15 January 2022: Database Analysis
Prediction of Patient Survival with Psoas Muscle Density Following Transjugular Intrahepatic Portosystemic Shunts: A Retrospective Cohort Study
Biyu Zhang1ABCDEF, Weimin Cai1ABCDEF, Feng Gao1BE, Xinran Lin1BC, Ting Qian1F, Kaier Gu1B, Bingxin Song1B, Tanzhou Chen1A*DOI: 10.12659/MSM.934057
Med Sci Monit 2022; 28:e934057
Abstract
BACKGROUND: Psoas muscle density (PMD) as a nutritional indicator is a tool to evaluate sarcopenia, which is commonly diagnosed in patients with liver cirrhosis. However, there are limited data on its role in patients who have received a transjugular intrahepatic portosystemic shunt (TIPS). We aimed to determine the utility of PMD in predicting mortality of patients with TIPS implantation and to compare the clinical value of PMD, Child-Pugh score, model for end-stage liver disease (MELD) score, and MELD paired with serum sodium measurement (MELD-Na) score in predicting post-TIPS survival in 1 year.
MATERIAL AND METHODS: This retrospective study included 273 patients who met the criteria for study inclusion. All participants underwent computed tomography (CT) scans, Child-Pugh score evaluation, MELD-Na scoring, and MELD scoring. Post-TIPS survival time was estimated using the Kaplan-Meier survival curve. The prognostic values of scoring models such as the Child-Pugh score, MELD, MELD-Na, and PMD were evaluated using receiver operating characteristic curves.
RESULTS: During the 1-year follow-up period, 31 of 273 (11.36%) post-TIPS patients died. Multivariate analysis identified PMD as an independent protective factor. PMD showed a good ability to predict the occurrence of an endpoint within 1 year after TIPS. The area under the receiver operating characteristic curves for PMD, Child-Pugh score, MELD score, and MELD-Na for predicting mortality were, respectively, 0.72 (95% confidence interval [CI]: 0.663-0.773), 0.59 (95% CI: 0.531-0.651), 0.60 (95% CI: 0.535-0.655), and 0.58 (95% CI: 0.487-0.608).
CONCLUSIONS: PMD has appreciable clinical value for predicting the mortality of patients with TIPS implantation. In addition, PMD is superior to established scoring systems for identifying high-risk patients with a poor prognosis.
Keywords: Liver Cirrhosis, Portasystemic Shunt, Transjugular Intrahepatic, Psoas Muscles, sarcopenia, Cohort Studies, Female, Follow-Up Studies, Humans, Liver, Male, Severity of Illness Index, Tomography, X-Ray Computed
Background
Sarcopenia is characterized as the generalized loss of skeletal muscle mass, strength, and physical function [1]. The major component of malnutrition in liver disease is the loss of skeletal muscle mass or sarcopenia [2]. The complication rate of sarcopenia in patients with liver cirrhosis (LC) was reported to be 30–70% [3,4], and in patients with end-stage liver disease, the proportion is 40–60% [5–7]. A variety of measurements have been applied to assess the nutritional status of patients with LC [1,8]. Although a number of studies have measured the psoas muscle cross-sectional area (PMA) at the L3 vertebra level by computed tomography (CT) to quantify nutritional status [9,10], it may not be comprehensive because the PMA measurements did not include muscle mass or fat infiltration. A recent review proposed that psoas muscle density (PMD) is a more accurate assessment of muscle mass and function [11]. In related studies, low skeletal muscle density, rather than PMA, was associated with poorer muscle function and higher mortality in patients after cardiovascular surgery [12]. PMD has been shown to have potential in the prediction of noncancer mortality in patients with prostate cancer [13], incidence of postoperative complications after operative fixation of acetabular fractures, and survival of gastrointestinal surgery [14–16]. Reduced PMD is associated with prolonged hospital stays in patients undergoing transcatheter aortic valve implantation [17].
Transjugular intrahepatic portosystemic shunt (TIPS), a side-to-side portacaval shunt, is a proven technique that can significantly reduce portal venous pressure and reduce the complications of decompensated LC patients [18]. In past decades, numerous studies identified a significant decrease in the incidence of recurrent variceal bleeding or other complications due to portal hypertension through the use of TIPS [19,20]. MELD and Child-Pugh scores are commonly used to evaluate the severity of chronic liver disease and predict the prognosis in various clinical situations [21]. The MELD score has also been used to predict the survival in patients who have undergone TIPS [22]. It has been found to be less influenced by subjective interpretation of variables than the Child-Pugh score [23] since it is based on 3 objective parameters, including serum creatinine, bilirubin, and international normalized ratio. Multiple studies have found that MELD-Na, a composite index including the MELD score and measurement of serum sodium, significantly improves the effectiveness in predicting mortality and postoperative complications rates after liver transplantation [24–28]. MELD-Na was officially applied in the United Network for Organ Sharing after 2016, and it was also shown to be an effective predictor of short-term mortality after TIPS [29–31]. However, MELD, MELD-Na, and Child-Pugh scores have a critical drawback because they do not include the assessment of the nutritional status of LC patients [32].
The MELD-Sarcopenia score performed better in predicting waiting list mortality in cirrhotic liver transplant candidates than the MELD score [33]. PMD may be a reliable, simple, quantitative, noninvasive, reproducible measurement method for predicting mortality in post-TIPS patients. Although PMD has shown its capability in predicting survival in LC patients, its applicability in patients with TIPS implantation has not yet been explored. Therefore, the aim of our study was to compare the clinical values of the MELD score, MELD-Na score, Child-Pugh score, and PMD for predicting the survival rates of patient with TIPS implantation.
Material and Methods
STUDY POPULATION:
The present retrospective cohort study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University, and the requirement for informed consent was waived. The following patients were excluded: (a) under the age of 18 years; (b) those without nonenhanced CT image; (c) those with lack of follow-up record in our institution; (d) those with skeletal muscle-related disease such as myasthenia gravis, muscular pseudohypertrophy, myodystrophy, or polio; and (e) recipients of a liver transplant less than 1 year after TIPS. After the exclusion criteria were applied, 273 LC patients who underwent TIPS at the First Affiliated Hospital of Wenzhou Medical University between November 2013 and March 2019 were finally enrolled in this study.
DATA COLLECTION:
Laboratory parameters and essential information such as sex, age, etiology of LC, diabetes mellitus, hypertension, splenectomy, indications of TIPS, and targeted puncture of TIPS were collected within 24 h of the patients’ admission. Ascites was evaluated by referring to guidelines [39], and hepatic encephalopathy was assessed and graded referring to the West-Haven classification criteria [40]. All subjects were assessed by the Child-Pugh score and the MELD score [41] in the first 24 h after admission. Survival data were recorded from medical charts or clinical correspondence.
CT imaging of the patients during hospitalization was analyzed and calculated by 2 research fellows trained with sliceOmatic software (Tomovision, Montreal, QC Canada). They were blinded to clinical data. All CT scans were performed in the same scanner. Areas of interest were outlining in a single cross-sectional CT image at the level of mid L3 [42]. To reduce the interference of adipose tissue and ascitic fluid, a threshold defined by −29 Hounsfield units (HU) and +150 HU was used [43]. This provided the values of area in square millimeters and density in HU of each psoas muscle at this level (Figure 1).
All subjects were regularly followed up via telephone, regular clinic visits, and outpatient or hospital medical records. Patients were followed up after the first month and then every 3 months after TIPS. Follow-up ended on March 1, 2020. The endpoint was defined as the occurrence of death or 1 year after TIPS.
STATISTICAL ANALYSIS:
Descriptive statistics are expressed as numbers and corresponding percentages or mean values (±SD), as appropriate. Continuous variables are expressed as means±SD or medians with interquartile ranges. The
Results
A total of 324 patients were screened, and 273 patients were ultimately included in this study. Fifty-one patients were excluded as follows: 2 patients underwent liver transplantation less than 1 year after TIPS; 39 patients had previously missed CT scans; and 10 patients were lost to follow-up. Thirty-one patients (11.36%) died during 1 year of follow-up. The major etiology of LC was virus infection (151/273). Among the remaining patients, 53 had alcoholism, 35 had a clinical history of both alcoholism and hepatitis, and 34 had a less common etiology. The participants included 194 men and 79 women aged 53.54±10.51 years. The vast majority of patients underwent TIPS because of esophageal gastric-fundus variceal bleeding (90.11%), and the remaining patients had refractory ascites (n=13) or thrombosis (n=7). Table 1 lists the baseline characteristics of these subjects.
Results of the univariate and multivariate Cox analysis are shown in Table 2. There were 6 indicators selected via univariate and multivariate Cox analysis, including age, sex, TIPS targeted puncture, albumin, total bilirubin, and PMD. Multivariate Cox regression analysis was performed with the forward LR (forward stepwise regression based on maximum likelihood estimation) method. Finally, PMD and total bilirubin were screened out, which were statistically significant (
The optimal PMD threshold for predicting survival was 49.92 HU (
PMD was negatively correlated with mortality (r=0.90,
Discussion
Over the past few years, sarcopenia, defined as a muscle mass that is 2 standard deviations or more below the healthy young adult mean value [46], has been the subject of extensive research in LC patients. Comprehensive systematic reviews and meta-analyses have shown that patients with LC and sarcopenia experience adverse clinical outcomes [47,48]. Cross-sectional imaging studies have also reported that the prevalence of sarcopenia is 30%–70% among patients with LC [49]. Ronald et al [50] and Shoreibah et al [51] have explored the role of combined muscle mass index in survival after TIPS and found that the combination of MELD and sarcopenia appeared to be superior in predicting survival as compared with the MELD score alone. It also has been demonstrated that the failure to reverse sarcopenia after TIPS implantation is associated with a decreased survival rate [52,53]. So, studies on sarcopenia and mortality in patients with TIPS are still insufficient. This study explored whether PMD is a reliable, simple quantitative indicator for predicting the mortality of patients with TIPS.
There are various imaging criteria for sarcopenia, but there is no universally accepted definition. The skeletal muscle index (SMI), which normalizes muscle area to patient height, is a commonly used nutritional index. North American liver transplant centers proposed cutoffs of SMI <50 cm2/m2 in male patients and <39 cm2/m2 in female patients listed for liver transplant [8]. In addition to SMI, several ways have been used to predict outcomes in patients with LC, including psoas muscle thickness, psoas muscle index, and PMA [11]. In fact, due to fluid retention in patients with LC, the above assessments cannot accurately differentiate body composition, which affects results [54]. In recent years, PMD has been widely used to predict the mortality [33,51,55–58].
In our study, the overall cumulative 1-year mortality rate was 11.36% (31/273). Different institutions have shown all-cause mortality in patients with TIPS ranging from 7% to 70% [37,59,60]. The survival rate was relatively high compared with other studies, and several possible explanations should be considered. First, the vast majority of patients were in Child-Pugh A/B class (233/273). Secondly, the drainage of the left branch of the intrahepatic portal vein (PV) (199/273) may have made a difference. A meta-analysis by Zuo et al [61] showed that TIPS conducted via the left PV was associated with decreased rates of postoperative hemorrhage and TIPS dysfunction. The pattern of muscle decay varies with age and sex, as shown in previous research [44]. In our study, PMD decreased with aging, and it differed by sex (Table 3). Levels of serum zinc, serum vitamin D, blood ammonia, and testosterone may cause variation in the muscle between individuals of different ages and sex [1]. Our study of 273 patients who underwent TIPS showed that MELD outperformed MELD-Na in predicting 1-year mortality. A small study of 69 subjects from Ahmed et al [62] showed that MELD-Na was superior to MELD in predicting 30-day mortality, but Young et al [31] reported the opposite conclusion. However, a considerable number of studies have shown that MELD-Na is a good predictor of prognosis in liver transplantation patients, and it has entered clinical practice in the United States. Nevertheless, there is still a limited amount of research on the predictive capacity of MELD-Na for 1-year mortality in TIPS patients [29,31,62,63]. The MELD score seemed no different between the low-PMD group and the high-PMD group (
At present, multiple tools are used for evaluating the nutritional condition of LC patients, but a clinically available tool is still lacking. PMD is an objective, noninvasive quantitative indicator that uses clinically common software. As a routine inspection item, CT does not increase hospitalization costs. Clinically, early screening of LC patients with a poor prognosis and a high risk of death will improve survival time and quality of life. In summary, PMD will be useful to clinicians as a reliable tool to help in treatment decision-making for LC patients in clinical practice. Sarcopenia-based TIPS may provide even less benefit. It may be a better option to give TIPS after improving the patient’s nutritional status. The review by Ebadi et al [11] has shown that early, planned multimodal interventions, including nutritional support, physical exercise, and pharmacological intervention, are necessary to prevent and/or treat sarcopenia. Whether exercise, medication, or other treatments decrease mortality in patients with TIPS remains uncertain. More related work is needed in the future.
Our current research has limitations. First, our study was a retrospective single-center study, and external validation is needed. Due to the technical difficulty of TIPS and the clinical characteristics of patients at different medical centers, prospective multicenter validation is needed to acquire further evidence to support the use of PMD in clinical practice.
Conclusions
In conclusion, PMD measurement is an easy, objective, economical approach with repeatable clinical value for predicting the 1-year mortality of patients with TIPS. Further multicenter studies with larger cohorts or prospective trials are needed to validate our findings.
Figures
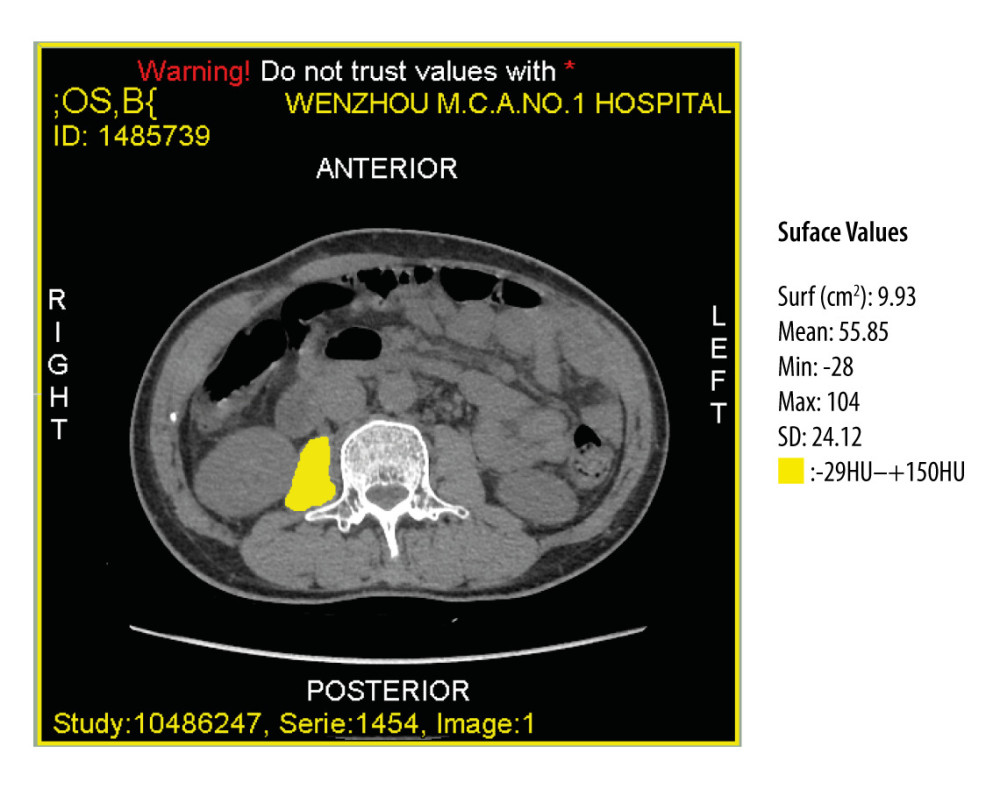 Figure 1. Computed tomography image at the midlevel of the third lumbar vertebra demonstrates a region of interest created by manually outlining the borders of both psoas muscles.
Figure 1. Computed tomography image at the midlevel of the third lumbar vertebra demonstrates a region of interest created by manually outlining the borders of both psoas muscles. 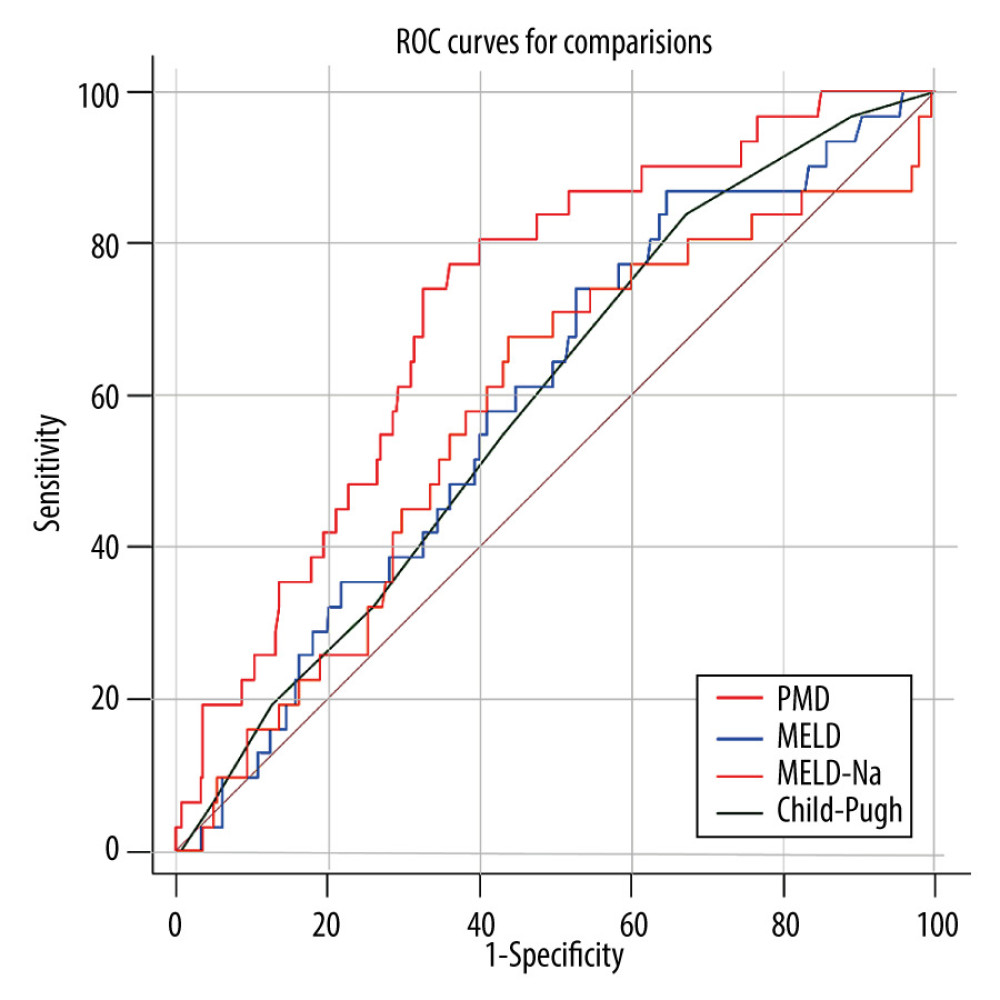 Figure 2. The receiver operating characteristic (ROC) curve of the predictive ability of psoas muscle density (PMD), Child-Pugh score, and model for end-stage liver disease (MELD) to predict post-transjugular intrahepatic portosystemic shunt death in 1 year.
Figure 2. The receiver operating characteristic (ROC) curve of the predictive ability of psoas muscle density (PMD), Child-Pugh score, and model for end-stage liver disease (MELD) to predict post-transjugular intrahepatic portosystemic shunt death in 1 year. 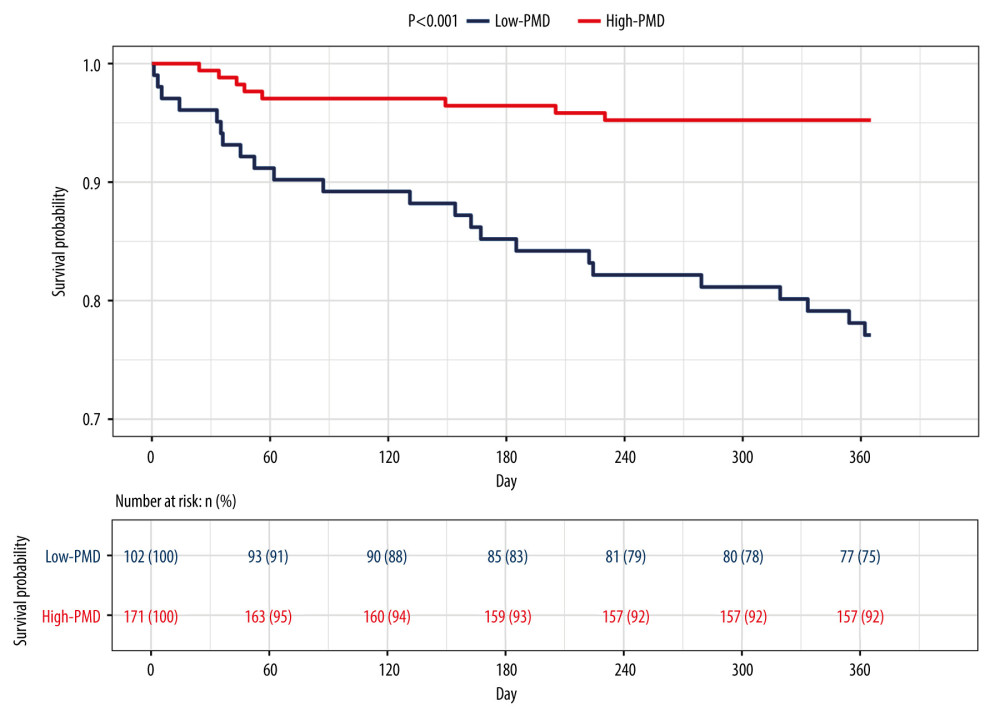 Figure 3. Kaplan-Meier survival curves stratified with psoas muscle density (PMD) level. Survival probability is greater when PMD is more than 49.92 Hounsfield units (HU).
Figure 3. Kaplan-Meier survival curves stratified with psoas muscle density (PMD) level. Survival probability is greater when PMD is more than 49.92 Hounsfield units (HU). 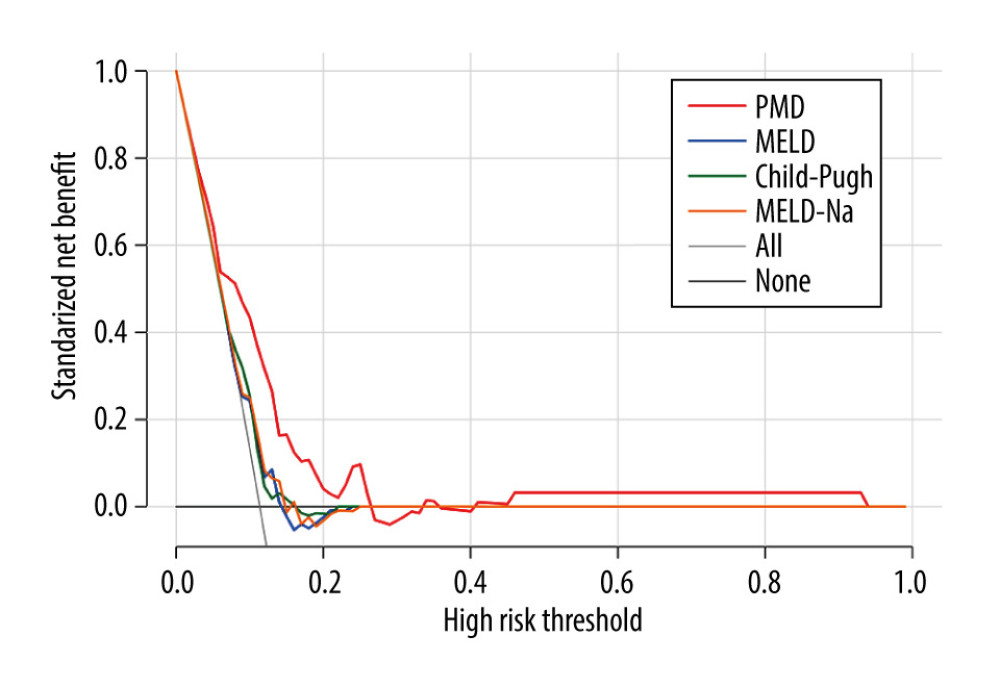 Figure 4. Decision curves for 3 ways to predict an event in the patients. MELD – model for end-stage liver disease; MELD-Na – composite of model for end-stage liver disease and serum sodium; PMD – psoas muscle density.
Figure 4. Decision curves for 3 ways to predict an event in the patients. MELD – model for end-stage liver disease; MELD-Na – composite of model for end-stage liver disease and serum sodium; PMD – psoas muscle density. 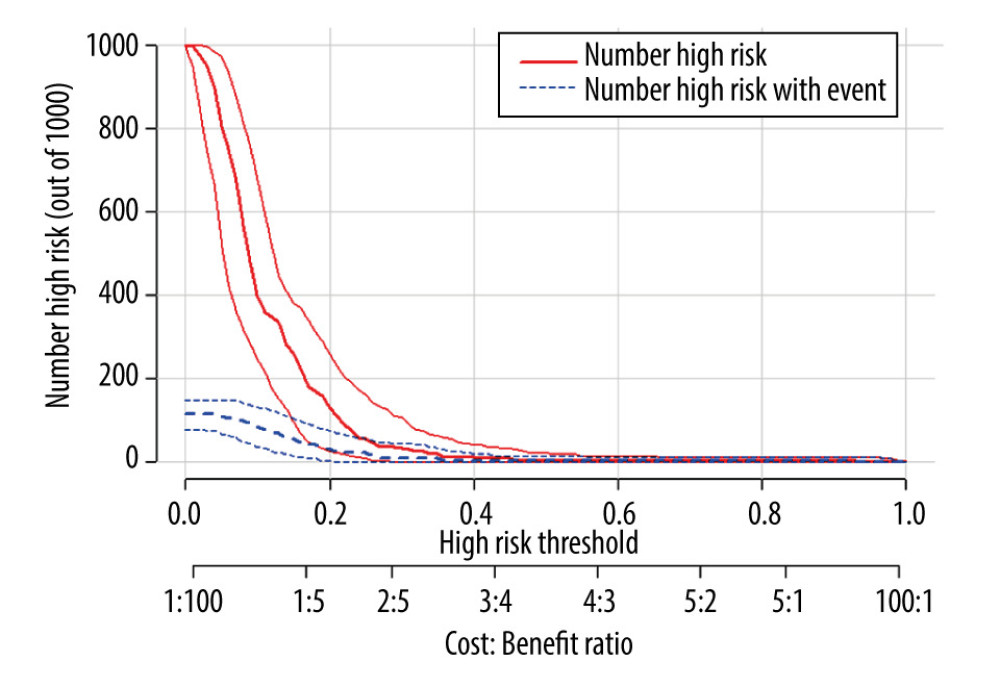 Figure 5. Clinical impact curve for the psoas muscle density (PMD) measurement.
Figure 5. Clinical impact curve for the psoas muscle density (PMD) measurement. 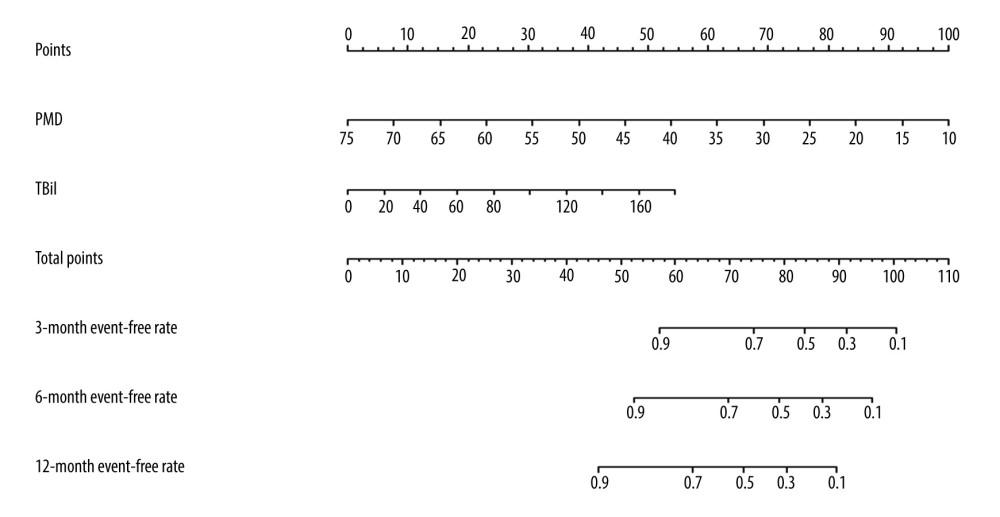 Figure 6. The nomogram of improved psoas muscle density (PMD) measurement. TBil – total bilirubin.
Figure 6. The nomogram of improved psoas muscle density (PMD) measurement. TBil – total bilirubin. Tables
Table 1. Characteristics of the study population, stratified by survival event.a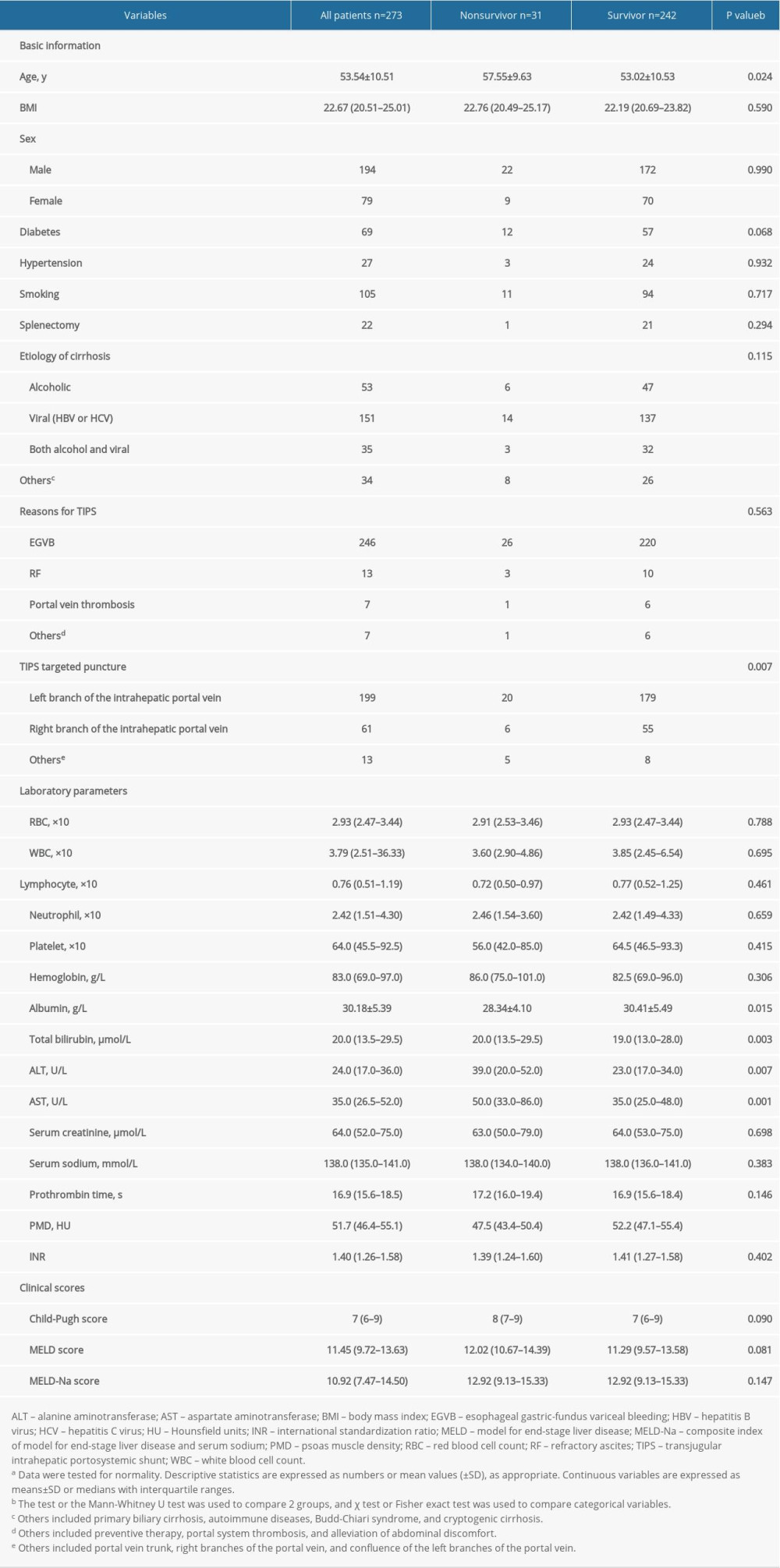 Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a
Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a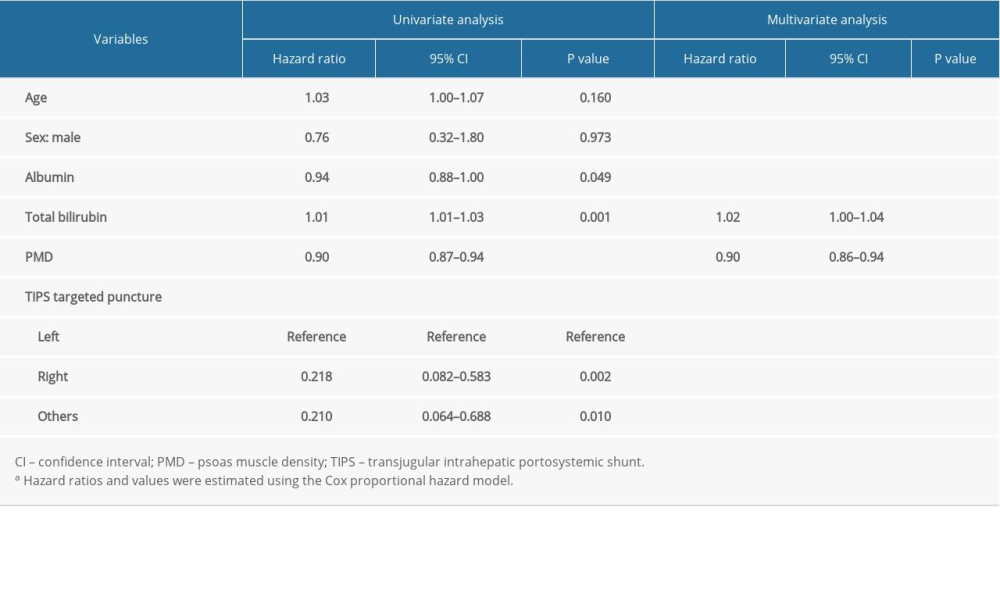 Table 3. Characteristics of the study population, stratified by PMD measurement.a
Table 3. Characteristics of the study population, stratified by PMD measurement.a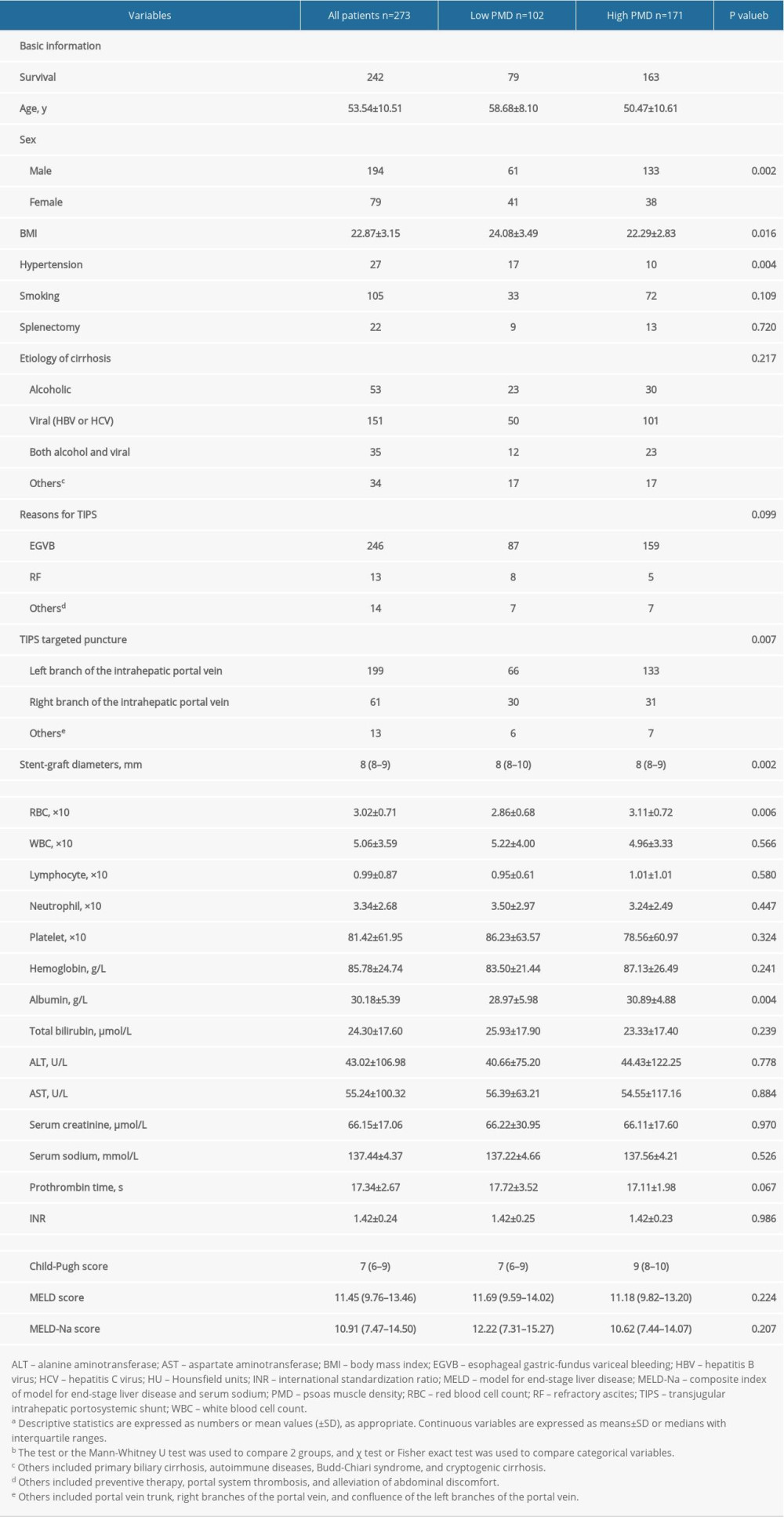 Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point.
Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point.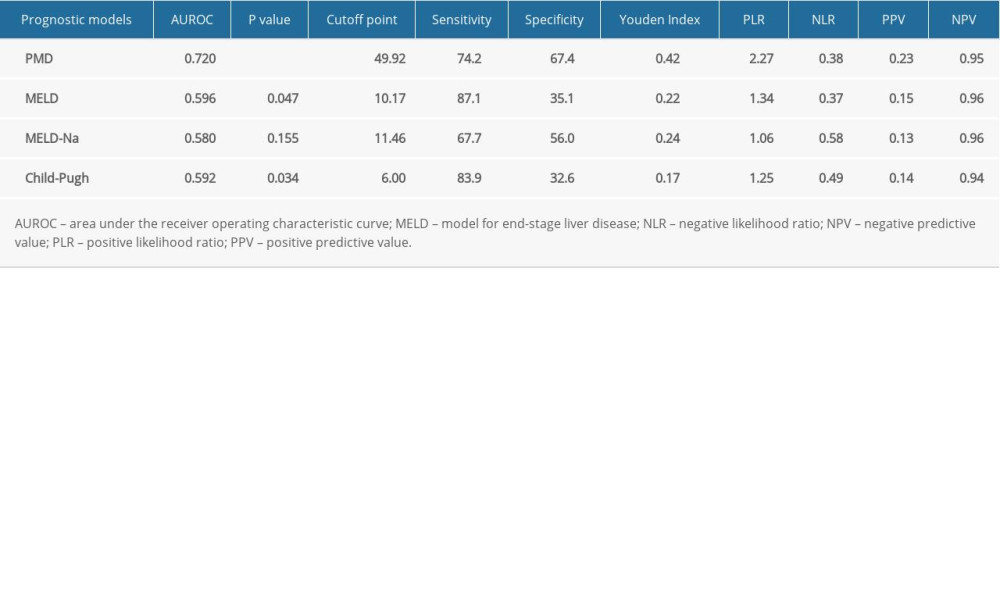 Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate.
Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate.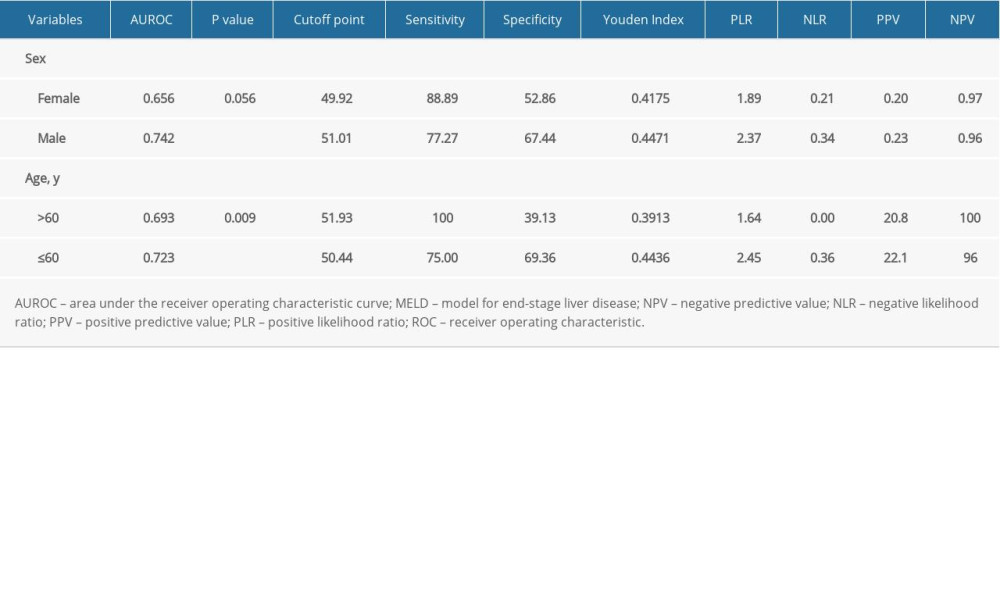
References
1. Nishikawa H, Fukunishi S, Asai A, Sarcopenia and frailty in liver cirrhosis: Life (Basel), 2021; 11(5); 399
2. Dasarathy S, Merli M, Sarcopenia from mechanism to diagnosis and treatment in liver disease: J Hepatol, 2016; 65; 1232-44
3. Scaglione S, Kliethermes S, Cao G, The epidemiology of cirrhosis in the United States: A population-based study: J Clin Gastroenterol, 2015; 49(8); 690-96
4. Dasarathy S, Consilience in sarcopenia of cirrhosis: J Cachexia Sarcopenia Muscle, 2012; 3; 225-37
5. Montano-Loza AJ, Meza-Junco J, Baracos VE, Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation: Liver Transpl, 2014; 20; 640-48
6. Montano-Loza AJ, Meza-Junco J, Prado CM, Muscle wasting is associated with mortality in patients with cirrhosis: Clin Gastroenterol Hepatol, 2012; 10; 166-73
7. Tandon P, Ney M, Irwin I, Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value: Liver Transpl, 2012; 18; 1209-16
8. Carey EJ, Lai JC, Wang CW, A multicenter study to define sarcopenia in patients with end-stage liver disease: Liver Transpl, 2017; 23; 625-33
9. Jones KI, Doleman B, Scott S, Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications: Colorectal Dis, 2015; 17; O20-26
10. Englesbe MJ, Patel SP, He K, Sarcopenia and mortality after liver transplantation: J Am Coll Surg, 2010; 211; 271-78
11. Ebadi M, Bhanji R, Tandon P, Review article: Prognostic significance of body composition abnormalities in patients with cirrhosis: Aliment Pharmacol Ther, 2020; 52; 600-18
12. , Warfarin reduces the incidence of portal vein thrombosis after transjugular intrahepatic portosystemic shunt: A prospective study: Gastroenterology, 2017; 152; S1049
13. McDonald A, Swain T, Mayhew D, CT measures of bone mineral density and muscle mass can be used to predict noncancer death in men with prostate cancer: Radiology, 2017; 282; 475-83
14. Chakedis J, Spolverato G, Beal EW, Pre-operative sarcopenia identifies patients at risk for poor survival after resection of biliary tract cancers: J Gastrointest Surg, 2018; 22(10); 1697-708
15. Cichos K, Mahmoud K, Spitler C, Risk factors for surgical site infection after operative fixation of acetabular fractures: Is psoas density a useful metric?: Clin Orthop Relat Res, 2020; 478; 1760-67
16. Herrod P, Boyd-Carson H, Doleman B, Quick and simple; Psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection: Tech Coloproctol, 2019; 23; 129-34
17. Tzeng Y, Wei J, Tsao T, Computed tomography-determined muscle quality rather than muscle quantity is a better determinant of prolonged hospital length of stay in patients undergoing transcatheter aortic valve implantation: Acad Radiol, 2020; 27; 381-88
18. García-Pagán JC, Saffo S, Where does TIPS fit in the management of patients with cirrhosis?: JHEP Rep, 2020; 2; 100122
19. Niekamp A, Kuban J, Lee S, Transjugular intrahepatic portosystemic shunts reduce variceal bleeding and improve survival in patients with cirrhosis: A population-based analysis: J Vasc Interv Radiol, 2020; 31; 1382-91
20. Trivedi PS, Rochon PJ, Durham JD, Ryu RK, National trends and outcomes of transjugular intrahepatic portosystemic shunt creation using the nationwide inpatient sample: J Vasc Interv Radiol, 2016; 27(6); 838-45
21. European Association for the Study of the Liver, EASL clinical practice guidelines: liver transplantation: J Hepatol, 2016; 64; 433-85
22. Al Sibae M, Cappell M, Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS: Dig Dis Sci, 2011; 56; 977-87
23. Kamath PS, Wiesner RH, Malinchoc M, A model to predict survival in patients with end-stage liver disease: Hepatology, 2001; 33; 464-70
24. Biggins SW, Use of serum sodium for liver transplant graft allocation: A decade in the making, now is it ready for primetime?: Liver Transpl, 2015; 21; 279-81
25. Biggins SW, Kim WR, Terrault NA, Evidence-based incorporation of serum sodium concentration into MELD: Gastroenterology, 2006; 130; 1652-60
26. Borroni G, Maggi A, Sangiovanni A, Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients: Dig Liver Dis, 2000; 32; 605-10
27. Freitas ACT, Rampim AT, Nunes CP, Coelho JCU, Impact of meld sodium on liver transplantation waiting list: Arq Bras Cir Dig, 2019; 32; e1460
28. Ruf AE, Kremers WK, Chavez LL, Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone: Liver Transpl, 2005; 11; 336-43
29. Gaba RC, Couture PM, Bui JT, Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation: J Vasc Interv Radiol, 2013; 24; 411-20 quiz 421
30. Allegretti AS, Frenk NE, Li DK, Evaluation of model performance to predict survival after transjugular intrahepatic portosystemic shunt placement: PLoS One, 2019; 14; e0217442
31. Young S, Rostambeigi N, Golzarian J, Lim N, MELD or sodium MELD: a comparison of the ability of two scoring systems to predict outcomes after transjugular intrahepatic portosystemic shunt placement: Am J Roentgenol, 2020; 215; 215-22
32. Montano-Loza A, Duarte-Rojo A, Meza-Junco J, Inclusion of sarcopenia within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis: Clin Transl Gastroenterol, 2015; 6; e102
33. van Vugt JLA, Alferink LJM, Buettner S, A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort: J Hepatol, 2018; 68; 707-14
34. Gleeson J, Barry J, O’Reilly S, Use of liver imaging and biopsy in clinical practice: N Engl J Med, 2017; 377; 2296
35. Rajesh S, George T, Philips CA, Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update: World J Gastroenterol, 2020; 26; 5561-96
36. Karasu Z, Gurakar A, Kerwin B, Effect of transjugular intrahepatic portosystemic shunt on thrombocytopenia associated with cirrhosis: Dig Dis Sci, 2000; 45; 1971-76
37. Vizzutti F, Schepis F, Arena U, Transjugular intrahepatic portosystemic shunt (TIPS): Current indications and strategies to improve the outcomes: Intern Emerg Med, 2020; 15; 37-48
38. Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases Practice Guidelines: The role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension: J Vasc Interv Radiol, 2005; 16; 615-29
39. Aithal G, Palaniyappan N, China L, Guidelines on the management of ascites in cirrhosis: Gut, 2021; 70; 9-29
40. Vilstrup H, Amodio P, Bajaj J, Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver: Hepatology, 2014; 60; 715-35
41. Durand F, Valla D, Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD: J Hepatol, 2005; 41(Suppl 1); S100-7
42. Shen W, Punyanitya M, Wang Z, Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image: J Appl Physiol (1985), 2004; 97(6); 2333-38
43. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography: J Appl Physiol (1985), 1998; 85(1); 115-22
44. Figueiredo P, Marques EA, Gudnason V, Computed tomography-based skeletal muscle and adipose tissue attenuation: Variations by age, sex, and muscle: Exp Gerontol, 2021; 149; 111306
45. Kerr KF, Brown MD, Zhu K, Janes H, Assessing the clinical impact of risk prediction models with decision curves: Guidance for correct interpretation and appropriate use: J Clin Oncol, 2016; 34; 2534-40
46. Baumgartner RN, Koehler KM, Gallagher D, Epidemiology of sarcopenia among the elderly in New Mexico: Am J Epidemiol, 1998; 147; 755-63
47. Kim G, Kang SH, Kim MY, Baik SK, Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis: PLoS One, 2017; 12; e0186990
48. van Vugt JL, Levolger S, de Bruin RW, Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation: Am J Transplant, 2016; 16(8); 2277-92
49. Kim HY, Jang JW, Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score: World J Gastroenterol, 2015; 21; 7637-47
50. Ronald J, Bozdogan E, Zaki IH, Relative sarcopenia with excess adiposity predicts survival after transjugular intrahepatic portosystemic shunt creation: Am J Roentgenol, 2020; 214; 2005
51. Shoreibah MG, Mahmoud K, Aboueldahab NA, Psoas muscle density in combination with model for end-stage liver disease score can improve survival predictability in transjugular intrahepatic portosystemic shunts: J Vasc Interv Radiol, 2019; 30; 154-61
52. Tsien C, Shah SN, McCullough AJ, Dasarathy S, Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent: Eur J Gastroenterol Hepatol, 2013; 25; 85-93
53. Praktiknjo M, Book M, Luetkens J, Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis: Hepatology, 2018; 67; 1014-26
54. Johnson TM, Overgard EB, Cohen AE, DiBaise JK, Nutrition assessment and management in advanced liver disease: Nutr Clin Pract, 2013; 28; 15-29
55. Daniel P, Kloeckner R, Koch S, Sarcopenia as prognostic factor for survival after orthotopic liver transplantation: Eur J Gastroenterol Hepatol, 2020; 32(5); 626-34
56. Ferreira JMM, Cunha P, Carneiro A, Sarcopenia as a prognostic factor in peripheral arterial disease: Descriptive review: Ann Vasc Surg, 2021; 74; 460-74
57. Von Geldern P, Salas C, Alvayay P, Nutritional assessment by subjective methods versus computed tomography to predict survival in oncology patients: Nutrition, 2021; 84; 111006
58. Ariën F, Baitar A, Perkisas S, The association between muscle mass and the degree of myosteatosis of the psoas muscle and mortality in older patients with cancer: J Geriatr Oncol, 2021; 12; 85-90
59. Shams T, Moschouri E, Denys AEarly TIPS: A practical review after 15 years of scientific evidence: Rev Med Suisse, 2020; 16(704); 1548-53 [in French]
60. Trebicka J, Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients?: Semin Liver Dis, 2018; 38; 87-96
61. Zuo K, Wang C, Wang J, Transjugular intrahepatic portosystemic shunt through left branch versus right branch of portal vein: A meta-analysis: Abdom Radiol (NY), 2021; 46; 1718-25
62. Ahmed R, Santhanam P, Rayyan Y, MELD-Na as a prognostic indicator of 30- and 90-day mortality in patients with end-stage liver disease after creation of transjugular intrahepatic portosystemic shunt: Eur J Gastroenterol Hepatol, 2015; 27; 1226-27
63. Guy J, Somsouk M, Shiboski S, New model for end stage liver disease improves prognostic capability after transjugular intrahepatic portosystemic shunt: Clin Gastroenterol Hepatol, 2009; 7; 1236-40
64. Golse N, Bucur PO, Ciacio O, A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation: Liver Transpl, 2017; 23; 143-54
Figures
 Figure 1. Computed tomography image at the midlevel of the third lumbar vertebra demonstrates a region of interest created by manually outlining the borders of both psoas muscles.
Figure 1. Computed tomography image at the midlevel of the third lumbar vertebra demonstrates a region of interest created by manually outlining the borders of both psoas muscles. Figure 2. The receiver operating characteristic (ROC) curve of the predictive ability of psoas muscle density (PMD), Child-Pugh score, and model for end-stage liver disease (MELD) to predict post-transjugular intrahepatic portosystemic shunt death in 1 year.
Figure 2. The receiver operating characteristic (ROC) curve of the predictive ability of psoas muscle density (PMD), Child-Pugh score, and model for end-stage liver disease (MELD) to predict post-transjugular intrahepatic portosystemic shunt death in 1 year. Figure 3. Kaplan-Meier survival curves stratified with psoas muscle density (PMD) level. Survival probability is greater when PMD is more than 49.92 Hounsfield units (HU).
Figure 3. Kaplan-Meier survival curves stratified with psoas muscle density (PMD) level. Survival probability is greater when PMD is more than 49.92 Hounsfield units (HU). Figure 4. Decision curves for 3 ways to predict an event in the patients. MELD – model for end-stage liver disease; MELD-Na – composite of model for end-stage liver disease and serum sodium; PMD – psoas muscle density.
Figure 4. Decision curves for 3 ways to predict an event in the patients. MELD – model for end-stage liver disease; MELD-Na – composite of model for end-stage liver disease and serum sodium; PMD – psoas muscle density. Figure 5. Clinical impact curve for the psoas muscle density (PMD) measurement.
Figure 5. Clinical impact curve for the psoas muscle density (PMD) measurement. Figure 6. The nomogram of improved psoas muscle density (PMD) measurement. TBil – total bilirubin.
Figure 6. The nomogram of improved psoas muscle density (PMD) measurement. TBil – total bilirubin. Tables
 Table 1. Characteristics of the study population, stratified by survival event.a
Table 1. Characteristics of the study population, stratified by survival event.a Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a
Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a Table 3. Characteristics of the study population, stratified by PMD measurement.a
Table 3. Characteristics of the study population, stratified by PMD measurement.a Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point.
Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point. Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate.
Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate. Table 1. Characteristics of the study population, stratified by survival event.a
Table 1. Characteristics of the study population, stratified by survival event.a Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a
Table 2. The results of univariate and multivariate Cox analysis of the association between clinical parameters and 1-year mortality rate.a Table 3. Characteristics of the study population, stratified by PMD measurement.a
Table 3. Characteristics of the study population, stratified by PMD measurement.a Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point.
Table 4. Diagnostic accuracy of different scoring systems in predicting post-transjugular intrahepatic portosystemic shunt mortality within 1-year at the optimal cutoff point. Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate.
Table 5. Subgroup ROC curves of the association between PMD and 1-year mortality rate. In Press
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








