13 July 2022: Clinical Research
Serum Calprotectin – a NET Product – as a Biomarker of Disease Activity in Patients with Systemic Lupus Erythematosus: A Single-Center Case-Control Study from Poland
Iwona Homa-Mlak1ACDEF*, Marcin Mazurek1BCEF, Aleksandra MajdanDOI: 10.12659/MSM.936534
Med Sci Monit 2022; 28:e936534
Abstract
BACKGROUND: Calprotectin (S100A8/A9 or myeloid-related protein 8/14) is a heterodimeric S100 complex expressed in leukocytes. Calprotectin participates in development of the inflammatory response by binding to receptors for advanced glycation end-products (RAGE) and Toll-like receptors (TLR). The clinical activity of systemic lupus erythematosus (SLE) is evaluated using the Systemic Lupus International Collaborating Clinics (SLICC) criteria and the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). This Polish single-center case-control study aimed to evaluate serum levels of calprotectin as a rapid diagnostic biomarker of SLE (59 patients with SLE were compared with 52 healthy controls).
MATERIAL AND METHODS: Calprotectin concentration was measured with the use of enzyme-linked immunosorbent assay (ELISA). The SLE activity of the patients was assessed by the SLEDAI scale. Statistical analysis of the results was carried out using MedCalc 15.8 software. P<0.05 was considered statistically significant.
RESULTS: A significantly higher concentration of calprotectin was found in the study group compared to the control group (medians: 3.11 vs 2.45 ng/ml; P=0.0013). We found that calprotectin has high sensitivity (89.83%) and specificity (53.85%) in differentiating between SLE patients and healthy volunteers. We found that calprotectin has very high sensitivity (100%) and specificity (82.46%) in detection of patients with moderate and severe SLE assessed using SLEDAI.
CONCLUSIONS: Consistent with previous studies, serum calprotectin level was revealed to have potential as a rapid diagnostic biomarker of disease activity in patients with SLE.
Keywords: Autoimmune Diseases, Lupus Erythematosus, Systemic, S-100 Calcium-Binding Protein Alpha Subunit, Calgranulin A, Case-Control Studies, Humans, Leukocyte L1 Antigen Complex, Poland, Severity of Illness Index
Background
Neutrophils are among the essential cells of the immune system. They play a key role in defending the body against pathogens by means of various mechanisms, including phagocytosis and degranulation of cytoplasmic granular proteins with bactericidal activity. The impaired neutrophil function makes the host susceptible to various types of pathogens, which can be life-threatening [1,2]. In addition to the above-mentioned mechanisms, neutrophils demonstrate their function by creating extracellular neutrophil traps (NETs) [1,3]. NETs are induced by reactive oxygen species (ROS) produced by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. This causes decondensation of chromatin induced by citrullinating peptidylarginine deiminase 4 (PAD-4) histones and the breakdown of intracellular membranes, which makes the mixing of nuclear and cytoplasmic components possible. Then the cytoplasmic membrane breaks and the NET structure is released [3–5]. The total NET composition is not yet thoroughly understood. It contains granular substances (eg, cathepsin G, myeloperoxidase, and lysozyme) derived from peroxisomes (catalase), glycolytic enzymes (alpha-enolase, transketolase), components of the cell nucleus (eg, histones H2A, H3, and H4), cytoplasm (S100A8, A9, and A12), and the cytoskeleton (eg, actin and plastin-2) [5].
NETs are a defense mechanism, but they can also exert toxic effects. In an inflammatory environment, they can stimulate an autoimmune reaction in the body by releasing autoantigens such as nucleic acids and proteins [3]. The proteins contained in NETs can constitute a target for the formation of immune complexes and autoantibodies, and they induce further NET formation, which results in positive feedback [3].
Calprotectin (S100A8/A9 or myeloid-related protein 8/14 – MRP8/14) is a heterodimeric S100 complex expressed in leukocytes [6]. It is released by phagocytes at the site of inflammation [7]. After being released from neutrophils and monocytes, it modulates the inflammatory response [8]. The extracellular calprotectin induces inflammation by binding to receptors for advanced glycation end-products (RAGE) and pattern recognition Toll-like receptors 4 (TLR4) located on the surface of leukocytes and endothelial cells [9]. This causes the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor (TNF-α) [9]. Recent studies indicate that calprotectin may be a useful biomarker in monitoring the activity of autoimmune diseases, including inflammatory bowel disease and rheumatoid arthritis (RA) [8].
Systemic lupus erythematosus (SLE) is an autoimmune multi-system disease. It is most common in women of childbearing age, but it is also increasingly prevalent in patients over the age of 40 years [10]. The concurrent inflammation can develop in various organs and the elevated level of autoantibodies against nuclear antigens (eg, anti-nuclear antibodies, ANA), double-stranded DNA (dsDNA), histones and proteins (eg, anti-thyroid peroxidase antibodies, and TPOAb) is also observed. In addition, in SLE patients excessive neutrophil activity is commonly observed, even at an early stage of the disease [11]. The EULAR/ACR 2019 criteria have a novel structure with use of anti-nuclear antibodies (ANA) as an (obligatory) entry criterion [12]. (Noninfectious) fever is the one new criterion. All criteria items now have individual weights (from 2 to 10) and are structured in domains, within which only the highest item is counted. There is one common attribution rule, counting criteria only if there is no more likely alternative explanation. Ten points are sufficient for maintaining the classification. The new criteria have reached a sensitivity of 96.1% and a specificity of 93.4%. Classification is not the same as diagnosis [13–15]. Diagnosis is not dependent on fulfilling classification criteria. All attempts to withhold therapies from SLE patients not fulfilling SLE classification criteria must be countered, since this would clearly be inappropriate and dangerous. Second, classification criteria are not meant for screening. They should only be employed if there is reason to believe a patient could have SLE. SLE is characterized by periods of remission and exacerbation. During exacerbations, the risk of premature death increases significantly, mainly due to infection or cardiovascular disorders. To measure disease activity in patients with SLE, certain clinical methods for evaluating disease activity can be used. One of them is SLEDAI-2K; the original version was introduced in 1985 [16,17]. In 2002, it was modified to reflect persistent active disease in those descriptors that had previously been considered new or recurrent occurrences (SLEDAI-2K) [17,18]. SLEDAI-2K is an index allowing for disease activity assessment and considers specific manifestations in 9 organs or systems that were noted in the last 10 days. In total 24 items are assessed: 8 items with 8 points each, 6 items with 4 points each, 7 items with 2 points each, and 3 items each with 1 point each. Therefore, patients can score from 0 to 105 points [18]. To capture those items of permanent change that has occurred in patients after a diagnosis of SLE, regardless of attribution, the Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology Damage Index (SDI) can be used [19,20]. It consists of 41 items covering 12 organ systems. Within each scale or system, there is a variable number of components (up to 6). Thirty-one items score 1 point each if present. Six items can score a maximum of 2 points each; 1 item can score a maximum of 3 points [20,21].
Therefore, this Polish single-center case-control study aimed to evaluate serum levels of calprotectin as a rapid diagnostic biomarker of SLE (52 healthy controls were compared with 59 patients with SLE treated in the Clinic of Rheumatology and Systemic Connective Tissue Diseases of the Medical University of Lublin).
Material and Methods
STATISTICAL ANALYSIS:
MedCalc 15.8 (MedCalc Software, Belgium) was used to perform statistical analysis. P<0.05 was considered statistically significant. Most scientific studies in medicine consider a P value of <0.05 as rejection of the null hypothesis, thus 0.05 was the value used for type I error (alpha). In the case of type II error (beta), we started with a cut-off of 0.2 to achieve 80% statistical power. The sample size calculation was based on previously published data. The calculations were based on a serum calprotectin concentration (expressed as means and corresponding standard deviations) comparison in SLE patients and healthy controls. Considering the difference in means and corresponding standard deviations, as well as the ratio of sample sizes in compared groups reported in the study quoted [13], the minimal number of subjects in our study was estimated to be 7 (4 in the study group and 3 in the control). Subsequently, considering that these are relatively small numbers, we decided to increase the power of the test to 99.999%. This change increased the sample size of the study group to 14 patients. Moreover, to achieve maximum reliability of obtained results, we decided to enlarge the study group by about 400%. Thus, we estimated that at least 56 subjects should be included in the study group. Quantitative variables and some demographic and clinical factors used to describe the study and control groups are presented by means of descriptive statistics: measures of central tendency and dispersion (median, range). Due to the non-normal distribution of the data in the case of analyzed continuous variables (tested with the use of the D’Agostino-Pearson test), in further analyses we used non-parametric tests. The Spearman rank correlation test was used to assess the correlation of calprotectin concentration and selected demographic, clinical and laboratory factors (continuous variables). Using the Mann-Whitney U test, analysis of the difference in the concentration of calprotectin in the control and the study group was performed, as well as within some subgroups of patients, in relation to the selected demographic and clinical factors: duration of disease or SLE degree of progression according to SLEDAI. The analysis based on ROC curves was used to assess the diagnostic utility of determining the level of calprotectin in SLE detection and in differentiation between specific clinical conditions.
Results
STUDY AND CONTROL GROUP CHARACTERISTICS:
The study group consisted of 59 patients, including 51 women (86.44%) and 8 men (13.56%). The median age in the study group was 36 years (range 19–72 years). SLE activity was assessed using SLEDAI; 86.4% of patients were classified as having mild SLE. In turn, 10.2% of patients had moderate SLE, while 3.4% had active SLE (Table 1). A panel of laboratory tests was performed on all patients and differences in calprotectin values in relation to morphological and biochemical factors were examined. The control group consisted of 52 healthy volunteers matched with respect to age (median 38 years, range 22–64 years) and sex (84.62% women and 15.38% men) (Table 1).
DIFFERENCES IN CALPROTECTIN CONCENTRATION IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS IN RELATION TO DEMOGRAPHIC AND CLINICAL FACTORS:
We examined the serum concentration of calprotectin in patients depending on sex, age, and duration and severity of disease. The level of calprotectin was not significantly associated with sex, age, duration of the disease, or its activity. A statistically significant higher serum calprotectin concentration was found in the study group as compared to the control (medians: 3.11 vs 2.45 ng/ml; P=0.0013) (Table 2).
THE ASSESSMENT OF THE DIAGNOSTIC USEFULNESS OF THE CALPROTECTIN IN THE DETECTION OF SYSTEMIC LUPUS ERYTHEMATOSUS AND SPECIFIC CLINICAL CONDITIONS USING ROC CURVE ANALYSIS:
We examined the assessment of the diagnostic usefulness of the calprotectin in the detection of systemic lupus erythematosus and specific clinical conditions. The calprotectin was characterized with 89.83% sensitivity and 53.85% specificity in the differentiation of patients with SLE and healthy people (cut-off >2.45, AUC=0.720, 95% CI=0.612–0.812 p=0.0001) (Figure 1, Table 3). The calprotectin was characterized with 100% sensitivity and 82.46% specificity in the detection of patients with moderate and severe SLE assessed using SLEDAI (cut-off ≤2.37, AUC=0.842, 95% CI=0.724–0.924; p<0.0001) (Figure 2, Table 3). The calprotectin was characterized with 100% sensitivity and 59.09% specificity in the detection of patients with elevated level of IgA antibodies (cut-off >3090, AUC=0.716, 95% CI=0.507–0.874; p=0.0296) (Figure 3, Table 3).
ASSESSMENT OF CORRELATION BETWEEN LEVEL OF CALPROTECTIN AND DEMOGRAPHIC AND CLINICAL FACTORS IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS:
We examined the correlation between the level of calprotectin and demographic and clinical factors in patients with SLE. A statistically significant but weak negative correlation was found between the level of calprotectin and the concentration of complement component 3 (C3) protein (rho=−0.429, P=0.0012; Figure 4, Table 4). A statistically significant but weak positive correlation was noted between the level of calprotectin and dsDNA (rho=0.325, P=0.0277; Figure 5, Table 4).
DIFFERENCES IN CALPROTECTIN CONCENTRATION IN RELATION TO CLINICAL MANIFESTATION OF SYSTEMIC LUPUS ERYTHEMATOSUS:
We examined the serum concentration of calprotectin depending on clinical manifestation of the disease. Only 1 result was statistically significant – higher serum calprotectin concentration was found in the SLE patients with myopathy as compared to the study group without myopathy (medians: 3.94 vs 3.09 ng/ml; P=0.0277) (Table 5).
Discussion
Neutrophils play an important role in the pathogenesis of autoimmune diseases (including SLE) and the associated organ damage. Cell death in the NET mechanism can be immunostimulatory, leading to activation of innate immune mechanisms. The elements of NETs have the ability to activate pDC (plasmacytoid dendritic cells), which can promote further activation of the immune system and increase INF type I synthesis, which is characteristic for SLE. The above mechanism may explain why infections are a common cause of SLE exacerbations [10,11,23,24]. One of the hypotheses is also based on the assertion that, in patients with SLE, the process of NET elements degradation is slower than in healthy people [25]. Hakkim et al indicated that the identification of patients in whom the degradation of NET elements is impaired can be effective in identifying patients who may be at risk of developing nephritis [25]. Leffler et al showed that the inability to degrade NETs was correlated with increased SLE activity (assessed using SLEDAI), and lower levels of C3 and C4 proteins. In addition, pleurisy and kidney involvement were more common in these patients [26]. In another study, Leffler et al confirmed the former study results and demonstrated that the reduced ability to degrade NETs was associated with increased disease activity assessed using SLEDAI, with the development of nephritis, increased dsDNA, and low C3 [27]. Slow degradation of NET elements may be associated with SLE pathogenesis and clinical symptoms. Potentially, drugs that promote faster degradation of NET elements may be effective in some SLE patients [25–27].
According to the available scientific reports, calprotectin was identified as one of the main components of NETs [5]. We noted higher calprotectin concentration in the study group as compared to the control. Moreover, we found, that calprotectin has high sensitivity (89.83%) and specificity (53.85%) in differentiating between SLE patients and healthy volunteers. We found that calprotectin has very high sensitivity (100%) and specificity (82.46%) in the detection of patients with moderate and severe SLE assessed using SLEDAI. We also performed basic morphological and biochemical tests on our patients, which are used for diagnosis and monitoring of the disease. We noted a statistically significant but weak negative correlation between the level of calprotectin and the concentration of complement component 3 (C3) protein. We also found a statistically significant but weak positive correlation between the level of calprotectin S100A8 protein and dsDNA.
The increased concentration of this protein is observed in SLE as well as in other autoimmune diseases, such as psoriasis and rheumatoid arthritis [3,28–30]. Significantly higher levels of calprotectin were detected in patients with SLE. Sumova et al evaluated the levels of S100A4, S100A8/9, and S100A12 proteins in 44 patients with SLE and 43 healthy volunteers. In the case of calprotectin, they obtained similar results and demonstrated a significantly higher level of calprotectin in patients with SLE [9]. Soyfoo et al also showed that serum calprotectin level were significantly higher in patients with SLE compared to healthy controls [24]. Moreover, Haga also showed a higher concentration of calprotectin in patients with SLE (n=100) compared to healthy controls (n=80) (3661.25 μg/l vs 1050.83 μg/l;
The limitations of the study include a relatively small study group and its heterogeneity in terms of disease exacerbation (assessed according to SLEDAI). To confirm our results, further research is needed. Other limitations include the single-center design and the observational nature of the study. Moreover, we did not analyze the correlation between calprotectin and some other supplementary laboratory parameters used in the diagnostics of SLE.
Studies using animal models of such autoimmune diseases as multiple sclerosis, SLE, RA, and inflammatory bowel disorders have shown that low molecular weight inhibitors of calprotectin lead to reduction in disease activity due to blocking of this particle [7]. NETs are recognized as being involved in the pathogenesis of autoimmune diseases. This information may help researchers to develop new therapies and establish new markers of disease development, assessment of its stage of progression, and the monitoring of treatment [3,32].
Conclusions
Patients with SLE demonstrate significantly higher serum calprotectin level as compared to healthy people. Calprotectin correlates with known markers of SLE exacerbation. Consistent with previous studies, serum calprotectin level was revealed to have potential as a rapid diagnostic biomarker of disease activity in patients with SLE.
Figures
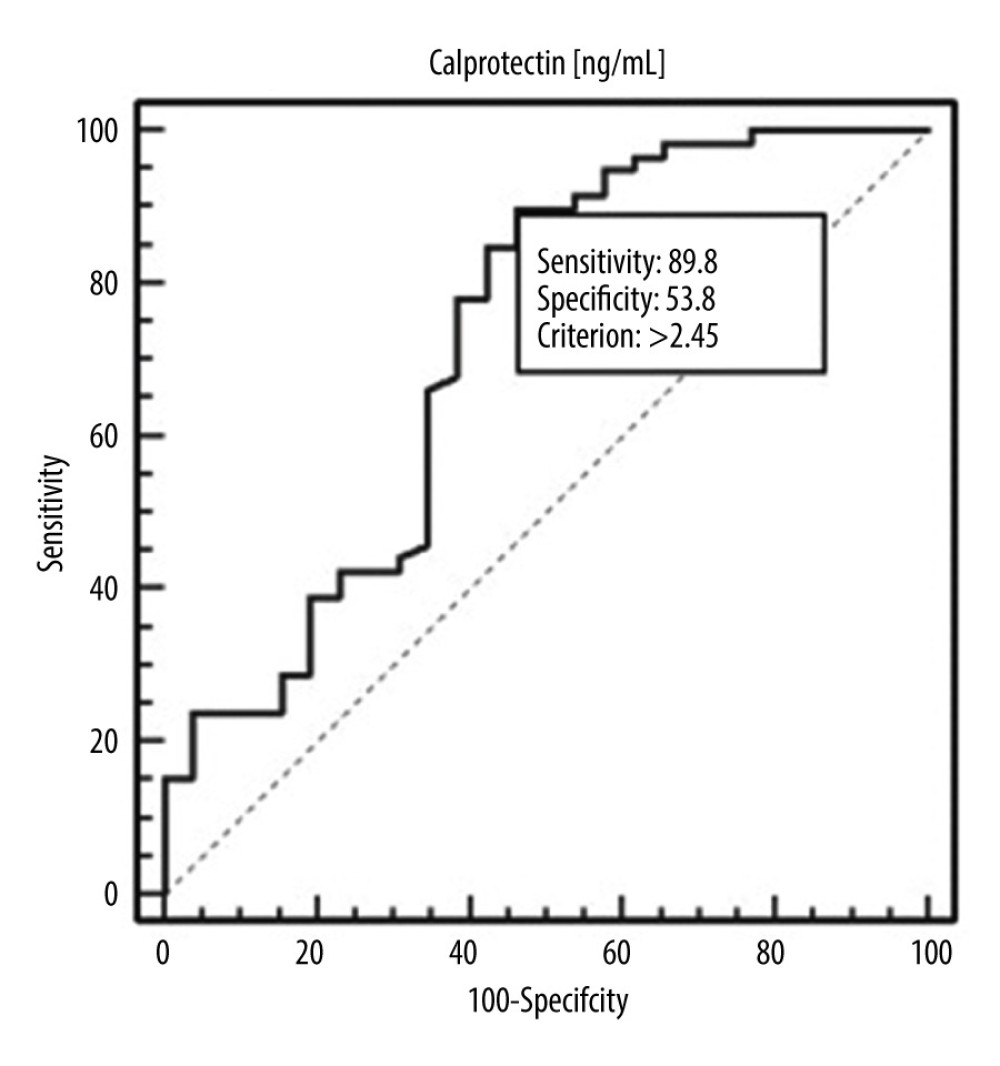 Figure 1. Receiver operating characteristic (ROC) curve – diagnostic utility of calprotectin concentration in detection of systemic lupus erythematosus (SLE).
Figure 1. Receiver operating characteristic (ROC) curve – diagnostic utility of calprotectin concentration in detection of systemic lupus erythematosus (SLE). 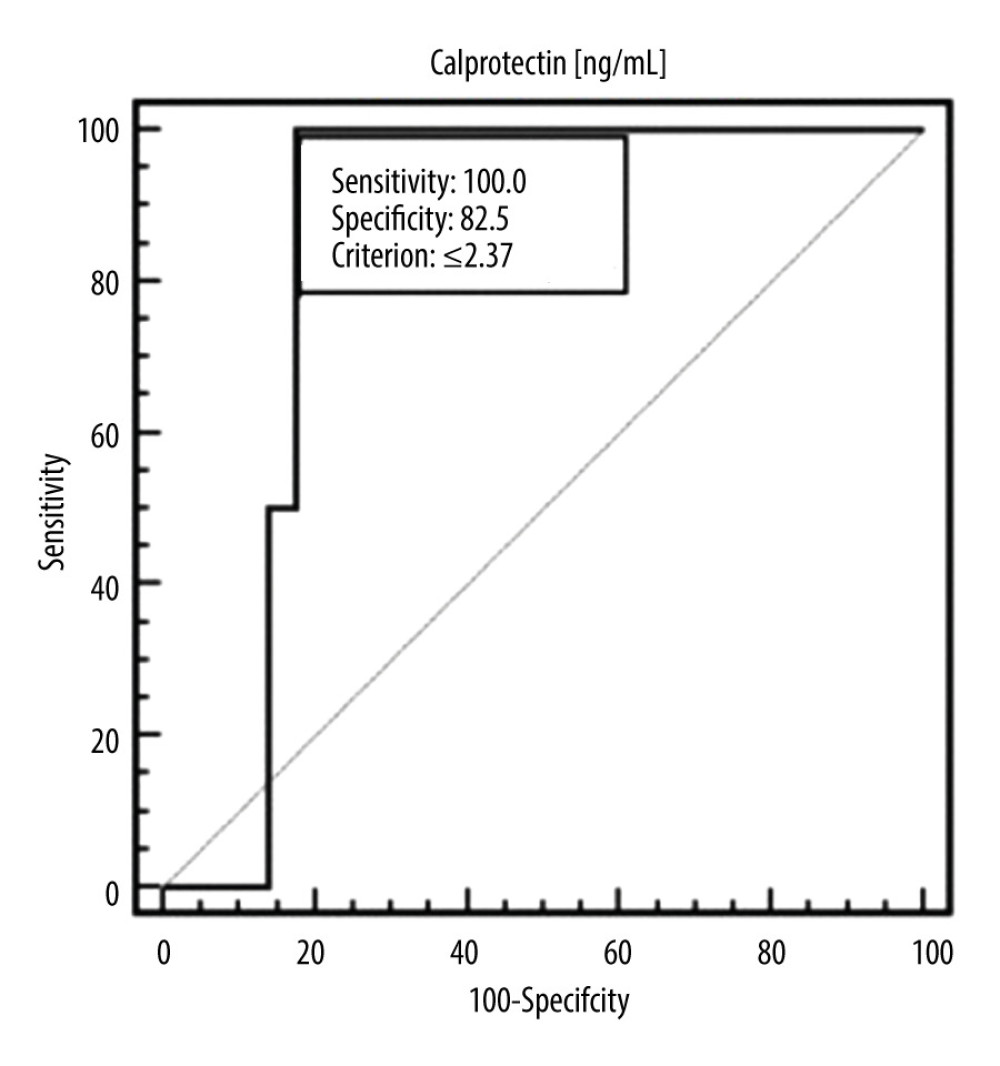 Figure 2. ROC curve – diagnostic utility of calprotectin concentration in detection of moderate or severe SLE (according to SLEDAI).
Figure 2. ROC curve – diagnostic utility of calprotectin concentration in detection of moderate or severe SLE (according to SLEDAI). 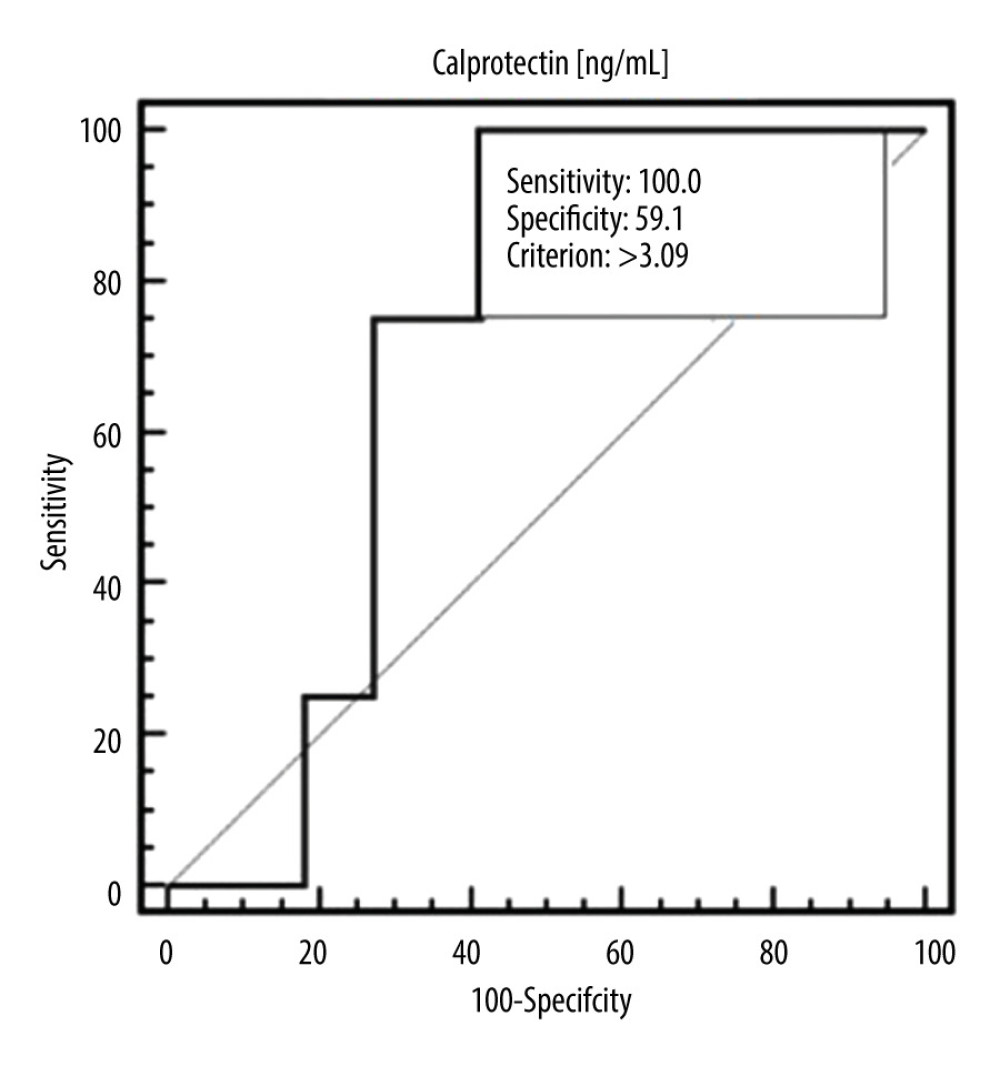 Figure 3. ROC curve – diagnostic utility of calprotectin concentration in detection of SLE with elevated level of immunoglobulin A (IgA) antibodies.
Figure 3. ROC curve – diagnostic utility of calprotectin concentration in detection of SLE with elevated level of immunoglobulin A (IgA) antibodies. 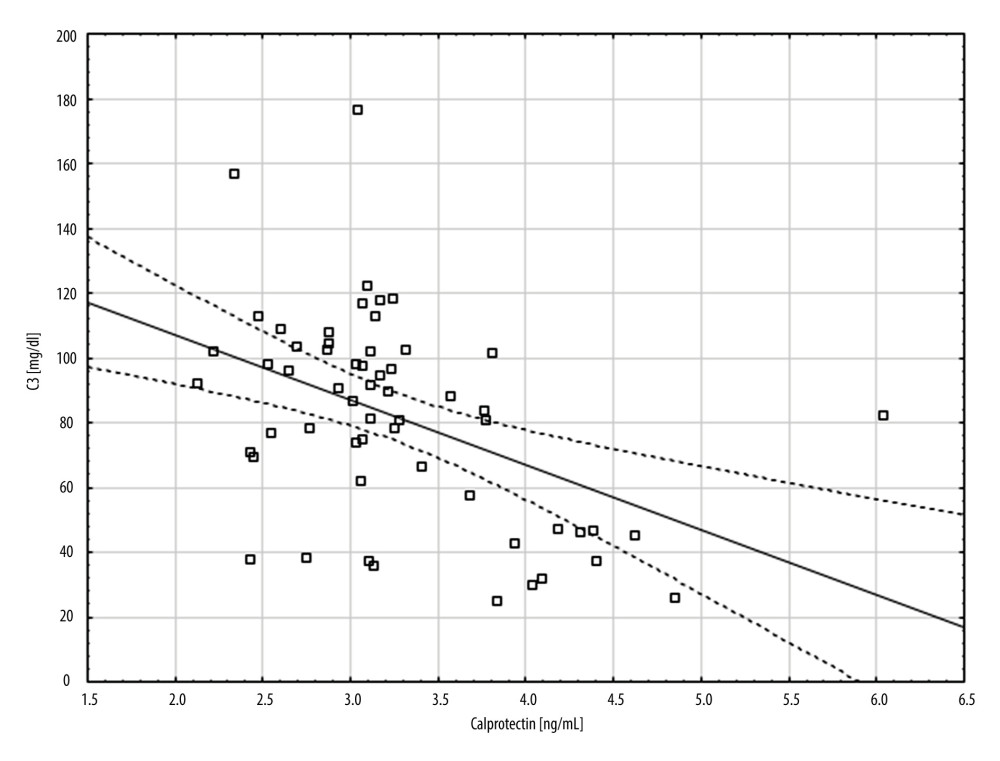 Figure 4. A scatter plot showing the correlation between complement component 3 (C3) (mg/dl) and calprotectin concentration (ng/ml).
Figure 4. A scatter plot showing the correlation between complement component 3 (C3) (mg/dl) and calprotectin concentration (ng/ml). 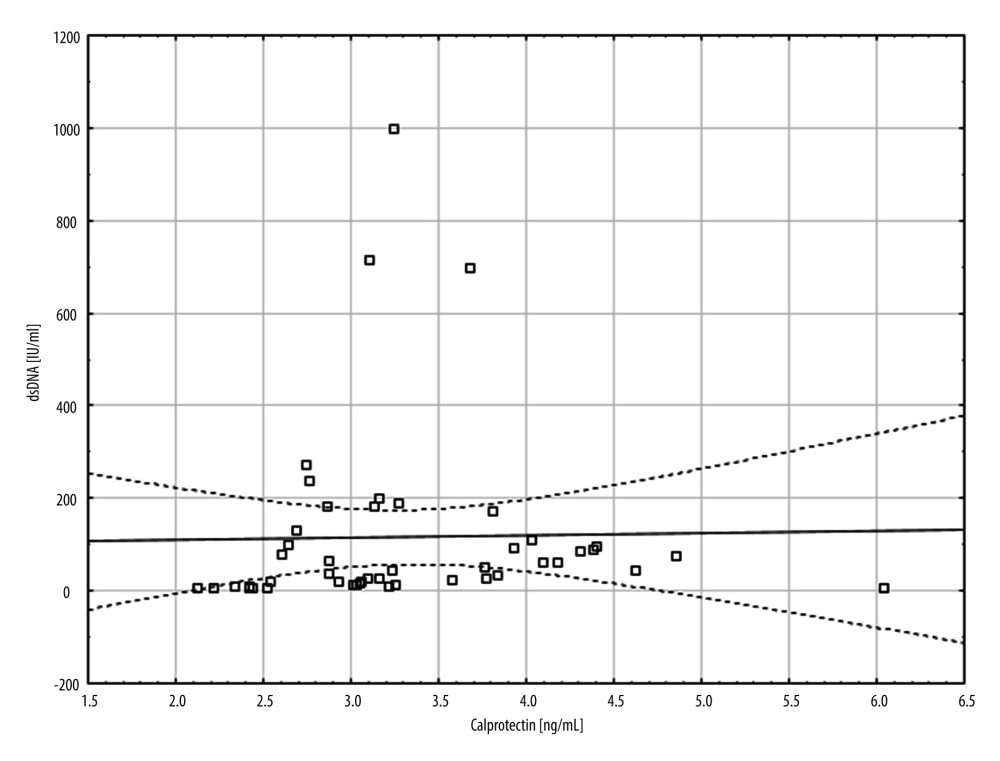 Figure 5. A scatter plot showing the correlation between double-stranded DNA (dsDNA) (IU/ml) and calprotectin concentration (ng/ml).
Figure 5. A scatter plot showing the correlation between double-stranded DNA (dsDNA) (IU/ml) and calprotectin concentration (ng/ml). Tables
Table 1. General characteristic of the study group.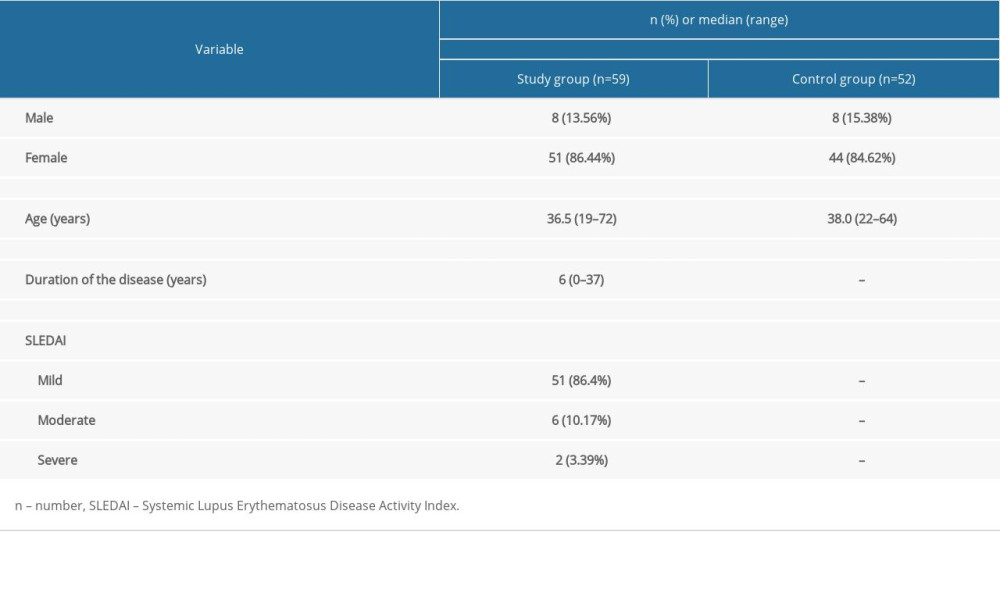 Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group.
Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group.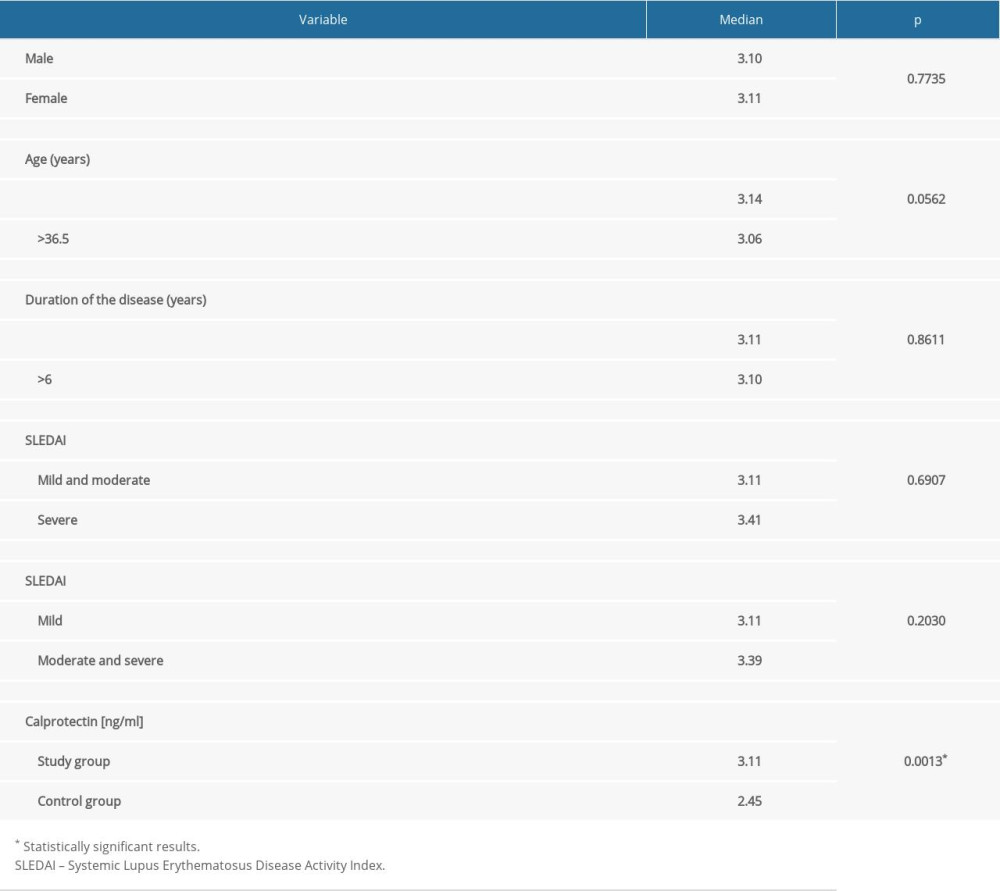 Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis.
Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis.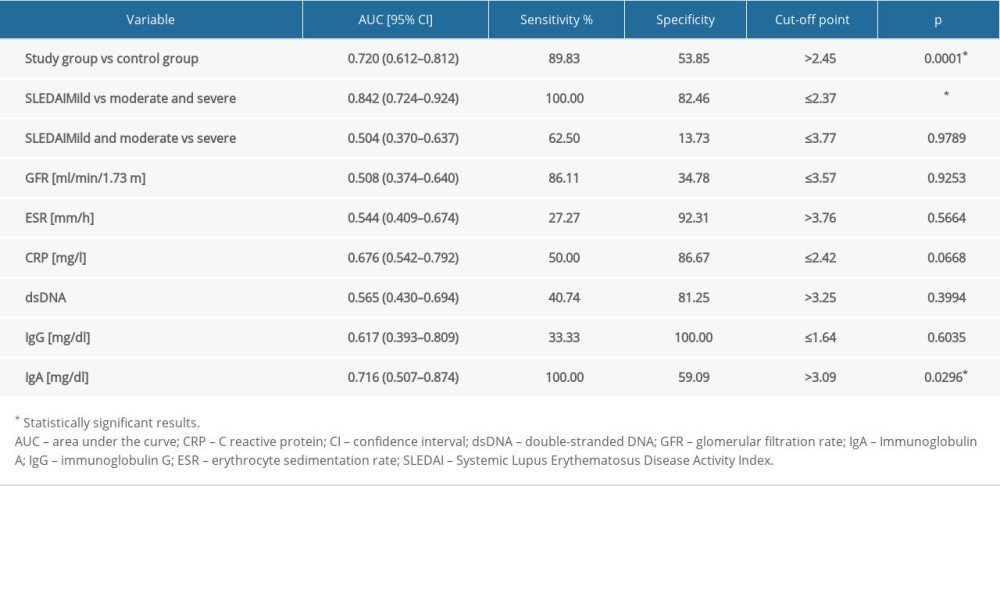 Table 4. Correlation between selected clinical and laboratory factors and calprotectin.
Table 4. Correlation between selected clinical and laboratory factors and calprotectin.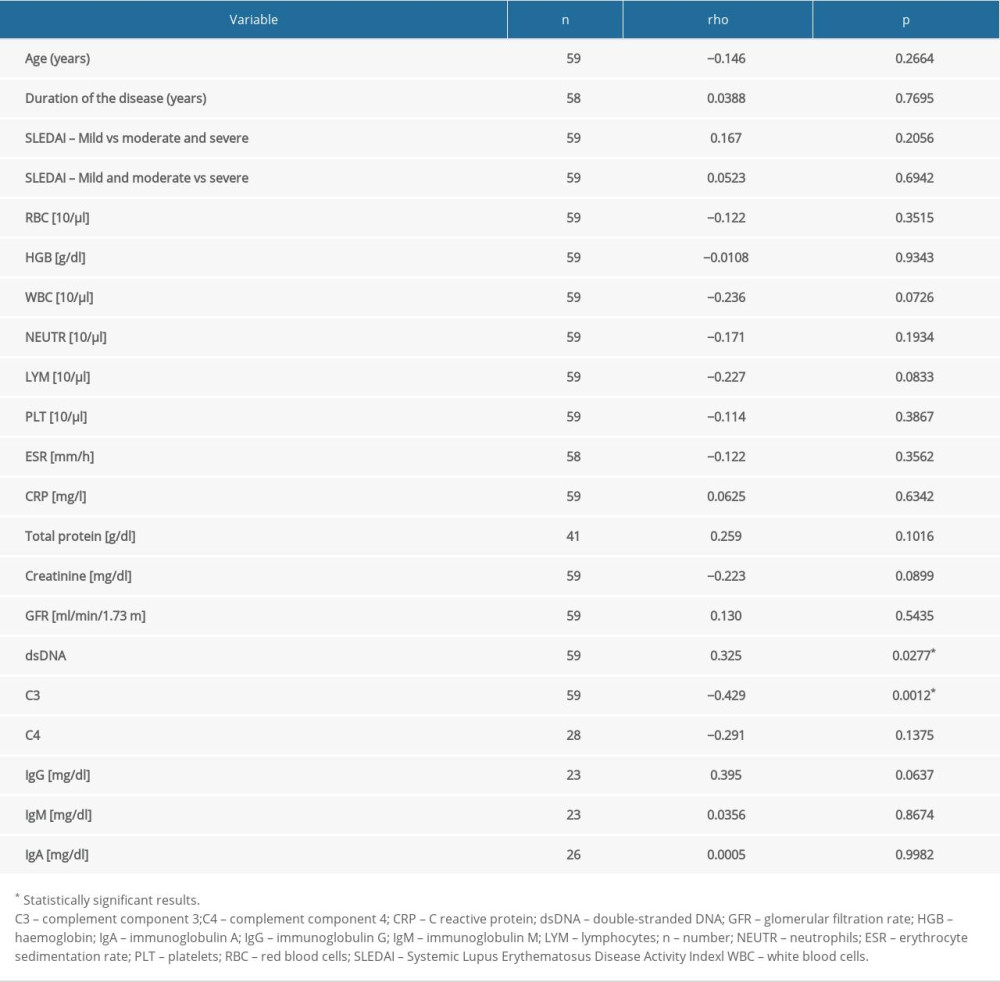 Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group.
Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group.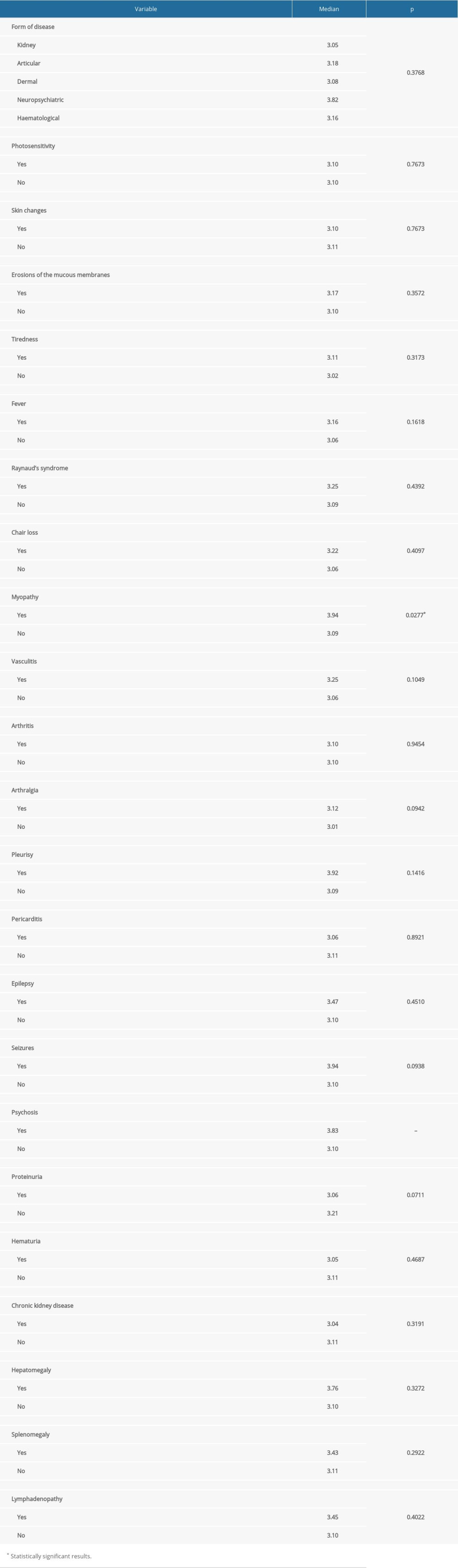
References
1. Brinkmann V, Reichard U, Goosmann C, Neutrophil extracellular traps kill bacteria: Science, 2004; 303(5663); 1532-35
2. Grayson PC, Kaplan MJ, At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases: J Leukoc Biol, 2016; 99(2); 253-64
3. Barnado A, Crofford LJ, Oates JC, At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases: J Leukoc Biol, 2016; 99(2); 265-78
4. Fuchs TA, Abed U, Goosmann C, Novel cell death program leads to neutrophil extracellular traps: J Cell Biol, 2007; 176(2); 231-41
5. Urban CF, Ermert D, Schmid M, Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans: PLoS Pathog, 2009; 5(10); e1000639
6. Kopeć-Mȩdrek M, Widuchowska M, Kucharz EJ, Calprotectin in rheumatic diseases: A review: Reumatologia, 2016; 54(6); 306-9
7. Lood C, Stenström M, Tydén H, Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus: Arthritis Res Ther, 2011; 13(2); R60
8. Pruenster M, Vogl T, Roth J, Sperandio M, S100A8/A9: From basic science to clinical application: Pharmacol Ther, 2016; 167; 120-31
9. Šumová B, Cerezo LA, Szczuková L, Circulating S100 proteins effectively discriminate SLE patients from healthy controls: A cross-sectional study: Rheumatol Int, 2019; 39(3); 469-78
10. Gordon C, Amissah-Arthur M-B, Gayed M, The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults: Rheumatology (Oxford), 2018; 57(1); e1-45
11. Tydén H, Lood C, Gullstrand B, Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus: Lupus, 2017; 26(2); 139-49
12. Aringer M, Costenbader K, Daikh D, 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus: Arthritis Rheumatol (Hoboken, NJ), 2019; 71(9); 1400-12
13. Aringer M, Dörner T, Leuchten N, Johnson SR, Toward new criteria for systemic lupus erythematosus – a standpoint: Lupus, 2016; 25(8); 805-11
14. Johnson SR, Goek ON, Singh-Grewal D, Classification criteria in rheumatic diseases: A review of methodologie properties: Arthritis Care Res, 2007; 57(7); 1119-33
15. Aringer M, Costenbader KH, Dörner T, Johnson SR, Response to: Do the 2019 EULAR/ACR SLE classification criteria close the door on certain groups of SLE patients?’ by Chi et al: Ann Rheum Dis, 2021; 80(8); 2021
16. Uribe AG, Vilá LM, McGwin GJ, The systemic lupus activity measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus: J Rheumatol Oct, 2004; 31(10); 1934-40
17. Jaffe RB, Combined oral contraceptives in women with systemic lupus erythematosus: Commentary: Obstet Gynecol Surv, 2006; 61(4); 251-53
18. Sánchez-Guerrero J, Uribe AG, Jiménez-Santana L, A trial of contraceptive methods in women with systemic lupus erythematosus: N Engl J Med, 2005; 353(24); 2539-49
19. Gladman D, Ginzler E, Goldsmith C, The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus: Arthritis Rheum, 1996; 39(3); 363-69
20. Stoll T, Seifert B, Isenberg DA, SLICC/ACR damage index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus: Br J Rheumatol, 1996; 35(3); 248-54
21. Rahman P, Gladman DD, Urowitz MB, Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus: Lupus, 2001; 10(2); 93-96
22. https://www.fn-test.com/product/eh1933/
23. Banchereau J, Pascual V, Type I interferon in systemic lupus erythematosus and other autoimmune diseases: Immunity, 2006; 25(3); 383-92
24. Soyfoo MS, Roth J, Vogl T, Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus: J Rheumatol, 2009; 36(10); 2190-94
25. Hakkim A, Fürnrohr BG, Amann K, Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis: Proc Natl Acad Sci USA, 2010; 107(21); 9813-18
26. Leffler J, Gullstrand B, Jönsen A, Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus: Arthritis Res Ther, 2013; 15(4); R84
27. Leffler J, Martin M, Gullstrand B, Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease: J Immunol, 2012; 188(7); 3522-31
28. Geven EJW, van den Bosch MHJ, Di Ceglie I, S100A8/A9, a potent serum and molecular imaging biomarker for synovial inflammation and joint destruction in seronegative experimental arthritis: Arthritis Res Ther, 2016; 18(1); 247
29. Lee KH, Kronbichler A, Park DD-Y, Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review: Autoimmun Rev, 2017; 16(11); 1160-73
30. van Lent PLEM, Blom AB, Schelbergen RFP, Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis: Arthritis Rheum, 2012; 64(5); 1466-76
31. Haga HJ, Brun JG, Berntzen HB, Calprotection in patients with systemic lupus erythematosus: Relation to clinical and laboratory parameters of disease activity: Lupus, 1993; 2(1); 47-50
32. Frangou E, Vassilopoulos D, Boletis J, Boumpas DT, An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment: Autoimmun Rev, 2019; 18(8); 751-60
Figures
 Figure 1. Receiver operating characteristic (ROC) curve – diagnostic utility of calprotectin concentration in detection of systemic lupus erythematosus (SLE).
Figure 1. Receiver operating characteristic (ROC) curve – diagnostic utility of calprotectin concentration in detection of systemic lupus erythematosus (SLE). Figure 2. ROC curve – diagnostic utility of calprotectin concentration in detection of moderate or severe SLE (according to SLEDAI).
Figure 2. ROC curve – diagnostic utility of calprotectin concentration in detection of moderate or severe SLE (according to SLEDAI). Figure 3. ROC curve – diagnostic utility of calprotectin concentration in detection of SLE with elevated level of immunoglobulin A (IgA) antibodies.
Figure 3. ROC curve – diagnostic utility of calprotectin concentration in detection of SLE with elevated level of immunoglobulin A (IgA) antibodies. Figure 4. A scatter plot showing the correlation between complement component 3 (C3) (mg/dl) and calprotectin concentration (ng/ml).
Figure 4. A scatter plot showing the correlation between complement component 3 (C3) (mg/dl) and calprotectin concentration (ng/ml). Figure 5. A scatter plot showing the correlation between double-stranded DNA (dsDNA) (IU/ml) and calprotectin concentration (ng/ml).
Figure 5. A scatter plot showing the correlation between double-stranded DNA (dsDNA) (IU/ml) and calprotectin concentration (ng/ml). Tables
 Table 1. General characteristic of the study group.
Table 1. General characteristic of the study group. Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group.
Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group. Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis.
Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis. Table 4. Correlation between selected clinical and laboratory factors and calprotectin.
Table 4. Correlation between selected clinical and laboratory factors and calprotectin. Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group.
Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group. Table 1. General characteristic of the study group.
Table 1. General characteristic of the study group. Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group.
Table 2. Differences in calprotectin value depending on demographic and clinical factors in the study group. Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis.
Table 3. Evaluation of diagnostic usefulness of calprotectin in detection of systemic lupus erythematosus (SLE) and specific clinical conditions by means of receiver operating characteristic (ROC) curve analysis. Table 4. Correlation between selected clinical and laboratory factors and calprotectin.
Table 4. Correlation between selected clinical and laboratory factors and calprotectin. Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group.
Table 5. Differences in calprotectin value depending on clinical manifestation of SLE in the study group. In Press
05 Mar 2024 : Clinical Research
Muscular Function Recovery from General Anesthesia in 132 Patients Undergoing Surgery with Acceleromyograph...Med Sci Monit In Press; DOI: 10.12659/MSM.942780
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








