19 June 2021: Review Articles
A Review of Neurological Involvement in Patients with SARS-CoV-2 Infection
Yidan Xu12EF, Yu Zhuang12EF, Lumei Kang13AG*DOI: 10.12659/MSM.932962
Med Sci Monit 2021; 27:e932962
Abstract
ABSTRACT: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative pathogen of the recent pandemic of coronavirus disease 19 (COVID-19). As the infection spreads, there is increasing evidence of neurological and psychiatric involvement in COVID-19. Headache, impaired consciousness, and olfactory and gustatory dysfunctions are common neurological manifestations described in the literature. Studies demonstrating more specific and more severe neurological involvement such as cerebrovascular insults, encephalitis and Guillain-Barré syndrome are also emerging. Respiratory failure, a significant condition that leads to mortality in COVID-19, is hypothesized to be partly due to brainstem impairment. Notably, some of these neurological complications seem to persist long after infection. This review aims to provide an update on what is currently known about neurological involvement in patients with COVID-19 due to SARS-CoV-2 infection. In this review, we demonstrate invasion routes of SARS-CoV-2, provide evidence to support the neurotropism hypothesis of the virus, and investigate the pathological mechanisms that underlie neurological complications associated with SARS-CoV-2.
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2, Nervous System, review, Ageusia, anosmia, COVID-19, Encephalitis, Headache, Nervous System Diseases, Neuroimmunomodulation, Pandemics, SARS-CoV-2, Stroke
Background
A spillover of the novel coronavirus from bats and other undetermined secondary hosts to humans in 2019 has caused a devastating wave of infectious disease around the world [1]. SARS-CoV-2, the causative agent of COVID-19, is highly contagious and even with countermeasures taken globally to break the chain of transmission, as of 22 May 2021 the number of people diagnosed with COVID-19 has surpassed 100 million, including 3 437 545 deaths [2]. This enveloped positive-stranded RNA virus shows pathogenicity similar to that of another human coronavirus (HCoV) [1]. Individuals infected with SARS-COV-2 demonstrate a broad spectrum of clinical manifestations, ranging from asymptomatic infection to lethal acute respiratory distress syndrome (ARDS), among which respiratory distress poses the most significant risk for death [3].
Although the primary target of the virus is the respiratory system, evidence of its neurological involvement is mounting. At the initial stage of the pandemic, a multicenter retrospective case series in Wuhan, China demonstrated that among 214 patients infected with SARS-CoV-2, 36.4% developed neurological complications, including olfactory and gustatory dysfunction, cerebrovascular involvement, and skeletal muscle injury [4]. As the infection spreads, cases of post-infectious neurological symptoms, such as Guillain-Barré syndrome (GBS), are also increasingly documented in the literature [5]. An overall clinical picture of the neurological involvement of SARS-CoV-2 has emerged from several recent systemic reviews. In a study including 68 361 COVID-19 patients, 21% showed neurological symptoms, with headache, psychiatric disorders, myopathy, unconsciousness, anosmia, and ageusia being most prominent. Despite several general non-specific symptoms, the specific manifestations, like stroke, are associated with severe disease outcome [6]. These neurological manifestations appear to be an aggregate of the direct damage caused by viral invasion, neuroinflammation secondary to systemic injuries, and non-specific clinical complications [5].
This review aims to provide an update on what is currently known about neurological involvement in patients with COVID-19 due to SARS-CoV-2 infection. In particular, we provide evidence to support the neurotropism hypothesis of the virus and investigate the pathological mechanisms that underlie neurological complications associated with SARS-CoV-2.
SARS-CoV-2
SARS-CoV-2, the 7th member of the Coronaviridae family, is closely related to SARS-CoV. The large genome of SARS-CoV-2 contains 14 open reading frames (ORFs). The 5′ end ORF1a and OPF1b encode for non-structural proteins. Structure proteins, including envelope (E), membrane (M), nucleocapsid (N), spike (S) protein, and other accessory proteins, are encoded by the remaining genome. S proteins consist of 2 subunits, S1 and S2, which provide the virus with membrane fusion potential. The viral intracellular life cycle initiates with the receptor-binding domains (RBDs) of S1 attachment to the ACE2 receptor [7]. After being primed by transmembrane protease serine (TMPRSS2) on the host cell membrane, S protein is activated to facilitate the fusion process. Then, the viral genomic RNA is released into the cytoplasm and starts replication [8].
Apart from its primary target, type 2 alveolar epithelial cells, ACE2 is abundantly expressed in endothelial cells throughout most of the body, suggesting that SARS-CoV-2 is capable of infecting various organs once it is present in the circulation. Studies also revealed this receptor expression in the nervous system, including neurons and glia in the striatum, cerebral cortex, substantia nigra, and brainstem, suggesting the neurotropic potential of the virus [9].
The S protein of SARS-CoV-2 possesses a sequence identity as high as 77% with that of SARS-CoV. Notably, SARS-CoV-2 S protein was found to have a much higher affinity for ACE2 than SARS-CoV [10]. Thus, this novel coronavirus could have a stronger transmissibility and neural pathogenicity.
Neurotropism of SARS-CoV-2
The neurotropic propensity has been described in several HCoVs, such as HCoV229E, HCoV-OC43, SARS-CoV, and MERS-CoV [11]. Earlier postmortem studies on SARS-CoV patients indicated the presence of viruses in the brain by real-time reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry (IHC), and electron microscopy [12]. Growing evidence demonstrated the presence of SARS-CoV-2 RNA in cerebrospinal fluid (CSF), supporting the neurotropism hypothesis of SARS-CoV-2 [13]. This raises the question of how SARS-CoV-2 spreads to the central nervous system (CNS). The virus can enter the CNS via 2 non-exclusive routes: retrograde neuronal dissemination and hematogenous dissemination [14,15] (Figure 1).
Retrograde Axonal Dissemination
The olfactory pathway is the most likely way SARS-CoV-2 disseminates to the CNS [14]. Axonal transmission via olfactory nerves initiates at the olfactory mucosa lining the roof of the nasal cavity [16]. The olfactory epithelium (OE), the major structure of the olfactory mucosa, consists of olfactory sensory neurons (OSN), sustentacular cells, Bowman’s glands, and epithelial cilia [17]. Protruding from the cribriform plate, the bipolar OSNs communicating between the OE and the olfactory bulb (OB). Mitral cells in the OB subsequently project to the olfactory cortex, hippocampus, and other brain regions [17]. Although the obligatory receptors for SARS-CoV-2 entrance, ACE2 and TMPRSS2, are not widespread in OSNs, they are expressed in premature OSN horizontal basal cells [18]. A complementary receptor, Neuropilin-1, which is highly expressed in the OB, is reported to interact with the other 2 receptors to facilitate SARS-CoV-2 entrance and infection [19]. This suggests that SARS-CoV-2 infects ACE2-expressing horizontal basal cells, which then mature into OSNs and subsequently invade the OB [18]. Subsequently, viral particles can either be transported actively by motor proteins (kinesin and dynein) and microtubules in neurons or diffuse passively to reach the CNS [20]. As such, viruses could spread rapidly throughout the brain by taking advantage of the neuroanatomy structures and cause damage [16].
This pathway has been shown in other neuro-invasive viruses. For instance, after intranasally infecting mice with mouse hepatitis virus, virus antigens and particles were observed in the OB and the anterior cortex [21]. After ablation of the OB, no sign of neuro-invasion was identified [22]. Experiments on transgenic mice revealed that after intranasal inoculation, HCoV-OC43 antigens were detected in the OB and later in the entire brain, indicating this strain of HCoV could invade susceptible brain regions by propagating along neuronal connections [20,23]. Supporting evidence comes from recent tissue-based studies revealing CNS changes in deceased COVID-19 patients. Meinhardt and colleagues detected the intact coronavirus particles as well as the viral RNA in the olfactory mucosa and in distinct brain regions where olfactory axons project in 33 deceased patients with COVID-19 [24]. Ultrastructural autopsy analysis of olfactory nerve, gyrus rectus, and medulla oblongata indicated diffuse impairment, including axons, glia, and myelin sheath [25]. Consistent with this, several studies using magnetic resonance imaging (MRI) demonstrated OB damage during SARS-CoV-2 infection [26]. Supporting this, in another brain MRI study, a medical worker who presented with mild symptoms and later progressed into severe anosmia showed signal alterations in cortical areas related to olfaction [27]. Therefore, one of the most common neurological manifestations of COVID-19, hypo/anosmia, can be attributed to this dissemination route.
The second putative neuroanatomical route for SARS-CoV-2 dissemination is the trigeminal nerve. It is hypothesized that SARS-CoV-2 can enter the CNS by invading the sensory axon of the trigeminal nerve in the nasal cavity. Three branches of its sensory axons (ophthalmic, maxillary, and mandibular nerve) extend to the trigeminal ganglion and terminate in the nucleus of pons [28]. One study detected a high level of SARS-CoV-2 RNA in the trigeminal ganglion of samples from deceased COVID-19 patients [24]. Following this, another postmortem study identified axonal degeneration and cell loss in the trigeminal nerve [29]. The trigeminal nuclei act as a transportation hub, communicating between the terminal nerve endings and the nucleus tractus solitarius in the brainstem, which may partly explain the occurrence of microvascular clotting in some COVID-19 patients [28].
Another putative transmitting route is the vagus nerve. As ACE2 are expressed extensively on the epithelium of the gastrointestinal tract, it is possible that viruses can invade from the enteric nerve plexus and ascend along the vagus nerve [28]. Several studies also suggest that SARS-CoV-2 can access the CNS via vagus nerve peripheral fibers in the lung, as observed in influenza [14,30]. A study examining the brainstem neuropathology detected SARS-CoV-2 in vagus nerve fibers by use of IHC [31]. However, the currently available literature suggests that genuine vagus nerve infection is limited and this route of transmissions needs to be further clarified.
Previous studies demonstrated that the brainstem, with which the vagus nerve is connected, is a frequent infection site for SARS-CoV [32]. Consistent with the above, the development of fatal respiratory distress in severe COVID-19 patients is in part caused by dysfunction of the cardiorespiratory center in this region [24]. Considering this, it should not be overlooked that the virus spreads retrogradely to invade the CNS via the abovementioned pathways.
Hematogenous Dissemination
The CNS is usually protected from various harmful substances by the highly selective filter of the blood-brain barrier (BBB). However, this tight barrier can be damaged by the virus, which can then allow its entrance [33]. Similar to other respiratory viruses, SARS-CoV-2 infects peripheral organs, especially those with abundant ACE2 expression, and can penetrate local epithelial barriers and enter the circulation. There, it can subsequently gain access to the CNS by damaging the BBB endothelial cells or the blood-CSF barrier in the choroid plexus [34]. The sluggish blood flow allows a full engagement of the S protein with ACE2 receptors on brain microvascular endothelium cells (BMECs), facilitating subsequent viral budding from the BBB into the CNS [35]. This has already been described in several recognized neuro-invasive virus, such as hepatitis E virus [36] and herpes simplex virus [37]. For instance, in vivo and in vitro experiments suggest that Zika virus can even replicate in the BMECs and destabilize tight junctions by downregulating expression of related genes [38]. A recent postmortem case report of a 74-year-old COVID-19 patient identified viral-like particles in BMECs and pericytes and the involvement of astrocytes in the frontal lobe, further corroborating this possible route [39]. Alternatively, SARS-CoV-2 can penetrate the BBB paracellularly. In severely infected individuals, exacerbated immune response secondary to systemic inflammation can impair the integrity of the BBB, rendering it more permeable [40]. Concordant with this, an experiment on in vitro models of BBB suggested that S proteins of SARS-CoV-2 can significantly alter BBB properties. Furthermore, damaged barrier integrity was observed in an advanced 3D microfluidic model of the BBB [41].
Viruses can hide in infected immune cells and move toward the BBB, referred to as the “Trojan horse” mechanism [42]. It is suggested that dendritic cells and macrophages are potential vehicles favoring viral entry into the CNS. Evidence has revealed viral antigens in macrophages of ACE2-expressing transgenic mice [43]. Thus, if immune cells serve as a viral pool for SARS-CoV-2, it is possible that invasion through the BBB could occur even after acute infection.
Neuroinflammation in the CNS
SARS-CoV-2 invading the CNS can dysregulate the neurological function through several mechanisms. First, being highly sensitive to the oxygen demand, the brain is vulnerable to neuron necrosis. Besides the alveolar impairment, ischemic stroke and coagulopathy of severe COVID-19 cases can lead to inadequate tissue oxygenation and a switch to anaerobic metabolism [6]. In normoxic conditions, the hypoxia-inducible factors-1 (HIF-1) triggers microglia to transform into a proinflammatory state, thus exhibiting their neuroprotective roles. On the other hand, hypoxia itself is a potent activator of HIF-1 [44, 45]. Thus, COVID-19-associated brain hypoxia and HIF-1 can exacerbate the microglia transformation into a proinflammatory phenotype and the production of inflammatory cytokines. As a result, microglia may be impeded to switch to an anti-inflammatory state, which is required for neuron repair, adding insult to the brain injury [45]. A well-organized immune response plays a fundamental role in neuroprotection, but it can be detrimental when exacerbated. COVID-19 patients have a higher level of proinflammatory cytokines in the circulation, such as interleukin-1β (IL-1β), interferon-γ (IFN-γ), IL-10, and monocyte chemotactic protein-1 (MCP-1), compared to healthy controls, which may induce cytokine release syndrome (CRS) [46]. In this condition, the BBB integrity decreases and subsequently permits damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), and immune cells to enter the CNS. Upon coronavirus infection, the infiltrating T cells can cause axonal injury and demyelination [47]. In response to DAMPS, PAMPs, and peripheral cytokines, resident microglia become reactive, changing morphology and producing cytokines (TNF-α and IL-6), which can also contribute to glutamate excitotoxicity. A sustained CRS can result in prolonging microglial reactivity, dysregulating the synaptic connections, and impeding the survivability of neurons [48]. Infiltrating neutrophils can also elicit neuronal damage by amplifying demyelination and contributing to oligodendrocyte apoptosis, as observed in virus-infected mouse models [49]. These mechanisms of brain insult are demonstrated in Figure 2.
Clinical Neurological Manifestations
Aside from common cold symptoms of fever, cough, fatigue, the clinical manifestations of COVID-19 also involve the CNS. Ghannam et al showed that in the early stage of the outbreak, among 82 cases of COVID-19 with neurological manifestations, 48.8% of subjects had cerebrovascular insults, 28% had a neuromuscular disorder, and 23% had encephalopathy [50]. Cagnazzo et al revealed neurological symptoms such as headache (5.4%), psychiatric issues (4.6%), decreased consciousness (2.8%), and acute cerebrovascular injuries in their meta-analysis of clinical manifestations of 68 361 COVID-19 patients [6]. It has been proposed that the neurological manifestations that occur at the initial stage of disease could be used as an indicator of infection or as a predictor of poor outcome [51].
Olfactory and Gustatory Manifestation
Impaired olfactory and gustatory functions are common manifestations in COVID-19 patients, especially for mild cases [52]. Though initially underestimated [53], the association of olfactory and gustatory impairment with COVID-19 has gradually gained worldwide attention. A meta-analysis indicated that the pooled prevalence of smell and taste dysfunction was 41.0% and 38.2%, respectively. The prevalence of these disorders varies widely in the literature. A much higher prevalence is reported in Europe, ranging from 70% to 85% [54]. A study in the United States also showed a similar prevalence [55]. However, lower incidences of smell and taste impairment were reported in Asia [56], suggesting a possible ethnic difference in this symptom. As the prevalence of chemosensory impairment during COVID-19 was mostly assessed by self-reporting questionnaires, it could be influenced by subjectivity. Two subsequent studies using standardized tests revealed a much higher than expected proportion of patients with olfactory dysfunctions [57].
It has been demonstrated that anosmia and hypogeusia are more frequently observed in subjects with milder COVID-19 disease [52]. Although a later study contradicted this finding, a recent meta-analysis revealed that patients with chemosensory dysfunctions were less likely to be associated with severe disease outcomes compared with patients without this symptom. Since it is the first overt symptom in many subjects, olfactory dysfunction could be used as a diagnostic parameter for prompt isolation or as a potential prognostic marker for COVID-19 [58].
Cerebrovascular Insult
Cerebrovascular insult, although rare in patients infected with SARS-CoV-2, is a critical risk factor for severe disease and mortality. A systemic review disclosed that, despite regular venous thrombosis prophylaxis, the pooled incidence of ischemic and hemorrhagic stroke in COVID-19 patients was 1.3% and 1.1%, respectively [59]. A retrospective study in Italy reported 2.5% (9/388) of hospitalized coronavirus patients had ischemic stroke [60]. An early single-center retrospective study (n=221) found that 6% of COVID-19 subjects developed acute cerebrovascular insult, with more cases of ischemic stroke (n=11) than of intracerebral hemorrhage (n=1) and venous sinus thrombosis (n=1) [61]. Consistent with the above, Ghannam et al demonstrated that ischemic stroke accounted for 42% of all identified cerebrovascular complications, with large-vessel occlusion being most prevalent; other reported cerebrovascular complications were cerebral vein thrombosis (n=2), intracerebral hemorrhages (n=2), and aneurysmal subarachnoid hemorrhage (n=1) [50]. Notably, both types of stroke are associated with a high mortality rate [62]. In addition, COVID-19 patients have an increased risk of ischemic stroke and cryptogenic stroke compared to contemporary uninfected controls [59].
Indeed, cerebrovascular insult is closely linked with COVID-19, as these 2 syndromes share similar risk factors such as hypertension and diabetes [63]. A multicenter study indicated that among in-hospital COVID-19 patients, those with a more severe disease status showed a higher relative risk for cerebrovascular diseases [64]. However, this condition is not limited to the elderly and those with comorbidities [63]. Studies on younger individuals also described 2 previously healthy children who later experienced disabling arterial ischemic strokes following SARS-CoV-2 infection. Among them, an 8-year-old girl had stroke in the bilateral middle cerebral artery due to recently formed clots and suffered re-occlusion soon after mechanical thrombectomy. Both cases showed evidence of systemic post-infectious arteritis [65].
Encephalitis and Meningoencephalitis
Several case reports demonstrated inflammatory manifestations of CNS such as encephalitis, myelitis, and meningoencephalitis in patients infected with SARS-CoV-2. Encephalitis, the acute and diffuse inflammation of the parenchyma, is a potentially devastating neurological deficit. Clinically, it is characterized by altered mental status (ranging from drowsiness to coma), fever, psychosis, and seizures [66].
Siow et al suggested that encephalitis is an uncommon symptom of COVID-19, with a pooled incidence of 0.215% [67]. Characteristic features of encephalitis were reported by Sohal et al in a 72-year-old infection-confirmed man who presented with weakness, breathing difficulty, and altered mental status. He later progressed to seizures 2 days after admission [68]. A severe form of this condition, acute disseminated encephalomyelitis (ADEM), which is a post-infectious demyelinating neurological disorder affecting the brain and spinal cord, was also reported. Parsons et al reported that a 51-year-old woman presented with coma and compromised oculocephalic response and was diagnosed with ADEM, as high signals in the cerebral white matter and diffuse demyelinating lesions were detected by MRI [69].
PNS Manifestations
In terms of neuropathies, studies describing the GBS and GBS variants in COVID-19 patients are emerging. However, the rarity of case reports of these conditions in such a huge infected population makes it difficult to attain a more accurate incidence rate. GBS is an acute peripheral neuropathy, characterized by weakness of the periphery, which can progress rapidly to cause acute paralysis throughout the body [70]. A case series in Italy observed the initial symptoms of GBS, including lower-extremities weakness, flaccid tetraparesis, and paresthesia, in patients with COVID-19; 4 patients later progressed into generalized or quadriplegia [71]. Similarly, a recent case report in Colombia demonstrated that a woman with typical GBS clinical symptoms then deteriorated into general areflexia and diaphragmatic weakness during hospitalization and was subsequently transferred to the intensive care unit [72]. A systemic review including 50 patients with GBS and COVID-19 suggested that acute inflammatory demyelinating polyneuropathy (AIDP) (33/50, 66%) was the most frequent GBS variant observed in COVID-19 patients [73]. A rare variant of GBS, Miller-Fisher syndrome, was also identified in several studies [72,74]. In a few cases of Miller-Fisher syndrome concomitant with COVID-19, commonly reported manifestations included perioral paresthesia, ataxia, impaired vision, ophthalmoplegia, and generalized areflexia [74]. Fortunately, two-thirds of patients showed improvement after administration of immunoglobulin [75].
The time lag between initial SARS-CoV-2 infection and development of typical symptoms of GBS suggests that these manifestations could be attributed to post-infectious mechanisms [51]. The findings of an earlier study prompted speculation that SARS-CoV-2 can initiate aberrant immune response against nerves and cause damage after a period of viral infection [76]. Of note, although rarely, in some patients with SARS-CoV-2, GBS was the first or only presentation of infection [50]. Thus, closer attention should be paid to identifying patients with severe neuropathies but without any flu-like symptoms.
Psychiatric Manifestations
Evidence from previous SARS epidemics suggests the impact of infectious disease is not limited to the physical body, but also adversely affects mental health, resulting in psychiatric disorders [77]. An observational study following SARS survivors for 4 years showed a significant increase in psychiatric morbidity (3.3% before infection versus 42.5% after infection). Posttraumatic stress disorder (PTSD) is the most frequently diagnosed disorder (54.5%), followed by depression, panic disorder, and obsessive-compulsive disorder [77]. It is pertinent that similar observations have been made in COVID-19 patients. To date, psychiatric involvement in COVID-19 includes depression, impulsivity, acute stress disorder, insomnia, and PTSD [78, 79]. One of the largest relevant cohort studies, including over 44 000 subjects, provided robust evidence of the bidirectional associations of COVID-19 with psychiatric disorders. Pre-existing psychiatric issues may predispose patients to COVID-19, while COVID-19 patients may experience worsening anxiety/depression conditions compared to non-infected controls [80]. In addition, it is suggested that older age, female gender, and severe disease status are associated with a higher incidence of psychiatric symptoms [81]. Socioeconomic issues also play a crucial role, especially during the largest pandemic ever in history [82]. Working in healthcare during the outbreak, detaching from the public during quarantine, and worrying about unemployment after recovery could adversely affect the mental health of COVID-19 patients and the general public [83], and these impacts can last for a long time, even after the pandemic.
It is proposed that systemic and neurological inflammation may underlie several psychiatric disorders. Elevated cytokine levels, especially IL-6, TNF-α, and IL-1β, were detected in the circulation in many psychiatric diseases, including depression, PTSD, and obsessive-compulsive disorder [84–86], which is consistent with data on COVID-19 patients [46]. Thus, viruses that invaded the CNS can interrupt glial reactivity and interfere with neuronal networks by triggering an immune response [78].
Mechanisms Related to Neurological Complications
The COVID-19-associated neurological manifestations appear to be an aggregate of direct damage caused by viral invasion and indirect damage secondary to systemic diseases and/or post-infectious immune disorders.
Direct Viral Damage
Direct viral invasion through the aforementioned axon dissemination routes accounts for several neurological complications in patients with COVID-19. The development of anosmia and ageusia can be attributed to impairment of OSNs. Laurendon and colleagues demonstrated bilateral olfactory bulb edema and mild olfactory clefts edema in a 27-year-old man with COVID-19-related complete anosmia and ageusia.
Currently, most studies suggest that COVID-19-associated anosmia is most likely due to the death of supporting cells, which subsequently impair OSN function. These cells are responsible for OSN metabolism and play a crucial role in regulating olfaction [87]. Little ACE2 is present on OSNs but it is abundantly expressed on sustentacular cells in the olfactory epithelium [18]. Of note, a murine experiment demonstrated that sustentacular cells were heavily infected after nasal inhalation of SARS-COV-2; by contrast, no damage to OSNs was identified [88]. Thus, their vulnerability to viral infections explains the high prevalence of anosmia and ageusia in COVID-19 patients.
Recently, some studies suggested that direct viral damage in the brainstem can partially explain the respiratory failure in COVID-19 patients. In support of this speculation, Bulfamante et al conducted neurohistopathology and IHC to examine the pathologic features in the brainstem-diseased COVID-19 patients who died of respiratory failure [31]. They observed significantly increased neuronal damage and corpora amylacea (suggestive of astrocyte activation) in the medullar oblongata compared with non-infected controls. SARS-CoV-2 nucleoprotein were also detected in brainstem neurons and glial cells.
Indirect Systemic Damage
Indirect CNS impairment secondary to pulmonary dysfunction or systemic inflammation also underlies many neurological manifestations observed in COVID-19 patients. Typically, the development of stroke can be attributed to these mechanisms. As in other respiratory viruses, SARS-CoV-2 infection is linked with hypercoagulation by activating a coagulation cascade [89]. This well-described condition, termed sepsis-induced coagulopathy, is associated with systemic inflammatory response and is characterized by increased inflammatory markers such as D-dimer and C-reaction proteins, as well as thrombocytopenia [90]. Moreover, SARS-CoV-2 infection induces exaggerated immune response with increased proinflammatory cytokine production that can exacerbate endothelium damage of arteries and veins, further triggering the development of thromboembolic events [91].
The ‘post-infectious’ theory proposed by Marchioni et al provided an explanation for the development of GBS and its variants [92]. Evidence suggested that SARS-CoV-2 induces GBS through a molecular mimicry mechanism; specifically, the virus expresses epitopes resembling gangliosides. As gangliosides play a critical role in axons and glia communication and receptor signal transduction, host-generated cross-reactive antibodies secondary to viral infection could mistakenly attack those nerve tissues, resulting in GBS [28,70]. The cytokine storm resulting from viral disturbance of the immune system could be why patients with COVID-19 are predisposed to neuropathies like GBS. In particular, Li et al recently revealed the underlying pathophysiology of GBS in COVID-19 by conducting bioinformatics analyses [93]. The genetic crosstalk between COVID-19 and GBS they observed shows the pivotal role of Th17 cells in increasing the risk of GBS in COVID-19 patients [93].
Long COVID? A Glimpse into the Neuronal System
There is a growing concern regarding the long-term sequelae of COVID-19, particularly involving the nervous system, as cases of patients recovering from initial symptoms while beginning to present new neurological manifestations are now emerging. It was demonstrated that some patients had double vision, hallucinations, disorientation, and impaired attention, quadriplegia, facial paralysis, and hypogeusia even 6 months after infection [75]. A prospective cohort study in France assessed the clinical status of COVID-19 patients 4 months after discharge. Researchers conducted telephone assessment and recorded symptoms of fatigue and cognitive decline in many discharged COVID-19 patients, with an incidence of 31% and 21%, respectively. In addition, symptoms of anxiety or depression and PTSD were identified in 94 previous ICU patients through extra evaluations [94]. These data are corroborated by an earlier large cohort study in China, following up 1733 discharged patients for about 6 months, which suggested that fatigue or muscle weakness were the most characteristic problems for COVID-19 survivors (63%, 1038/1655). Others may suffer from sleep difficulties and anxiety or depression in the long term [95]. This finding is consistent with a follow-up study showing that there were new-onset neurologic symptoms even 3 months after infection, including hyposmia/anosmia, myopathy, mild encephalopathy, parkinsonism, GBS, and ischemic stroke [96].
Nevertheless, since control groups and pre-COVID evaluations were limited in these studies, it remains a significant challenge to form conclusions on the association of these observations with the long-term outcome of COVID-19 [94]. Surprisingly, a case-control study provided the first suggestion of the hallmarks of long COVID based on [18F] FDG-PET/CT. Long COVID patients (with at least 1 long-lasting symptom more than 1 month after recovery) presented with a lower metabolization rate in the right parahippocampal gyrus and thalamus than in melanoma controls. It is noteworthy that the hypometabolism in these brain regions was similar to that of subjects with long-lasting fatigue and anosmia/ageusia [97]. Although there are relatively few reports on patients with long COVID, considering the large scale of the pandemic, there could be many COVID-19 patients with persistent neurological issues. Moreover, the lingering neurological symptoms, such as long-lasting alteration in chemosensory functions, chronic fatigue syndrome, and post-pandemic mental problems, could be more than just a temporary setback. Studies proposed that if the virus remains latent in the nervous system and causes irreversible damage, it could have a considerable long-term impact on patients’ daily lives. Thus, understanding of the full spectrum of long-term consequences of COVID-19 and undertaking possible preventive interventions are required, even as vaccination coverage is expanding among populations.
Conclusions
With an emphasis on respiratory symptoms at the initial stage of the outbreak, previous studies may have underestimated the prevalence of neurological involvement in COVID-19 patients. As SARS-CoV-2 continues to spread, COVID-19-associated neurological and psychiatric involvement are increasingly described in the literature. However, it remains difficult to establish a close link between these neurological manifestations and underlying mechanisms. Here, we updated current evidence on its neurological involvement and concluded that SARS-CoV-2-induced neurological manifestations are caused by an aggregate of direct viral damage, secondary systemic diseases, and/or post-infectious immune disorders. Moreover, although patients with long-lasting neurological symptoms have surfaced in the past few months, the exact mechanisms remain unclear. Therefore, healthcare workers should be aware that some neurological manifestations could proceed the classic respiratory symptom of COVID-19, and understanding of these symptoms is helpful for predicting the prognosis of patients. Additional research on the pathology and at the cellular level is warranted to ascertain the pathophysiological mechanisms of long-lasting neurological symptoms of COVID-19.
Figures
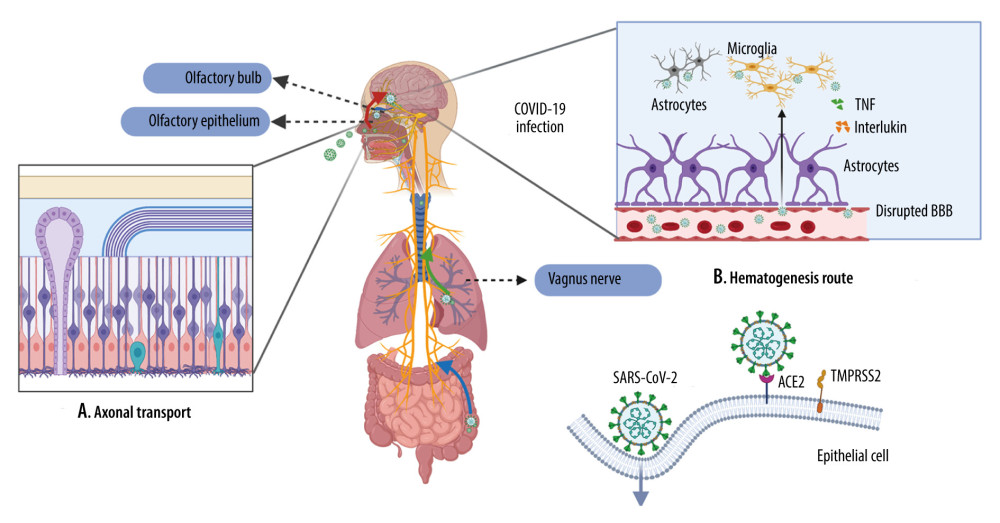 Figure 1. Viral journey to the central nervous system (CNS)A. Axonal transport. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown in the olfactory epithelium, where they can disseminate along the olfactory nerve, ascending from the cribriform plate into the olfactory bulb, then disseminate throughout the CNS via interconnected neurons. SARS-CoV-2 is also presented in the lungs and the gastrointestinal tract, where they can spread along the vagus nerve to reach the CNS, including the brainstem. B. Hematogenesis dissemination. SARS-CoV-2 first invades peripheral epithelial cells, which express ACE2 and TMPRSS2. Once inside the bloodstream, the virus penetrates through the impaired BBB and invades the CNS. (Created with BioRender.com).
Figure 1. Viral journey to the central nervous system (CNS)A. Axonal transport. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown in the olfactory epithelium, where they can disseminate along the olfactory nerve, ascending from the cribriform plate into the olfactory bulb, then disseminate throughout the CNS via interconnected neurons. SARS-CoV-2 is also presented in the lungs and the gastrointestinal tract, where they can spread along the vagus nerve to reach the CNS, including the brainstem. B. Hematogenesis dissemination. SARS-CoV-2 first invades peripheral epithelial cells, which express ACE2 and TMPRSS2. Once inside the bloodstream, the virus penetrates through the impaired BBB and invades the CNS. (Created with BioRender.com). 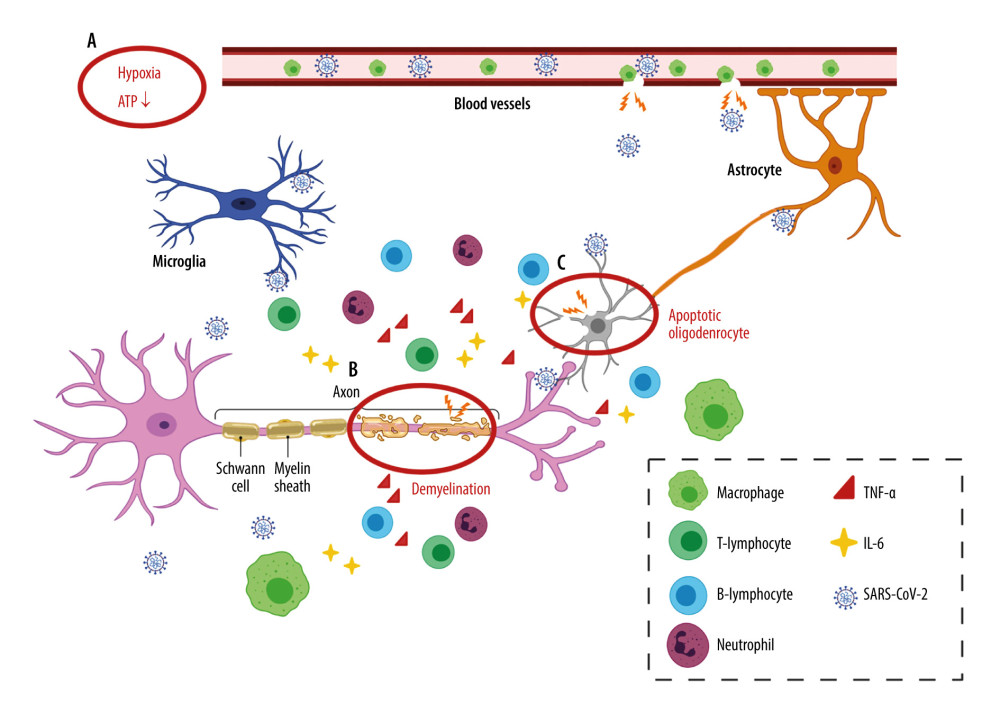 Figure 2. Neuroinflammation in the CNSA. Hypoxia. Inadequate oxygen supply to the brain leads to ATP crisis and exacerbated inflammatory state. B. Demyelination. A leaky BBB allows the entrance of virus and peripheral lymphocytes into the CNS, resulting in activation of microglia. Reactivated microglia release inflammatory cytokines (TNF-α and IL-6), contributing to demyelination and neuron death. C. Oligodendrocyte apoptosis. Infiltrating neutrophils adversely impact neuronal function by inducing oligodendrocyte apoptosis. (Created with BioRender.com).
Figure 2. Neuroinflammation in the CNSA. Hypoxia. Inadequate oxygen supply to the brain leads to ATP crisis and exacerbated inflammatory state. B. Demyelination. A leaky BBB allows the entrance of virus and peripheral lymphocytes into the CNS, resulting in activation of microglia. Reactivated microglia release inflammatory cytokines (TNF-α and IL-6), contributing to demyelination and neuron death. C. Oligodendrocyte apoptosis. Infiltrating neutrophils adversely impact neuronal function by inducing oligodendrocyte apoptosis. (Created with BioRender.com). References
1. Zhou P, Yang XL, Wang XG, A pneumonia outbreak associated with a new coronavirus of probable bat origin: Nature, 2020; 579(7798); 270-73
2. WHO: WHO Official Updates – Coronavirus Disease 2019, 2021
3. Wang D, Hu B, Hu C, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China: JAMA, 2020; 323(11); 1061-69
4. Mao L, Jin H, Wang M, Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China: JAMA Neurol, 2020; 77(6); 683-90
5. Scoppettuolo P, Borrelli S, Naeije G, Neurological involvement in SARS-CoV-2 infection: A clinical systematic review: Brain Behav Immun Health, 2020; 5; 100094
6. Cagnazzo F, Arquizan C, Derraz I, Neurological manifestations of patients infected with the SARS-CoV-2:A systematic review of the literature: J Neurol, 2020; 15; 3
7. Ke Z, Oton J, Qu K, Structures and distributions of SARS-CoV-2 spike proteins on intact virions: Nature, 2020; 588(7838); 498-502
8. Xiu S, Dick A, Ju H, Inhibitors of SARS-CoV-2 entry:Current and future opportunities: J Med Chem, 2020; 63(21); 12256-74
9. Lavoie JL, Cassell MD, Gross KW, Sigmund CD, Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model: Hypertension, 2004; 43(5); 1116-19
10. Hassanzadeh K, Perez Pena H, Dragotto J, Considerations around the SARS-CoV-2 spike protein with particular attention to COVID-19 brain infection and neurological symptoms: ACS Chem Neurosci, 2020; 11(15); 2361-69
11. Nagu P, Parashar A, Behl T, Mehta V, CNS implications of COVID-19:A comprehensive review: Rev Neurosci, 2021; 32(2); 219-34
12. Ding Y, He L, Zhang Q, Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients:Implications for pathogenesis and virus transmission pathways: J Pathol, 2004; 203(2); 622-30
13. Lewis A, Frontera J, Placantonakis DG, Cerebrospinal fluid in COVID-19:A systematic review of the literature: J Neurol Sci, 2021; 421; 117316
14. Lima M, Siokas V, Aloizou AM, Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system: Curr Treat Options Neurol, 2020; 22(11); 37
15. Bougakov D, Podell K, Goldberg E, Multiple neuroinvasive pathways in COVID-19: Mol Neurobiol, 2021; 58(2); 564-75
16. Burks SM, Rosas-Hernandez H, Alejandro Ramirez-Lee M, Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier?: Brain Behav Immun, 2021; 95; 7-14
17. Suzuki Y, Takeda M, Obara N, Olfactory epithelium consisting of supporting cells and horizontal basal cells in the posterior nasal cavity of mice: Cell Tissue Res, 2000; 299(3); 313-25
18. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R, Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium:Identification of cell types and trends with age: ACS Chem Neurosci, 2020; 11(11); 1555-62
19. Mayi BS, Leibowitz JA, Woods AT, The role of Neuropilin-1 in COVID-19: PLoS Pathog, 2021; 17(1); e1009153
20. Dube M, Le Coupanec A, Wong AHM, Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43: J Virol, 2018; 92(17); e00404-18
21. Barthold SW, Olfactory neural pathway in mouse hepatitis virus nasoencephalitis: Acta Neuropathol, 1988; 76(5); 502-6
22. Perlman S, Jacobsen G, Afifi A, Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory nerves: Virology, 1989; 170(2); 556-60
23. Jacomy H, St-Jean JR, Brison E, Mutations in the spike glycoprotein of human coronavirus OC43 modulate disease in BALB/c mice from encephalitis to flaccid paralysis and demyelination: J Neurovirol, 2010; 16(4); 279-93
24. Meinhardt J, Radke J, Dittmayer C, Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19: Nat Neurosci, 2021; 24(2); 168-75
25. Bulfamante G, Chiumello D, Canevini MP, First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem: Minerva Anestesiol, 2020; 86(6); 678-79
26. Aragao M, Leal MC, Cartaxo Filho OQ, Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI: Am J Neuroradiol, 2020; 41(9); 1703-6
27. Politi LS, Salsano E, Grimaldi M, Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia: JAMA Neurol, 2020; 77(8); 1028-29
28. Satarker S, Nampoothiri M, Involvement of the nervous system in COVID-19:The bell should toll in the brain: Life Sci, 2020; 262; 118568
29. von Weyhern CH, Kaufmann I, Neff F, Kremer M, Early evidence of pronounced brain involvement in fatal COVID-19 outcomes: Lancet, 2020; 395(10241); e109
30. Matsuda K, Park CH, Sunden Y, The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice: Vet Pathol, 2004; 41(2); 101-7
31. Bulfamante G, Bocci T, Falleni M, Brainstem neuropathology in two cases of COVID-19:SARS-CoV-2 trafficking between brain and lung: J Neurol, 2021 [Online ahead of print]
32. McCray PB, Pewe L, Wohlford-Lenane C, Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus: J Virol, 2007; 81(2); 813-21
33. Spindler KR, Hsu TH, Viral disruption of the blood-brain barrier: Trends Microbiol, 2012; 20(6); 282-90
34. Pezzini A, Padovani A, Lifting the mask on neurological manifestations of COVID-19: Nat Rev Neurol, 2020; 16(11); 636-44
35. Baig AM, Khaleeq A, Ali U, Syeda H, Evidence of the COVID-19 virus targeting the CNS:Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms: ACS Chem Neurosci, 2020; 11(7); 995-98
36. Tian J, Shi R, Liu T, Brain infection by hepatitis E virus probably via damage of the blood–brain barrier due to alterations of tight junction proteins: Front Cell Infect Microbiol, 2019; 9; 52
37. He Q, Liu H, Huang C, Herpes simplex cirus 1-induced blood–brain barrier damage involves apoptosis associated with GM130-mediated golgi stress: Front Mol Neurosci, 2020; 13; 2
38. Chiu CF, Chu LW, Liao IC, The mechanism of the Zika virus crossing the placental barrier and the blood–brain barrier: Front Microbiol, 2020; 11; 214
39. Paniz-Mondolfi A, Bryce C, Grimes Z, Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): J Med Virol, 2020; 92(7); 699-702
40. Hu J, Jolkkonen J, Zhao C, Neurotropism of SARS-CoV-2 and its neuropathological alterations:Similarities with other coronaviruses: Neurosci Biobehav Rev, 2020; 119; 184-93
41. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier: Neurobiol Dis, 2020; 146; 105131
42. Izquierdo-Useros N, Naranjo-Gomez M, Erkizia I, HIV and mature dendritic cells:Trojan exosomes riding the Trojan horse?: PLoS Pathog, 2010; 6(3); e1000740
43. Bao L, Deng W, Huang B, The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice: Nature, 2020; 583(7818); 830-33
44. Mukandala G, Tynan R, Lanigan S, O’Connor JJ, The effects of hypoxia and inflammation on synaptic signaling in the CNS: Brain Sci, 2016; 6(1); 6
45. Ferraro E, Germano M, Mollace R, HIF-1, the Warburg effect, and macrophage/microglia polarization potential role in COVID-19 pathogenesis: Oxid Med Cell Longev, 2021; 2021; 8841911
46. Xu Z, Shi L, Wang Y, Pathological findings of COVID-19 associated with acute respiratory distress syndrome: Lancet Respir Med, 2020; 8(4); 420-22
47. Dandekar AA, Wu GF, Pewe L, Perlman S, Axonal damage is T cell mediated and occurs concomitantly with demyelination in mice infected with a neurotropic coronavirus: J Virol, 2001; 75(13); 6115-20
48. Goncalves de Andrade E, Simoncicova E, Carrier M, Microglia fighting for neurological and mental health:On the central nervous system frontline of COVID-19 pandemic: Front Cell Neurosci, 2021; 15; 647378
49. Septyaningtrias DE, Susilowati R, Neurological involvement of COVID-19:From neuroinvasion and neuroimmune crosstalk to long-term consequences: Rev Neurosci, 2021; 32(4); 427-42
50. Ghannam M, Alshaer Q, Al-Chalabi M, Neurological involvement of coronavirus disease 2019:A systematic review: J Neurol, 2020; 267(11); 3135-53
51. Whittaker A, Anson M, Harky A, Neurological manifestations of COVID-19:A systematic review and current update: Acta Neurol Scand, 2020; 142(1); 14-22
52. Boscolo-Rizzo P, Borsetto D, Fabbris C, Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19: JAMA Otolaryngol Head Neck Surg, 2020; 146(8); 729-32
53. Mao L, Jin H, Wang M, Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China: JAMA Neurol, 2020; 77(6); 683-90
54. Villarreal IM, Morato M, Martinez-RuizCoello M, Olfactory and taste disorders in healthcare workers with COVID-19 infection: Eur Arch Otorhinolaryngol, 2021; 278(6); 2123-27
55. Yan CH, Faraji F, Prajapati DP, Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms: Int Forum Allergy Rhinol, 2020; 10(7); 806-13
56. Song J, Deng YK, Wang H, Self-reported taste and smell disorders in patients with COVID-19:Distinct features in China: Curr Med Sci, 2021; 41(1); 14-23
57. Vaira LA, Deiana G, Fois AG, Objective evaluation of anosmia and ageusia in COVID-19 patients:Single-center experience on 72 cases: Head Neck, 2020; 42(6); 1252-58
58. Brandao Neto D, Fornazieri MA, Dib C, Chemosensory dysfunction in COVID-19:Prevalences, recovery rates, and clinical associations on a large Brazilian sample: Otolaryngol Head Neck Surg, 2021; 164(3); 512-18
59. Katsanos AH, Palaiodimou L, Zand R, The impact of SARS-CoV-2 on stroke epidemiology and care:A meta-analysis: Ann Neurol, 2021; 89(2); 380-88
60. Lodigiani C, Iapichino G, Carenzo L, Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy: Thromb Res, 2020; 191; 9-14
61. Li Y, Li M, Wang M, Acute cerebrovascular disease following COVID-19:A single center, retrospective, observational study: Stroke Vasc Neurol, 2020; 5(3); 279-84
62. Syahrul S, Maliga HA, Ilmawan M, Hemorrhagic and ischemic stroke in patients with coronavirus disease 2019:Incidence, risk factors, and pathogenesis – a systematic review and meta-analysis: F1000Res, 2021; 10; 34
63. Guan WJ, Liang WH, Zhao Y, Comorbidity and its impact on 1590 patients with COVID-19 in China:A nationwide analysis: Eur Respir J, 2020; 55(5); 2000547
64. Le TT, Gutierrez-Sacristan A, Son J, Multinational prevalence of neurological phenotypes in patients hospitalized with COVID-19: medRxiv, 2021 2021:012721249817 [Preprint]
65. Appavu B, Deng D, Dowling MM, Arteritis and large vessel occlusive strokes in children after COVID-19 infection: Pediatrics, 2021; 147(3); e2020023440
66. Tyler KL, Acute viral encephalitis: N Engl J Med, 2018; 379(6); 557-66
67. Siow I, Lee KS, Zhang JJY, Encephalitis as neurological complication of COVID-19:A systematic review and meta analysis of incidence, outcomes and predictors: Eur J Neurol, 2021 [Online ahead of print]
68. Ellul MA, Benjamin L, Singh B, Neurological associations of COVID-19: Lancet Neurol, 2020; 19(9); 767-83
69. Parsons T, Banks S, Bae C, COVID-19-associated acute disseminated encephalomyelitis (ADEM): J Neurol, 2020; 267(10); 2799-802
70. van den Berg B, Walgaard C, Guillain-Barre syndrome:Pathogenesis, diagnosis, treatment and prognosis: Nat Rev Neurol, 2014; 10(8); 469-82
71. Toscano G, Palmerini F, Ravaglia S, Guillain-Barre syndrome associated with SARS-CoV-2: N Engl J Med, 2020; 382(26); 2574-76
72. Mackenzie N, Lopez-Coronel E, Dau A, Concomitant Guillain-Barre syndrome with COVID-19:A case report: BMC Neurol, 2021; 21(1); 135
73. Sriwastava S, Kataria S, Tandon M, Guillain Barre syndrome and its variants as a manifestation of COVID-19:A systematic review of case reports and case series: J Neurol Sci, 2021; 420; 117263
74. Li Z, Li X, Shen J, Miller Fisher syndrome associated with COVID-19:An up-to-date systematic review: Environ Sci Pollut Res Int, 2021; 28(17); 20939-44
75. Karuppan MKM, Devadoss D, Nair M, SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism: Mol Neurobiol, 2021; 58(6); 2465-80
76. Willison HJ, Jacobs BC, van Doorn PA, Guillain-Barré syndrome: Lancet, 2016; 388(10045); 717-27
77. Lam MH, Wing YK, Yu MW, Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors:Long-term follow-up: Arch Intern Med, 2009; 169(22); 2142-47
78. Steardo L, Steardo L, Verkhratsky A, Psychiatric face of COVID-19: Transl Psychiatry, 2020; 10(1); 261
79. Cao W, Fang Z, Hou G, The psychological impact of the COVID-19 epidemic on college students in China: Psychiatry Res, 2020; 287; 112934
80. Taquet M, Luciano S, Geddes JR, Harrison PJ, Bidirectional associations between COVID-19 and psychiatric disorder:Retrospective cohort studies of 62 354 COVID-19 cases in the USA: Lancet Psychiatry, 2021; 8(2); 130-40
81. Vindegaard N, Benros ME, COVID-19 pandemic and mental health consequences:Systematic review of the current evidence: Brain Behav Immun, 2020; 89; 531-42
82. Holmes EA, O’Connor RC, Perry VH, Multidisciplinary research priorities for the COVID-19 pandemic:A call for action for mental health science: Lancet Psychiatry, 2020; 7(6); 547-60
83. Szczesniak D, Gladka A, Misiak B, The SARS-CoV-2 and mental health:From biological mechanisms to social consequences: Prog Neuropsychopharmacol Biol Psychiatry, 2021; 104; 110046
84. Ma X, Reynolds SL, Baker BJ, Li X, IL-17 enhancement of the IL-6 signaling cascade in astrocytes: J Immunol, 2010; 184(9); 4898-906
85. Lindqvist D, Dhabhar FS, Mellon SH, Increased pro-inflammatory milieu in combat related PTSD – A new cohort replication study: Brain Behav Immun, 2017; 59; 260-64
86. Karaguzel EO, Arslan FC, Uysal EK, Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder: Compr Psychiatry, 2019; 89; 61-66
87. Liang F, Sustentacular cell enwrapment of olfactory receptor neuronal dendrites:An update: Genes (Basel), 2020; 11(5); 493
88. Bryche B, St Albin A, Murri S, Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters: Brain Behav Immun, 2020; 89; 579-86
89. Harapan BN, Yoo HJ, Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19): J Neurol, 2021 [Online ahead of print]
90. Han H, Yang L, Liu R, Prominent changes in blood coagulation of patients with SARS-CoV-2 infection: Clin Chem Lab Med, 2020; 58(7); 1116-20
91. Yaghi S, Ishida K, Torres J, SARS-CoV-2 and stroke in a New York Healthcare System: Stroke, 2020; 51(7); 2002-11
92. Marchioni E, Ravaglia S, Montomoli C, Postinfectious neurologic syndromes: A prospective cohort study, 2013; 80(10); 882-89
93. Li Z, Huang Z, Li X, Bioinformatic analyses hinted at augmented T helper 17 cell differentiation and cytokine response as the central mechanism of COVID-19-associated Guillain-Barre syndrome: Cell Prolif, 2021; 54(5); e13024
94. Morin L, Savale L, Pham TWriting Committee for the COMEBAC Study Group, Four-month clinical status of a cohort of patients after hospitalization for COVID-19: JAMA, 2021; 325(15); 1525-34
95. Huang C, Wang Y, Li X, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China: Lancet, 2020; 395(10223); 497-506
96. Rass V, Beer R, Schiefecker AJ, Neurological outcome and quality of life 3 months after COVID-19:A prospective observational cohort study: Eur J Neurol, 2021 [Online ahead of print]
97. Sollini M, Morbelli S, Ciccarelli M, Long COVID hallmarks on [18F]FDG-PET/CT:A case-control study: Eur J Nucl Med Mol Imaging, 2021 [Online ahead of print]
Figures
 Figure 1. Viral journey to the central nervous system (CNS)A. Axonal transport. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown in the olfactory epithelium, where they can disseminate along the olfactory nerve, ascending from the cribriform plate into the olfactory bulb, then disseminate throughout the CNS via interconnected neurons. SARS-CoV-2 is also presented in the lungs and the gastrointestinal tract, where they can spread along the vagus nerve to reach the CNS, including the brainstem. B. Hematogenesis dissemination. SARS-CoV-2 first invades peripheral epithelial cells, which express ACE2 and TMPRSS2. Once inside the bloodstream, the virus penetrates through the impaired BBB and invades the CNS. (Created with BioRender.com).
Figure 1. Viral journey to the central nervous system (CNS)A. Axonal transport. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been shown in the olfactory epithelium, where they can disseminate along the olfactory nerve, ascending from the cribriform plate into the olfactory bulb, then disseminate throughout the CNS via interconnected neurons. SARS-CoV-2 is also presented in the lungs and the gastrointestinal tract, where they can spread along the vagus nerve to reach the CNS, including the brainstem. B. Hematogenesis dissemination. SARS-CoV-2 first invades peripheral epithelial cells, which express ACE2 and TMPRSS2. Once inside the bloodstream, the virus penetrates through the impaired BBB and invades the CNS. (Created with BioRender.com). Figure 2. Neuroinflammation in the CNSA. Hypoxia. Inadequate oxygen supply to the brain leads to ATP crisis and exacerbated inflammatory state. B. Demyelination. A leaky BBB allows the entrance of virus and peripheral lymphocytes into the CNS, resulting in activation of microglia. Reactivated microglia release inflammatory cytokines (TNF-α and IL-6), contributing to demyelination and neuron death. C. Oligodendrocyte apoptosis. Infiltrating neutrophils adversely impact neuronal function by inducing oligodendrocyte apoptosis. (Created with BioRender.com).
Figure 2. Neuroinflammation in the CNSA. Hypoxia. Inadequate oxygen supply to the brain leads to ATP crisis and exacerbated inflammatory state. B. Demyelination. A leaky BBB allows the entrance of virus and peripheral lymphocytes into the CNS, resulting in activation of microglia. Reactivated microglia release inflammatory cytokines (TNF-α and IL-6), contributing to demyelination and neuron death. C. Oligodendrocyte apoptosis. Infiltrating neutrophils adversely impact neuronal function by inducing oligodendrocyte apoptosis. (Created with BioRender.com). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








