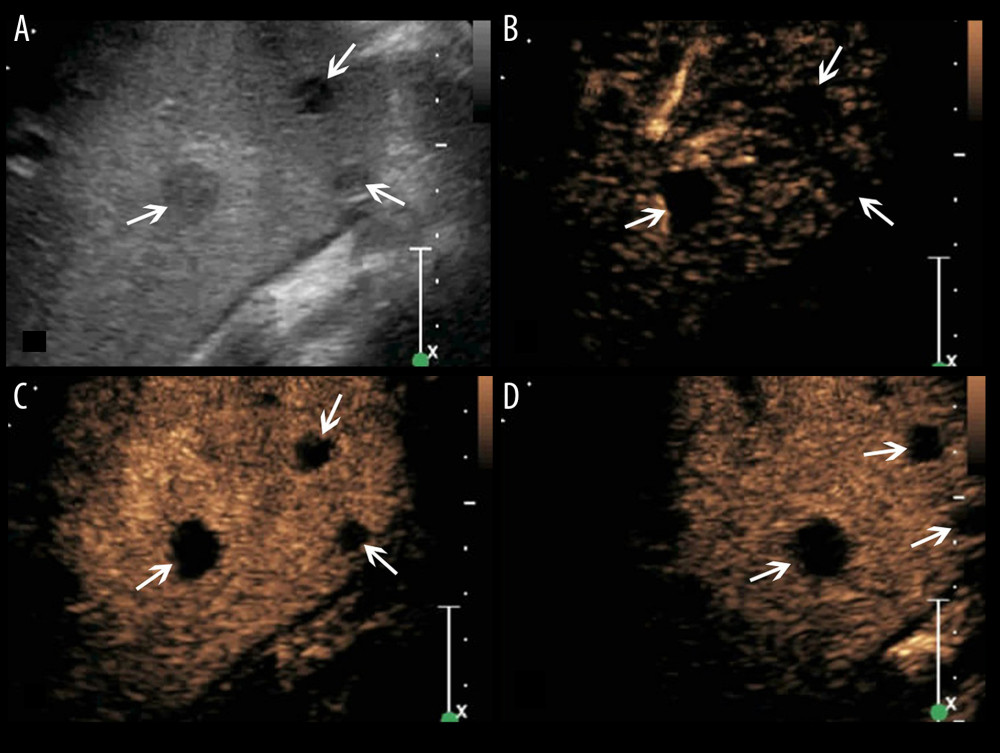 Figure 5. A splenic TB case with multiple non-enhanced splenic lesions. (A) Conventional US demonstrates 3 hypoechoic splenic lesions with relatively ill-defined borders (arrows); (B–D) CEUS demonstrates the 3 lesions show non-enhancement during all phases (arrows).
Figure 5. A splenic TB case with multiple non-enhanced splenic lesions. (A) Conventional US demonstrates 3 hypoechoic splenic lesions with relatively ill-defined borders (arrows); (B–D) CEUS demonstrates the 3 lesions show non-enhancement during all phases (arrows). References
1. Azzam NA, Splenic tuberculosis presenting as fever of unknown origin with severe neutropenia: Ann Clin Microbiol Antimicrob, 2013; 12; 13
2. Lin SF, Zheng L, Zhou L, Solitary splenic tuberculosis: A case report and review of the literature: World J Surg Oncol, 2016; 14; 154
3. Lestari DA, Rahadiani N, Syaiful RA, Isolated spleen tuberculosis in an immunocompetent patient, a rare case report: Int J Surg Case Rep, 2021; 83; 105966
4. Raviraj S, Gogia A, Kakar A, Isolated splenic tuberculosis without any radiological focal lesion: Case Rep Med, 2015; 2015; 130209
5. Grover S, Arya Y, Gaba S, Isolated splenic tuberculosis: A diagnostic conundrum: Cureus, 2021; 13; e12958
6. De Backer AI, Vanhoenacker FM, Mortele KJ, MRI features of focal splenic lesions in patients with disseminated tuberculosis: Am J Roentgenol, 2006; 186; 1097-102
7. Vanhoenacker FM, De Backer AI, Op de BB, Imaging of gastrointestinal and abdominal tuberculosis: Eur Radiol, 2004; 14(Suppl 3); E103-15
8. Kumar S, Pai AG, Tungenwar PN, Isolated primary tuberculosis of spleen – a rare entity in the immuno-competent patient: Int J Surg Case Rep, 2017; 30; 93-96
9. Schwarze V, Lindner F, Marschner C, Single-center study: The diagnostic performance of contrast-enhanced ultrasound (CEUS) for assessing focal splenic lesions compared to CT and MRI: Clin Hemorheol Microcirc, 2019; 73; 65-71
10. Yang R, Lu Q, Xu J, Value of contrast-enhanced ultrasound in the differential diagnosis of focal splenic lesions: Cancer Manag Res, 2021; 13; 2947-58
11. Li XZ, Song J, Sun ZX, Conventional ultrasound and contrast-enhanced ultrasound in the diagnosis of splenic diseases: A systematic review and meta-analysis: J Ultrasound Med, 2020; 39; 1687-94
12. Metlo A, Shah SI, Rehan A, Solitary splenic tuberculosis in an immunocompetent child: A case report: Cureu, 2019; 11; e5210
13. Weledji EP, Benefits and risks of splenectomy: Int J Surg, 2014; 12; 113-19
14. Taibbi A, Bartolotta TV, Matranga D, Splenic hemangiomas: Contrast-enhanced sonographic findings: J Ultrasound Med, 2012; 31; 543-53
15. Nishina S, Sakai H, Kawakami T: J Infect Chemother, 2021; 27; 354-58
16. Gupta P, Dhaka N, Rohilla M, Isolated splenic tuberculosis presenting as an unusual splenic mass: Int J Mycobacteriol, 2018; 7; 397-98
17. Sahoo S, Naik S, Mishra B, Tuberculous splenic abscess in the immunocompetent host: A report and review of literature: Monaldi Arch Chest Dis, 2020; 90; 79-85
18. Bachmann C, Gorg C, Color Doppler sonographic findings in focal spleen lesions: Eur J Radiol, 2005; 56; 386-90
19. Zavariz JD, Konstantatou E, Deganello A, Common and uncommon features of focal splenic lesions on contrast-enhanced ultrasound: A pictorial review: Radiol Bras, 2017; 50; 395-404
20. Stang A, Keles H, Hentschke S, Incidentally detected splenic lesions in ultrasound: Does contrast-enhanced ultrasonography improve the differentiation of benign hemangioma/hamartoma from malignant lesions?: Ultraschall Med, 2011; 32; 582-92
21. Stang A, Keles H, Hentschke S, Differentiation of benign from malignant focal splenic lesions using sulfur hexafluoride-filled microbubble contrast-enhanced pulse-inversion sonography: Am Roentgenol, 2009; 193; 709-21
22. Lim J, Yu JS, Hong SW, A case of mass-forming splenic tuberculosis: MRI findings with emphasis of diffusion-weighted imaging characteristics: J Korean Med Sci, 2011; 26; 457-60
23. Cao BS, Li XL, Li N, The nodular form of hepatic tuberculosis: Contrast-enhanced ultrasonographic findings with pathologic correlation: J Ultrasound Med, 2010; 29; 881-88
24. Ioanitescu ES, Copaci I, Mindrut E, Various aspects of Contrast-enhanced Ultrasonography in splenic lesions – a pictorial essay: Med Ultrason, 2020; 22; 2521
Figures
 Figure 1. A splenic TB lesion with hyper-enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a relatively well-defined border (arrows); (B–D), CEUS demonstrates that the lesion become diffuse enhancement at 8 s after injection of SonoVue (B), followed by homogeneous enhancement at 10 s after injection (C, arrows) and hypo-enhancement in the late parenchymal phase (D, arrows).
Figure 1. A splenic TB lesion with hyper-enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a relatively well-defined border (arrows); (B–D), CEUS demonstrates that the lesion become diffuse enhancement at 8 s after injection of SonoVue (B), followed by homogeneous enhancement at 10 s after injection (C, arrows) and hypo-enhancement in the late parenchymal phase (D, arrows). Figure 2. A splenic TB lesion with rim-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with relatively well-defined boundary (arrows); (B–D), CEUS demonstrates that the lesion began to enhance from the margin at 12 s after injection of the SonoVue (B, arrows) and presents rim-like enhancement at 25 s (C, arrows), followed by hypo-enhancement compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows).
Figure 2. A splenic TB lesion with rim-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with relatively well-defined boundary (arrows); (B–D), CEUS demonstrates that the lesion began to enhance from the margin at 12 s after injection of the SonoVue (B, arrows) and presents rim-like enhancement at 25 s (C, arrows), followed by hypo-enhancement compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows). Figure 3. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a well-defined border and heterogeneous(arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the margin at 13 s after injection of SonoVue (B, arrows) to the internal in a septation-like manner at 30 s (C, head arrows). The internal septation-like enhancement is washed out and the marginal tissues become iso-enhanced compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows).
Figure 3. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoechoic lesion with a well-defined border and heterogeneous(arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the margin at 13 s after injection of SonoVue (B, arrows) to the internal in a septation-like manner at 30 s (C, head arrows). The internal septation-like enhancement is washed out and the marginal tissues become iso-enhanced compared with the peripheral splenic parenchyma in the late parenchymal phase (D, arrows). Figure 4. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoehoic lesion in the splenic envelope with a well-defined border (arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the splenic envelope at 30 s after injection of SonoVue (B, arrow) toward the peritoneum. Its internal area is presented with septation-like enhancement at 32 s (C, head arrows), which is completely washed out in the late parenchymal phase. The marginal area is iso-enhanced compared with the peripheral splenic parenchyma (D, arrows).
Figure 4. A splenic TB lesion with septation-like enhancement. (A) Conventional US demonstrates a hypoehoic lesion in the splenic envelope with a well-defined border (arrows); (B–D) CEUS demonstrates that the lesion begins to enhance from the splenic envelope at 30 s after injection of SonoVue (B, arrow) toward the peritoneum. Its internal area is presented with septation-like enhancement at 32 s (C, head arrows), which is completely washed out in the late parenchymal phase. The marginal area is iso-enhanced compared with the peripheral splenic parenchyma (D, arrows). Figure 5. A splenic TB case with multiple non-enhanced splenic lesions. (A) Conventional US demonstrates 3 hypoechoic splenic lesions with relatively ill-defined borders (arrows); (B–D) CEUS demonstrates the 3 lesions show non-enhancement during all phases (arrows).
Figure 5. A splenic TB case with multiple non-enhanced splenic lesions. (A) Conventional US demonstrates 3 hypoechoic splenic lesions with relatively ill-defined borders (arrows); (B–D) CEUS demonstrates the 3 lesions show non-enhancement during all phases (arrows). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








