21 February 2022: Clinical Research
Effect of Shengbai Decoction on Chemotherapy-Induced Myelosuppression and Survival of Gastric Cancer Patients After Radical Resection: A Retrospective Study
Linhua Yao1ABCEF, Wenming Feng2ABEF, Yulong Tao2ABCEF, Chengwu Tang2ABCDEF*DOI: 10.12659/MSM.935936
Med Sci Monit 2022; 28:e935936
Abstract
BACKGROUND: Myelosuppression is one of the most common chemotherapy-induced adverse events and results in a series of clinical symptoms. This study aimed to evaluate the effect of Shengbai decoction (SD) on chemotherapy-induced myelosuppression and survival of gastric cancer (GC) patients after radical resection.
MATERIAL AND METHODS: We retrospectively analyzed data from 115 patients with stage II-III GC who underwent adjuvant chemotherapy after radical resection between May 2015 and June 2017 in our hospital. Among these patients, 57 received Shengbai decoction along with adjuvant chemotherapy (SD group), while 58 received adjuvant chemotherapy alone (control group). Medical records, including adverse events, the treatment completion rate of adjuvant chemotherapy, 3-year overall survival (OS), and 3-year recurrence-free survival (RFS), were compared.
RESULTS: Patient characteristics did not differ significantly between the 2 groups. No adverse events related to Shengbai decoction were reported in the SD group. Patients in the SD group had less neutropenia (P=0.0430), thrombocytopenia (P=0.0323), and anemia (P=0.0497). The SD group had a significantly lower probability of dose reduction (P=0.0448). The completion rate of adjuvant chemotherapy of the SD group was considerably higher than that of the control group (P=0.0398). The SD group had a significantly better 3-year RFS (P=0.0369) and 3-year OS (P=0.0455) than the control group.
CONCLUSIONS: Shengbai decoction effectively improved postoperative survival of patients with GC by alleviating chemotherapy-induced myelosuppression and improving the completion rate of adjuvant chemotherapy.
Keywords: Medicine, Chinese Traditional, Survival, Chemotherapy, Adjuvant, Adolescent, Adult, Antineoplastic Agents, Drugs, Chinese Herbal, Female, Humans, Male, Stomach Neoplasms, young adult
Background
Gastric cancer (GC), one of the most prevalent malignancies, caused nearly 770 000 deaths in 2020 [1]. D2 gastrectomy is considered a standardized surgical strategy for localized GC in Asian countries [2]. It is also widely proposed by treatment guidelines of Western countries due to the 15-year findings of the Dutch D1/D2 study [3–5]. Nevertheless, approximately 40% of patients develop recurrence within 2 years despite radical resection [6–8]. Various adjuvant chemotherapy regimens have been implemented to control postoperative relapse and improve long-term survival over the past 40 years [9].
However, all the chemotherapy regimens cause toxic adverse events, which harm patient quality of life and treatment compliance [10]. Myelosuppression is one of the most common chemotherapy-induced adverse events and results in a series of clinical symptoms, such as anemia, thrombocytopenia, and neutropenia [11]. Severe myelosuppression even leads to treatment dose reduction or discontinuation, which limit treatment efficacy.
Chinese herbal medicine (CHM) has been widely used in cancer treatment as integrative therapy [12]. Several studies revealed that CHM can prevent chemotherapy-induced myelosuppression [13–15]. However, the efficacy of CHM varies greatly with the composition [16]. This retrospective study was performed to assess the effect of Shengbai decoction on chemotherapy-induced myelosuppression and survival of GC patients after radical resection.
Material and Methods
PATIENTS:
A total of 115 patients with stage II–III GC who underwent adjuvant chemotherapy after radical resection between May 2015 and June 2017 in our hospital were retrospectively analyzed. Among these patients, 57 received Shengbai decoction along with adjuvant chemotherapy (SD group), and 58 received adjuvant chemotherapy alone (control group).
Inclusion criteria were: undergoing adjuvant chemotherapy after D2 gastrectomy, pathological TNM stage II–III GC [17], ECOG 0–1, age 18–75 years, no previous anti-cancer treatment, adequate organ function, and complete follow-up data. Exclusion criteria were: allergic to any ingredient of the Shengbai decoction, recurrence or death within 6 months after surgery, a history of other malignant tumors, and lost to follow-up.
We conducted this study following the principles of the Declaration of Helsinki and “Good Clinical Practice” guidelines. The Institutional Review Board of the college approved this study (approval no. HZYY-2020100811). All patients signed the informed consent.
ADMINISTRATION OF ADJUVANT CHEMOTHERAPY AND SHENGBAI DECOCTION:
Postoperative chemotherapy was started within 3 weeks after surgery. The regimens of chemotherapy referred to the NCCN guidelines for GC. Patients decided by themselves whether to receive Shengbai decoction after doctors clarified the potential risks and benefits. Patients signed an additional consent form if they chose to receive Shengbai decoction. The cost of the Shengbai decoction was covered by medical insurance.
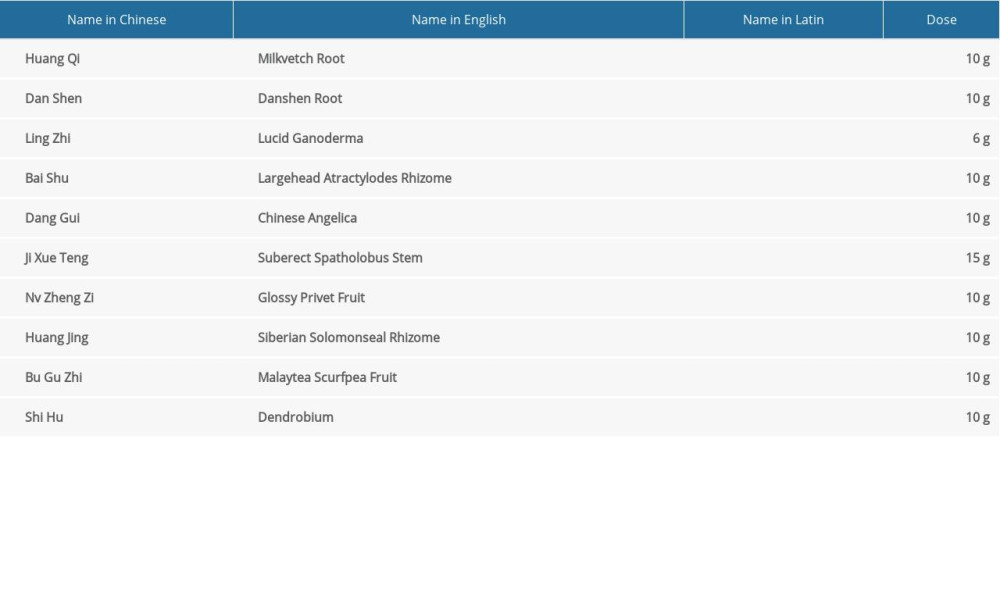
The Shengbai decoction was administered concurrently with the chemotherapy to the patients of the SD group (taken morning and evening, 30 min after meals, 100 mL per time). The formula of Shengbai decoction consisted of Radix Astragali 10 g, Radix Salviae Miltiorrhizae 10 g, Ganoderma Lucidumseu Sinensis 6 g, Rhizoma Atractylodis Macrocephalae 10 g, Radix Angelicae Sinensis 10 g, Caulis Spatholobi 15 g, Fructus Ligustri Lucidi 10 g, Rhizoma Polygonati 10 g, Fructus Psoraleae 10 g and Herba Dendrobii 10 g (Table 1).
Treatment-related adverse events were estimated based on the Common Terminology Criteria for Adverse Events (CTCAE v4.0). The treatment dose would be reduced to 75% in subsequent treatment courses in case of grade 3–4 hematologic or acute non-hematologic adverse events or grade 2–3 hand-foot syndrome (HFS). Treatments for myelosuppression were not implemented until grade 3 or worse hematologic adverse events occurred. Chemotherapy was discontinued in the event of disease progression or life-threatening adverse events, or patients’ request for discontinuation. Patients who received fewer than half of the scheduled cycles of chemotherapy were excluded from analysis.
ASSESSMENT AND FOLLOW-UP:
All patients underwent comprehensive disease assessment and health monitoring during the chemotherapy phase and the follow-up phase. Follow-up visits were initiated after the chemotherapy ended. In the first postoperative year, patients underwent monthly follow-up visits and then every 3 months until death or last follow-up.
Recurrence was diagnosed by imaging examination and, if necessary, cytologic analysis or biopsy. Once recurrence was identified, chemotherapy, radiofrequency ablation, or palliative care were implemented.
STATISTICAL ANALYSIS:
Statistical analysis and visualization were performed using MedCalc software (version 15.2.2, MedCalc Software, Ltd). Quantitative variables were analyzed using the
Results
PATIENT CHARACTERISTICS:
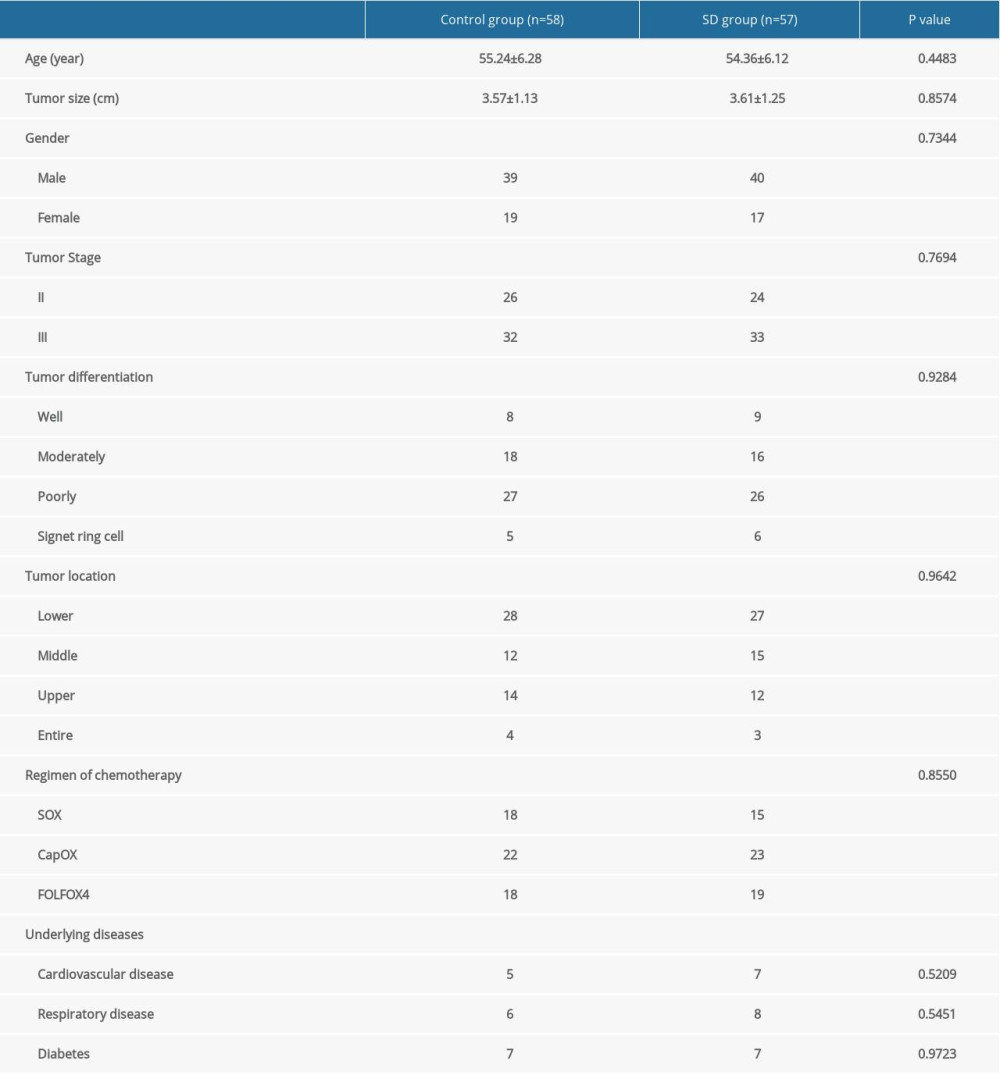
Of these patients, 50 had stage II disease and 65 had stage III disease. Patient characteristics did not differ significantly between the 2 groups (Table 2).
ADVERSE EVENTS AND TREATMENT COMPLETION RATE:
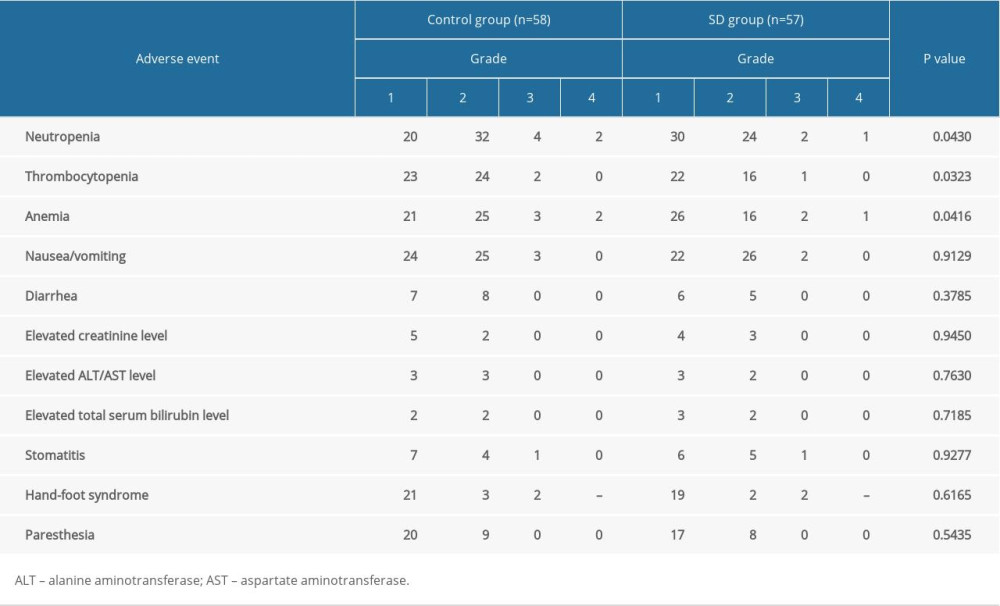
Treatment-related adverse events were shown in Table 3. There were no significant differences found in non-hematologic adverse events between the 2 groups. No adverse events related to Shengbai decoction were reported in the SD group. Shengbai decoction significantly relieved chemotherapy-induced myelosuppression. Patients in the Shengbai decoction group had less neutropenia (P=0.0430), thrombocytopenia (P=0.0323), and anemia (P=0.0497). No treatment-related deaths occurred. Most of the adverse events were controlled by symptomatic treatment and dose reduction. Dose reduction was documented in 16 patients from the control group and 7 patients from the SD group. The SD group had a significantly lower probability of dose reduction (P=0.0448). Despite administration of dose reduction and thorough monitoring and symptomatic treatment, chemotherapy was finally discontinued in 9 patients (8 from the control group and 1 from the SD group). The completion rate of adjuvant chemotherapy of the SD group was significantly higher than that of the control group (56/57 vs 50/58, P=0.0398).
SURVIVAL OUTCOMES:
In 30 patients (20 from the control group and 10 from the SD group), recurrence was reported within the first 3 postoperative years. The 3-year RFS rate was 65.52% and 82.76% for the control group and SD group, respectively. The SD group had a significantly better 3-year RFS than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355] (Figure 1).
There were 25 deaths reported (17 from the control group and 8 from the SD group) within the first 3 postoperative years. The 3-year OS rate was 70.18% and 85.96% for the control group and SD group, respectively. The SD group had a significantly better 3-year OS than the control group (P=0.0455; HR for death, 0.4369; 95% CI, 0.1994 to 0.9575) (Figure 2).
Discussion
China is one of the countries with the highest incidence rates of GC, and about half of the world’s GC-related deaths occur in this country [1]. Due to the inadequacy of nationwide screening programs for cancer, nearly 80% of GC cases are metastatic or locally advanced at the time of diagnosis [18]. Therefore, the prognosis of GC in China is poor despite the advancement of surgical technique.
Postoperative chemotherapy improves the survival of advanced GC but also causes various adverse events, which result in poor quality of life and low treatment compliance. Dose reduction is consistently implemented when severe adverse events are reported, and overwhelming adverse events can even lead to discontinuation of chemotherapy. Myelosuppression is one of the most common chemotherapy-induced adverse events, characterized by anemia, thrombocytopenia, and neutropenia.
Without appropriate treatment, myelosuppression will cause serious consequences [19]. Currently, the main treatments for chemotherapy-induced myelosuppression include dose adjustment, blood transfusion, and recombinant human stimulating factors. However, these treatments can cause organ injuries and vascular events, and they can even contribute to cancer progression [20,21].
Chinese herbal medicine is one of the treatment strategies for myelosuppression and is widely used in China. The Shengbai decoction used in this study was composed of 10 kinds of herbal plants. In the formula,
Our results showed that the incidence and severity of myelosuppression were significantly reduced by Shengbai decoction. Patients in the SD group experienced significantly less neutropenia (
Our study has several limitations. First, bias in patient selection was inevitable for this non-randomized and retrospective study. Secondly, this study was performed at a single medical center in China with a limited sample size. Therefore, we are preparing a random clinical trial with a larger sample size to confirm the results of this study. Moreover, further basic research is needed to elucidate the mechanism underlying the therapeutic effects of Shengbai decoction on chemotherapy-induced myelosuppression.
Conclusions
Our results suggest that Shengbai decoction can effectively improve postoperative survival of patients with GC by alleviating chemotherapy-induced myelosuppression and improving the completion rate of adjuvant chemotherapy.
Figures
![Comparison of 3-year recurrence-free survival between the 2 groups. Recurrence was reported in 30 patients (20 from the control group and 10 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year recurrence-free survival than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355]. MedCalc software (version 15.2.2, MedCalc Software Ltd.) was used to create the figure.](https://jours.isi-science.com/imageXml.php?i=medscimonit-28-e935936-g001.jpg&idArt=935936&w=1000) Figure 1. Comparison of 3-year recurrence-free survival between the 2 groups. Recurrence was reported in 30 patients (20 from the control group and 10 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year recurrence-free survival than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355]. MedCalc software (version 15.2.2, MedCalc Software Ltd.) was used to create the figure.
Figure 1. Comparison of 3-year recurrence-free survival between the 2 groups. Recurrence was reported in 30 patients (20 from the control group and 10 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year recurrence-free survival than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355]. MedCalc software (version 15.2.2, MedCalc Software Ltd.) was used to create the figure. 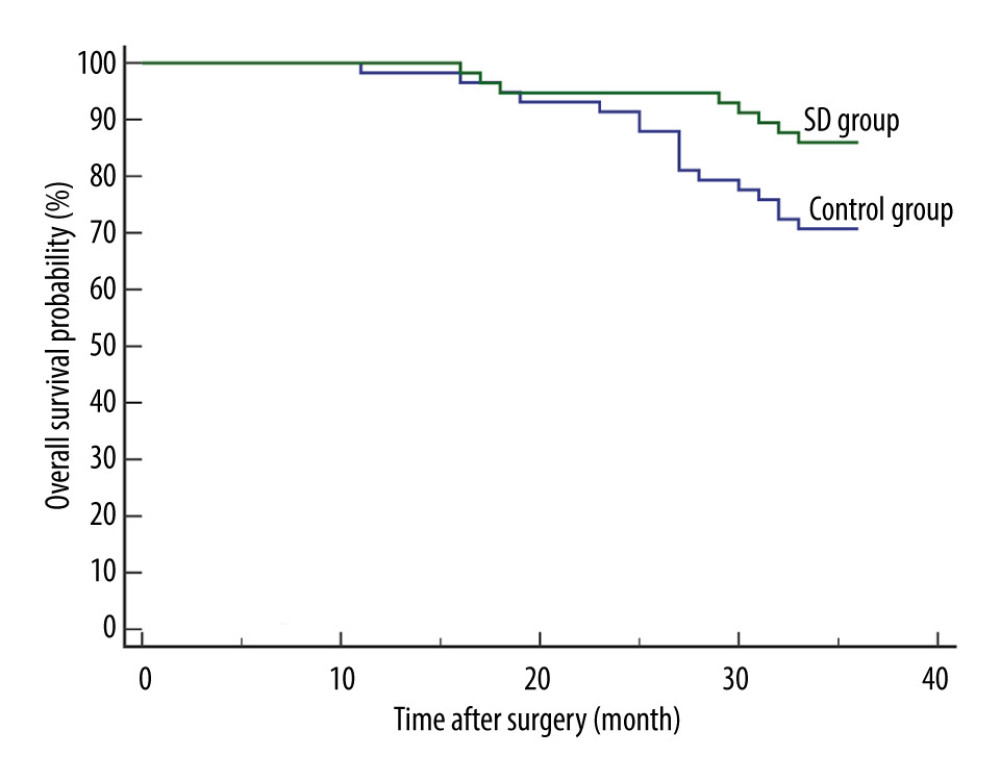 Figure 2. Comparison of 3-year overall survival between the 2 groups. There were 25 deaths reported (17 from the control group and 8 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year overall survival than the control group (P=0.0455; HR for death, 0.4369; 95% CI, 0.1994 to 0.9575). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 2. Comparison of 3-year overall survival between the 2 groups. There were 25 deaths reported (17 from the control group and 8 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year overall survival than the control group (P=0.0455; HR for death, 0.4369; 95% CI, 0.1994 to 0.9575). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. References
1. Sung H, Ferlay J, Siegel RL, Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Cancer J Clin, 2021; 71(3); 209-49
2. Sasako M, Inoue M, Lin JT, Gastric Cancer Working Group report: Jpn J Clin Oncol, 2010; 40(Suppl 1); i28-37
3. Coburn N, Cosby R, Klein L, Staging and surgical approaches in gastric cancer: A clinical practice guideline: Curr Oncol, 2017; 24(5); 324-31
4. Coburn N, Cosby R, Klein L, Staging and surgical approaches in gastric cancer: A systematic review: Cancer Treat Rev, 2018; 63; 104-15
5. Songun I, Putter H, Kranenbarg EM, Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial: Lancet Oncol, 2010; 11(5); 439-49
6. Cunningham D, Allum WH, Stenning SP, Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer: N Engl J Med, 2006; 355(1); 11-20
7. D’Angelica M, Gonen M, Brennan MF, Patterns of initial recurrence in completely resected gastric adenocarcinoma: Ann Surg, 2004; 240(5); 808-16
8. Ychou M, Boige V, Pignon JP, Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial: J Clin Oncol, 2011; 29(13); 1715-21
9. Kilic L, Ordu C, Yildiz I, Current adjuvant treatment modalities for gastric cancer: From history to the future: World J Gastrointest Oncol, 2016; 8(5); 439-49
10. Zhang Z, Zhang Y, Chen G, Olanzapine-based triple regimens versus neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy: A network meta-analysis: Oncologist, 2018; 23(5); 603-16
11. Hou B, Liu R, Qin Z, Oral Chinese herbal medicine as an adjuvant treatment for chemotherapy, or radiotherapy, induced myelosuppression: A systematic review and meta-analysis of randomized controlled trials: Evid Based Complement Alternat Med, 2017; 2017; 3432750
12. Shih WT, Yang PR, Shen YC, Traditional Chinese medicine enhances survival in patients with gastric cancer after surgery and adjuvant chemotherapy in taiwan: A nationwide matched cohort study: Evid Based Complement Alternat Med, 2021; 2021; 7584631
13. Chen T, Shen HM, Deng ZY, A herbal formula, SYKT, reverses doxorubicininduced myelosuppression and cardiotoxicity by inhibiting ROSmediated apoptosis: Mol Med Rep, 2017; 15(4); 2057-66
14. Wang C, Gao H, Cai E: Biomed Pharmacother, 2019; 109; 2062-69
15. Wang LF, Xu ZY, Wang ZQ, Clinical observation of Shuanghuang Shengbai Granule on prevention and treatment of myelosuppression caused by chemotherapy in cancer patients: Chin J Integr Med, 2017; 23(2); 105-9
16. Hong J, Chen X, Huang J, Danggui buxue decoction, a classical formula of traditional Chinese medicine, fails to prevent myelosuppression in breast cancer patients treated with adjuvant chemotherapy: A prospective study: Integr Cancer Ther, 2017; 16(3); 406-13
17. Edge SB, Compton CC: Ann Surg Oncol, 2010; 17(6); 1471-74
18. Yang K, Hu JK, Gastric cancer treatment: Similarity and difference between China and Korea: Transl Gastroenterol Hepatol, 2017; 2; 36
19. Yang S, Che H, Xiao L, Traditional Chinese medicine on treating myelosuppression after chemotherapy: A protocol for systematic review and meta-analysis: Medicine, 2021; 100(4); e24307
20. Hung JY, Horn D, Woodruff K, Colony-stimulating factor 1 potentiates lung cancer bone metastasis: Lab Invest, 2014; 94(4); 371-81
21. Tigue CC, McKoy JM, Evens AM, Granulocyte-colony stimulating factor administration to healthy individuals and persons with chronic neutropenia or cancer: An overview of safety considerations from the Research on Adverse Drug Events and Reports project: Bone Marrow Transplant, 2007; 40(3); 185-92
22. Fujitani K, Kurokawa Y, Takeno A, Time to initiation or duration of S-1 adjuvant chemotherapy; Which really impacts on survival in stage II and III gastric cancer?: Gastric Cancer, 2018; 21(3); 446-52
23. Jang SH, Jung YJ, Kim MG, Kwon SJ, The prognostic significance of compliance with postoperative adjuvant chemotherapy in patients with stage III gastric cancer: An observational study: J Gastric Cancer, 2018; 18(1); 48-57
Figures
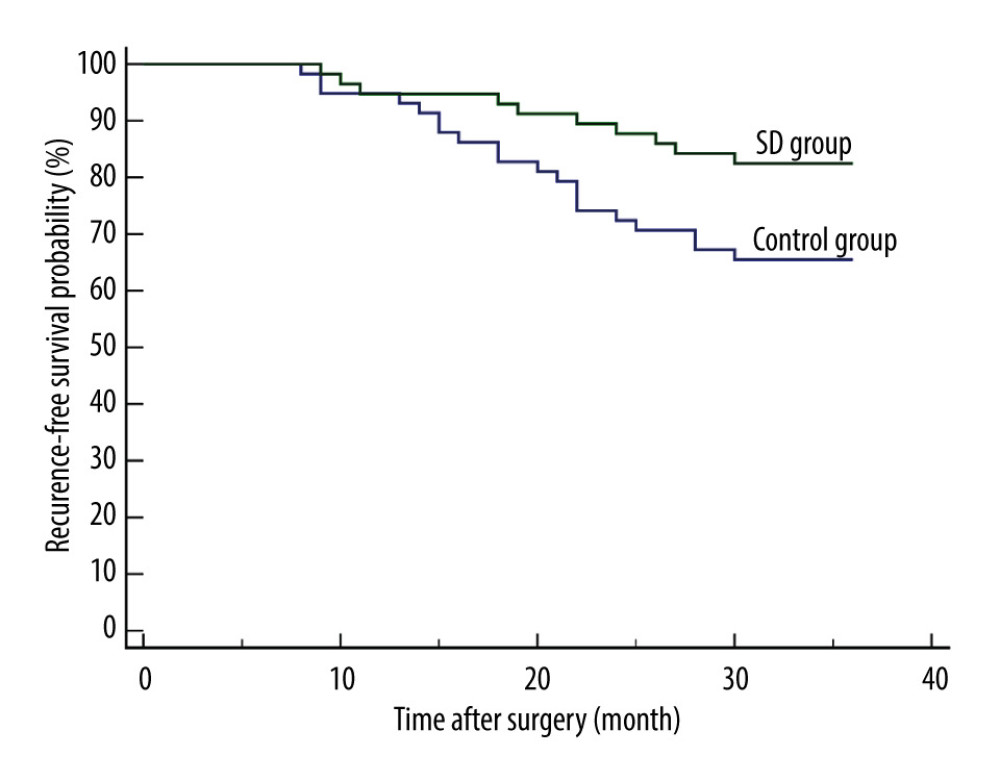 Figure 1. Comparison of 3-year recurrence-free survival between the 2 groups. Recurrence was reported in 30 patients (20 from the control group and 10 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year recurrence-free survival than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355]. MedCalc software (version 15.2.2, MedCalc Software Ltd.) was used to create the figure.
Figure 1. Comparison of 3-year recurrence-free survival between the 2 groups. Recurrence was reported in 30 patients (20 from the control group and 10 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year recurrence-free survival than the control group [P=0.0369; hazard ratio (HR) for recurrence, 0.4570; 95% confidence interval (CI), 0.2232 to 0.9355]. MedCalc software (version 15.2.2, MedCalc Software Ltd.) was used to create the figure. Figure 2. Comparison of 3-year overall survival between the 2 groups. There were 25 deaths reported (17 from the control group and 8 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year overall survival than the control group (P=0.0455; HR for death, 0.4369; 95% CI, 0.1994 to 0.9575). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure.
Figure 2. Comparison of 3-year overall survival between the 2 groups. There were 25 deaths reported (17 from the control group and 8 from the SD group) within the first 3 postoperative years. The SD group had a significantly better 3-year overall survival than the control group (P=0.0455; HR for death, 0.4369; 95% CI, 0.1994 to 0.9575). MedCalc software (version 15.2.2, MedCalc Software, Ltd.) was used to create the figure. In Press
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952