02 June 2022: Database Analysis
Effect of Clemastine Fumarate on Perioperative Hemodynamic Instability Mediated by Anaphylaxis During Cardiopulmonary Bypass Surgery
Lijuan Tian1ABCDEF, Yue Liu1ACDE, Yuda Fei2DF, Hong Lv1BF, Fuxia Yan1BF, Lihuan Li1ABDEF, Jia Shi1ABDF*DOI: 10.12659/MSM.936367
Med Sci Monit 2022; 28:e936367
Abstract
BACKGROUND: Perioperative hemodynamic instability mediated by anaphylaxis is a life-threatening complication in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). This study aimed to evaluate the effect of clemastine fumarate in this specific patient population.
MATERIAL AND METHODS: We enrolled 100 participants who met the inclusion criteria and randomly allocated them to the treatment group and the placebo group. Participants in the treatment group and the placebo group were treated separately with an injection of clemastine fumarate and saline, respectively. Plasma histamine concentration and blood pressure were quantified at 5 timepoints during the perioperative period, and differences between the 2 groups were assessed by repeated-measures ANOVA. The postoperative complications and in-hospital mortality also were evaluated. All participants were followed up for 7 days after cardiac surgery.
RESULTS: Plasma histamine concentrations increased in both groups but were statistically significantly lower in the treatment group during the perioperative period (P=0.007). Diastolic blood pressure (P=0.014) and mean arterial pressure (P=0.024) in the treatment group were significantly higher than in the placebo group during the perioperative period. The coefficients of variation for systolic (13.9±4.2% vs 17.2±4.4%, P<0.01) and diastolic (12.9±4.9% vs 15.3±5.2%, P=0.02) blood pressure were significantly lower in the treatment group compared with the placebo group.
CONCLUSIONS: Pretreatment with clemastine fumarate restrains the increase in histamine concentration and provides safer hemodynamics in patients undergoing cardiac surgery with CPB.
Keywords: Anaphylaxis, Cardiopulmonary Bypass, Clemastine, Hemodynamics, Histamine, Humans, Perioperative Care, Vascular Diseases
Background
Perioperative anaphylaxis is a life-threatening complication in cardiac surgery with cardiopulmonary bypass (CPB) [1], leading to increased mortality ranging from 3% to 9% [2]. Many drugs commonly used in the perioperative period such as neuromuscular blocking agents, antibiotics, and opiates can trigger allergic reactions [3]. In cardiovascular surgery, heparin and protamine are routinely used as anticoagulants and neutralizers, respectively. However, protamine poses an allergenic hazard. Protamine sulfate reduces systemic vascular resistance and increases pulmonary vascular resistance by directly activating mast cells to release histamine [4–6]. Some studies suggested that plasma histamine concentrations correlate with the severity of hypersensitivity reactions [7–9]. The cardiovascular system represents a major target of anaphylaxis [10]. Histamine produces a spectrum of symptoms by binding to specific receptors widely distributed in blood vessels and cardiac tissues [11–13]. Significant hemodynamic instability caused by anaphylaxis is one risk to patients undergoing cardiac surgery [14,15]. Preventive measures to decrease the frequency and intensity of perioperative anaphylaxis are essential for these patients. Although previous studies have explored perioperative anaphylaxis in terms of its epidemiology, manifestation, management, and outcome [1,7,8,16], the effects of antihistamines on perioperative anaphylaxis remain to be explored. Clemastine fumarate is a second-generation H1 receptor antagonist, currently approved in many countries for the treatment of allergic disorders. The present pilot study aimed to evaluate the effects of clemastine fumarate on perioperative hemodynamics in patients undergoing cardiac surgery with CPB.
Material and Methods
STUDY DESIGN:
This was a prospective, double-blinded, randomized, controlled trial. The study was approved by the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (protocol number 2016-810) and written informed consent was obtained from all participants. The trial was registered before patient enrollment at
ENROLLMENT:
The inclusion criteria were as follows: (1) age between 18 and 75 years; (2) undergoing elective coronary artery bypass grafting (CABG), congenital heart surgery, or valve replacement surgery with CPB. The exclusion criteria were as follows: (1) a previous history of cardiac surgery; (2) allergy to clemastine fumarate, other antihistamines with similar chemical characteristics, or any excipients, including sorbitol, sodium citrate, propylene glycol, or ethanol; (3) a history of myasthenia gravis, porphyria, or bronchial asthma, or currently on monoamine oxidase inhibitor treatment; (4) ongoing any known allergic reactivity; (5) pregnancy or lactation; (6) intellectual or legal disability; and (7) other serious diseases that might impede entry or affect survival, such as cancer.
RANDOMIZATION AND BLINDING:
Eligible participants were randomized at a 1: 1 ratio to the treatment group or the placebo group by computer-generated randomization. The results of the assignment were sealed in opaque envelopes to ensure concealment. Patient recruitment, randomization, and blinding were conducted and supervised by an independent committee. Syringes containing transparent preparations of equal volume were administered to participants in both the treatment group and the placebo group. Drugs were prepared by a dedicated nurse. Neither the investigators nor the participants were aware of treatment allocation until discharge of the last patient, except in emergency cases.
INTERVENTION:
Participants in the treatment group received intramuscular injection of clemastine fumarate (2 mg/2 ml, Limin Biopharmaceutical Co., Ltd., Jinan, China) in the gluteus maximus 20 minutes before anesthetic induction, while participants in the placebo group received the same volume of saline at the same timepoint. General anesthesia was induced with 0.05 mg·kg−1 of midazolam (5 mg/1 ml, Nhwa Pharmaceutical Co., Ltd, Jiangsu, China), 0.2–0.3 mg·kg−1 of etomidate (20 mg/10 ml, Nhwa Pharmaceutical Co., Ltd, Jiangsu, China), 0.2 mg·kg−1 of cis-atracurium (10 mg/5 ml, Hengrui Pharmaceutical Co., Ltd, Jiangsu, China), and 1–2 μg·kg−1 of sufentanil (50 μg/1 ml, Yichang Humanwell Pharmaceutical Co., Ltd, Hubei, China), and maintained with infusion of sufentanil (0.5–1 μg·kg−1·h−1), cis-atracurium (0.05–0.15 mg·kg−1·h−1), and 2–4 mg·kg−1·h−1 of propofol (500 mg/50 ml, AstraZeneca UK Limited, Italy). After endotracheal intubation, mechanical ventilation was performed with a tidal volume of 8–10 ml·kg−1 and a respiratory rate of 12–14 breaths·min−1. A full dose (400 IU·kg−1) of heparin (12 500 IU/2 ml, SPH NO.1 Biochemical and pharmaceutical Co., Ltd, Shanghai, China) was delivered, and the adequacy of heparin anticoagulation during CABG was monitored by an activated clotting time of >450 seconds. At the end of extracorporeal circulation, heparin was neutralized with protamine sulfate (50 mg/5 ml, SPH NO.1 Biochemical and pharmaceutical Co., Ltd, Shanghai, China) at a ratio of 1: 1.5–2.0 (heparin: protamine). Intravenous norepinephrine (2 mg/1 ml, Jinyao Pharmaceutical Co., Ltd, Tianjin, China) was administered in case of a systolic blood pressure (SBP) of <80 mmHg. After surgery, patients were transferred to the intensive care unit for postoperative care.
OUTCOMES:
Blood samples were collected from the radial artery at the following time points: before induction of anesthesia (T0), 5 minutes after heparinization (T1), 5 minutes after neutralization (T2), at the end of surgery (T3), and 24 hours postoperatively (T4). The primary outcome was differences in plasma histamine concentration between the treatment group and the placebo group during perioperative period. Blood samples were centrifuged for 15 minutes (4°C, 3500 r/min), and plasma was stored at −80°C for further laboratory testing. Plasma concentrations of histamine were evaluated using human histamine enzyme-linked immunosorbent assay kits (IBL International, RE59221, Morrisville, NC, U.S.A).
The secondary outcomes were perioperative hemodynamic parameters, perioperative cutaneous manifestations, and adverse events. Perioperative blood pressure (BP) and heart rate (HR) were recorded and compared at the timepoints: before induction of anesthesia (T0), 5 minutes after heparinization (T1), 5 minutes after neutralization (T2), at the end of surgery (T3), and 24 hours postoperatively (T4). In the current study, BP and HR coefficients of variation (CVs) served as measures of interpatient variability and were defined as the ratio of the standard deviation (SD) to the arithmetic mean for all points of surgery. Perioperative cutaneous manifestation was classified as follows: grade 0: no significant change; grade 1: <120 s of skin flushing; grade 2: >120 s of skin flushing; grade 3: skin erythema; grade 4: wheals.
FOLLOW UP:
All participants were followed up until 7 days after surgery. The investigator performing the follow-up visit was blinded to treatment group allocation. Data on drug-related adverse events were collected. A physical examination, 12-lead electrocardiography, chest X-ray, and echocardiography were performed and analyzed. Complete blood counts and basic metabolic panels were measured and analyzed. In-hospital mortality and subsequent adverse events were recorded and analyzed, including drowsiness, dizziness, and headache.
STATISTICAL CONSIDERATIONS:
To calculate the minimum sample size that would ensure the smallest margin of error, we used the mean and SD of the differences in plasma histamine concentrations between the treatment group and the placebo group of the first 20 participants. Five minutes after administration of protamine, the mean histamine concentrations in the placebo group and the treatment group were 4.588 ng/ml and 3.828 ng/ml, respectively, with a SD of 1.145. Using the PASS 11.0 software (NCSS, Inc., Kaysville, UT, U.S.A.), it was determined that 48 was the smallest sample size required to obtain an error of 0.05 and a power of 90%. Therefore, 50 participants were required in each group. Clinical data of participants are summarized by groups. The Shapiro-Wilk normality test was used for normality test. For continuous variables, data that obeyed normal distribution were presented as mean and standard deviation and compared by the student’s t-test between groups. Data that did not follow a normal distribution were presented as median (interquartile range) and analyzed by using non-parametric test. Repeated measures two-way ANOVA were employed for the data that were repeated measured, such as plasma histamine, BP, HR. Categorical variables were described as percentages and were compared using the chi-squared test or Fisher’s exact test, as appropriate. SPSS 26.0 software (IBM Corporation, New York, U.S.A.) was used for all statistical analyses, and a
Results
FLOWCHART:
From February 20th to July 31st, 2019, a total of 4120 patients underwent cardiac surgery in the hospital. Among them, 1279 patients did not meet the inclusion criteria, 2483 patients who were involved in other clinical trials were excluded, and 258 patients refused to participate. Finally, 100 participants who underwent cardiac surgery with CPB were included in the study (Figure 1). All participants provided written informed consent to participate in the study. Therefore, 50 participants in the treatment group and 50 participants in the placebo group were assessed.
DEMOGRAPHIC AND SURGICAL DATA:
There were no significant differences between the 2 groups in Demographic and surgical data (Table 1). The treatment group and the placebo group were comparable at baseline. Although the rate of norepinephrine treatment due to SBP <80 mmHg in the treatment group was lower compared with the placebo group, there was no significant difference in the rate of norepinephrine treatment between the 2 groups (30.0% vs 46.0%, respectively, P=0.15).
PRIMARY OUTCOME:
Both treatment group and placebo group exhibited significantly increased compared with baseline at T1, T2, T3and T4 with a peak level on T1 (Figure 2A). There were significant differences in plasma histamine concentration between the treatment group and the placebo group during perioperative period (P=0.007). The Sidak multiple-comparison test revealed significant differences at T1 and T2 between the 2 groups (P=0.006 and P=0.009) (Supplementary Table 1). Further, a significant main effect for time (P<0.001) was observed along with a significant group×time interaction effect (P=0.036). Statistical analysis also showed a difference in increase of plasma histamine concentration from T0 to T1 between the treatment group and the placebo group (0.89 ng/ml [0.17, 1.60 ng/ml] vs 1.42 ng/ml [0.37, 3.05 ng/ml], P=0.03).
SECONDARY OUTCOMES:
Perioperative blood pressure fluctuations were shown in Figure 2B–2D. There were significant differences in Diastolic blood pressure (DBP) (P=0.014) and mean arterial pressure (MAP) (P=0.024) between the treatment group and the placebo group during perioperative period. The Sidak multiple-comparison test revealed significant difference in DBP at T1 (P=0.047) and significant difference in MAP at T2 (P=0.038) between the 2 groups (Supplementary Tables 2 and 3). While there were differences in SBP and HR, these differences were not statistically significant (Supplementary Tables 2 and 4). A significantly lower variability was observed in CVSBP (13.9±4.2% vs 17.2±4.4%, respectively, P<0.01) and CVDBP (12.9±4.9% vs 15.3±5.2%, respectively, P=0.02) in the treatment group compared with the placebo group (Table 2). In the treatment group, the cutaneous manifestation changes were less frequent. However, no significant difference was observed between the 2 groups (P=0.27). One patient in the placebo group experienced wheals (Table 2).
FOLLOW UP:
There were no in-hospital deaths in the present study. In terms of the incidence of adverse events, including dizziness, headache, and nausea, no significant difference was observed between the 2 groups (Table 3).
Discussion
In this pilot study, we found that pretreatment with clemastine fumarate significantly reduced histamine release in patients undergoing cardiovascular surgery with CPB. Furthermore, participants in the treatment group maintained higher BP and had lower variability of BP.
Because of the wide distribution of specific receptors in blood vessels and cardiac tissues, histamine can lead to tachycardia, telangiectasia, hypotension, myocardial infarction and delayed atrioventricular conduction, while the smooth muscle contraction effect leads to bronchospasm and gastrointestinal colic [13]. Anaphylaxis events created acute decrease in preload, decrease afterload, increase the heart rate and increase myocardial contractility that could exacerbate cardiovascular dysfunction. Clemastine fumarate is a potent anti-allergy and anti-inflammatory agent [17]. A recent study confirmed that clemastine fumarate protected cardiomyocytes through inhibition of the TLR4/PI3K/Akt signaling pathway [18]. This result showed that lower plasma histamine level may be correlated with a more stable hemodynamic homeostasis.
The results in the present study were consistent with a previous report that suggested an association between clemastine fumarate and more stable hemodynamics after cardiac surgery with CPB. In the prospective study conducted by Lango et al suggested that clemastine fumarate can accelerate the normalization of arterial BP during protamine administration after CPB during CABG [19]. A retrospective cohort study of 2376 patients with allergic reactions in emergency department, H1 receptor antagonist administration was associated with a lower likelihood of progression to anaphylaxis. Their results have indicated that early H1 receptor antagonist treatment in the emergency department or prehospital setting may decrease progression to anaphylaxis [20]. Zhou et al, in their prospective study, demonstrated that pretreatment with promethazine (an H1 receptor antagonist) significantly decreased mivacurium-induced histamine release in children and provided stable hemodynamics during administration of anesthesia [21]. Results from the 6th National Audit Project imply that prophylactic use of antihistamines might reduce the severity of anaphylaxis, but might not prevent it [7]. However, the study focused on chlorphenamine, which is a first-generation H1 receptor antagonist, rather than second-generation H1 receptor antagonist.
One of the advantages of this study compared to the abovementioned previous studies is that the determination of plasma histamine concentration was matched for the collection of hemodynamic indicators. This study design helped better reveal the correlations between plasma histamine concentration and hemodynamics. Beyond the indicators of hemodynamics, we observed that blood pressure decreased dramatically as plasma histamine concentration increased during perioperative period. Previous studies have addressed the effects of various H1 antihistamines (were mainly first-generation) in various study populations. Compare with first-generation H1 antihistamines, second-generation H1 antihistamines have a safety profile with reduced capacity to induce central nervous system related adverse effects as they have a low potential to cross the blood–brain barrier, and provide selective H1 receptor blockade without anticholinergic activity. This study specifically focused on preventive use of clemastine fumarate before cardiopulmonary bypass surgery. Based on previous research, compared with oral tablets, intramuscular clemastine fumarate is absorbed more rapidly with longer persistence and a more stable plasma concentration [22,23]. Considering previous research [19] and guidelines on anaphylaxis treatment [24], a dose of 2 mg (2 ml) clemastine fumarate or the same volume of saline was injected intramuscularly 20 minutes before anesthetic induction in the treatment group and the placebo group, respectively.
It is now well established that BP lability is the strongest predictor of morbidity after CABG [25]. Perioperative hypotension, which is caused by hypersensitivity reactions, leads to organic hypoperfusion, thereby resulting in ischemia of the heart, brain, and other organs, and ultimately leading to an elevated incidence of postoperative complications. Our study confirmed that preoperative pretreatment with clemastine provided more stable and safer hemodynamics during cardiopulmonary bypass surgery. Although clemastine fumarate is widely prescribed in clinical practice as an H1 antihistamine for prophylactic use, at present, there are few large prospective studies on perioperative allergies. Due to the lack of sufficient evidence, the safety and clinical efficacy of preventive use of antihistamines during the perioperative period are still controversial. Results from this study provides a reference for a more comprehensive and large-scale clinical trials in the future.
The current trial was a single-center pilot study with a relatively small sample size, which might limit the expansibility of the conclusion. The sample size may not be sufficient to detect more differences between the 2 groups. Anaphylaxis demonstrates a complicated manifestation in clinical practice with certain mediators other than histamine. Laboratory tests to identify more specific mediators might be useful in future studies. Unstable BP is one of the characteristics of anaphylaxis and is interfered with by various factors in surgical procedures. But this study was a randomized controlled trial, not a descriptive study. Strict randomization and blinding can ensure random variability, systematic bias and confounding factors are reasonably excluded.
Conclusions
The current pilot study revealed that the preventive use of clemastine fumarate could restrain the increase in plasma histamine concentration and contribute to safer hemodynamics in patients undergoing cardiac surgery with CPB. Further large-scale clinical trials are needed to reinforce the efficacy of clemastine fumarate as a histamine H1 receptor blocker in cardiovascular surgery.
Figures
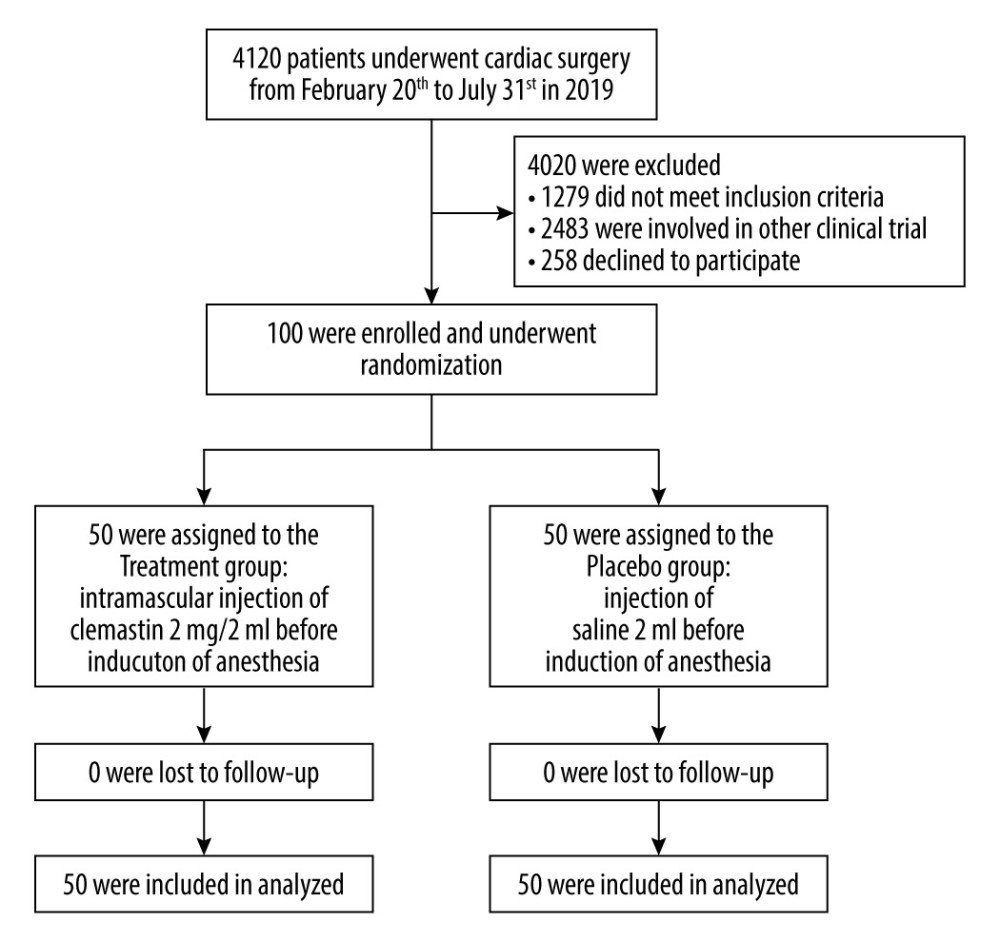 Figure 1. CONSORT Flow chart of chosen participants.
Figure 1. CONSORT Flow chart of chosen participants. 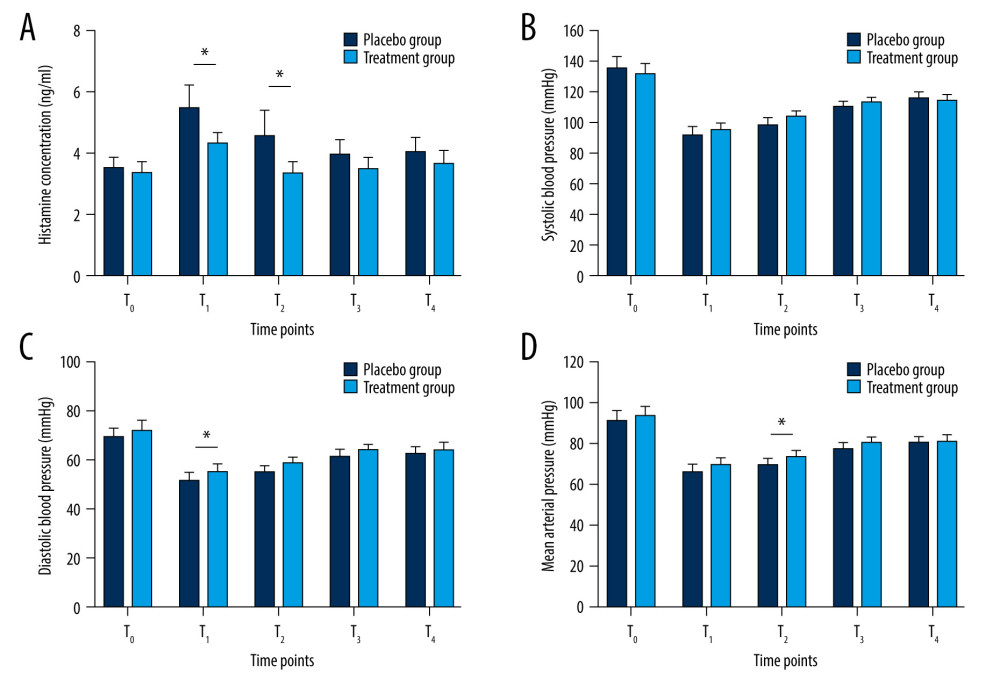 Figure 2. Plasma histamine concentrations (A) and systolic (B), diastolic (C), mean (D) blood pressure at each time point. Error bar indicates 95% CI. T0 – before induction of anesthesia, T1 – 5 minutes after heparinization, T2 – 5 minutes after neutralization, T3 – at the end of surgery, T4 – 24 hours postoperatively.
Figure 2. Plasma histamine concentrations (A) and systolic (B), diastolic (C), mean (D) blood pressure at each time point. Error bar indicates 95% CI. T0 – before induction of anesthesia, T1 – 5 minutes after heparinization, T2 – 5 minutes after neutralization, T3 – at the end of surgery, T4 – 24 hours postoperatively. Tables
Table 1. Comparison of demographic and surgical data.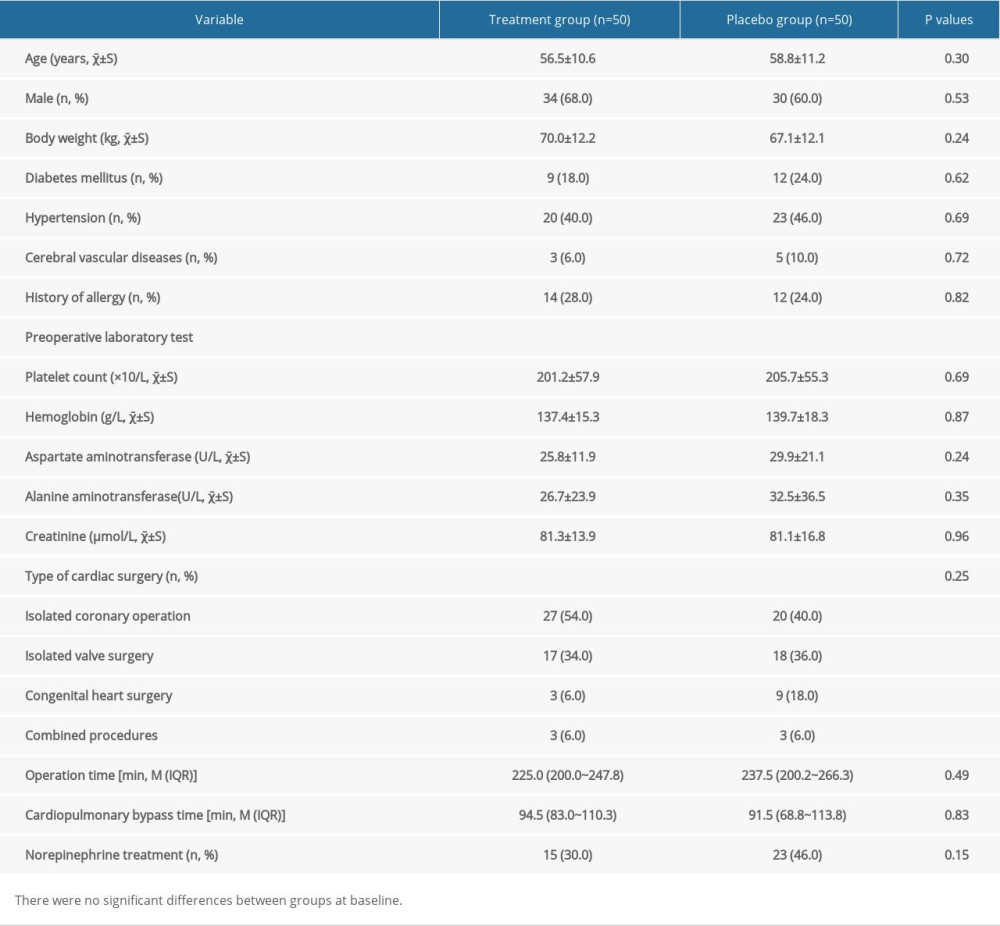 Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations.
Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations.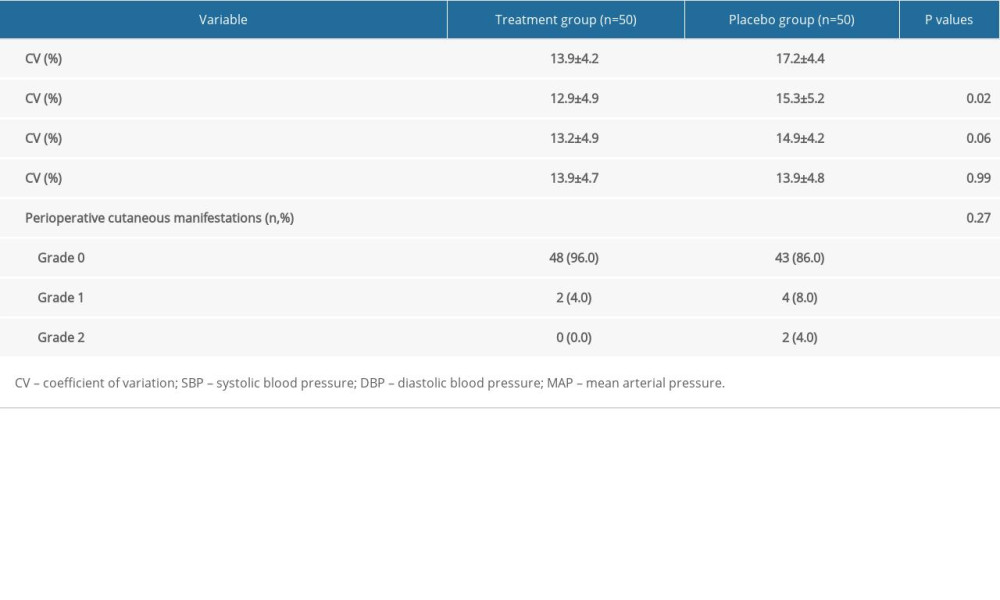 Table 3. Follow-up data.
Table 3. Follow-up data.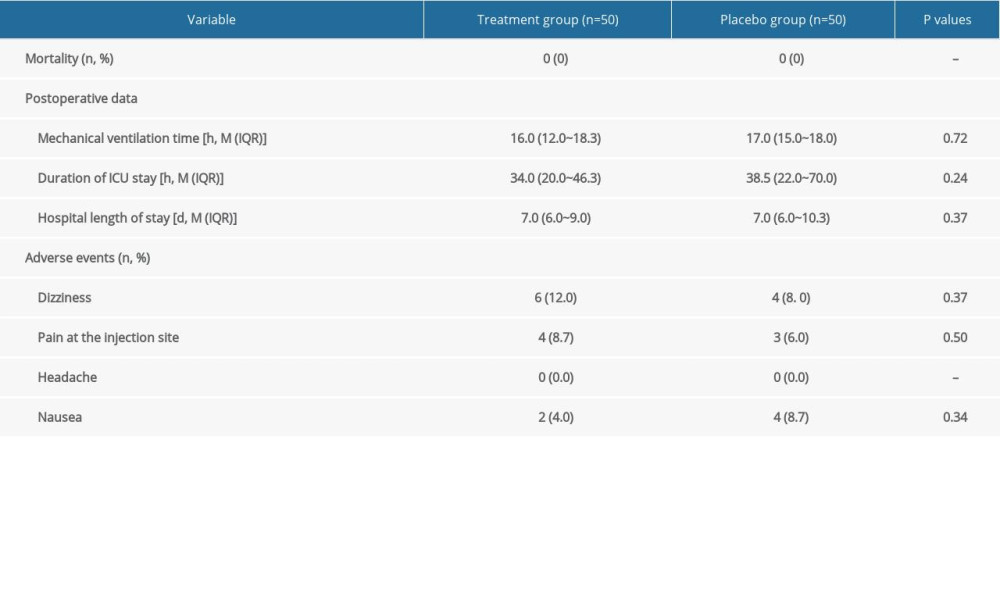 Supplementary Table 1. Multiple-comparison result of plasma histamine concentration (ng/ml, χ̄±S).
Supplementary Table 1. Multiple-comparison result of plasma histamine concentration (ng/ml, χ̄±S).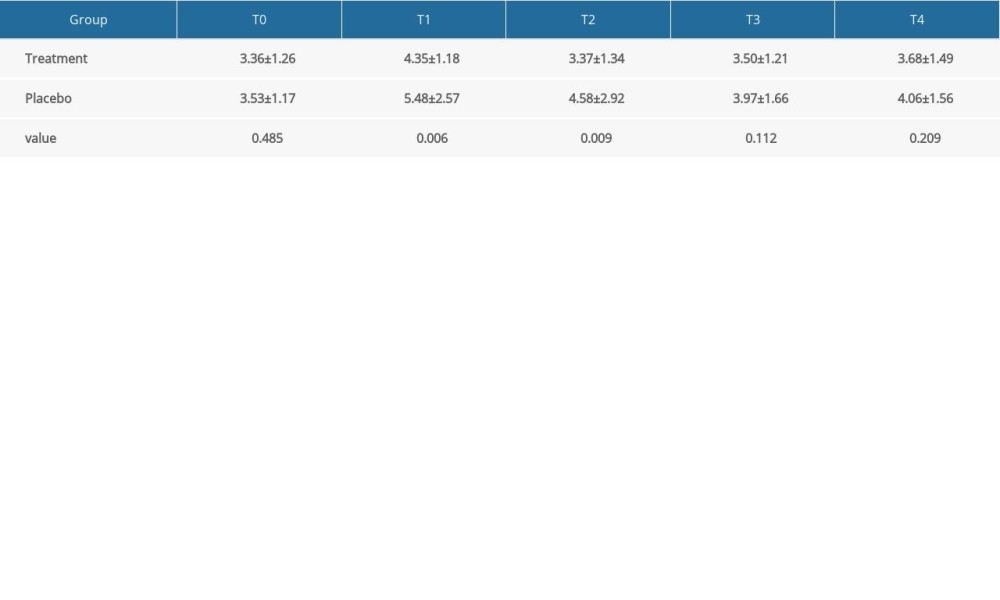 Supplementary Table 2. Multiple-comparison result of systolic blood pressure (mmHg, χ̄±S).
Supplementary Table 2. Multiple-comparison result of systolic blood pressure (mmHg, χ̄±S).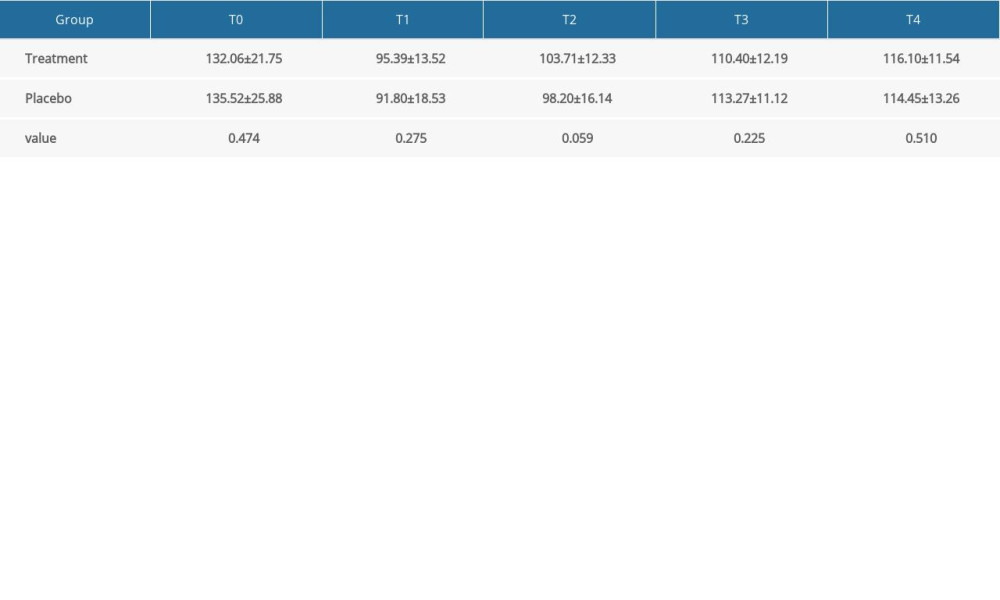 Supplementary Table 3. Multiple-comparison result of diastolic blood pressure (mmHg, χ̄±S).
Supplementary Table 3. Multiple-comparison result of diastolic blood pressure (mmHg, χ̄±S).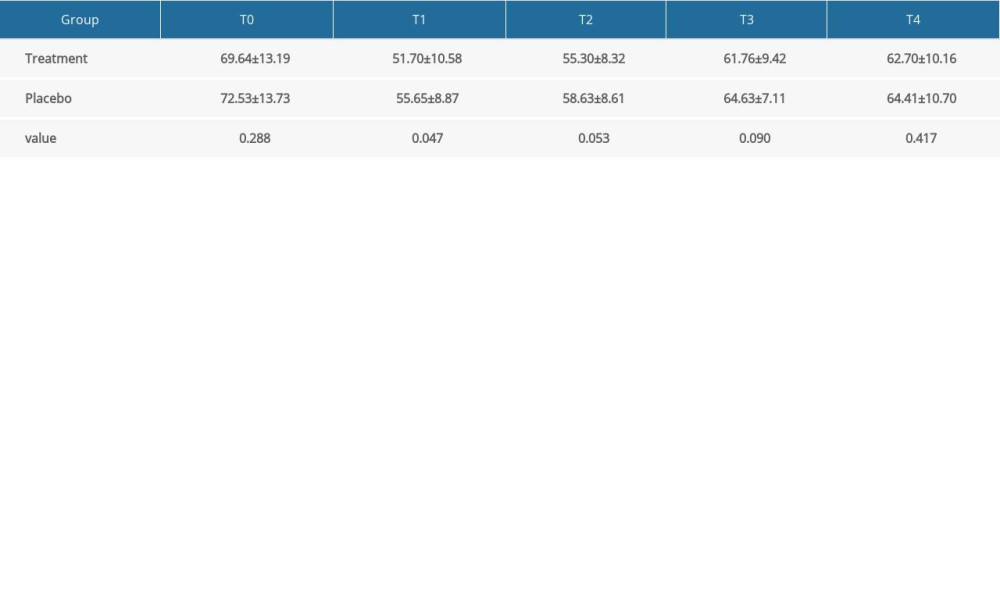 Supplementary Table 4. Multiple-comparison result of mean arterial pressure (mmHg, χ̄±S).
Supplementary Table 4. Multiple-comparison result of mean arterial pressure (mmHg, χ̄±S).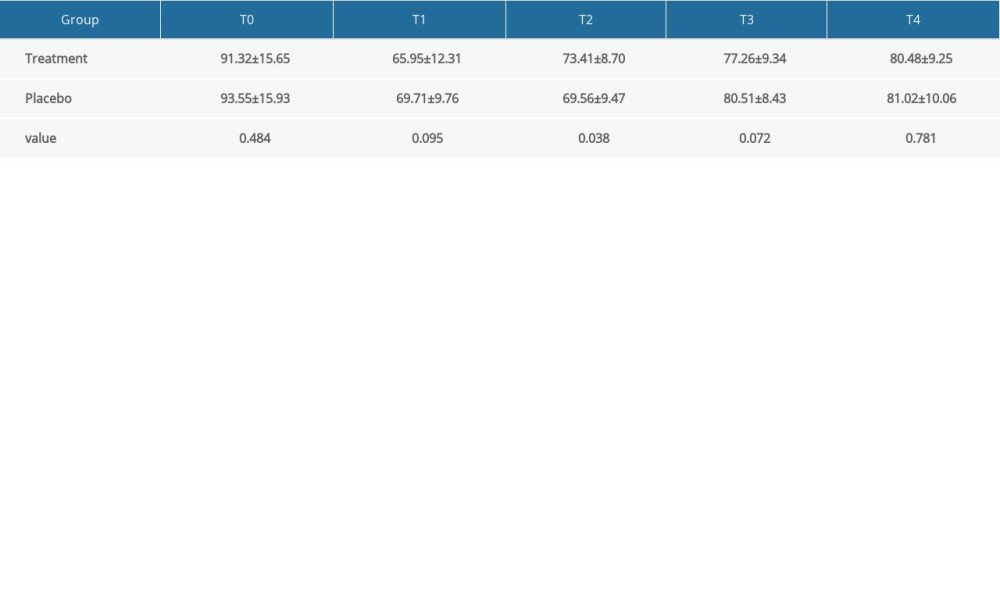
References
1. Anipindi S, Anupuba S, Norawat R, Incidence and outcome of anaphylaxis in cardiac surgical patients – should we proceed to cardiopulmonary bypass and complete surgery?: Br J Anaesth, 2018; 120; e13
2. Caimmi S, Caimmi D, Bernardini R, Perioperative anaphylaxis: Epidemiology: Int J Immunopathol Pharmacol, 2011; 24; S21-26
3. Che L, Li X, Zhang X, The nature and reported incidence of suspected perioperative allergic reactions: A cross-sectional survey: J Clin Anesth, 2021; 74; 110404
4. Ovrum E, Lindberg H, Holen EA, Systemic and pulmonary circulatory effects of protamine following cardiopulmonary bypass in man: Scand J Thorac Cardiovasc Surg, 1991; 25; 19-24
5. Shapira N, Schaff HV, Piehler JM, Cardiovascular effects of protamine sulfate in man: Thorac Cardiovasc Surg, 1982; 84; 505-14
6. Tiwari D, Saraogi A, Gehlot R, Life-threatening protamine allergic reaction in a patient with extra-adrenal pheochromocytoma undergoing off-pump coronary artery bypass grafting: Ann Card Anaesth, 2016; 19; 351-53
7. Harper NJN, Cook TM, Garcez T: Br J Anaesth, 2018; 121; 159-71
8. Berroa F, Lafuente A, Javaloyes G, The usefulness of plasma histamine and different tryptase cut-off points in the diagnosis of peranaesthetic hypersensitivity reactions: Clin Exp Allergy, 2014; 44; 270-77
9. Kay AB, Allergy and allergic diseases. First of two parts: N Engl J Med, 2001; 344; 30-37
10. Matucci A, Vultaggio A, Fassio F, Heart as the early main target of severe anaphylactic reactions: Two case reports: Intern Emerg Med, 2011; 6; 467-69
11. Low I, Stables S, Anaphylactic deaths in Auckland, New Zealand: A review of coronial autopsies from 1985 to 2005: Pathology, 2006; 38; 328-32
12. Kuda Y, Kurata Y, Wang M, Major contribution of vasospasm-induced coronary blood flow reduction to anaphylactic ventricular dysfunction assessed in isolated blood-perfused rat heart: Cardiol J, 2014; 21; 11-17
13. Kounis NG, Cervellin G, Koniari I, Anaphylactic cardiovascular collapse and Kounis syndrome: Systemic vasodilation or coronary vasoconstriction?: Ann Transl Med, 2018; 6; 332
14. Chang TI, Reboussin DM, Chertow GM, Visit-to-visit office blood pressure variability and cardiovascular outcomes in SPRINT (Systolic Blood Pressure Intervention Trial): Hypertension, 2017; 70; 751-58
15. Dyke CM, Benz CL, Taggart CM, Systolic and diastolic blood pressure variability as risk factors for adverse events after coronary artery bypass grafting: JAMA Surg, 2019; 154; 92-94
16. Berroa F, Lafuente A, Javaloyes G, The incidence of perioperative hypersensitivity reactions: A single-center, prospective, cohort study: Anesth Analg, 2015; 121; 117-23
17. Li H, Xiao Y, Li Q, The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1: Cancer Cell, 2022; 40; 36-52e9
18. Yuan X, Juan Z, Zhang R, Clemastine fumarate protects against myocardial ischemia reperfusion injury by activating the TLR4/PI3K/Akt signaling pathway: Front Pharmacol, 2020; 11; 28
19. Lango R, Mroziński P, Wujtewicz M, Administration of clemastine – H1 histamine receptor blocker in the prevention of haemodynamic disorders after protamine sulfate administration in patients subjected to coronary artery bypass grafting in extracorporeal circulation: Med Sci Monit, 2000; 6; 769-75
20. Kawano T, Scheuermeyer FX, Gibo K, H1-antihistamines reduce progression to anaphylaxis among Emergency Department patients with allergic reactions: Acad Emerg Med, 2017; 24; 733-41
21. Xiang Z, Yan-Liang Q, Xiao-Yang S, Effects of promethazine or dexamethasone pretreatment on mivacurium-induced histamine release in children: Paediatr Anaesth, 2014; 24; 322-26
22. Xie Z, Liao Q, Li Z, Development and full validation of a sensitive quantitative assay for the determination of clemastine in human plasma by liquid chromatography-tandem mass spectrometry: J Pharm Biomed Anal, 2007; 44; 924-30
23. Ma Z, Qiao Y, Peng J, Study on the pharmacokinetics of clemastine fumarate injection in healthy volunteers: China Phamlacist, 2011; 14; 620-24
24. Kroigaard M, Garvey LH, Gillberg L, Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia: Acta Anaesthesiol Scand, 2007; 51; 655-70
25. Fontes ML, Aronson S, Mathew JP, Pulse pressure and risk of adverse outcome in coronary bypass surgery: Anesth Analg, 2008; 107; 1122-29
Figures
 Figure 1. CONSORT Flow chart of chosen participants.
Figure 1. CONSORT Flow chart of chosen participants. Figure 2. Plasma histamine concentrations (A) and systolic (B), diastolic (C), mean (D) blood pressure at each time point. Error bar indicates 95% CI. T0 – before induction of anesthesia, T1 – 5 minutes after heparinization, T2 – 5 minutes after neutralization, T3 – at the end of surgery, T4 – 24 hours postoperatively.
Figure 2. Plasma histamine concentrations (A) and systolic (B), diastolic (C), mean (D) blood pressure at each time point. Error bar indicates 95% CI. T0 – before induction of anesthesia, T1 – 5 minutes after heparinization, T2 – 5 minutes after neutralization, T3 – at the end of surgery, T4 – 24 hours postoperatively. Tables
 Table 1. Comparison of demographic and surgical data.
Table 1. Comparison of demographic and surgical data. Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations.
Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations. Table 3. Follow-up data.
Table 3. Follow-up data. Table 1. Comparison of demographic and surgical data.
Table 1. Comparison of demographic and surgical data. Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations.
Table 2. Perioperative hemodynamic parameters and perioperative cutaneous manifestations. Table 3. Follow-up data.
Table 3. Follow-up data. Supplementary Table 1. Multiple-comparison result of plasma histamine concentration (ng/ml, χ̄±S).
Supplementary Table 1. Multiple-comparison result of plasma histamine concentration (ng/ml, χ̄±S). Supplementary Table 2. Multiple-comparison result of systolic blood pressure (mmHg, χ̄±S).
Supplementary Table 2. Multiple-comparison result of systolic blood pressure (mmHg, χ̄±S). Supplementary Table 3. Multiple-comparison result of diastolic blood pressure (mmHg, χ̄±S).
Supplementary Table 3. Multiple-comparison result of diastolic blood pressure (mmHg, χ̄±S). Supplementary Table 4. Multiple-comparison result of mean arterial pressure (mmHg, χ̄±S).
Supplementary Table 4. Multiple-comparison result of mean arterial pressure (mmHg, χ̄±S). In Press
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
06 Mar 2024 : Clinical Research
Prevalence and Variation of Medical Comorbidities in Oral Surgery Patients: A Retrospective Study at Jazan ...Med Sci Monit In Press; DOI: 10.12659/MSM.943884
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








