11 February 2022: Clinical Research
Estimation of 24-h Urine Protein Excretion Using Urine Albumin-to-Creatinine Ratio from an In-Hospital Population
Xin Liu12BCDEF, Yonghong Zhao3CDE, Yunlin Feng24ACDEF*DOI: 10.12659/MSM.934307
Med Sci Monit 2022; 28:e934307
Abstract
BACKGROUND: There is little information available on quantitative description of the relationship between urine albumin-to-creatinine ratio (ACR) and 24-h urine protein excretion (24-h UPE). Here, we developed a calculation tool for 24-h UPE using the urine ACR and limited information on the request form.
MATERIAL AND METHODS: This was a retrospective and observational study. All individuals with same-day urine ACR and 24-h UPE tests in Sichuan Provincial People’s Hospital from September 1, 2018 to December 31, 2019 were enrolled. Correlation and agreement between urine ACR and 24-h UPE were evaluated using correlation analysis and an intraclass correlation coefficient, respectively. The Durbin-Watson test and ANOVA were used to assess the performance of the calculation tool, and reliability of the prediction equation was evaluated in the validation group using residual error analysis.
RESULTS: A total of 906 participants were enrolled, including 639 participants in the development group and 267 in the validation group. Natural logarithm transformation was applied to remove skewness. Natural logarithm-transformed urine ACR correlated well with natural-logarithm-transformed 24-h UPE (Pearson coefficient=0.908; P<0.001) and the agreement was consistently good (overall ICC=0.938; 95% CI: 0.928-0.947; P<0.001). The multivariable regression model had good performance (R²=0.864) and high accuracy, demonstrated by results of residual error analysis.
CONCLUSIONS: We provide a practical calculation tool to estimate total protein excretion using urine ACR and readily accessible variables. However, 24-h UPE is still mandatory when proteinuria is over 10 g/day or when most proteinuria may not be of glomerular origin.
Keywords: Logistic Models, Proteinuria, Renal Insufficiency, Chronic, Albuminuria, China, Creatinine, Female, Humans, Inpatients, Male, Reproducibility of Results, Urinalysis
Background
Proteinuria is not only one of the most important manifestations of kidney diseases, it is also an essential measurement in monitoring the progress and prognosis of various glomerular diseases [1]. Therefore, its quantification constitutes an indispensable part of the daily practice of nephrologists, and 24-h urine protein excretion (24-h UPE) is considered the criterion standard method. However, this test can be cumbersome for patients and can have a high risk of inaccuracy due to inadequate sample collection [2].
Other well-accepted approaches to evaluate proteinuria are the urine protein-to-creatinine ratio (PCR) and urine albumin-to-creatinine ratio (ACR) in spot morning urine samples [3], whose collection is much simpler than that of 24-h urine collection. Although there are a number of studies using urine PCR to predict 24-h UPE [2–5], investigating the correlation between PCR and ACR [6], and demonstrating that ACR and PCR in random urine are strongly correlated [7,8], the quantitative description of the relationship between urine ACR and 24-h UPE has not been well described [4]. In addition, there is ongoing debate on the utility and practicality of replacing 24-h urine collection with a spot morning urine sample [9,10].
The routine proteinuria examinations carried out in our hospital include the 24-h UPE and urine ACR tests. However, we have found the 24-h urine collection procedure to be poorly understood by patients, especially those with lower levels of education, which often causes errors in the results. Therefore, we developed a simple tool which can be easily used for patients who present for the first time and do not have time to collect a 24-h urine sample to estimate 24-h UPE using urine ACR and limited information on the request form. We hope this tool can provide more information to facilitate clinical practice.
Material and Methods
STUDY POPULATION:
This was a retrospective and observational study of all individuals who had proteinuria assessment by urine ACR and 24-h UPE on the same day from September 1, 2018, to December 31, 2019, in Sichuan Provincial People’s Hospital. Data were extracted from the electronic system of the Department of Laboratory Medicine instead of the medical record system to ensure accuracy. All patients who had been prescribed proteinuria examinations were instructed verbally and by printed instructions on the correct procedure of urine sample collection as part of a hospital-wide standardized program launched June 1, 2018, which was adopted from the study by Smith et al [8]. Briefly, the urine ACR sample was the spot morning mid-stream urine sample. To collect the 24-h urine samples, patients were instructed to empty the bladder in the morning and discard the urine, and from that point onward for 24 h, all urine was to be saved in a clean container. The last urine sample was to be collected at the end of that 24-h period when the bladder was emptied. Once the collection was completed, patients were instructed to measure the total amount of urine and adequately mix the urine sample by stirring with a clean stick or by gently inverting the container several times. After that, a sample of 3 to 5 mL of urine was to be sent to the laboratory with the total amount marked. This program encouraged clinicians to order the 24-h UPE and urine ACR simultaneously for urine protein examination candidates. The Nephrology Department also held 2 lectures on screening for chronic kidney diseases to popularize the program. To avoid confusion from overflow proteinuria, patients with a confirmed diagnosis of hematologic diseases were excluded, as were those with an obvious mismatch of urine ACR and 24-h UPE, which was defined as urine ACR <500 μg/mg plus 24-h UPE >5 g or urine ACR >5000 μg/mg plus 24-h UPE <1 g.
This study was approved by the local Ethics Committee of Sichuan Provincial People’s Hospital (no. 2017.124). The Institutional Review Board waived the need for informed consent since this was an observational analysis of de-identified data. The study was conducted in compliance with local ethics specifications and the principles of the Helsinki Declaration.
MEASUREMENT OF PROTEINURIA:
All urine specimens were handled and tested at our central laboratory, and appropriate measurements of preservation and shipment of urine samples were applied. Urinary albumin, urinary creatinine, and total urinary protein were tested on a Beckman Coulter AU5841 chemical analyzer (Beckman Coulter, CA, USA) using a turbidimetric immunoassay, sarcosine oxidase assay, and pyrogallol red assay, respectively. Three commercial test kits were traced to the standard reference materials NIST ERM DA470k, SRM 967a, and SRM 927d, respectively. Urine ACR (μg/mg) was calculated as the ratio of urine albumin concentration to urine creatinine concentration. The linear range of urine albumin, urine creatinine, and 24-h UPE tests were 4 to 300 mg/mL, 2.4 to 8840 μmol/L, and 0 to 200 mg/L, respectively. All 3 assessments were validated and certified by the National Center for Clinical Laboratories and compared against the secondary reference laboratory results. All tests were completed within 4 h of urine collection.
DATA COLLECTION:
Basic information on the request form, including age, sex, source, request department, and primary diagnosis, was collected alongside the results of urine ACR and 24-h UPE. The source of samples was classified as either out-patient or in-patient. Requesting departments were classified into Internal Medicine and “others”, which consisted of Surgery, Pediatrics, Gynecology and Obstetrics, Emergency Department, and others. Primary diagnoses were classified into routine examination and medical condition-related diagnosis, which included proteinuria with unknown origin, nephrotic syndrome, non-dialysis-dependent chronic kidney disease, dialysis-dependent chronic kidney disease, connective tissue disease, and others.
STATISTICAL ANALYSIS:
The Kolmogorov-Smirnov test was used to test the normality of distribution for all continuous data. Natural logarithm (LN) transformation was applied to move skewness when needed. Descriptive data were expressed in terms of the median (interquartile range) or mean±standard deviation for continuous data and number (percentage) for categorical data. To investigate the correlation between urine ACR and 24-h UPE, the correlation coefficient was determined and interpreted as follows [11]: 0.00 to 0.29, negligible; 0.3 to 0.49, low; 0.5 to 0.7, moderate; 0.7 to 0.9, high; and 0.9 to 0.99, very high. Intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals (CIs) were calculated based on an absolute-agreement, 2-way mixed-effects model [12] to evaluate the agreement between urine ACR and 24-h UPE. An ICC ≥0.85 was interpreted as good agreement [13].
To develop and validate an equation to calculate 24-h UPE, we followed a 4-step procedure. First, the population was randomly divided at a ratio of 7: 3 into a development group and a validation group. Differences in variables between the development and validation groups were tested using the
Statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, NY, USA), with statistical significance set at
Results
CORRELATION AND AGREEMENT:
Correlation analysis showed a strong correlation between LN 24-h UPE and LN urine ACR in the overall population and different subgroups (Pearson coefficients: 0.908, 0.925, 0.876 in the overall, development, and validation groups, respectively; all
PREDICTION EQUATION OF 24-H UPE:
Qualitative evaluation revealed potential significant factors for LN 24-h UPE, including source (χ2 test: t=7.550, P<0.001), primary diagnosis (χ2 test: t=5.942, P<0.001), sex (χ2 test: t=3.285, P=0.001), age (Spearman r=0.087, P=0.027), and LN urine ACR (Pearson r=0.908, P<0.001). Significant predictors included in the multivariable linear regression equation were LN urine ACR, source, and sex (Table 2). Results of the Durbin-Watson test (1.661) and ANOVA (F=1357.608, P=0.000) demonstrated the prediction equation met the requirement of independence. A standardized residual error histogram and residual error plot demonstrated that the prediction equation met the requirement of normality and homogeneity (Figure 2). The detailed equation was as follows:
The equation was then back-transformed and translated into an online calculator for the readers’ convenience (scan the QR code in Supplementary Figure 3 in Supplementary data or visit: http://redcap.samsph.com: 16866/surveys/ and enter the code: H7XKTXXDW).
The residual error evaluation of the prediction equation in the validation group also supported the good calibration (Supplementary Figure 4). Subgroup analyses indicated out-patients and female patients tended to have more positive residual errors (Supplementary Figures 5, 6). The prediction equation performed best for 24-h UPE ≤3.5 g/day, as the mean of standard errors was the lowest among the 3 groups (Supplementary Table 1 and Supplementary Figure 7).
Discussion
This study demonstrated good correlation and agreement between spot urine ACR and 24-h UPE in patients having these 2 examinations simultaneously. Our multivariable regression model of 24-h UPE had a good performance (R2=0.864) and high accuracy demonstrated by results of residual error analysis. However, the present equation is not suitable for overflow proteinuria when the main reason for urine protein is not glomerular diseases.
In 2009, Eknoyan et al proposed the limitations of different methods for urine albumin detection [14]. Bachmann et al compared the bias between 16 commonly used detection methods and showed that there were sizable differences in the urine albumin detection results of different reagent manufacturers [15]. The diversity of albumin in urine presents a great challenge for its accurate quantification [16]. ERM DA470k, developed by the International Federation of Clinical Chemistry, is used as a candidate reference substance for urine albumin [17]. It is especially important to evaluate the consistency of different methods to ensure the applicability of the method. Urine albumin, urine creatinine, and urine total protein in the present study can be traced back to NIST ERM DA470k, SRM 967a, and SRM 927d in their respective specifications, ensuring the application scenarios of the results of this research in other regions.
Owing to the difficulty in adequately collecting 24-h urine, especially in patients with lower levels of education and/or with poor compliance, there has long been an effort to use results from spot urine to replace 24-h UPE during clinical decision making [18–20]. Our findings of a strong correlation between urine ACR and 24-h UPE were consistent with previous reports on the correlation between these 2 variables in primary glomerular disease as well as other diseases with secondary proteinuria [21–23]. Atkins et al also reported a strong correlation between urine albumin excretion and total protein excretion in the Australian adult population, particularly among the elderly and patients with comorbidities, but concluded a urine albumin measurement should not replace the total protein excretion test [24]. Teo et al reported similar results in patients with chronic kidney disease and provided an equation to calculate 24-h UPE using urine ACR [25]. Our study examined the correlation between spot urine protein and 24-h UPE in a larger population, including patients with internal medicine diseases and patients who presented for a routine check-up. The primary care system is not yet well established in China; therefore, many people come to hospitals for examinations without symptoms. This can be reflected by the medians of urine ACR and 24-h UPE, which were both below the significant pathological level in the present study. The equation also benefits from the limited number of predictors, making it very easy and convenient to use. Therefore, our results might help us to conveniently estimate total protein excretion in a more general situation, especially in the clinic. In addition, the web-based survey tool can continuously collect data, and we look forward to its future updates. With more data collected, we are hoping to further improve the applicability of the equation.
Be that as it may, the use of urine ACR as a sole indicator for quantification of proteinuria has not reached a consensus [9,10]. Huang et al reported that spot urine ACR was a simple and convenient indicator of significant proteinuria in women with pre-eclampsia [19]; however, by querying the US nationwide laboratory network, Katayev et al found that testing for only urine (micro)albumin can miss up to 40% of female patients and 30.8% of male patients with gross proteinuria [26]. The difference might result from the different study populations. It is important to note that total protein excretion estimated by albuminuria cannot be used when most protein excreted is not mainly due to the impaired filtration, but rather is due to over-excretion or impaired absorption [27]. Estimating 24-h UPE by urine ACR is suitable for urine protein of glomerular origin. Therefore, in situations when the major component of proteinuria is from a tubular origin, collecting 24-h urine is still mandatory.
Subgroup analysis indicated the prediction equation performed best for 24-h UPE of ≤3.5 g/day. The discrepancy between predicted and observed 24-h UPE values increased with the amount of 24-h UPE. This result was consistent with our clinical observation. However, it should also be noted that there were far fewer participants in the high proteinuria group than in the low proteinuria group. Another result from the subgroup analysis was that the prediction equation tended to over-estimate 24-h UPE for out-patients and female patients, reflected by the negative signs in front of the 2 corresponding variables in the equation. Further improvement is expected since continuous data collection in ongoing.
The study has some limitations. First, the study was based on a retrospective analysis of participants who had both urine ACR and 24-h UPE tests at the same time from a single center. Selection bias could not be avoided since patients presenting with proteinuria but having 24-h UPE and urine ACR tested separately were excluded. As mentioned earlier, we tried to reduce the bias through a hospital-wide program and internal lectures, which encourage clinicians to order both tests at the same time. In addition, the equation should improve with continuous data collection. To be generalized to a wider population, these results still need further validation. Second, the prediction model has an inherent limitation of not being suitable for proteinuria of tubular origin, as mentioned earlier. Therefore, we need to keep this in mind when interpreting the results. Third, since we did not routinely measure body weight in our clinic, we were not able to evaluate the adequacy of urine collection, which was usually assessed by comparing the creatinine from the 24-h urine sample collection with the expected creatinine content [1]. Including samples from under-collection might have interfered with the accuracy in assessing the correlation and agreement between urine ACR and 24-h UPE. To overcome this limitation, we provided patients with instructions on collecting urine specimens according to a hospital-wide standardized program and enrolled every patient with paired urine ACR and 24-h UPE results to reduce possible bias. With more data collected, the performance of the estimation tool is hoped to improve.
Conclusions
In conclusion, urine ACR was correlated with 24-h UPE in a general population. Based on our results, we offer a practical calculation tool to estimate total protein excretion using urine ACR and readily accessible variables. However, 24-h UPE is still mandatory when proteinuria is over 10 g/day or when most proteinuria may not be of glomerular origin.
Figures
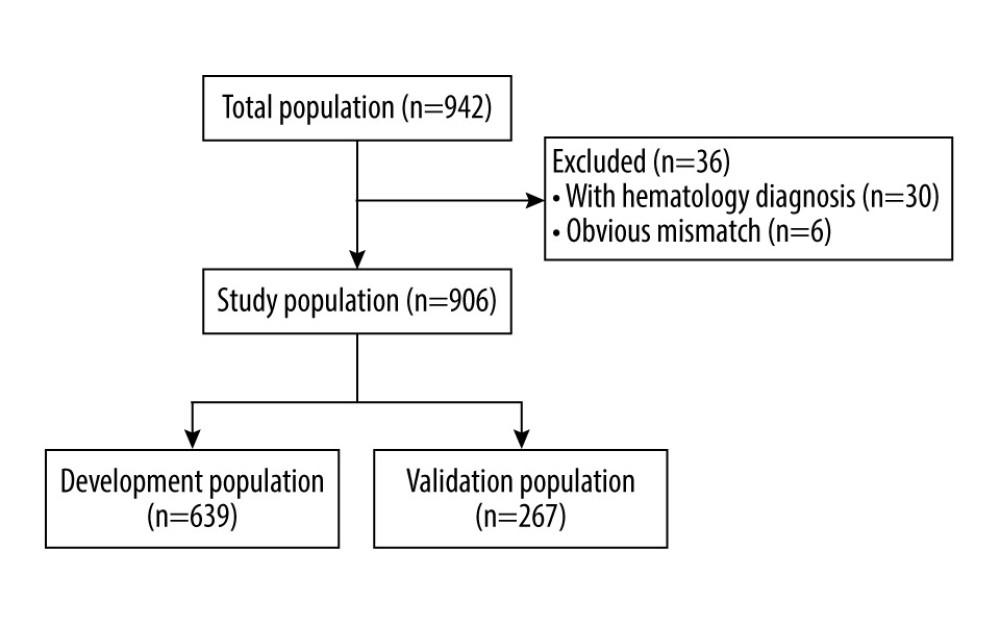 Figure 1. Flow chart of study population. The figure was created by PowerPoint 2010 for Windows (Microsoft, WA, USA).
Figure 1. Flow chart of study population. The figure was created by PowerPoint 2010 for Windows (Microsoft, WA, USA). 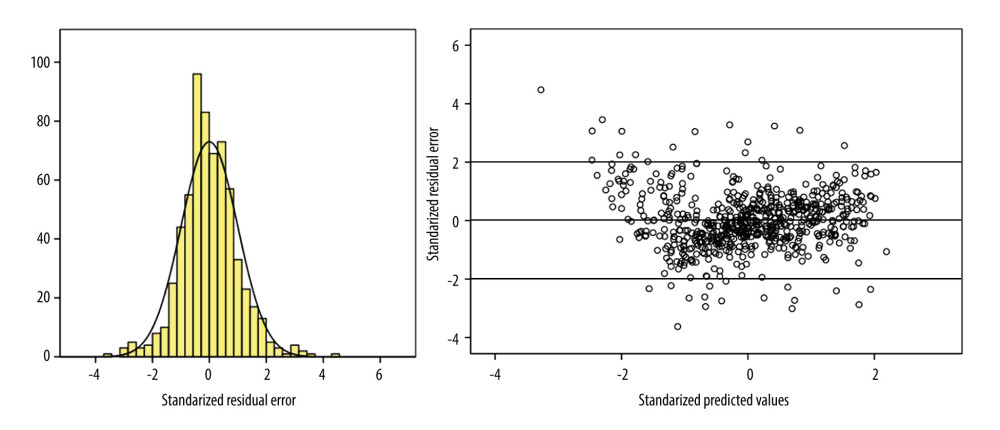 Figure 2. Illustration of performance evaluation in the development group. (Left) Histogram of standardized residual error in the development group, demonstrating the linear regression met the requirement of normality. The horizontal coordinates represent standardized residual errors between predicted and observed results. The vertical coordinates represent percentages. (Right) Plot of residual errors in the development group, demonstrating the linear regression met the requirement of homogeneity and independence. The horizontal coordinates represent predicted natural logarithm-transformed 24-h urine protein excretion. The vertical coordinates represent residual errors between predicted and observed results. The figure was created by SPSS version 22.0 (IBM Corp, Armonk, NY, USA).
Figure 2. Illustration of performance evaluation in the development group. (Left) Histogram of standardized residual error in the development group, demonstrating the linear regression met the requirement of normality. The horizontal coordinates represent standardized residual errors between predicted and observed results. The vertical coordinates represent percentages. (Right) Plot of residual errors in the development group, demonstrating the linear regression met the requirement of homogeneity and independence. The horizontal coordinates represent predicted natural logarithm-transformed 24-h urine protein excretion. The vertical coordinates represent residual errors between predicted and observed results. The figure was created by SPSS version 22.0 (IBM Corp, Armonk, NY, USA). References
1. Gansevoort RT, de Jong PE, Postma MJ, Cost-effectiveness of screening for proteinuria: JAMA, 2004; 291(12); 1442-43
2. Mitchell SC, Sheldon TA, Shaw AB, Quantification of proteinuria: A re-evaluation of the protein/creatinine ratio for elderly participants: Age Ageing, 1993; 22(6); 443-49
3. Jensen JS, Clausen P, Borch-Johnsen K, Detecting microalbuminuria by urinary albumin/creatinine concentration ratio: Nephrol Dial Transplant, 1997; 12(Suppl 2); 6-9
4. Gansevoort RT, Verhave JC, Hillege HL, The validity of screening based on spot morning urine samples to detect participants with microalbuminuria in the general population: Kidney Int Suppl, 2005(94); S28-35
5. Akbari A, White CA, Shahbazi N, Spot urine protein measurements: Are these accurate in kidney transplant recipients?: Transplantation, 2012; 94(4); 389-95
6. Weaver RG, James MT, Ravani P, estimating urine albumin-to-creatinine ratio from protein-to-creatinine ratio: development of equations using same-day measurements: J Am Soc Nephrol, 2020; 31(3); 591-601
7. Kim SM, Lee CH, Lee JP, The association between albumin to creatinine ratio and total protein to creatinine ratio in patients with chronic kidney disease: Clin Nephrol, 2012; 78(5); 346-52
8. Smith ER, Cai MM, McMahon LP, The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients: Nephrol Dial Transplant, 2012; 27(4); 1534-41
9. Townsend JC, Albumin to creatinine ratio: An unreliable index of 24 h albumin excretion in healthy adults: N Z Med J, 1987; 100(817); 66-67
10. Derhaschnig U, Kittler H, Woisetschlager C, Microalbumin measurement alone or calculation of the albumin/creatinine ratio for the screening of hypertension patients?: Nephrol Dial Transplant, 2002; 17(1); 81-85
11. Mukaka MM, Statistics corner: A guide to appropriate use of correlation coefficient in medical research: Malawi Med J, 2012; 24(3); 69-71
12. Donner A, Koval JJ, The estimation of intraclass correlation in the analysis of family data: Biometrics, 1980; 36(1); 19-25
13. Streiner DL, Norman GR: Reliability, generalizability theory and validity Health measurement scales: A practical guide to their development and use, 2008, New York, Oxford University Press
14. Eknoyan G, Lameire N, Eckardt KU, KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: Kidney Int, 2013; 3(4); S1-150
15. Bachmann LM, Nilsson G, Bruns DE, State of the art for measurement of urine albumin: Comparison of routine measurement procedures to isotope dilution tandem mass spectrometry: Clin Chem, 2014; 60(3); 471-80
16. Sviridov D, Drake SK, Hortin GL, Reactivity of urinary albumin (microalbumin) assays with fragmented or modified albumin: Clin Chem, 2008; 54(1); 61-68
17. Lieske JC, Bondar O, Miller WG, A reference system for urinary albumin: Current status: Clinical Chem Lab Med, 2013; 51(5); 981-89
18. Ginsberg JM, Chang BS, Matarese RA, Garella S, Use of single voided urine samples to estimate quantitative proteinuria: N Engl J Med, 1983; 309(25); 1543-46
19. Huang Q, Gao Y, Yu Y, Urinary spot albumin: Creatinine ratio for documenting proteinuria in women with preeclampsia: Rev Obstet Gynecol, 2012; 5(1); 9-15
20. Methven S, MacGregor MS, Traynor JP, Comparison of urinary albumin and urinary total protein as predictors of patient outcomes in CKD: Am J Kidney Dis, 2011; 57(1); 21-28
21. Zhao YF, Zhu L, Liu LJ, Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy: Clin J Am Soc Nephrol, 2016; 11(6); 947-55
22. Wilkinson C, Lappin D, Vellinga A, Spot urinary protein analysis for excluding significant proteinuria in pregnancy: J Obstet Gynaecol, 2013; 33(1); 24-27
23. Collier G, Greenan MC, Brady JJ, A study of the relationship between albuminuria, proteinuria and urinary reagent strips: Ann Clin Biochem, 2009; 46(Pt 3); 247-49
24. Atkins RC, Briganti EM, Zimmet PZ, Chadban SJ, Association between albuminuria and proteinuria in the general population: the AusDiab Study: Nephrol Dial Transplant, 2003; 18(10); 2170-74
25. Teo BW, Loh PT, Wong WK, Spot urine estimations are equivalent to 24-hour urine assessments of urine protein excretion for predicting clinical outcomes: Int J Nephrol, 2015; 2015; 156484
26. Katayev A, Zebelman AM, Sharp TM, Prevalence of isolated non-albumin proteinuria in the US population tested for both, urine total protein and urine albumin: An unexpected discovery: Clin Biochem, 2017; 50(6); 262-69
27. Stevens PE, Levin AKidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members, Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline: Ann Intern Med, 2013; 158(11); 825-30
Figures
 Figure 1. Flow chart of study population. The figure was created by PowerPoint 2010 for Windows (Microsoft, WA, USA).
Figure 1. Flow chart of study population. The figure was created by PowerPoint 2010 for Windows (Microsoft, WA, USA). Figure 2. Illustration of performance evaluation in the development group. (Left) Histogram of standardized residual error in the development group, demonstrating the linear regression met the requirement of normality. The horizontal coordinates represent standardized residual errors between predicted and observed results. The vertical coordinates represent percentages. (Right) Plot of residual errors in the development group, demonstrating the linear regression met the requirement of homogeneity and independence. The horizontal coordinates represent predicted natural logarithm-transformed 24-h urine protein excretion. The vertical coordinates represent residual errors between predicted and observed results. The figure was created by SPSS version 22.0 (IBM Corp, Armonk, NY, USA).
Figure 2. Illustration of performance evaluation in the development group. (Left) Histogram of standardized residual error in the development group, demonstrating the linear regression met the requirement of normality. The horizontal coordinates represent standardized residual errors between predicted and observed results. The vertical coordinates represent percentages. (Right) Plot of residual errors in the development group, demonstrating the linear regression met the requirement of homogeneity and independence. The horizontal coordinates represent predicted natural logarithm-transformed 24-h urine protein excretion. The vertical coordinates represent residual errors between predicted and observed results. The figure was created by SPSS version 22.0 (IBM Corp, Armonk, NY, USA). Tables
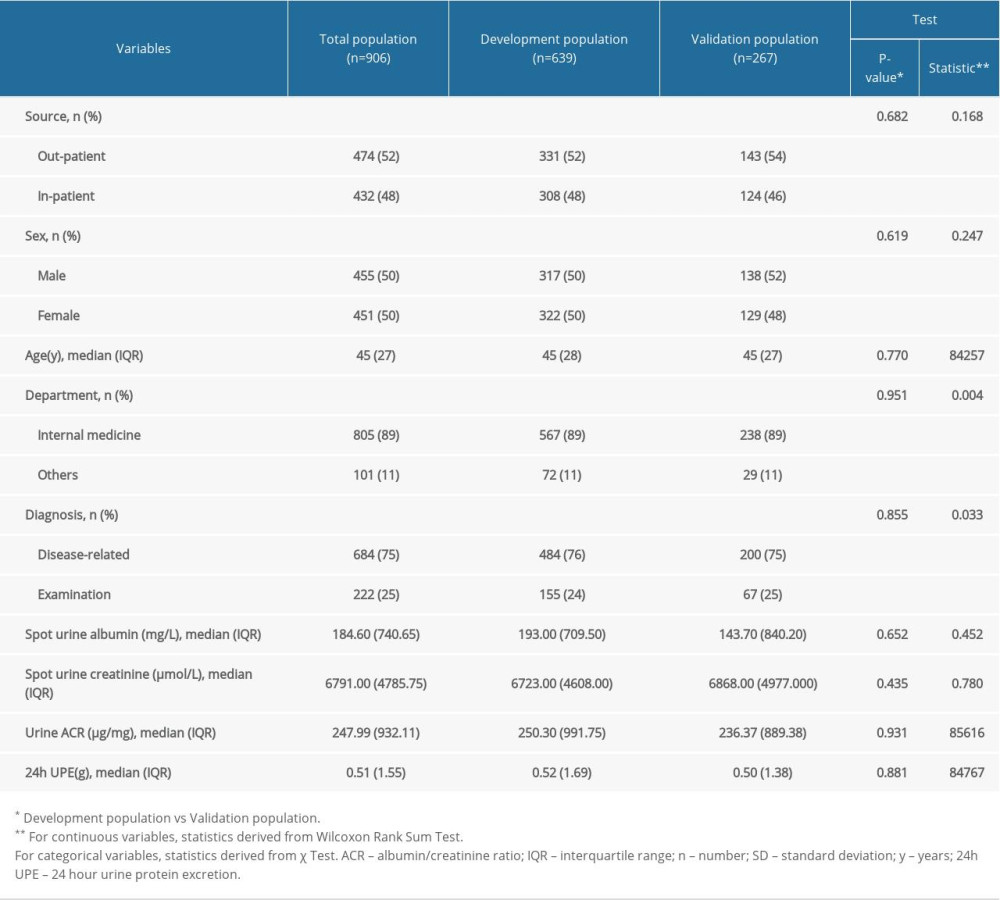 Table 1. Characteristics of the study population.
Table 1. Characteristics of the study population.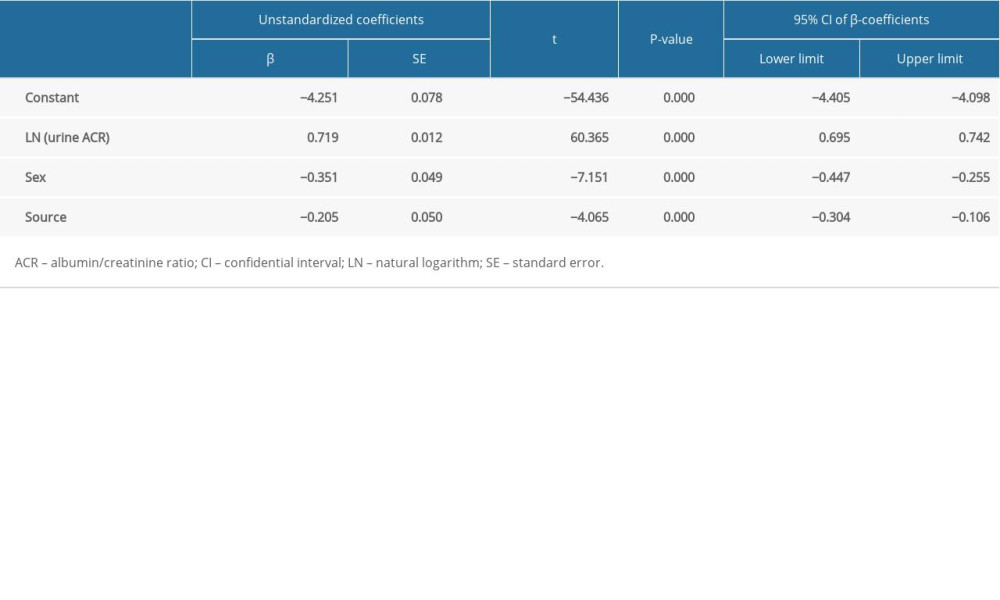 Table 2. Multivariable linear regression results of the prediction model.
Table 2. Multivariable linear regression results of the prediction model. Table 1. Characteristics of the study population.
Table 1. Characteristics of the study population. Table 2. Multivariable linear regression results of the prediction model.
Table 2. Multivariable linear regression results of the prediction model.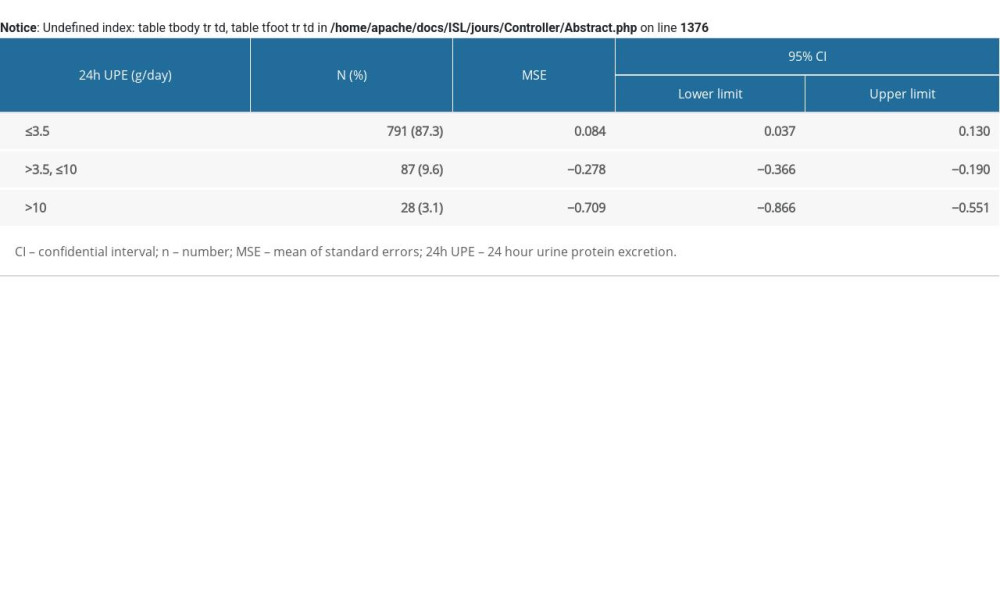 Supplementary Table 1. The accuracy of the prediction equation for different ranges of 24-h urine protein excretion in the validation group.
Supplementary Table 1. The accuracy of the prediction equation for different ranges of 24-h urine protein excretion in the validation group. In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








