22 May 2022: Clinical Research
A Retrospective Study from a Single Center of 208 Patients with Unilateral Chronic Subdural Hematoma to Compare Outcomes Following Burr Hole Craniotomy and Hematoma Drainage Within 48 Hours and Between 48 Hours and 5 Days
In-Hyoung LeeDOI: 10.12659/MSM.936774
Med Sci Monit 2022; 28:e936774
Abstract
BACKGROUND: This retrospective study from a single center aimed to compare patient outcomes following burr hole craniotomy (BHC) and hematoma drainage within 48 hours and between 48 hours and 5 days in 208 patients with unilateral chronic subdural hematoma (CSDH).
MATERIAL AND METHODS: Patients were divided into 2 groups according to the duration of drainage: early (1 or 2 days, n=100) and late (3-5 days, n=108) drain removal. We compared the clinical outcomes, recurrence rate, time to recurrence, and complications between the groups. Quantitative analysis of computed tomography parameters at various time points (postoperative, day of drain removal, and postoperative day 7) was also performed to compare radiologic outcomes.
RESULTS: Clinical outcomes and recurrence rate were similar in both groups. We found no significant differences in radiologic outcomes of both groups through all chronologies, although the total drainage volume was significantly greater in the late removal group (P<0.001). The incidence of surgical complications was significantly higher in the late drain removal group (8% vs 22.2%, P=0.007). Specifically, contralateral subdural effusion (CSE) tended to occur more frequently in the late removal group (2% vs 8.3%, P=0.038).
CONCLUSIONS: Following BHC and hematoma drainage for unilateral CSDH, maintaining the subdural drain for more than 48 hours did not improve patient outcomes or reduce the recurrence of hematoma, but increased the surgical complications, including CSE.
Keywords: Drainage, chronic subdural hematoma, Postoperative Complications, Subdural Effusion, Treatment Outcome, Craniotomy, Hematoma, Subdural, Chronic, Humans, Trephining
Background
Chronic subdural hematoma (CSDH) is commonly encountered by neurosurgeons in a clinical setting, and its incidence is significantly higher in the elderly [1,2]. With the recent progressive aging of the population, its incidence has been markedly increased [3,4].
Previous studies have proposed surgical evacuation with burr hole craniotomy (BHC) and closed-system drainage as the mainstay of treatment for patients with symptomatic CSDH [5,6]. If adequately treated with surgery, CSDH has a favorable prognosis as a benign disease [7,8]. However, it remains problematic that it recurs at a higher incidence of 5–33%, thereby warranting repeated surgical evacuations [6–9]. Repeated surgeries are associated with a risk of complications, posing a challenge for neurosurgeons. Therefore, it is necessary to reduce the risk of postoperative complications and recurrence, which are inevitable in treating CSDH.
Risk factors for CSDH recurrence include patient-related and radiologic factors [8–10]. However, there is a paucity of previous literature on surgery-related risk factors; only the lack of closed-system drainage has been described as a possible risk factor, and other parameters remain controversial [10–13]. Few studies have clarified the relationship between the duration of postoperative drainage and treatment outcomes and recurrence [14,15]. Moreover, no quantitative studies have assessed the effects of the duration of catheter placement on radiological parameters, and there is no established consensus on the ideal drainage duration.
Therefore, this retrospective study from a single center aimed to compare patient outcomes following BHC and hematoma drainage within 48 hours and between 48 hours and 5 days in 208 patients with unilateral CSDH by quantitative analysis of computed tomography (CT) parameters at various time points (postoperative, day of drain removal, and postoperative day 7) and comprehensive evaluation of the clinical outcomes, recurrence rate, time to recurrence, and postoperative complications.
Material and Methods
PATIENT ENROLLMENT:
This current study was approved by the Institutional Review Board of our institution (HKS 2021-12-013), and the requirement for informed consent was waived due to the study’s retrospective design. We retrospectively analyzed a consecutive series of the patients with CSDH who underwent surgical treatment at our institution between January 2016 and December 2020.
The exclusion criteria were as follows: (1) bilateral CSDHs or contralateral subdural effusion (CSE); (2) previous drainage of ipsilateral CSDH (recurrent cases); (3) underwent intracranial operation within 6 months prior to admission; (4) CSDH caused by cerebrospinal fluid (CSF) shunt or arachnoid cyst; (5) age <18 years; (6) BHC with no drain placement or subperiosteal drain placement because of intraoperative definite brain re-expansion; (7) postoperative drain duration <24 hours; and (8) small craniotomy performed because of the presence of multiple separated membranes.
Eight patients who were lost to follow-up or lacked appropriate follow-up imaging and 2 patients who underwent craniotomy due to acute intracranial hemorrhage (AIH) in the postoperative stage were excluded from the study. In addition, 10 patients who presented with persistent initial clinical symptoms 24 hours after surgical treatment were excluded because early drain removal might not be sufficient to remove the residual hematoma. Thus, this study was conducted only on patients who showed symptom improvement within 24 hours after surgical treatment, suggesting that increased intracranial pressure (ICP) was sufficiently resolved by the surgical procedure.
Finally, 208 eligible patients with primary unilateral CSDH who underwent BHC with subdural drain insertion were included in the current study. The patients were divided into 2 groups based on the duration of drain placement: early (1 or 2 days, n=100) and late (3–5 days, n=108) drain removal groups (Figure 1).
BASELINE DATA COLLECTION:
We retrieved the following baseline medical variables and surgical data of the patients from the electronic medical database: age and sex; platelet count and coagulation time in the blood test; pre-existing comorbidities; a history of using antiplatelet and/or anticoagulant agents; a history of sustaining a recent head trauma (within 3 months); preoperative clinical symptoms; patients’ preoperative Glasgow Coma Scale (GCS), modified Rankin scale (mRS) score on admission, single or double burr hole trephination, duration of drain placement, and total drainage volume.
QUANTITATIVE ANALYSIS OF THE RADIOLOGIC PARAMETERS:
All CT data were assessed independently by 2 neurosurgeons and 1 neuroradiologist, all of whom were blinded to patients’ details. The following baseline radiological parameters based on preoperative CT were identified: hematoma laterality, hematoma density appearance, maximal hematoma thickness (HT), midline shift (MLS), and hematoma volume. Hematoma density was classified based on the difference in density between brain tissue and the hematoma (hypodense, isodense, hyperdense, or mixed density) [11]. HT, MLS, hematoma volume, and volume of trapped air were measured based on followed up CT scans performed in all patients immediately after surgery, the day of subdural drainage catheter removal, and postoperative day 7.
Volumetric analysis was performed for both the hematoma and trapped air using INFINITT PACS M6 software (INFINITT Healthcare Co., Ltd., Seoul, Korea). The two-dimensional area of the hematoma and postoperative pneumocephalus were manually outlined and then calculated in each image cut and multiplied by the thickness between the image cuts (5 mm). Thus, the volume of the hematoma and trapped air was obtained. The total volume of the hematoma and trapped air was calculated by summing the volume of each CT slice. In addition, we defined the depressed brain volume as the sum of the hematoma and trapped air volume. CSE is defined as a newly emerging fluid collection on the contralateral subdural area with a CT density equal to that of the CSF [16].
The quantitative measurements of radiological parameters are summarized in Figure 2.
SURGICAL TREATMENT AND POSTOPERATIVE MANAGEMENT:
All the patients were surgically treated using a single or double BHC, according to the surgeon’s preference. A single BHC is commonly employed at our institution. The hematoma was evacuated, followed by irrigation of the subdural cavity with warm saline until the drained fluid was clear. One 10.5 French subdural drainage catheter tube (Yushin Medical, Seoul, Korea) was inserted into the subdural space toward the frontal direction. It was connected to a closed drainage system and passive drainage was performed using natural pressure gradients. The duration of drain tube placement was determined according to the surgeon’s experience and preference, but it did not exceed 5 days. Mobilization was restricted during the maintenance period of the closed drainage system. Antiplatelet or anticoagulant agents were discontinued upon diagnosis in all patients. They usually resumed variable timing (2–4 weeks) postoperatively according to the patients’ status, not standardized [17]. Additionally, although the patients received single-dose prophylactic intravenous antibiotics postoperatively, no prophylactic antiepileptic medication was administered.
PATIENT EVALUATION AND DEFINITION OF CSDH RECURRENCE:
Clinical outcome measures included postoperative medical and surgical complications, length of hospital stay, postoperative GCS, degree of improvement in GCS, mRS scores at discharge and 3 and 6 months postoperatively, and mortality. We recorded the patients’ clinical outcomes in the outpatient department at least 6 months after surgery. During follow-up, serial CT scans were taken when there was a persistent presence or recurrence of initial symptoms or the onset of new neurologic deterioration.
“CSDH recurrence” is defined as the re-accumulation of ipsilateral hematoma confirmed on follow-up CT scans within 6 months postoperatively without additional head trauma, causing neurological deficits requiring reoperation [11]. We also recorded the time interval from initial surgery to diagnosis of recurrence.
STATISTICAL ANALYSIS:
Continuous variables are expressed as mean±standard deviations (range), and categorical variables as numbers (percentages). Univariate analysis was performed to compare baseline characteristics, outcomes, recurrence, and complications between the 2 groups, for which the independent
Results
COMPARISON OF THE CLINICAL AND RADIOLOGIC OUTCOMES:
Clinical outcomes of each group are summarized in Table 2. During the 6-month follow-up period, there were no significant differences in the clinical outcomes between the 2 groups (P>0.05).
A comparison of the radiological parameters between the 2 groups at various time points is presented in Table 3 and Figure 3. No significant differences in radiologic outcomes were identified between the groups (P>0.05).
RECURRENCE RATE AND TIME TO RECURRENCE ACCORDING TO DRAINAGE DURATION:
In our study, 26 patients experienced recurrences. After excluding the 5 deaths, the overall recurrence rate was 12.81%, which was comparable between the groups (12.3% vs 13.3%, P=0.818). The mean time interval from initial surgery to diagnosis of recurrence was 27.5±25.9 days; there was no significant difference with regard to the duration of drain placement (25.5±23.9 vs 29.2±27.4 days, P=0.728) (Figure 4).
POSTOPERATIVE COMPLICATIONS:
The incidence of postoperative complications is shown in Table 4. Overall, surgical complications were significantly higher in the late drain removal group than in the early drain removal group (P=0.007). In particular, CSE tended to occur more frequently in the late removal group and was approximately 4 times higher (P=0.038). Representative surgery-related complication – CSE – is depicted in Figure 5.
Discussion
This study demonstrated that the duration of subdural drain placement did not correlate with clinical and radiologic outcomes, recurrence rate, or time to recurrence in patients who showed symptom improvement within 24 hours after BHC and hematoma drainage for unilateral CSDH. Instead, early removal of closed-system drainage was more advantageous in lowering the incidence of surgical complications, especially CSE occurrence, than was late drain removal.
Similar to the vast majority of studies on CSDH treated with BHC with closed-system drainage, which presents a favorable prognosis, the overall clinical outcomes in the present study were favorable [7,8]. There was no significant difference in the rate of achievement of the favorable outcome (mRS score 0–3) at discharge in either group (75.3% vs 71.0%,
According to some previous studies that analyzed the recurrence rate of unilateral CSDH, it was reported from 9.6% to 16%, which is comparable to the results of our study reported a recurrence rate of 12.81% [19–21]. In the current study, the mean interval from the initial surgical treatment to reoperation was 27.5 days, which was within the mean time to recurrence reported in previous studies [8,21,22].
To date, there is a paucity of literature estimating the impact of drain duration on the recurrence and treatment outcomes of CSDH after BHC. Previous studies have reported conflicting results. According to a previous retrospective study conducted on 97 patients (121 cases of CSDH) who had been treated with BHC, a duration of 3 days for drainage was necessary for preventing CSDH recurrence [14]. However, the above study did not consider the patients’ initial radiological and clinical features between the 2 groups. According to Kale et al [23], the rate of recurrence was significantly lower in patients receiving drainage for 5–7 days than in those receiving drainage for 2–4 days, without increasing the risk of complications. However, these authors noted that there was no significant difference in the recurrence rate requiring reoperation between the 2 groups.
Except for the above 2 studies, recent studies were skeptical about extending the duration of drainage beyond 2 days; several studies have reported that the duration of drainage had no significant correlation with postoperative recurrence. According to Jeong et al [24], there was no significant correlation between the rate of CSDH recurrence and the duration of <2 days and ≥2 days after BHC. In a prospective randomized study, the recurrence rate was compared between 48 and 96 hours after twist-drill craniotomy in patients with CSDH. The authors found that a longer drain duration could not guarantee a low recurrence rate, but increased overall complications (10.7% vs 26.9%,
There are only a few studies on the correlation between the total drainage volume and postoperative recurrence, and the findings were conflicting. In a previous retrospective study, the recurrence rate increased with excessive drainage volume [25]. In contrast, in a prospective randomized trial that compared 48 hours with 96 hours of subdural drainage, the drainage volume was approximately half in the 48 hours group, but the recurrence rate was not significantly different [26]. A recent national multicenter randomized study showed that recurrence and mortality were irrelevant to postoperative drain production, which agrees with our results [27].
The discrepancy between total drainage volume and radiologic outcomes in the late removal group might imply that the proportion of “pure” hematoma of drained fluids decreased markedly from postoperative day 2; therefore, it can be assumed that the critical period of subdural drainage to remove “pure” hematomas might be the first 2 days after surgery. According to the neurosurgeon’s preference, the drainage tube may be maintained for an extended period to minimize residual hematoma, even if the clinical symptoms improve immediately. However, we found that extending the duration of drainage did not reduce the greater volume of residual hematoma compared to early drain removal. Therefore, neurosurgeons should reconsider maintaining the subdural drain for more than 2 days. In addition, further studies on the quantitative measurement of “pure” hematoma volume in the drained fluid could strengthen our theory.
In this study, 32 surgery-related complications occurred in 208 patients; the complication rate of 15.4% was comparable to previous reports [5,26,28]. To the best of our knowledge, no previous study has described CSE as a postoperative complication in patients undergoing BHC. The exact incidence and pathophysiological mechanisms of this complication after BHC have not yet been reported. Instead, several possible mechanisms have been proposed for the development of CSE after decompressive craniectomy [16]. It has been hypothesized that CSE occurs as a result of the difference in the pressure gradient between the 2 hemispheres because of rapid reduction in ICP and diminished CSF absorption due to tearing of the arachnoid layer. However, the above 2 hypotheses are insufficient to explain the difference in CSE incidence according to drain duration. Considering the underlying pathomechanism of CSDH, which is derived from CSF leaking into the subdural space through an arachnoid tear that acts as a one-way flap valve [29], it is speculated that prolonged drainage induces continuous leakage of CSF by a persistent pressure gradient, which impedes the achievement of static pressure between the subarachnoid and subdural spaces. Therefore, the primary mechanism of CSE after BHC is speculated to be caused by the shrinkage of the ipsilateral brain due to over-drainage of CSF originating from prolonged drain duration causing brain shift, leading to enlargement of the contralateral subdural space.
CSE should not be overlooked because of its potential for progression to CSDH despite the lack of immediate notable neurologic symptoms [30,31]. Concomitant delayed resorption of accompanying CSE and tearing of the bridging veins derived from subdural space enlargement results in hemorrhage into the subdural space, leading to CSDH [30,32]. In our cohorts, conversion of CSE to CSDH was found in 5 of 11 patients during a 6-month follow-up period, which is consistent with previous studies that reported the rate of CSDH preceded by traumatic subdural hygroma (SDG) was 4% to 58% [33]. The need for BHC for contralateral CSDH derived from postoperative CSE without additional trauma was found in 4 of 5 patients. In a recent study, 17 symptomatic patients of 20 (85%) who developed CSDH originating from traumatic SDG underwent surgical treatment, which is similar to the findings of the present study [34].
We observed that overall surgical complications were significantly higher in the late drain removal group than in the early drain removal group, which is also in line with the findings of previous studies [15,26]. There is a possibility that direct brain injury, which can provoke AIH or seizure, could be reduced by early drain removal after BHC and subdural drain insertion. Further large-scale cohort studies are warranted to clarify the correlation between prolonged drainage and various surgical complications.
This study has several limitations. First, the duration of drain tube placement was determined based on the surgeon’s preference owing to the study’s retrospective nature. Therefore, the potential advantage of prolonged drainage may be masked in patients with large residual hematoma volume. Second, the boundary between the hematoma and brain tissue may be unclear in isodense CSDH and contribute to data acquisition bias. The strengths of this study include its relatively large sample size compared with previous studies conducted on drain duration, and the quantitative assessment of radiological parameters. A well-defined prospective randomized controlled study is needed to clarify the ideal duration of drain placement.
Conclusions
In conclusion, following BHC and hematoma drainage for unilateral CSDH, maintaining the subdural drain for more than 48 hours did not improve patient outcomes or reduce the recurrence of hematoma, but increased the surgical complications, including CSE.
Figures
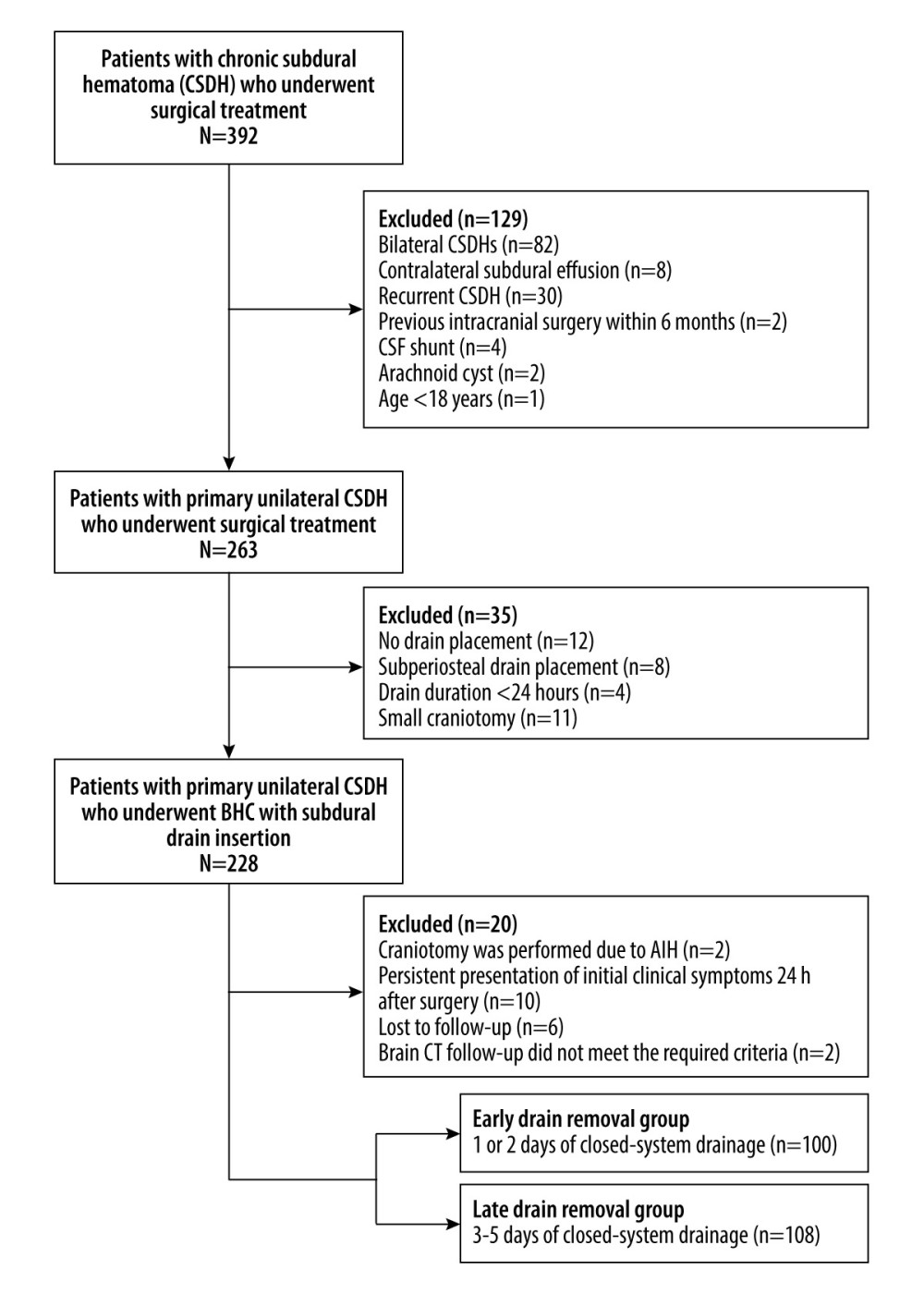 Figure 1. Flow diagram of patient selection CSF – cerebrospinal fluid; BHC – burr hole craniotomy; AIH – acute intracranial hemorrhage; CT – computed tomography. (Microsoft Office Word 2010, Microsoft, USA).
Figure 1. Flow diagram of patient selection CSF – cerebrospinal fluid; BHC – burr hole craniotomy; AIH – acute intracranial hemorrhage; CT – computed tomography. (Microsoft Office Word 2010, Microsoft, USA). 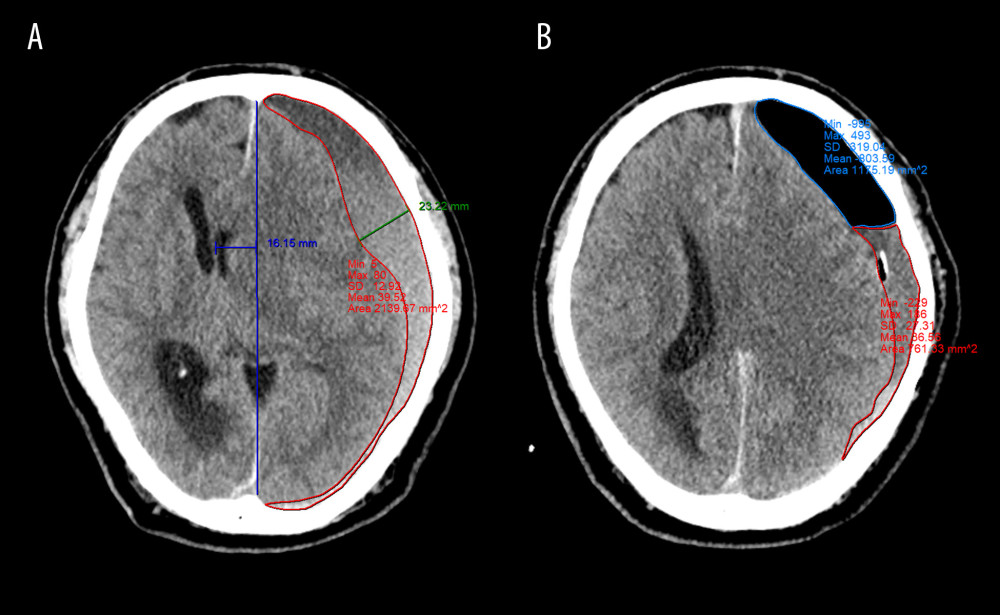 Figure 2. Quantitative measurement of radiological parameters on axial computed tomography (CT) scans(A) The midline shift on CT scans was measured as the distance from the septum pellucidum to the virtual perpendicular drawn midline (deep blue line). The maximal thickness of the hematoma was measured as the largest diameter between the brain’s surface and the inner table of the skull (green line). The boundary of the hematoma was manually traced and then calculated in each image slice and multiplied by 5 mm to assess the volume of preoperative hematoma (red line). (B) In the postoperative period, the above hematoma volume measurement method was used to estimate the volume of residual hematoma (red line) and trapped air (light blue line). (FastStone capture 9.0, FastStone Soft, USA).
Figure 2. Quantitative measurement of radiological parameters on axial computed tomography (CT) scans(A) The midline shift on CT scans was measured as the distance from the septum pellucidum to the virtual perpendicular drawn midline (deep blue line). The maximal thickness of the hematoma was measured as the largest diameter between the brain’s surface and the inner table of the skull (green line). The boundary of the hematoma was manually traced and then calculated in each image slice and multiplied by 5 mm to assess the volume of preoperative hematoma (red line). (B) In the postoperative period, the above hematoma volume measurement method was used to estimate the volume of residual hematoma (red line) and trapped air (light blue line). (FastStone capture 9.0, FastStone Soft, USA). 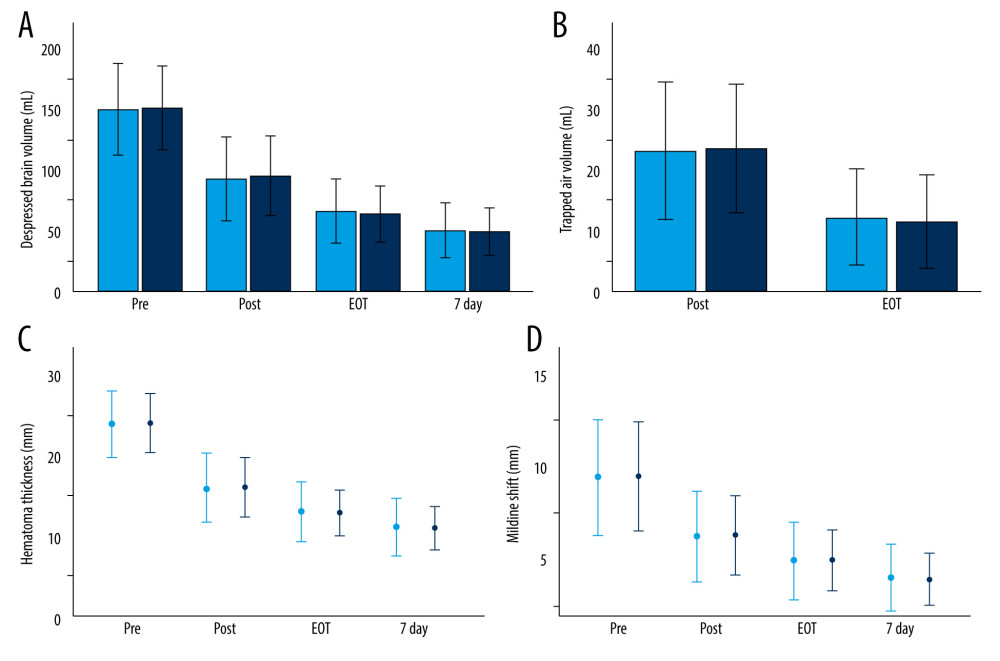 Figure 3. Radiological parameters at various time points in the early removal (light blue bars and dots) and late removal (deep blue bars and dots) groupsNo significant differences in depressed brain volume (A), trapped air volume (B), hematoma thickness (C), or midline shift (D) were observed between the 2 groups. Pre – preoperative; Post – postoperative; EOT – endpoint of treatment; 7 day – postoperative day 7. (SPSS 27.0 software, IBM, USA).
Figure 3. Radiological parameters at various time points in the early removal (light blue bars and dots) and late removal (deep blue bars and dots) groupsNo significant differences in depressed brain volume (A), trapped air volume (B), hematoma thickness (C), or midline shift (D) were observed between the 2 groups. Pre – preoperative; Post – postoperative; EOT – endpoint of treatment; 7 day – postoperative day 7. (SPSS 27.0 software, IBM, USA). 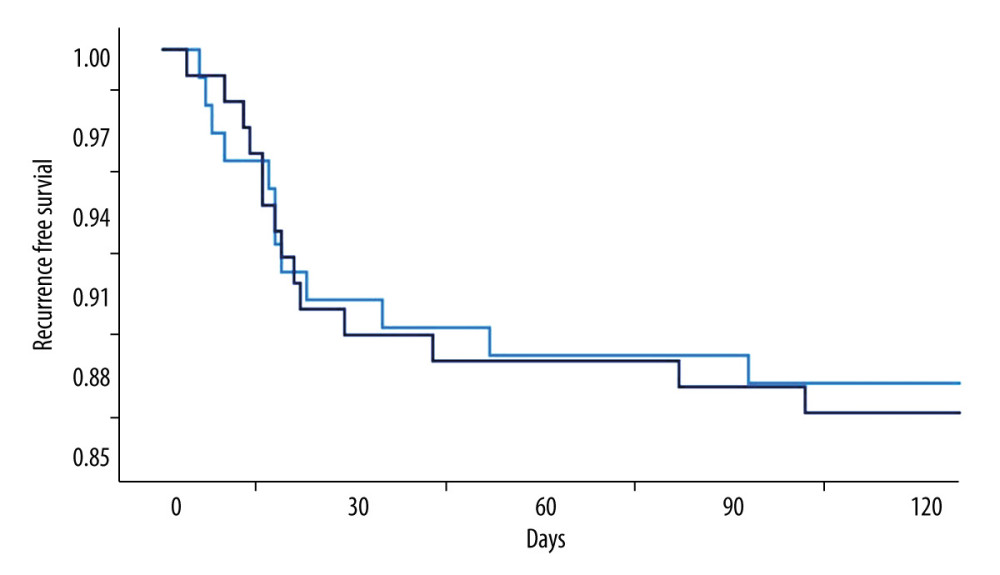 Figure 4. Kaplan-Meier curves of recurrence-free survival following burr hole craniotomy and hematoma drainageThere were no significant differences in recurrence rate and time to recurrence between the early (light blue line) and late removal (deep blue line) groups. (SPSS 27.0 software, IBM, USA).
Figure 4. Kaplan-Meier curves of recurrence-free survival following burr hole craniotomy and hematoma drainageThere were no significant differences in recurrence rate and time to recurrence between the early (light blue line) and late removal (deep blue line) groups. (SPSS 27.0 software, IBM, USA). 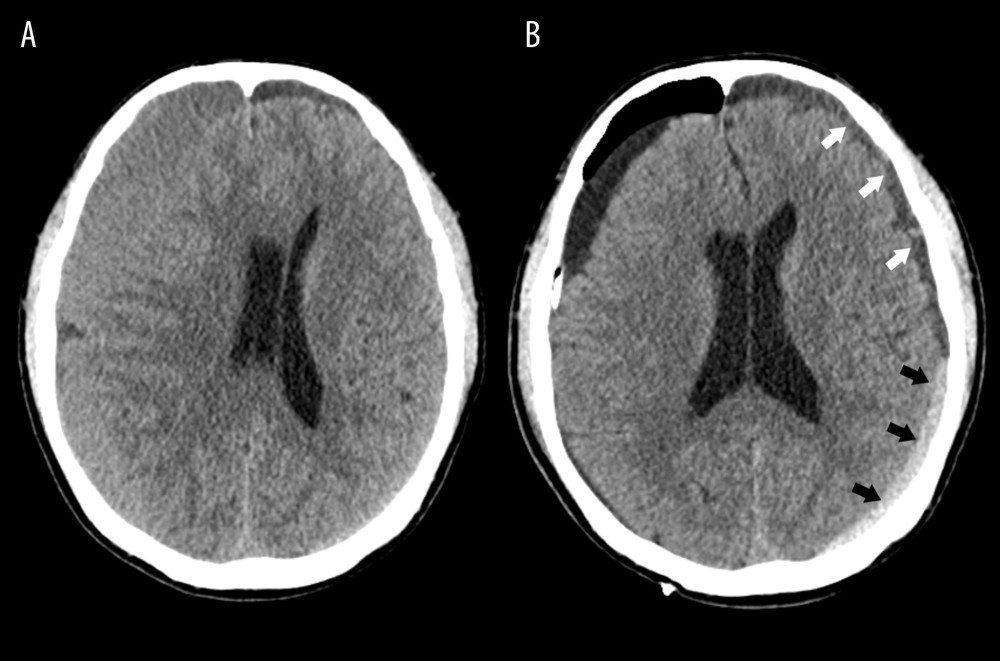 Figure 5. Representative image of contralateral subdural effusion (CSE) on axial computed tomography (CT) scans(A) Preoperative CT scan demonstrating isodense chronic subdural hematoma on the right-side causing a mass effect. (B) Postoperative day 4 follow-up CT scan showing remarkable resolution of the hematoma and a mass effect, but CSE (white arrows) containing separated acute hemorrhage (black arrows) was revealed. (FastStone capture 9.0, FastStone Soft, USA).
Figure 5. Representative image of contralateral subdural effusion (CSE) on axial computed tomography (CT) scans(A) Preoperative CT scan demonstrating isodense chronic subdural hematoma on the right-side causing a mass effect. (B) Postoperative day 4 follow-up CT scan showing remarkable resolution of the hematoma and a mass effect, but CSE (white arrows) containing separated acute hemorrhage (black arrows) was revealed. (FastStone capture 9.0, FastStone Soft, USA). References
1. Santarius T, Hutchinson PJ, Chronic subdural haematoma: Time to rationalize treatment?: Br J Neurosurg, 2004; 18(4); 328-32
2. Hsieh CT, Su IC, Hsu SK, Chronic subdural hematoma: Differences between unilateral and bilateral occurrence: J Clin Neurosci, 2016; 34; 252-58
3. Rauhala M, Luoto TM, Huhtala H, The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population: J Neurosurg, 2019; 132(4); 1147-57
4. Adhiyaman V, Chattopadhyay I, Irshad F, Increasing incidence of chronic subdural haematoma in the elderly: QJM, 2017; 110(6); 375-78
5. Ducruet AF, Grobelny BT, Zacharia BE, The surgical management of chronic subdural hematoma: Neurosurg Rev, 2012; 35(2); 155-69
6. Weigel R, Schmiedek P, Krauss J, Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review: J Neurol Neurosurg Psychiatry, 2003; 74(7); 937-43
7. Borger V, Vatter H, Oszvald Á, Chronic subdural haematoma in elderly patients: A retrospective analysis of 322 patients between the ages of 65–94 years: Acta Neurochir (Wien), 2012; 154(9); 1549-54
8. Mori K, Maeda M, Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate: Neurol Med Chir (Tokyo), 2001; 41(8); 371-81
9. Yadav YR, Parihar V, Namdev H, Bajaj J, Chronic subdural hematoma: Asian J Neurosurg, 2016; 11(4); 330-42
10. Desai VR, Scranton RA, Britz GW, Management of recurrent subdural hematomas: Neurosurg Clin N Am, 2017; 28(2); 279-86
11. Santarius T, Kirkpatrick PJ, Ganesan D, Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial: Lancet, 2009; 374(9695); 1067-73
12. Peng D, Zhu Y, External drains versus no drains after burr-hole evacuation for the treatment of chronic subdural hematoma in adults: Cochrane Database Syst Rev, 2016; 2016(8); CD011402
13. Liu W, Bakker NA, Groen RJ, Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures: J Neurosurg, 2014; 121(3); 665-73
14. Yu GJ, Han CZ, Zhang M, Prolonged drainage reduces the recurrence of chronic subdural hematoma: Br J Neurosurg, 2009; 23(6); 606-11
15. Sindou M, Ibrahim I, Maarrawi J, Chronic sub-dural hematomas: Twist drill craniostomy with a closed system of drainage, for 48 hours only, is a valuable surgical treatment: Acta Neurochir (Wien), 2010; 152(3); 545-46
16. Ling H, Yang L, Huang Z, Contralateral subdural effusion after decompressive craniectomy: What is the optimal treatment?: Clin Neurol Neurosurg, 2021; 210; 106950
17. Nassiri F, Hachem LD, Wang JZ, Reinitiation of anticoagulation after surgical evacuation of subdural hematomas: World Neurosurg, 2020; 135; e616-22
18. Glancz LJ, Poon MTC, Coulter IC, Does drain position and duration influence outcomes in patients undergoing burr-hole evacuation of chronic subdural hematoma? Lessons from a UK multicenter prospective cohort study: Neurosurgery, 2019; 85(4); 486-93
19. Huang YH, Yang KY, Lee TC, Liao CC, Bilateral chronic subdural hematoma: What is the clinical significance?: Int J Surg, 2013; 11(7); 544-48
20. Tsai TH, Lieu AS, Hwang SL, A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: Precipitating factors and postoperative outcomes: J Trauma, 2010; 68(3); 571-75
21. Torihashi K, Sadamasa N, Yoshida K, Independent predictors for recurrence of chronic subdural hematoma: A review of 343 consecutive surgical cases: Neurosurgery, 2008; 63(6); 1125-29
22. Lutz K, Kamenova M, Schaedelin S, Time to and possible risk factors for recurrence after burr-hole drainage of chronic subdural hematoma: A subanalysis of the cSDH-drain randomized controlled trial: World Neurosurg, 2019; 132; e283-89
23. Kale A, Öz İİ, Gün EG, Is the recurrence rate of chronic subdural hematomas dependent on the duration of drainage?: Neurol Res, 2017; 39(5); 399-402
24. Jeong SI, Kim SO, Won YS, Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination: Korean J Neurotrauma, 2014; 10(1); 15-21
25. Matsumoto K, Akagi K, Abekura M, Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage: Neurol Res, 1999; 21(3); 277-80
26. Ibrahim I, Maarrawi J, Jouanneau EEvacuation of chronic subdural hematomas with the Twist-Drill technique: Results of a randomized prospective study comparing 48-h and 96-h drainage duration: Neurochirurgie, 2010; 56(1); 23-27 [in French]
27. Jensen TSR, Haldrup M, Hjortdal Grønhøj M, National randomized clinical trial on subdural drainage time after chronic subdural hematoma evacuation: J Neurosurg, 2021 [Online ahead of print]
28. Wu Q, Liu Q, Chen D, Subdural drainage techniques for single burr-hole evacuation of chronic subdural hematoma: Two drains frontal-occipital position versus one drain frontal position: Br J Neurosurg, 2021; 35(3); 324-28
29. Kristof RA, Grimm JM, Stoffel-Wagner B, Cerebrospinal fluid leakage into the subdural space: Possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma: J Neurosurg, 2008; 108(2); 275-80
30. Lee KS, Bae WK, Doh JW, Origin of chronic subdural haematoma and relation to traumatic subdural lesions: Brain Inj, 1998; 12(11); 901-10
31. Lee KS, Bae WK, Park YT, Yun IG, The pathogenesis and fate of traumatic subdural hygroma: Br J Neurosurg, 1994; 8(5); 551-58
32. Lee KS, Review natural history of chronic subdural haematoma: Brain Inj, 2004; 18(4); 351-58
33. Lee KS, Bae WK, Bae HG, Yun IG, The fate of traumatic subdural hygroma in serial computed tomographic scans: J Korean Med Sci, 2000; 15(5); 560-68
34. Ahn JH, Jun HS, Kim JH, Analysis of risk factor for the development of chronic subdural hematoma in patients with traumatic subdural hygroma: J Korean Neurosurg Soc, 2016; 59(6); 622-27
Figures
 Figure 1. Flow diagram of patient selection CSF – cerebrospinal fluid; BHC – burr hole craniotomy; AIH – acute intracranial hemorrhage; CT – computed tomography. (Microsoft Office Word 2010, Microsoft, USA).
Figure 1. Flow diagram of patient selection CSF – cerebrospinal fluid; BHC – burr hole craniotomy; AIH – acute intracranial hemorrhage; CT – computed tomography. (Microsoft Office Word 2010, Microsoft, USA). Figure 2. Quantitative measurement of radiological parameters on axial computed tomography (CT) scans(A) The midline shift on CT scans was measured as the distance from the septum pellucidum to the virtual perpendicular drawn midline (deep blue line). The maximal thickness of the hematoma was measured as the largest diameter between the brain’s surface and the inner table of the skull (green line). The boundary of the hematoma was manually traced and then calculated in each image slice and multiplied by 5 mm to assess the volume of preoperative hematoma (red line). (B) In the postoperative period, the above hematoma volume measurement method was used to estimate the volume of residual hematoma (red line) and trapped air (light blue line). (FastStone capture 9.0, FastStone Soft, USA).
Figure 2. Quantitative measurement of radiological parameters on axial computed tomography (CT) scans(A) The midline shift on CT scans was measured as the distance from the septum pellucidum to the virtual perpendicular drawn midline (deep blue line). The maximal thickness of the hematoma was measured as the largest diameter between the brain’s surface and the inner table of the skull (green line). The boundary of the hematoma was manually traced and then calculated in each image slice and multiplied by 5 mm to assess the volume of preoperative hematoma (red line). (B) In the postoperative period, the above hematoma volume measurement method was used to estimate the volume of residual hematoma (red line) and trapped air (light blue line). (FastStone capture 9.0, FastStone Soft, USA). Figure 3. Radiological parameters at various time points in the early removal (light blue bars and dots) and late removal (deep blue bars and dots) groupsNo significant differences in depressed brain volume (A), trapped air volume (B), hematoma thickness (C), or midline shift (D) were observed between the 2 groups. Pre – preoperative; Post – postoperative; EOT – endpoint of treatment; 7 day – postoperative day 7. (SPSS 27.0 software, IBM, USA).
Figure 3. Radiological parameters at various time points in the early removal (light blue bars and dots) and late removal (deep blue bars and dots) groupsNo significant differences in depressed brain volume (A), trapped air volume (B), hematoma thickness (C), or midline shift (D) were observed between the 2 groups. Pre – preoperative; Post – postoperative; EOT – endpoint of treatment; 7 day – postoperative day 7. (SPSS 27.0 software, IBM, USA). Figure 4. Kaplan-Meier curves of recurrence-free survival following burr hole craniotomy and hematoma drainageThere were no significant differences in recurrence rate and time to recurrence between the early (light blue line) and late removal (deep blue line) groups. (SPSS 27.0 software, IBM, USA).
Figure 4. Kaplan-Meier curves of recurrence-free survival following burr hole craniotomy and hematoma drainageThere were no significant differences in recurrence rate and time to recurrence between the early (light blue line) and late removal (deep blue line) groups. (SPSS 27.0 software, IBM, USA). Figure 5. Representative image of contralateral subdural effusion (CSE) on axial computed tomography (CT) scans(A) Preoperative CT scan demonstrating isodense chronic subdural hematoma on the right-side causing a mass effect. (B) Postoperative day 4 follow-up CT scan showing remarkable resolution of the hematoma and a mass effect, but CSE (white arrows) containing separated acute hemorrhage (black arrows) was revealed. (FastStone capture 9.0, FastStone Soft, USA).
Figure 5. Representative image of contralateral subdural effusion (CSE) on axial computed tomography (CT) scans(A) Preoperative CT scan demonstrating isodense chronic subdural hematoma on the right-side causing a mass effect. (B) Postoperative day 4 follow-up CT scan showing remarkable resolution of the hematoma and a mass effect, but CSE (white arrows) containing separated acute hemorrhage (black arrows) was revealed. (FastStone capture 9.0, FastStone Soft, USA). Tables
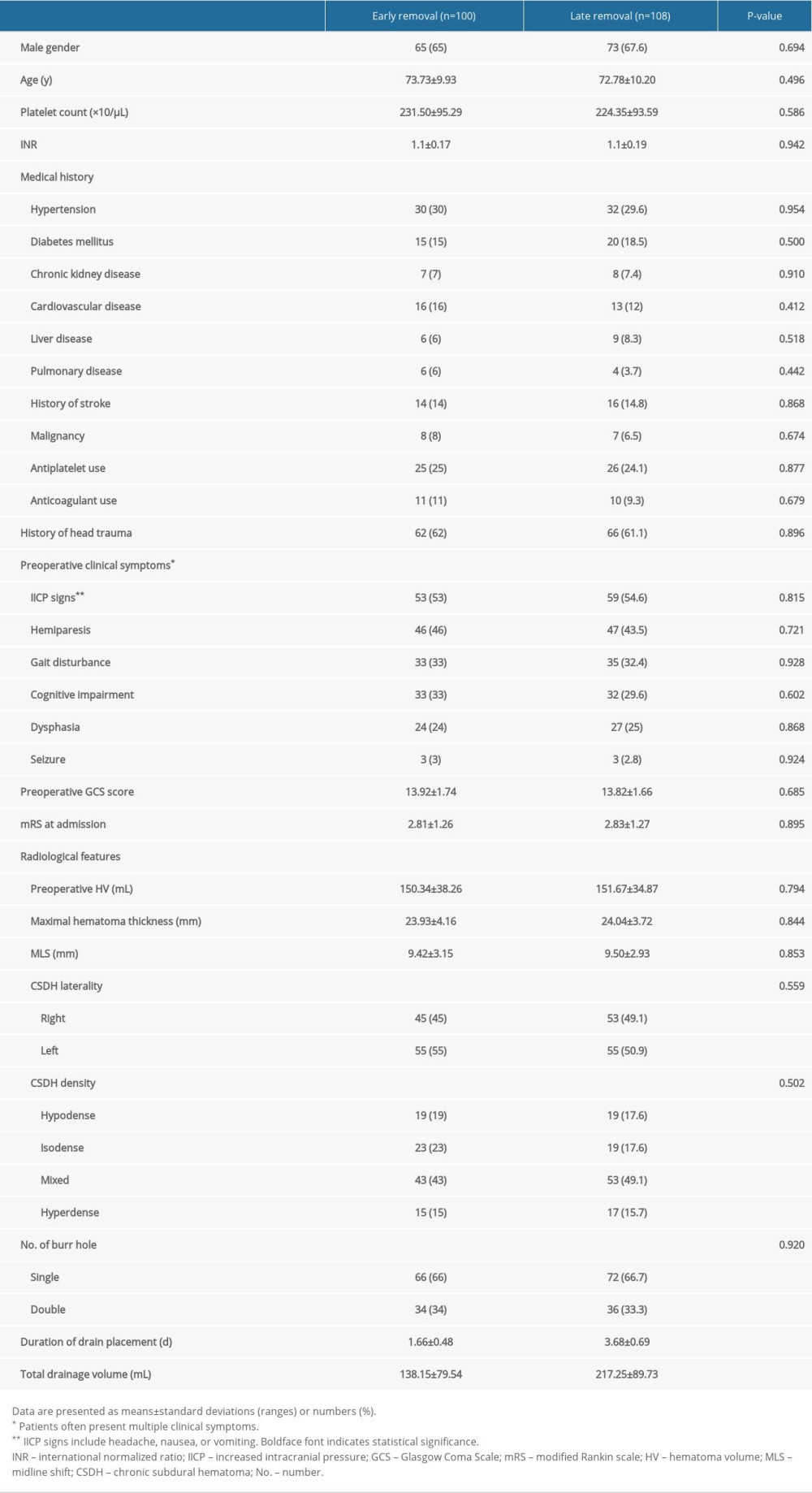 Table 1. Patient characteristics.
Table 1. Patient characteristics.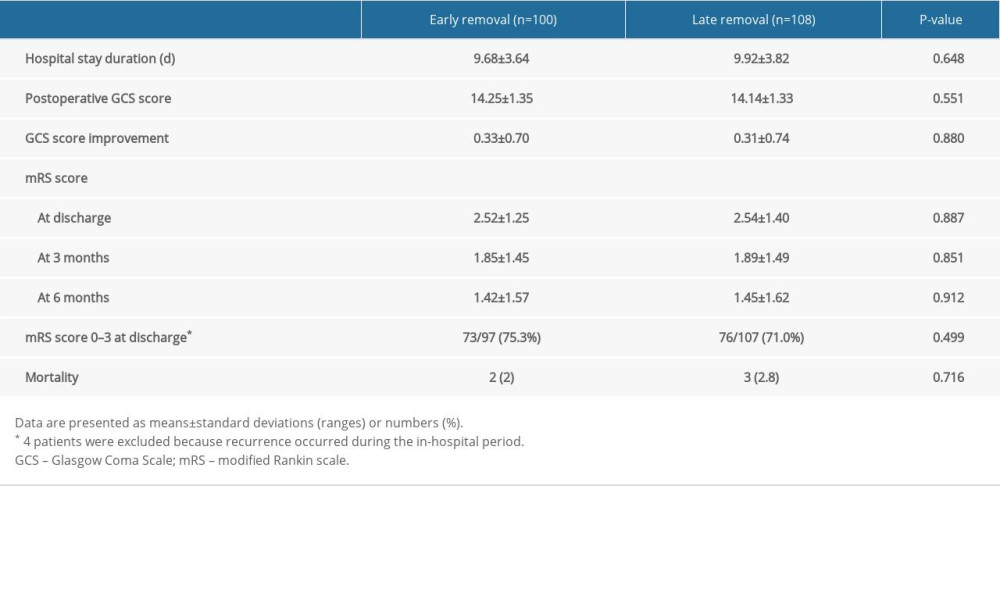 Table 2. Comparison of the clinical outcomes.
Table 2. Comparison of the clinical outcomes.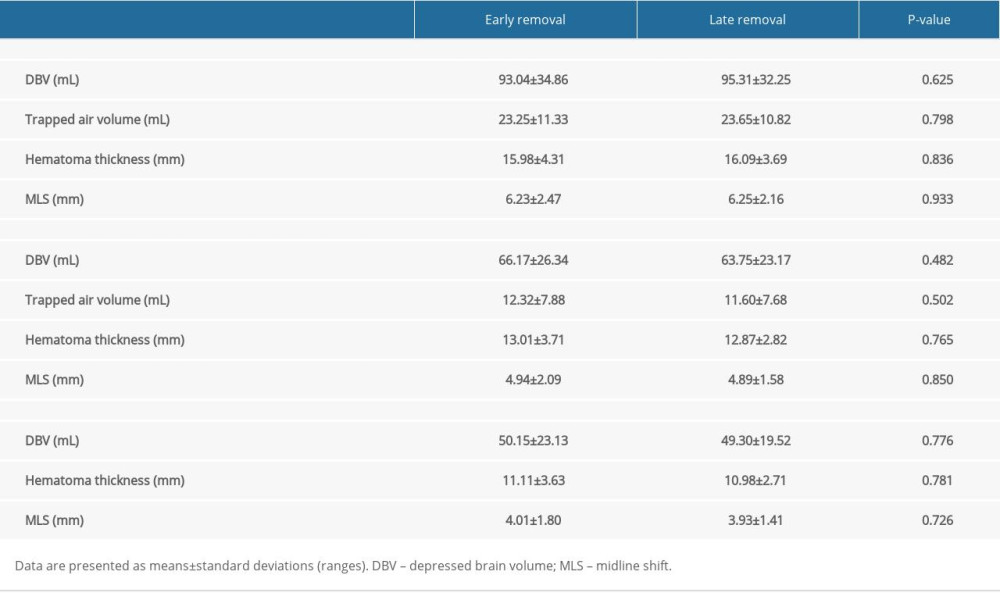 Table 3. Comparison of the radiologic outcomes at various time points.
Table 3. Comparison of the radiologic outcomes at various time points.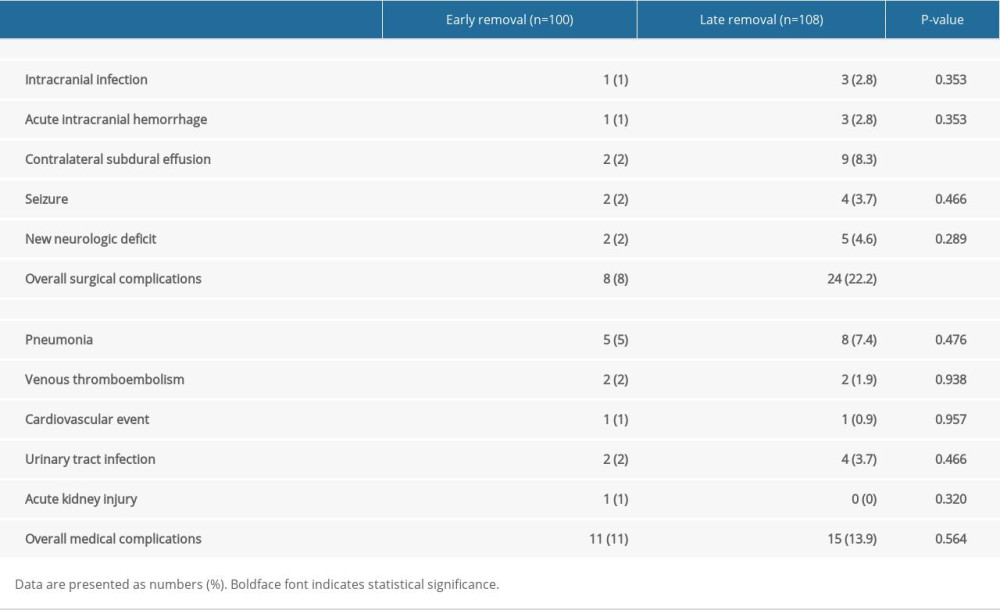 Table 4. Comparison of the postoperative complications.
Table 4. Comparison of the postoperative complications. Table 1. Patient characteristics.
Table 1. Patient characteristics. Table 2. Comparison of the clinical outcomes.
Table 2. Comparison of the clinical outcomes. Table 3. Comparison of the radiologic outcomes at various time points.
Table 3. Comparison of the radiologic outcomes at various time points. Table 4. Comparison of the postoperative complications.
Table 4. Comparison of the postoperative complications. In Press
08 Mar 2024 : Clinical Research
Evaluation of Foot Structure in Preschool Children Based on Body MassMed Sci Monit In Press; DOI: 10.12659/MSM.943765
15 Apr 2024 : Laboratory Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in OsteoarthritisMed Sci Monit In Press; DOI: 10.12659/MSM.943738
07 Mar 2024 : Clinical Research
Knowledge of and Attitudes Toward Clinical Trials: A Questionnaire-Based Study of 179 Male Third- and Fourt...Med Sci Monit In Press; DOI: 10.12659/MSM.943468
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








