20 May 2022: Clinical Research
Importance of Influenza Anti-Hemagglutinin Antibodies During the SARS-CoV-2 Pandemic in the 2019/2020 Epidemic Season in Poland
Lidia B. Brydak1ADEFG, Karol Szymański1BF, Katarzyna Kondratiuk1BF, Anna Poznańska2CDE, Adam Kolondra1B, Ewelina Hallmann1BDEF*DOI: 10.12659/MSM.936495
Med Sci Monit 2022; 28:e936495
Abstract
BACKGROUND: The aim of this study was to determine the level of anti-hemagglutinin antibodies in the serum of recovered patients during the SARS-CoV-2 pandemic in the 2019/2020 epidemic season in Poland, and the course of COVID-19.
MATERIAL AND METHODS: The material for the study consisted of the sera of COVID-19 convalescents obtained from the following 9 Regional Blood Donation and Blood Supply Centers located in 8 voivodeships. The hemagglutination inhibition reaction assay (HAI) using 8 viral hemagglutination units was used to determine antibody levels, in accordance with WHO recommendations.
RESULTS: This research confirms that a patient’s declared severity of the course of SARS-CoV-2 infection is influenced by the patient’s age and concomitant diseases. There was no statistically significant correlation between the level of anti-hemagglutinin antibodies and the severity of the course of a SARS-CoV-2 infection. Based on the serological tests conducted, it can be unequivocally concluded that both vaccinated and influenza-infected patients had a response rate in line with the requirements of the European Commission and the Committee for Medicinal Products for Human Use hemagglutinin antibodies for 4 influenza virus antigens tested.
CONCLUSIONS: Patients who confirmed their antibody levels with the Commission of the European Communities and the Committee for Propriety Medicinal Products (CPMP) requirements had a mild COVID-19 course. The results of our research emphasize the role of anti-hemagglutinin antibodies in the course of SARS-CoV-2 infection. COVID-19 convalescents have a higher response rate against all 4 types of anti-hemagglutinin antibodies analyzed.
Keywords: COVID-19, Influenza B virus, Influenza A virus, Antibodies, Viral, Hemagglutinins, Humans, Influenza, Human, Pandemics, Poland, SARS-CoV-2, Seasons
Background
In the 20th century, there were 3 pandemics caused by different subtypes of influenza A virus. Spanish flu, which caused 50–100 million deaths in 1918–1919, was caused by influenza virus subtype A/H1N1/. The 1918 influenza pandemic had an unusually high death rate, especially among adults, and was very widespread, affecting even Alaska. Deciphering this virus has become possible only thanks to the progress in scientific research (ie, molecular biology), as well as scientific expeditions aimed at collecting lung tissue samples of victims of this deadly influenza, which are stored frozen to the present day, as in Alaska. Professor Jeffrey Taubenberger, together with the team of the Department of Molecular Pathology of the Armed Forces Institute of Pathology in Washington, initiated research on the molecular pathology of Spanish flu virus. Then, Professor John Oxford of Barts & London Hospital and his team teamed up to continue the molecular research. Photographs of post-mortem autopsy cases included various descriptions of influenza pneumonia with acute massive alveolar bleeding, influenza pneumonia with severe acute pulmonary edema; bronchopneumonia, and influenza pneumonia with focal bronchiolitis and alveolitis [1].
The isolation of influenza virus in humans in 1933 by English researchers was the cornerstone of multidirectional research into such a highly variable pathogen, which, without exaggeration, can be called the master of metamorphosis. The next pandemic, the 1957–1958 Asian flu pandemic, was caused by the A/H2N2/subtype, but caused far fewer deaths (1–4 million). People who had survived the Spanish flu pandemic saw the value of influenza vaccination and were willing to be vaccinated. The first authorization for use of influenza vaccines in humans was given in 1941, for vaccines sponsored by the United States Armed Forces. These vaccinations had numerous adverse effects, but advances in technology and science contributed to their improvement [2]. The next 20th century flu pandemic was the 1968–1969 Hong Kong flu pandemic, which caused about 1–4 million deaths. In 1947 the World Health Organization (WHO), aware of the effects and threats of influenza infection, proposed a global program for epidemiological and virological research in the form of influenza surveillance at the 4th International Congress of Microbiology in Copenhagen [3], now referred to as the Global Influenza Surveillance and Response System. Poland has participated in it through the National Influenza Center since 1957 and has participated in virological and epidemiological
The rapid spread of an infectious disease such as influenza is caused, on the one hand, by the rapid movement of people, especially today, and, on the other hand, by the existence of animal reservoirs [5]. Influenza infects people regardless of age, time of year, latitude, political views, and religious beliefs. Prior to the COVID-19 pandemic, the WHO reported that around 5–10% of adults and 20–30% of children are infected with influenza each year; there are reports of 3–5 million acute cases of the disease and about 250 000–630 000 people die due to complications triggered by influenza, including 28–111.5 thousand children under 5 years of age [6]. Currently, there are many types of influenza vaccine and next-generation anti-influenza drugs [2,7–9]. Poland had the lowest influenza vaccination rate in Europe, especially in children under 14 years of age. Influenza vaccination is the most effective and cheapest method of influenza prevention. Influenza complications should be considered not only in health-related, but also in economic terms, which the country needs to pay for using tax revenues [10,11]. Increasing preventive measures (ie, increasing Polish vaccination rates, will consequently reduce the number of post-influenza complications and deaths. Providing concrete examples should be helpful in promoting prevention and encouraging healthcare professionals to protect not only patients, but also their relatives, and to debunk numerous myths related to vaccinations. The Department of Influenza Research, National Influenza Center in NIPH NIH-NRI, in collaboration with clinicians, has published over 200 articles assessing the humoral response after influenza vaccination in high-risk groups [10–14]. In children, determination of the humoral response was performed at 6–35 months of age, 3–8 years of age, 9–12 years of age, and 13–20 years of age, with acute lymphoblastic leukemia (ALL), vaccinated at various times from the end of treatment, with severe and mild hemophilia, with bronchopulmonary dysplasia, with glomerulonephritis, with chronic renal failure, subjected to continuous ambulatory peritoneal dialysis, hemodialysis, and with chronic renal failure, vaccinated once and twice, infected with HIV – after splenectomy: vaccinated in age groups 0–5, 6–10, 11–15, 16 years of age, (
Material and Methods
STATISTICAL ANALYSIS:
A response factor (percentage of respondents) was determined for all antibodies with an appropriate titer of ≥40 (according to the criteria of the Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Harmonization for Influenza vaccine (CPMP/BWP/214/96, 12 march, 1997, 20–21) people aged 18–60) and the average level of antibodies (their geometric mean in this group of participants – GMT) [24,25]. These values were calculated (with their 95% confidence intervals – 95% CI) for the total number of respondents and in subgroups distinguished based on sex, age, and vaccination against influenza in the 2019/2020 season, as well as for comparative purposes for people aged 15–64 years from the national serological review in the 2019/2020 season. The frequency of occurrence of qualitative variables for the total number of respondents and in the selected subgroups was compared using the chi-square test. Cases with incomplete data (summarized in Table 1) were excluded from the analyses to an appropriate extent (the results are presented without them). The distributions of quantitative variables (age and antibody titers) in the 2 groups were compared using the Mann-Whitney test. A significance level of 0.05 was adopted in all statistical analyses. The analyses were performed using SPSS 12.PL statistical software.
Results
The entire study was concerned with young and middle-aged people aged 18–64 years (their average age was 38) (Table 1). Most of the participants were men (71% men vs 29% women).
Information on influenza vaccination in the 2019/2020 epidemic season was obtained in 912 cases; 266 (29.2%) patients were vaccinated and 646 (79.8%) were unvaccinated.
The level of vaccination of the respondents in individual voivodeships varied significantly (statistically significant differences,
Both groups of respondents (vaccinated and unvaccinated) had a significantly higher proportion of men (77.1% and 69.1%, respectively), and the difference is statistically significant (
As can be seen from the data presented in Table 3, in the case of the A/Brisbane/02/2018 (H1N1)pdm09-like virus, people vaccinated significantly more often (
When it comes to the A/Kansas/14/2017 (H3N2) virus, vaccinated subjects significantly more often (
On the other hand, in the case of the B/Colorado/06/2017 Victoria lineage virus, vaccinated persons significantly more often (
People vaccinated against the B/Phuket/3073/2013 Yamagata lineage virus significantly more often (
The response rate did not depend on the sex of the subjects for the 3 types of antibodies, and only for the anti-B/Colorado/06/2017 (Victoria lineage) was it significantly higher in men than in women (34.5 [30.9–38.2]% vs 26.7 [21.4–32.0]%,
Influenza vaccination raises the levels of antibodies against all 4 viruses in the quadrivalent vaccine, but the levels depend on age as well as the patient’s immune memory.
In our study of people up to 40 years of age, the anti-A/Brisbane/02/2018 (H1N1)pdm09 antibody levels ≥40 were recorded in 65.1 [57.9–72.3]% of the vaccinated and 37.2 [32.4–42.1]% of unvaccinated persons (
In people up to 40 years of age, the anti-A/Kansas/14/2017 (H3N2) antibodies at a level of ≥40 were recorded in 75.1 [68.6–81.7]% of the vaccinated and 59.6 [54.7–64.5]% of the unvaccinated subjects (
In people up to 40 years of age, the anti-B/Colorado/06/2017 (B/Victoria lineage) antibodies at a level ≥40 were detected in 40.8 [33.4–48.2]% of the vaccinated and 17.7 [13.9–21.5]% of the unvaccinated subjects (
In people under the age of 40, the anti-B/Phuket/3073/2013 (B/Yamagata lineage) antibodies at a level of ≥40 were recorded in 82.8 [77.2–88.5]% of the vaccinated and 60.9 [56.1–65.8]% of the unvaccinated (
Among both vaccinated and unvaccinated subjects, the response rate did not show any relationship with their sex. The only exception was the anti-B/Colorado/06/2017 (Victoria lineage) antibodies among unvaccinated people – at a level of ≥40, they were found in 27.9 [23.7–32.0]% of men and 19.6 [14.1–25.1]% of women (
COVID-19 convalescents had a higher response rate for all the 4 analyzed antibodies; in 2 cases (type B), the differences are statistically significant: for B/Colorado/06/2017-Victoria lineage (32.3 [29.3–35.3]% vs 4.3 [2.0–6.6]%) and for B/Phuket-Yamagata lineage (61.6 [58.4–64.7]% vs 31.7 [26.4–36.9]%). The mean antibody titer (geometric mean) was higher in convalescents for 3 antigens; in 2 cases, the differences were statistically significant: for A/H1N1/pdm09 (99.7 [90.6–108.7] vs 69.0 [58.4–79.5]) and for B/Colorado/06/2017-Victoria lineage (80.7 [74.1–87.3] vs 52.2 [38.4–66.1]). For B/Phuket/3073/2013-Yamagata lineage, the antibody titers in both groups were almost equal.
Discussion
The scientific objective of this study was to assess the relationship between the presence of anti-hemagglutinin antibodies in the serum of convalescents and parameters such as: sex and age, vaccination against influenza, as well as the influence of detected anti-hemagglutinin antibodies on the subjectively perceived course of a COVID-19 infection. Anti-hemagglutinin antibodies are antibodies produced in the body following influenza vaccination or exposure to influenza virus. So far, research on the anti-hemagglutinin antibody level has been conducted in healthy and high-risk patients [10,2–20]. Due to the fact that SARS-CoV-2 is a new respiratory virus attacking humans, it is worth investigating the issue of the presence of anti-hemagglutinin antibodies in COVID-19 convalescents. SARS-CoV-2 appeared in Poland during the peak of the 2019/2020 flu epidemic season. Circulation of respiratory viruses in the population requires the use of effective diagnostic methods to confirm infections not only with influenza viruses, but also with influenza-like viruses. Apart from molecular biology methods, monitoring the level of anti-hemagglutinin antibodies in patients’ sera is also important for diagnostic purposes. When an infection is caused by influenza virus, the body produces antibodies against the virus surface glycoproteins (ie, hemagglutinin (HA) and neuraminidase [NA]). These antigens are different for type A viruses and type B viruses. Hemagglutinin is responsible for the adsorption of a viral particle to a cell receptor in the host body. This glycoprotein is capable of fusing cell membranes, thus enabling the integration of the viral envelope with the membranes of the attacked cell, which, as a result, leads to the penetration of the virion into the cytoplasm of the cell and its release within the internal structures. Haemagglutinin makes up 80% of the viral envelope proteins, is encoded by segment 4 of the genome, and takes the form of a trimer, with each monomer consisting of a spherical main domain and a stem. The host’s respiratory proteases are capable of cleaving HA into HA1 and HA2 subunits, rendering the virus infectious. The HA1 and HA2 subunits are linked to each other by disulfide bonds. The HA1 subunit is a sialic acid binding site. Proteins are found in this part. There are antigenic determinants (epitopes) against which the host’s immune system produces immunoglobulins during an infection. The HA2 subunit, on the other hand, forms a transmembrane part that anchors the protein in the viral lipid envelope and participates in fusion between the viral envelope and the endosome membrane. The hemagglutinin cleavage site is amino acid sequence-dependent and is only mediated by extracellular trypsin-like proteases in the upper respiratory tract or in the gastrointestinal tract, resulting in only a local infection. Located on the surface, the HA virus membrane is inactive (referred to as HA0). The activation of HA takes place inside the endosome, thanks to lower pH that changes the structure of the protein. X-ray studies have shown that, inside the endosome, each of the 3 HA0 polypeptide chains is cleaved into 2 fragments by host proteases present in the endosome. After the cleavage, 2 HA1 and HA2 protein fragments are formed, linked by a disulfide bridge. One of the HA2 fragments is referred to as the fusion peptide because it has the ability to bind to the endosome membrane. Under favorable conditions, the HA2 fusion fragment becomes exposed and migrates about 10 nm toward the endosome membrane. Studies have shown that acidification of the endosome and the rate of changes in the structure of hemagglutinin and the exposure of the fusion peptide are temperature-dependent and take place faster with increasing temperature. Therefore, the body’s usual response to an infection is a factor contributing to its faster elimination. The administration of an influenza vaccine produces specific influenza vaccine antibodies. The level of these antibodies is measured using the staining inhibition of hemagglutination (HAI). In this study, turkey blood or dust was used [22]. There are antibodies in the environment that bind to the shaft of the HA glycoprotein, but they are rare in humans, and there is no way of detecting them and their titer using the NI test. These immunoglobulins prevent changes in the conformation of the hemagglutinin structure caused by a change in endosome pH, which prevents the virus from attaching to the cell. The ability of anti-hemagglutinin antibodies to cross-react is important, blocking the proliferation and release from respiratory epithelial cells of a different subtype of influenza virus. For this reason, it is important to perform flu vaccinations every season, which contributes to increased immunity and thus reduces the risk of post-influenza complications. The obtained results will allow us to learn about the SARS-CoV-2 virus, which in many respects is still unknown. This type of research is also important for developing influenza vaccination recommendations with people at a higher risk in mind, such as healthcare professionals, patients with comorbidities, or the elderly, as these recommendations may reduce the mortality caused by COVID-19. In Poland, in the 2019/2020 epidemic season, only 65 deaths were recorded due to influenza complications, which is clearly an underestimation. Numerous studies by multiple authors support the role of influenza vaccination in children and people aged 65 regarding decreased spread of clinical expression of COVID-19 [26–31].
A study by Green et al showed structural similarities between influenza viruses and coronaviruses in their binding receptors and concluded that influenza vaccine could induce cross-resistance [27]. Based on the research they conducted in 2 subsequent epidemic seasons, 2018/2019 and 2019/2020, the authors indicate a relationship between earlier influenza vaccinations and reduced susceptibility to a SARS-CoV-2 infection, a milder form of COVID-19 disease. These studies were conducted using the computerized patient database of Leumit Health Services, a large health maintenance organization (HMO), from demographic data of 715 164 patients dispersed throughout the country, including medical visits, medical diagnoses, hospitalizations, and diagnostic tests. The authors also, based on their research, emphasized the importance of vaccination recommendation against influenza, especially in light of the current SARS-CoV-2 pandemic. Although Italian research by Amato et al [29] concerned the population of people aged 65 and over, influenza vaccination was shown to play a role in preventing and minimizing the spread of COVID-19 cases. Studies by Zanettini et al confirmed that COVID-19-related mortality is higher in the elderly and people with pre-existing chronic conditions [30]. It is also known that it is associated with increased morbidity and mortality in the elderly due to seasonal influenza infection; therefore, seasonal influenza vaccination is recommended for them, which was also confirmed by Polish studies [10]. Research conducted by American authors, based on analyses of various periods, suggests that influenza vaccination protects against COVID-19 mortality.
Research by Conlon et al in 27–201 people demonstrated that influenza vaccination was associated with a reduced number of positive results of COVID-19 molecular biology tests in people who were vaccinated against influenza [28]. The obtained results clearly indicate that influenza vaccinations should be recommended to reduce mortality, length of hospitalization, need for intensive care, and the use of mechanical ventilation. Patwardhan and Ohler conducted research on over 900 children who were tested positive for SARS-CoV-2 between February and August 2020 [26]. To quote the authors’ statement: “The twindemic of influenza and SARS-CoV-2 may become a public health nightmare because of the same seasonal occurrence and similarity in symptoms apart from interactions between the viruses and their host responses”, as long as we confirm the infection using molecular biology methods. The authors’ research showed that children who had a positive SARS-CoV-2 result in the analyzed influenza season had a lower chance of developing symptoms, especially breathing problems or severe disease symptoms. The authors concluded that SARS-CoV-2-positive children who were vaccinated against pneumococci were also less likely to develop the disease. For decades, children have been regarded as superspreaders, especially for respiratory viruses [9]. Unexpectedly, the correlation of the level of anti-hemagglutinin antibodies with the severity of the SARS-CoV-2 course has not been statistically confirmed. It could be associated with the age-limited group of patients, including prime-aged people (on average 38 years), analyzed in the 2019/2020 epidemic season. The study group did not include the elderly and fatal cases. Please note that this was the beginning of a pandemic, with fewer coronavirus variants occurring. Furthermore, the patients enrolled in the study subjectively assessed the severity of the disease course. Based on serological studies, it can be unequivocally concluded that both vaccinated patients and those who contracted influenza infection had a response rate of ≥40 in accordance with the requirements of the European Commission and the Committee for Medicinal Products for Human Use [24,25].
Vaccination significantly increased the response rate against all 4 types of anti-hemagglutinin antibodies analyzed (anti-A/Brisbane (H1N1)pdm09, anti-A/Kansas/14/2017 (H3N2), anti-B/Colorado/06/2017 Victoria lineage, and anti-B/Phuket/3073/2013 Yamagata lineage) as well as the level of anti-haemagglutinin antibodies in the subjects for 3 of them.
In addition, a study carried out in the Netherlands in 2020 by Debisarun et al showed that quadrivalent influenza vaccine induces a better cytokine response after stimulating immune cells with SARS-CoV-2 [31].
Conclusions
The serum level of anti-hemagglutinin antibodies in convalescents showed values greater than ≥40 the response levels as required by the Commission of the European Communities and the Committee for Propriety Medicinal Products (CPMP).
Patients who had their antibody levels confirmed with the EC and CPMP requirements had a mild COVID-19 course. There were no deaths among the studied group of patients.
Influenza vaccination helped all patients, regardless of age.
The results of our research emphasize the role of anti-hemagglutinin antibodies in the course of SARS-CoV-2 infection. It would be worth extending the study to a group of patients with a physician-confirmed, severe course of COVID-19 because, despite the proven effect of vaccination on the response rate, due to patients’ subjective assessments of the infection course, its influence on the infection severity was not statistically confirmed.
COVID-19 convalescents have a higher response rate against all 4 types of anti-hemagglutinin antibodies analyzed.
Figures
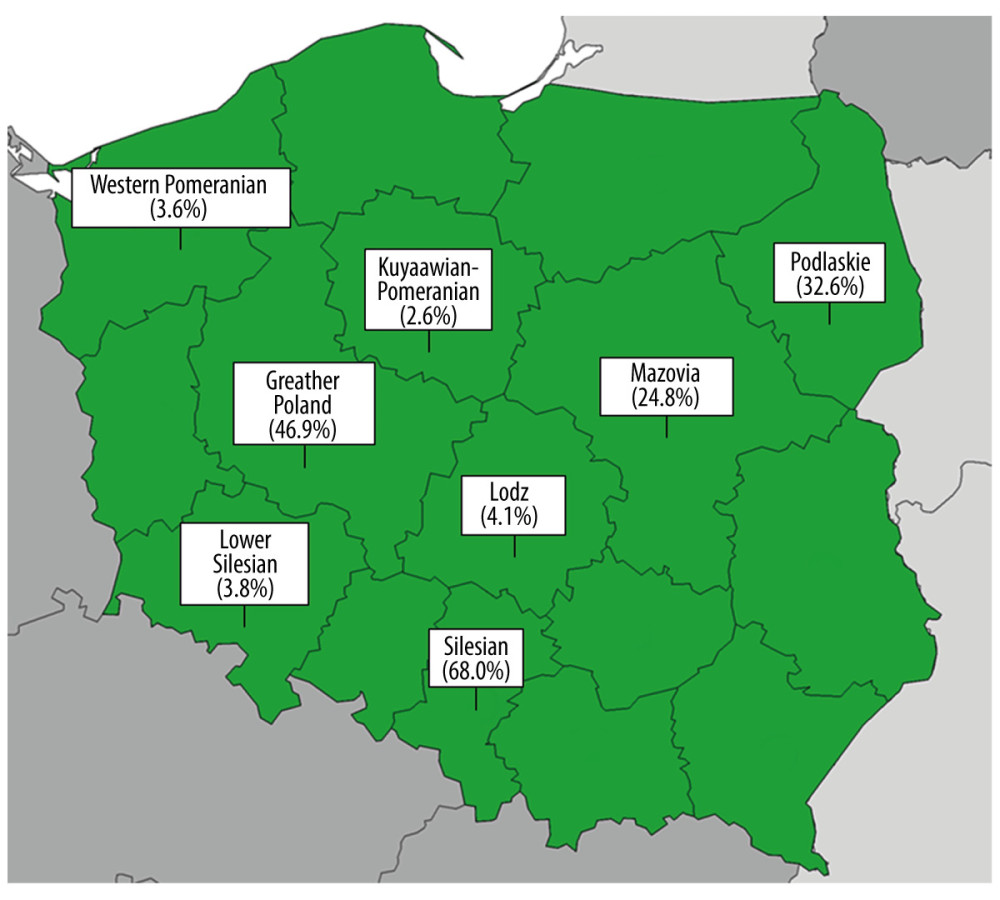 Figure. 1. The percentage of study subjects vaccinated against influenza in the 2019/2020 season in the 8 voivodeships included.
Figure. 1. The percentage of study subjects vaccinated against influenza in the 2019/2020 season in the 8 voivodeships included. 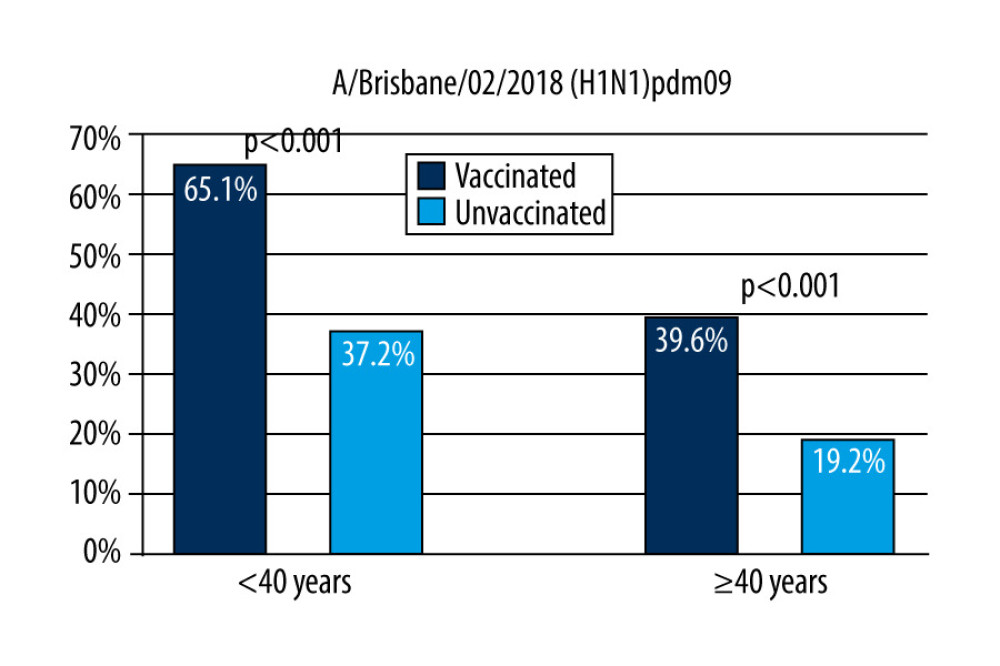 Figure 2. The response factor is therefore similar to that found in older unvaccinated participants and in younger unvaccinated participants after vaccination.
Figure 2. The response factor is therefore similar to that found in older unvaccinated participants and in younger unvaccinated participants after vaccination. 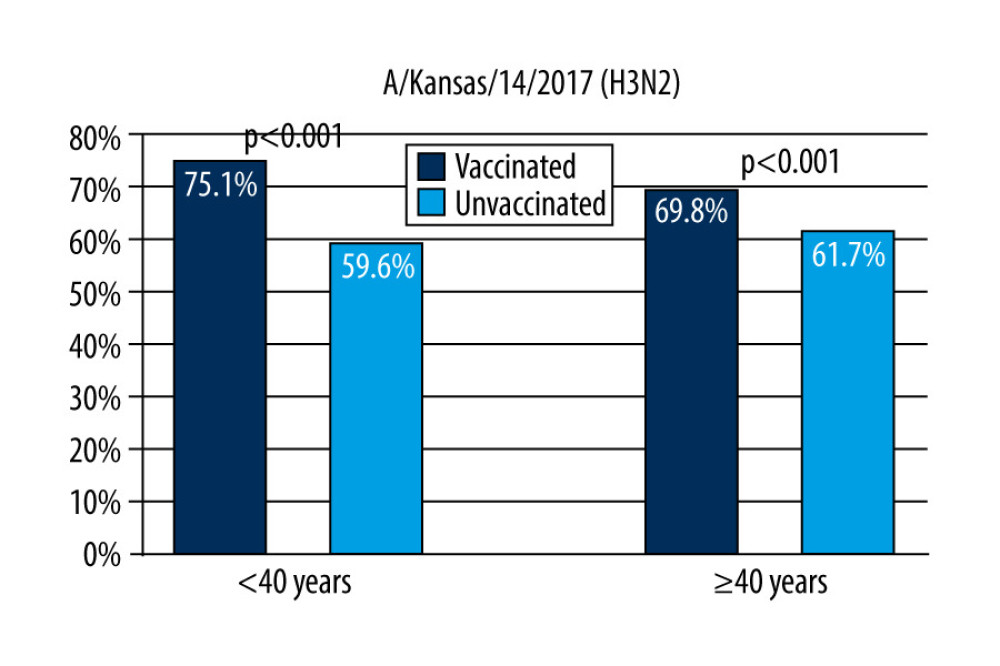 Figure 3. Response rate for anti-A/Kansas/14/2017 (H3N2) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 3. Response rate for anti-A/Kansas/14/2017 (H3N2) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. 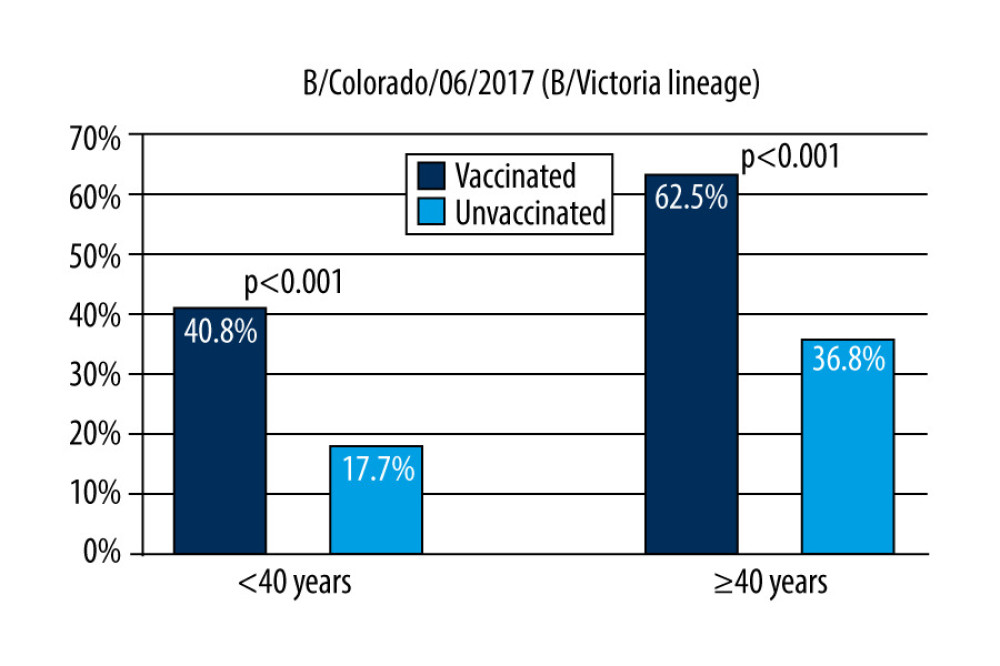 Figure 4. Response rate for anti-B/Colorado/06/2017 (B/Victoria lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 4. Response rate for anti-B/Colorado/06/2017 (B/Victoria lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. 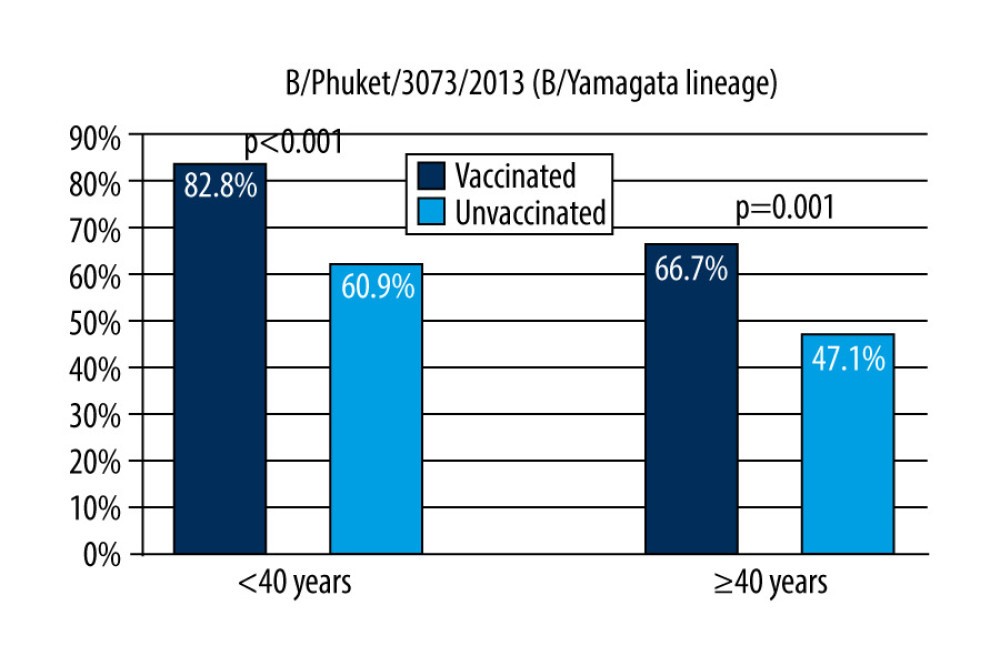 Figure 5. Response rate for anti-B/Phuket/3073/2013 (B/Yamagata lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 5. Response rate for anti-B/Phuket/3073/2013 (B/Yamagata lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. Tables
Table 1. The most important characteristics of the studied influenza.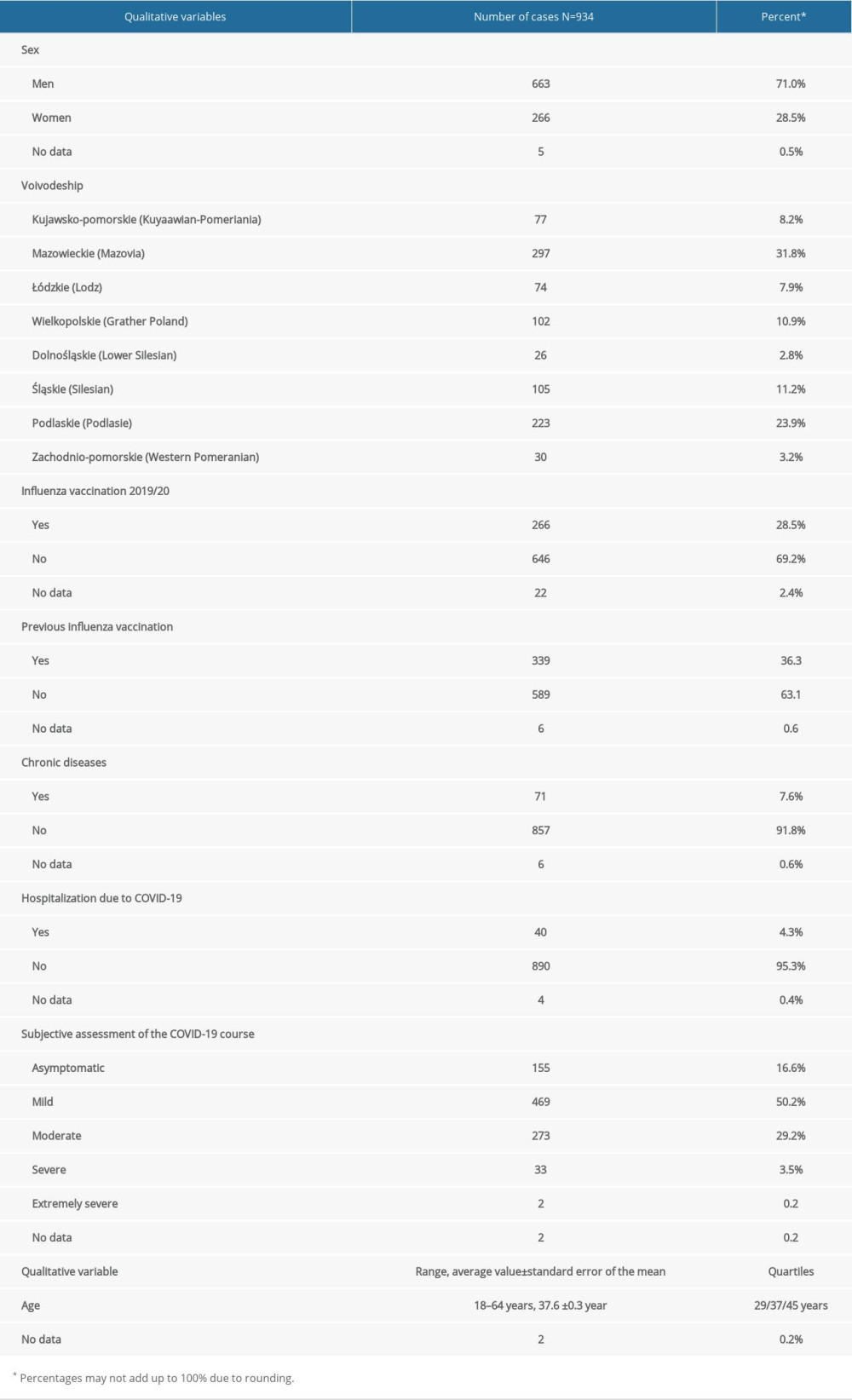 Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season.
Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season.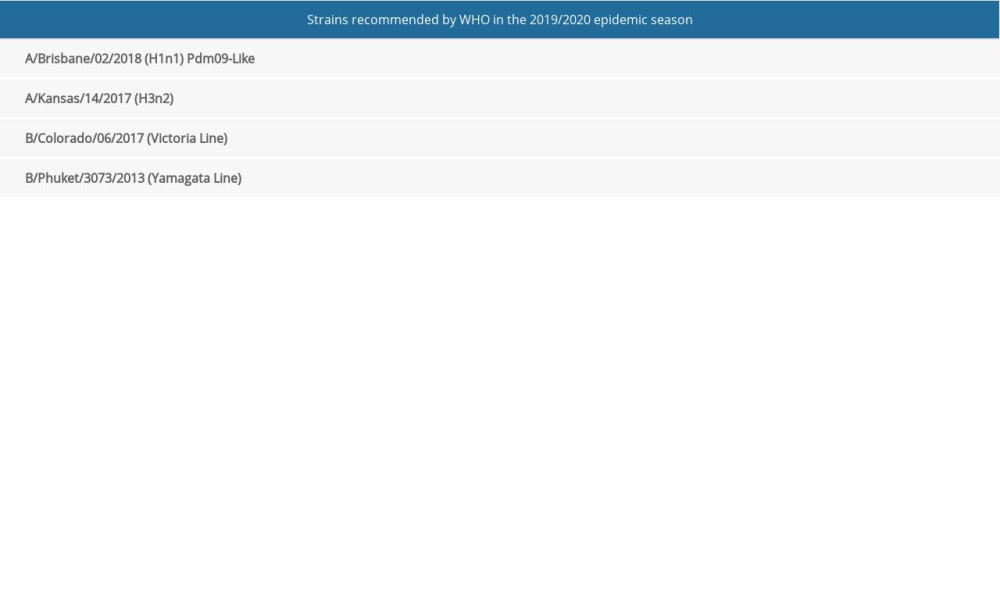 Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences.
Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences.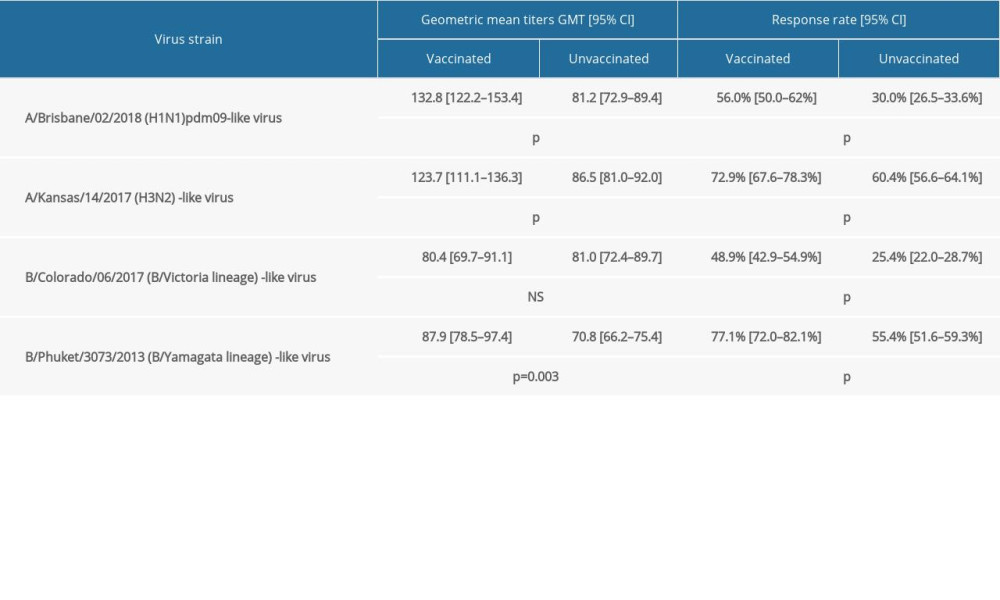 Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people).
Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people).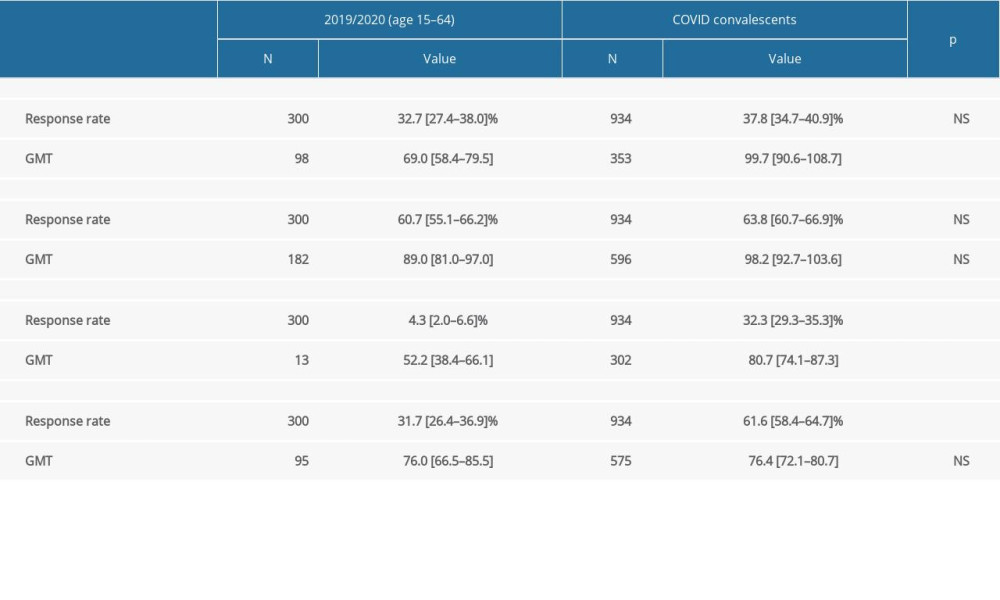
References
1. Brydak LB, 2008; 9-34, Warsaw, Rytm [in Polish]
2. Brydak LB, 2008; 193-252, Warsaw, Rytm [in Polish]
3. Brydak LB, 2008; 165-92, Warsaw, Rytm [in Polish]
4. Bednarska K, Hallmann-Szelińska E, Kondratiuk KInnovations in influenza surveillance: Probl Hig Epidemiol, 2016; 97(2); 101-5 [in Polish]
5. Brydak LB, 2008; 35-48, Warsaw, Rytm [in Polish]
6. World Health Organization (WHO) Available from: https://www.who.int/en
7. Brydak LB, 2008; 283-418, Warsaw, Rytm [in Polish]
8. Brydak LB, 2008; 253-82, Warsaw, Rytm [in Polish]
9. Ison MG, Antivirals and resistance: Influenza virus: Curr Opin Virol, 2011; 1(6); 563-73
10. Brydak LBInfluenza – prevention and treatment in children and adolescent: Standardy Medyczne Pediatrii, 2019; 2(16); 162-71 [in Polish]
11. Brydak LBHealth and economic effects of influenza infections in terms of public health in Poland: Pharmacoeconomics in the management of health care resources, 2018; 274-83, Warsaw, Kluwer [in Polish]
12. Mastalerz-Migas A, Bujnowaska-Fedak M, Brydak LB, Immune efficacy of first and repeat trivalent influenza vaccine in healthy subjects and hemodialysis patients: Adv Exp Med Biol, 2015; 836; 47-54
13. Mastalerz-Migas A, Pokorski M, Kiliś-Pstrusińśka K, Cytokines and toll-like receptors in the immune response to influenza vaccination: Adv Exp Med Biol, 2015; 836; 35-40
14. Mastalerz Migas A, Steciwko A, Brydak LB, Immune response to influenza vaccine in hemodialysis patients with chronic renal failure: Ads Exp Med Biol, 2013; 756; 285-90
15. Krzywański J, Nitsch-Osuch A, Mikulski T, Antibody response to trivalent influenza vaccine in the northern and the southern hemisphere in elite athletes: Adv Exp Med Biol, 2018; 1108; 49-54
16. Nitsch-Osuch A, Gołebiak I, Wyszkowska D, Influenza vaccination coverage among polish patients with chronic diseases: Adv Exp Med Biol, 2017; 968; 19-34
17. Gańczak M, Dubiel P, Drozd-Dąbrowska M, Hallmann-Szelińska E, Quadrivalent influenza vaccine-induced antibody response and influencing determinants in patients >55 years of age in the 2018/2019 season: Int J Environ Res Public Health, 2019; 16(22); 4489
18. Jagielska AM, Brydak LB, Nitsch-Osuch AS, Immunogenicity of split inactivated quadrivalent influenza vaccine in adults with obesity in the 2017/2018 season: Med Sci Monit, 2021; 27; e929572
19. Susło R, Pobrotyn P, Brydak LB, Seasonal influenza and low flu vaccination coverage as important factors modifying the costs and availability of hospital services in Poland: A retrospective comparative study: Int J Environ Res Public Health, 2021; 18(10); 5173
20. Sławin A, Brydak LB, Doniec Z, Serum vitamin D and immunogenicity of influenza vaccination in the elderly: Adv Exp Med Biol, 2021; 1324; 21-28
21. WHO Global Influenza Surveillance Network: Manual for the laboratory diagnosis and virological surveillance of influenza, 2011; 2.E; 43-57, World Health Organization
22. Commission of the European Committees: Harmonization of requirements for influenza vaccines EEC document III/3188/91-EN
23. Committee for Proprietary Medicinal Products (CPMP): Note for guidance on harmonization of requirements fi influenza vaccine (CPMP/BWP/214/96) march 12, 1997; 20-21
24. , Morbidity and Mortality Weekly Report: Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2020–21 influenza season, 2020; 69(8); 1-24
25. Patwarhan A, Ohler A, The flu vaccination may have a protective effect on the course of COVID-19 in the pediatric population: When does severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) meet influenza?: Cureus, 2021; 13(1); e12533
26. Green I, Ashkenazi S, Merzon E, The association of previous influenza vaccination and coronavirus disease-2019: HumVaccin Immunother, 2021; 17(7); 2169-75
27. Conlon A, Ashur C, Washer L, Impact of the influenza vaccine on COVID-19 infection rates and severity: Am J Infect Control, 2021; 49; 694-700
28. Amato M, Werba JP, Frigerio B, Relationship between influenza vaccination coverage rate and COVID-19 outbreak: An Italian ecological study: Vaccines, 2020; 8(3); 535
29. Zanettini C, Omar M, Dinalankara W, Influenza vaccination and COVID-19 mortality in the USA: medRxiv, 2020; 2020; 20129817
30. Debisarun PA, Struycken P, Dominguez-Andres J, The effect of influenza vaccination on trained immunity: Impact on COVID-19: medRxiv, 2020; 2020; 20212498
31. Brydak LB: Influenza – pandemic yesterday and today September 23–24, 2021, Warsaw, Medical University of Warsaw [in Polish]
Figures
 Figure. 1. The percentage of study subjects vaccinated against influenza in the 2019/2020 season in the 8 voivodeships included.
Figure. 1. The percentage of study subjects vaccinated against influenza in the 2019/2020 season in the 8 voivodeships included. Figure 2. The response factor is therefore similar to that found in older unvaccinated participants and in younger unvaccinated participants after vaccination.
Figure 2. The response factor is therefore similar to that found in older unvaccinated participants and in younger unvaccinated participants after vaccination. Figure 3. Response rate for anti-A/Kansas/14/2017 (H3N2) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 3. Response rate for anti-A/Kansas/14/2017 (H3N2) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. Figure 4. Response rate for anti-B/Colorado/06/2017 (B/Victoria lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 4. Response rate for anti-B/Colorado/06/2017 (B/Victoria lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. Figure 5. Response rate for anti-B/Phuket/3073/2013 (B/Yamagata lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season.
Figure 5. Response rate for anti-B/Phuket/3073/2013 (B/Yamagata lineage) antibodies by age of respondents and their influenza vaccination status in the 2019/2020 season. Tables
 Table 1. The most important characteristics of the studied influenza.
Table 1. The most important characteristics of the studied influenza. Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season.
Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season. Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences.
Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences. Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people).
Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people). Table 1. The most important characteristics of the studied influenza.
Table 1. The most important characteristics of the studied influenza. Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season.
Table 2. Strains of influenza viruses that are components of the vaccine for the 2019/2020 epidemic season. Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences.
Table 3. Mean antibody level (GMT) for the analyzed virus strains and the response rate with 95% confidence intervals in the study subjects divided into vaccinated and unvaccinated against influenza in the 2019/2020 season; statistical significance of observed differences. Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people).
Table 4. Comparison of parameters of 300 people aged 15–64 years from the 2019/2020 serological review (3 combined age groups) with those of COVID convalescents (934 people). In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








