01 September 2022: Clinical Research
High Level of Irisin as a Marker of Malnutrition in Head and Neck Cancer Patients Subjected to Radiotherapy
Iwona Homa-Mlak1ACDEF*, Radosław Mlak1ACDEF, Anna Brzozowska2BDF, Marcin Mazurek1CEF, Tomasz Powrózek1CDE, Monika Prendecka-Wróbel1CEF, Aneta Szudy-Szczyrek2ADE, Piotr Dreher3CEF, Katarzyna Kamińska4F, Teresa Małecka-Massalska1ACEF, Alicja Wójcik-Załuska5CEGDOI: 10.12659/MSM.936857
Med Sci Monit 2022; 28:e936857
Abstract
BACKGROUND: Head and neck cancers (HNC) are the 7th most prevalent neoplasms in the world. In 50% of these patients, body weight loss and malnutrition are observed before the beginning of therapy. It is known that an important role in the pathomechanism of malnutrition and cachexia is played by the development of inflammation, degradation of muscle fibers, and browning of white adipose tissue (WAT). It was demonstrated that even a slight increase in irisin concentration leads to browning of WAT.
MATERIAL AND METHODS: The study group consisted of 50 patients with HNC. The nutritional status of the patients was assessed by the Nutritional Risk Score 2002 (NRS 2002) and Subjective Global Assessment (SGA) scales. Using bioelectrical impedance analysis (BIA), the parameters fat mass (FM) and fat-free mass (FFM) were obtained.
RESULTS: Higher irisin values (1.57 vs 1.18 [ng/ml], P=0.0004) were observed in patients with higher nutritional risk (≥3) evaluated according to the NRS scale. In patients assessed as B or C on the SGA scale, higher values of irisin concentration (1.38 vs 1.07 [ng/ml], P=0.0139) were noted. It was also observed that the level of irisin before treatment was negatively correlated (rho=-0.30, p=0.0350) with FM% and was positively correlated (rho=0.30, p=0.0340) with FFM% in BIA measurements performed after the 7th cycle of RTH.
CONCLUSIONS: Based on these results, we conclude that patients with malnutrition tend to have higher irisin values compared to normally nourished patients. A high level of irisin may be a useful marker of malnutrition in patients with HNC.
Keywords: Cachexia, FNDC5 Protein, Human, Head and Neck Neoplasms, malnutrition, Electric Impedance, Fibronectins, Humans, Nutrition Assessment, Nutritional Status
Background
Head and neck cancers (HNC) are the 7th most prevalent neoplasms in the world. Among them, the majority (about 90%) are squamous cell carcinomas (SCCHN). In 50% of these patients, body weight loss and malnutrition occur even before the beginning of therapy. The main methods of SCCHN treatment are surgery, radiotherapy (RTH), chemotherapy (CTH), and combination of these methods. It is estimated that in the course of therapy the proportion of malnourished patients increases to 90%. Nutritional problems in patients with SCCHN are multifactorial. The definition of malnutrition includes progressive loss of muscle and fat tissue. Nutritional problems involve socio-psychological factors, complications of treatment (including dysphagia, xerostomia), or symptoms associated with tumor location. The symptoms associated with tumor location include gastrointestinal obstruction and odynophagia [1,2]. In patients with neoplastic malnutrition or cachexia, various degrees of body weight loss may occur in association with metabolic disturbances, lean body mass loss, and increased lipolysis [2,3]. It should be noted that not all cancer patients, including those with the tumors of the head and neck area (at the same stage of disease, subject to the same treatment method), develop malnutrition or cachexia [3]. Early detection of nutritional disturbances, especially before significant body weight loss, is associated with improved quality of life, longer time to disease progression, and better overall survival (OS) [2,4]. Identification of patients at risk of nutritional disorders may allow for the implementation of appropriate nutritional treatment. Therefore, the search for predictors of malnutrition and/or cachexia seems to be warranted.
Cancer cachexia is not always present in malnourished patients but all cachectic patients experience nutritional disturbances4[5]. Cancer cachexia is a multifactorial syndrome characterized by appetite disorders, body weight loss, metabolic changes, and intensified inflammation [5,6]. Lean and fatty body mass atrophy and skeletal muscle function loss are also observed. These changes lead to steady body weight loss [5]. Knowledge concerning the pathomechanism of malnutrition and cachexia has been expanding in the recent years. However, its complexity still leaves many aspects of these conditions unexplained. It is known that an important role is played by the development of inflammation, degradation of muscle fibers, and browning of white adipose tissue (WAT). One of the most important myokines is irisin, which is a fragment of extracellular domain of FNDC5 protein [5,7]. Irisin is regulated by proliferator-activated receptor-γ coactivator-1α (PGC1α) and the muscles can excrete it into the bloodstream. As a result, irisin can activate adipose tissue thermogenesis and cause browning of WAT [8]. The relationship between irisin and neoplasms is unclear. The level of irisin in patients with different types of cancer may be lower or higher compared to healthy people [9–11]. However, the major aim of research has not been to correlate the level of irisin with the nutritional status of these patients. Perhaps the differences in irisin levels were due to the nutritional status of these patients [11]. Therefore, because irisin is involved in hydrolysis triacylglycerols in WAT and inhibits de novo lipogenesis [12], we hypothesized that irisin may be associated with the development of malnutrition or cachexia in patients with HNC.
The aim of the study was to evaluate the level of serum irisin in HNC patients subjected to RTH and to assess its correlation with the occurrence of malnutrition.
Material and Methods
STATISTICAL ANALYSIS:
All data were collected in the Excel database and then imported into the MedCalc statistical program (MedCalc Software, Belgium). Since there were no similar studies comparing irisin levels in patients with different nutritional statuses, the sample size was calculated in the acquired data set retrospectively. We decided to use NRS status as a primary outcome. As input data, we used arithmetic means and corresponding standard deviations (SD) of irisin serum concentration. In most medical studies,
Results
No statistically significant differences in the level of irisin were observed in relation to the presence of the analysed demographic and clinical factors (Table 2). However, higher irisin values (1.57 vs 1.18 [ng/ml],
Discussion
Many factors influence the development of malnutrition and cachexia. The initial mechanism leading to hypercatabolism is a systemic inflammation characterized by, among other features, increased secretion of pro-inflammatory cytokines (interferon-γ, TNF-α, NFκβ, IL), catabolic mediators and reactive forms of oxygen, both by the cells of the host and cancer cells [16,17]. Chronic inflammation can cause changes in the metabolism of carbohydrates, fats, and proteins. The main changes in the metabolism of carbohydrates result from the lack of tolerance to glucose, resistance to insulin, and increase of gluconeogenesis. In patients, we can observe an increase of factors, which directly cause lipolysis (including the lipid-mobilizing factor (LMF) and zinc-alpha-2glycoprotein) and raise sensitivity to lipolytic factors, thus contributing to the loss of fat tissue [7,16]. However, one of the main reasons for the decrease of FFM (mainly muscular) is the decreased synthesis of new proteins with simultaneous increase of degradation of the existing ones [7,16]. The observed decrease of body weight in malnourished and cachectic patients is mainly associated with lipolysis (mostly WAT) in response to negative energetic balance and anorexia related to the development of cancer [18]. The influence of lipolysis on the development of cancer cachexia was demonstrated in a study in which the inhibition of lipid mobilization improved the patients’ condition [19]. Lipolysis of WAT causes loss of adipose tissue and contributes to decreased mass of skeletal muscles. In turn, WAT browning increases the expression of uncoupling protein 1 (UCP1), which leads to increased energy expenditure. Likewise, the activated BAT increases the expression of UCP1 and also increases energy expenditure. Both these processes cause negative energy balance, which contributes to the development and progression of malnutrition and cancer cachexia [19]. PGC1α Participates in many pathways regulating metabolic transformations and energy expenditure. PGC1α stimulates the expression of
The development of malnutrition and cachexia is associated with the decrease of fat-free and fat body mass as well as a change from WAT to BAT. An active inflammatory process, which is strongly induced at the time of cachexia progression, contributes to the degradation of muscle fibers [24]. The studies that have been conducted so far prove that irisin has a strong impact on the transformation of WAT into BAT. The present study has some limitations. To assess the nutritional status, we used methods such as the NRS and SGA scales, the results of laboratory tests, and BIA to assess body composition. We did not use any subjective anthropometric methods to evaluate muscle mass. We did not evaluate white and brown fat tissue in the patients. We used BIA method to evaluate the fat mass, percent of fat mass, fat-free mass, and percent of fat-free mass.
Conclusions
Patients with cachexia tend to have higher irisin values compared to normally nourished patients. Low level of irisin is associated with low risk of malnutrition in patients with HNC subjected to RTH. High level of irisin may be a useful marker of malnutrition in patients with HNC subjected to RTH.
Figures
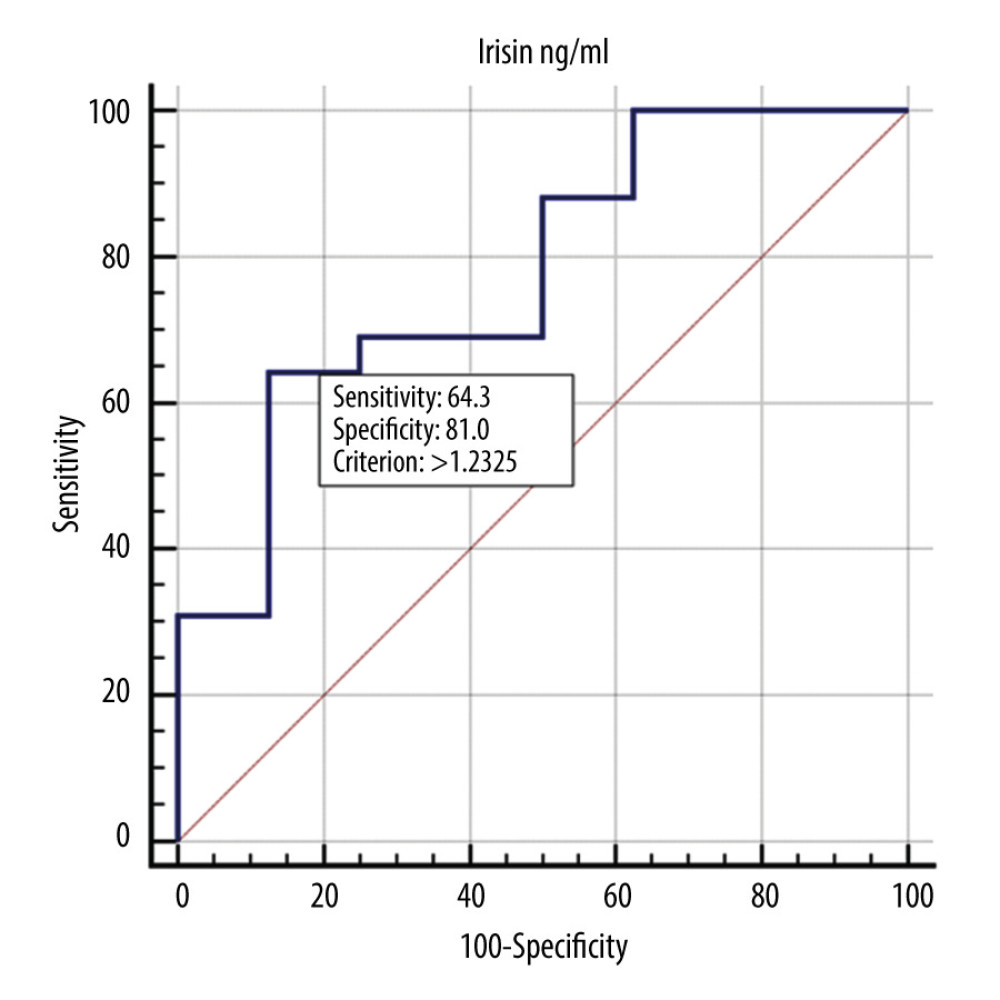 Figure 1. ROC curve showing the diagnostic usefulness of irisin in detecting malnutrition (B or C according to the SGA scale).
Figure 1. ROC curve showing the diagnostic usefulness of irisin in detecting malnutrition (B or C according to the SGA scale). 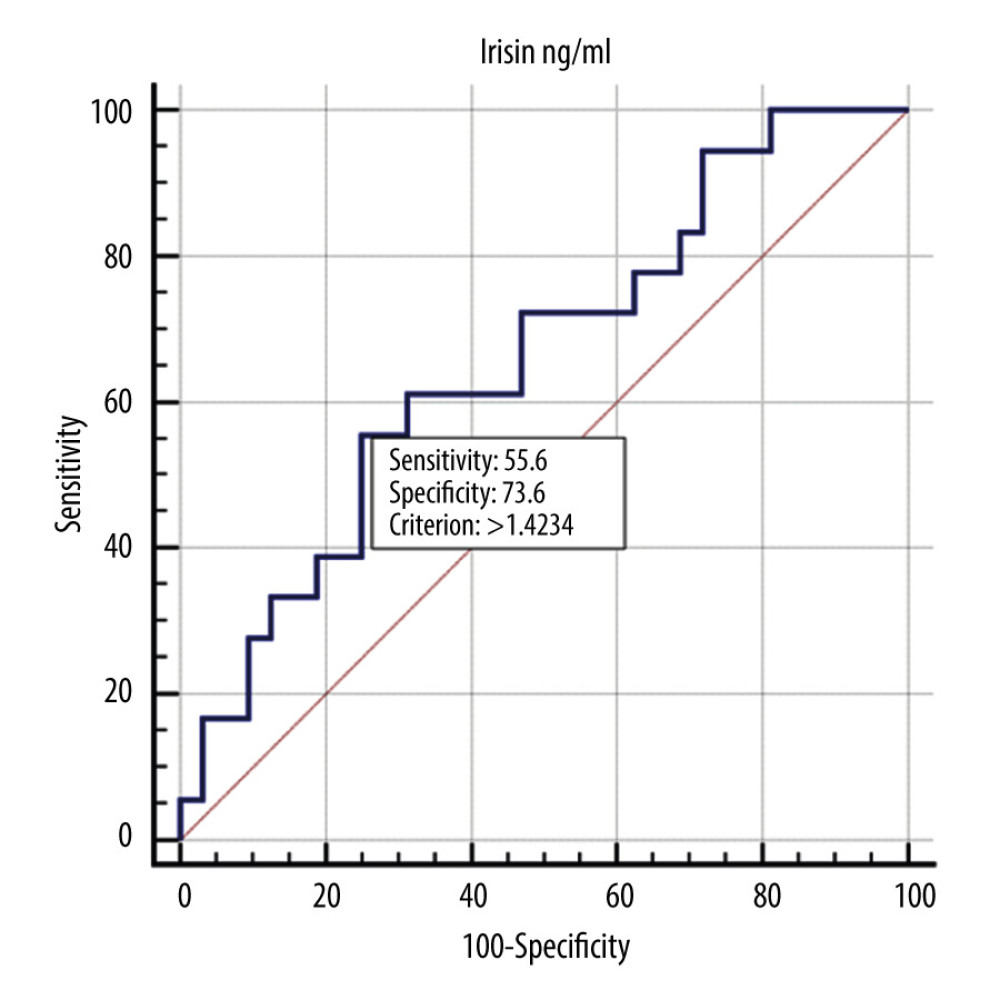 Figure 2. ROC curve showing the diagnostic usefulness of irisin in detecting severe malnutrition (C according to the SGA scale).
Figure 2. ROC curve showing the diagnostic usefulness of irisin in detecting severe malnutrition (C according to the SGA scale). 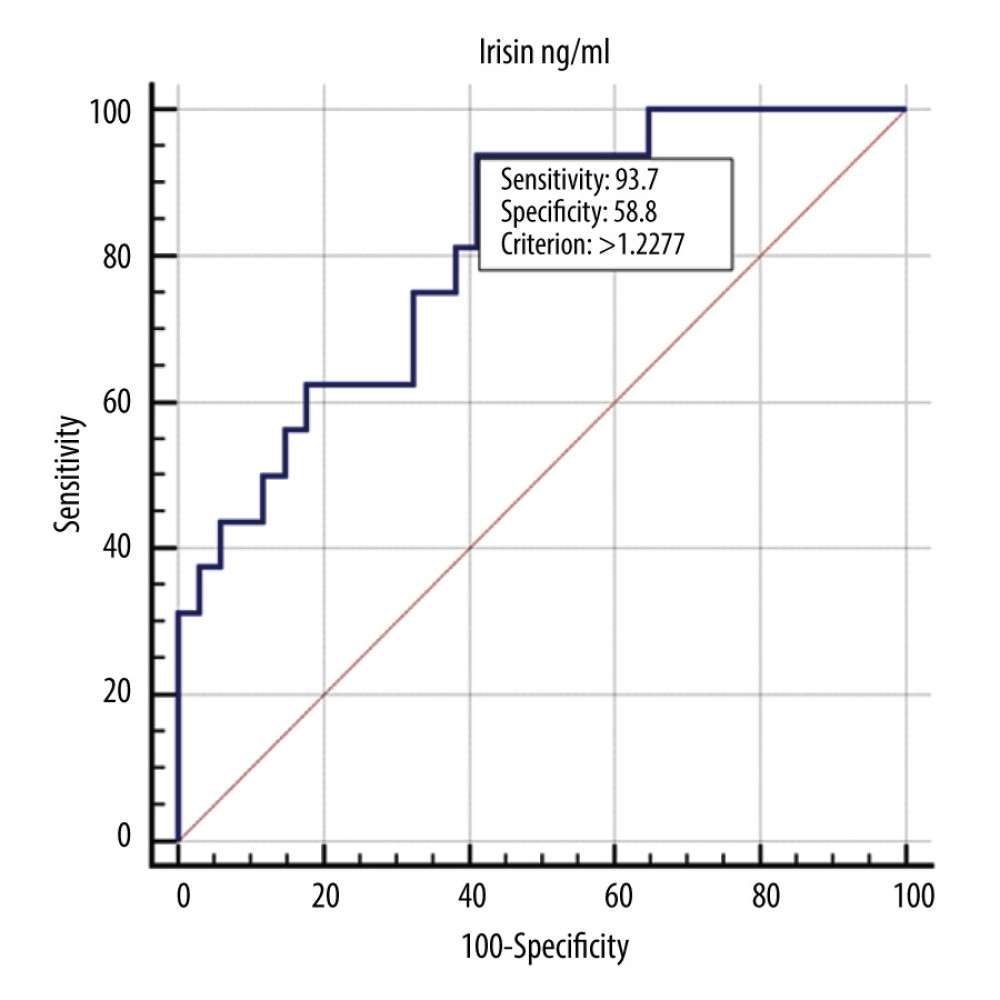 Figure 3. ROC curve showing the diagnostic usefulness of irisin in detecting nutritional risk (according to the NRS scale).
Figure 3. ROC curve showing the diagnostic usefulness of irisin in detecting nutritional risk (according to the NRS scale). Tables
Table 1. Characteristic of the study group.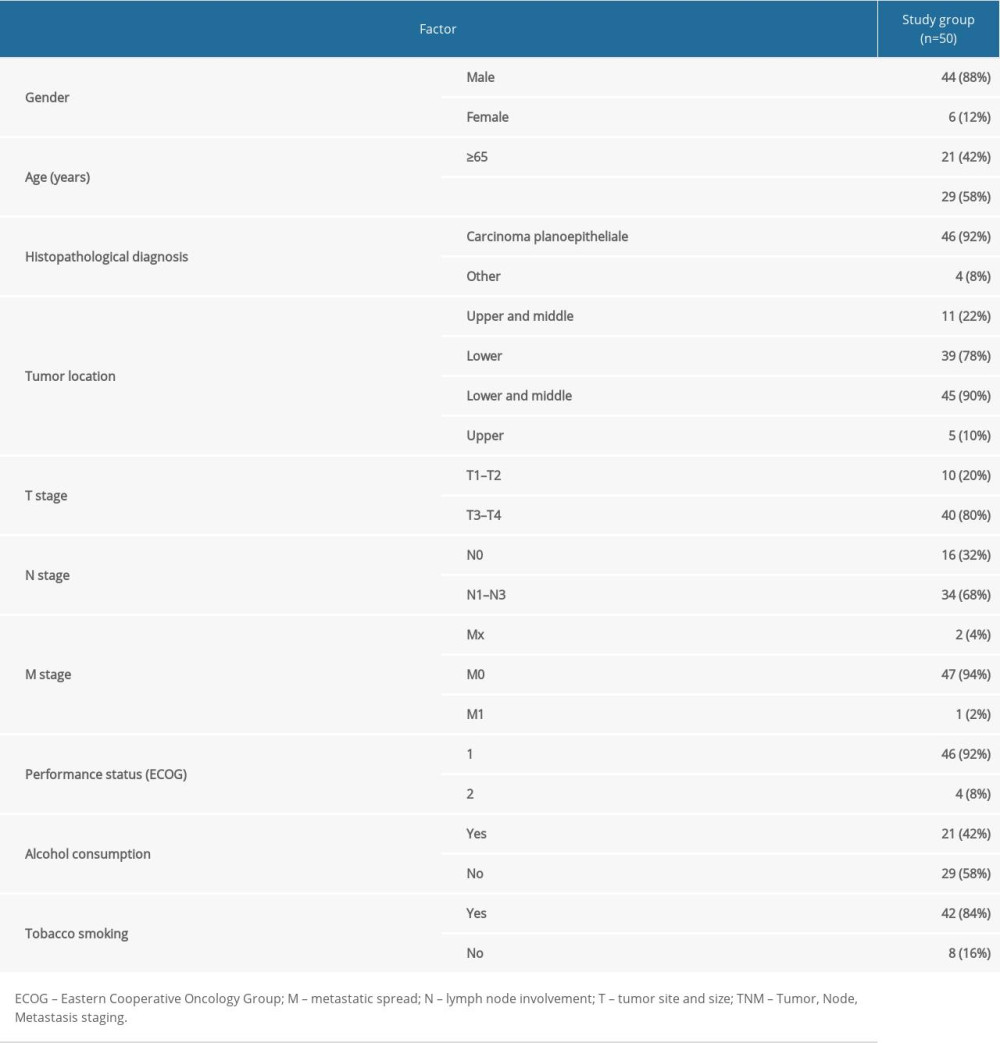 Table 2. Influence of demographic and clinical factors on irisin level in HNC patients.
Table 2. Influence of demographic and clinical factors on irisin level in HNC patients.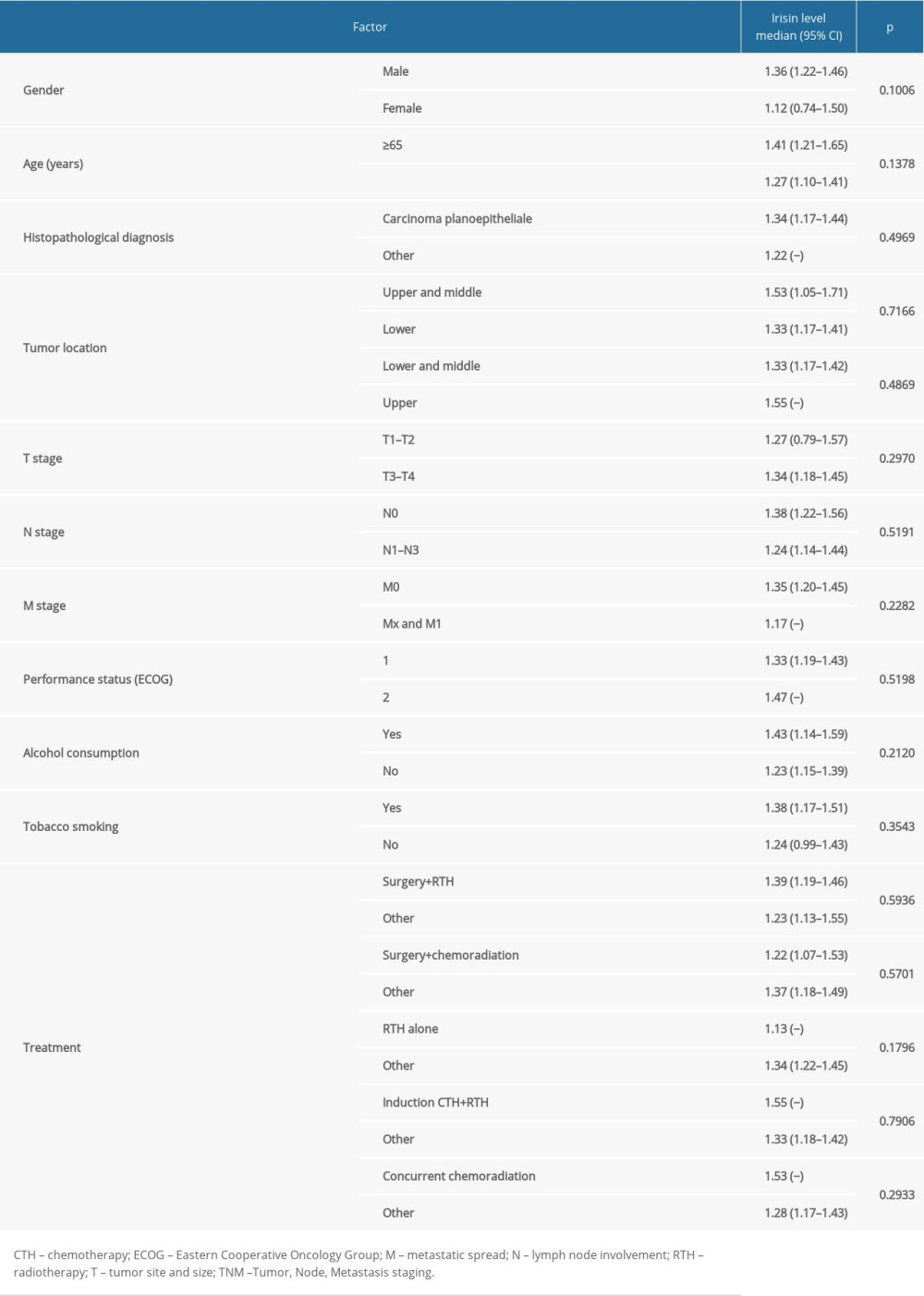 Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients.
Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients.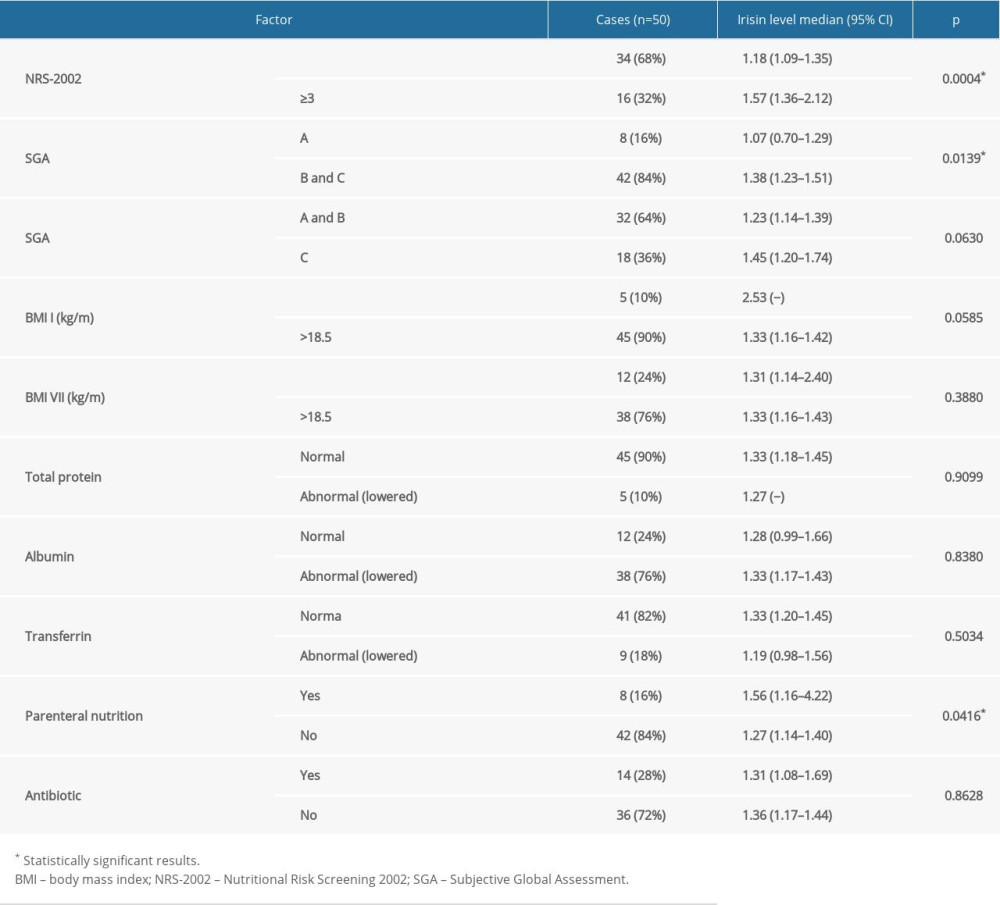 Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients.
Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients.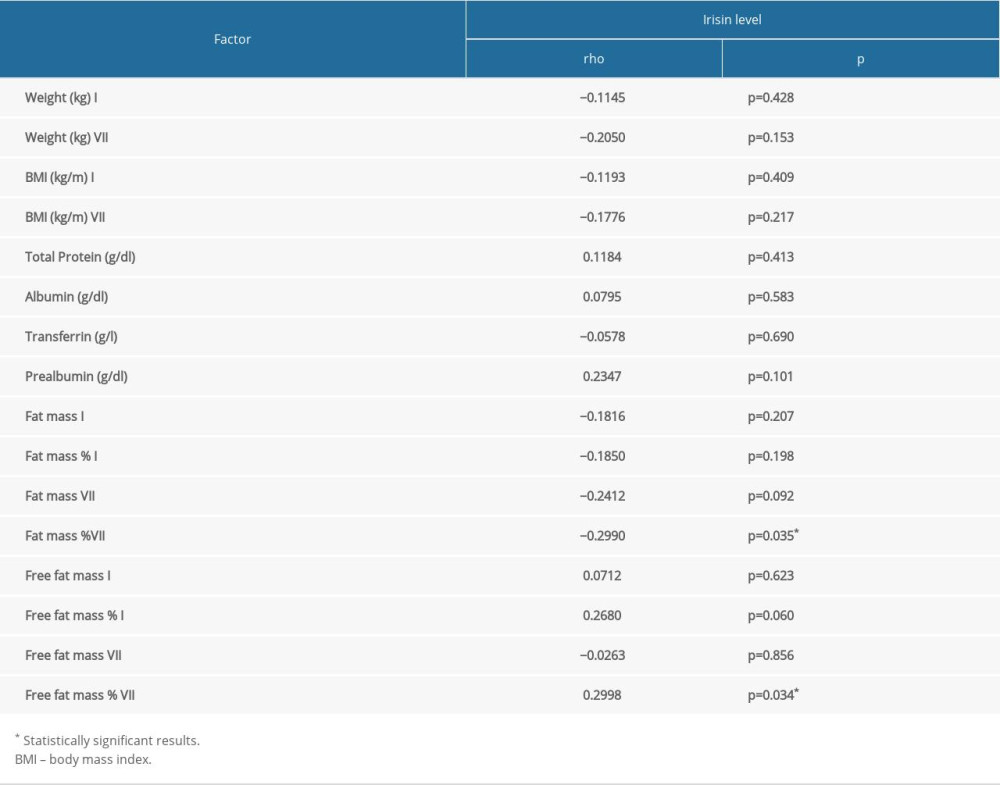 Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients.
Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients.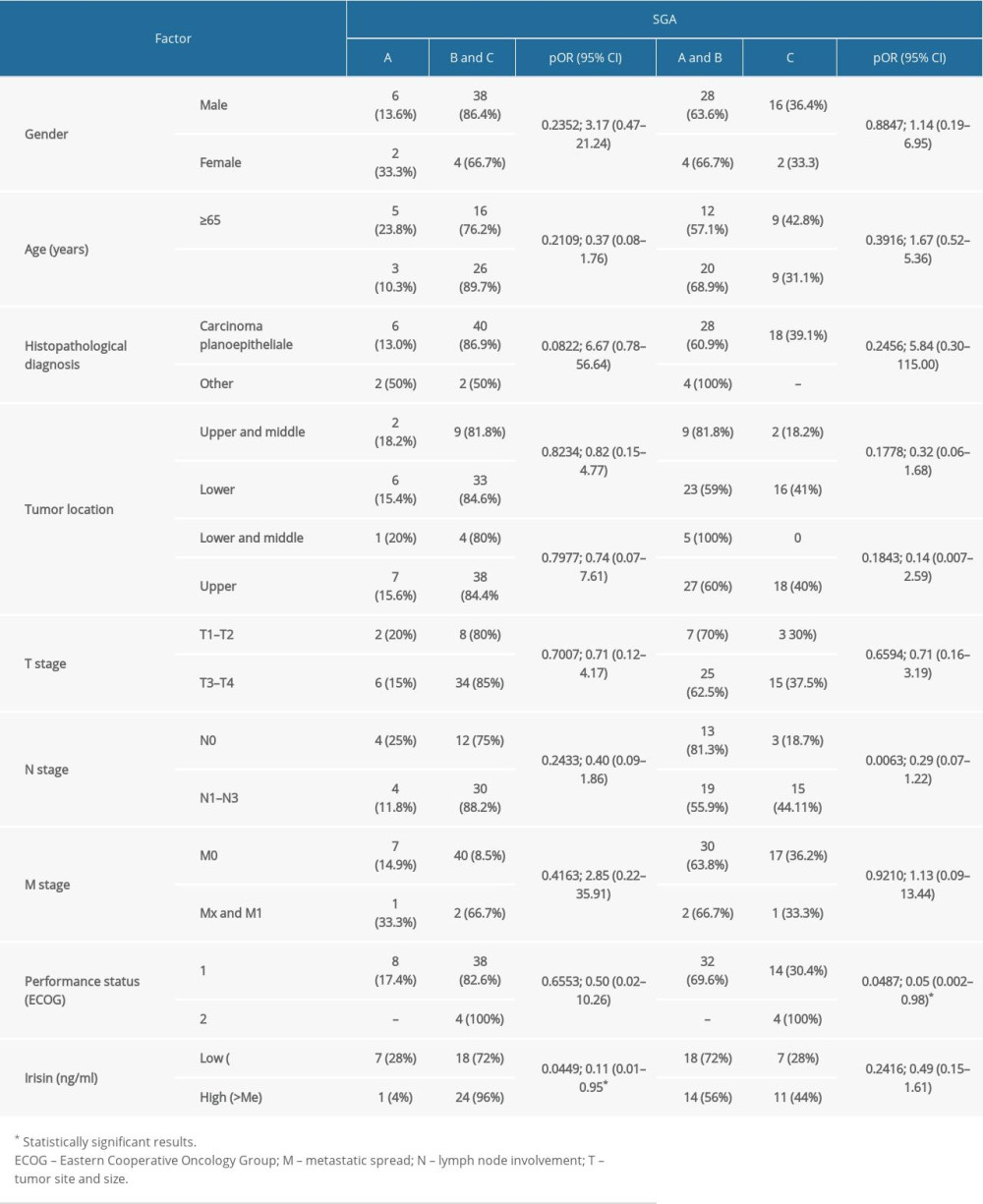 Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients.
Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients.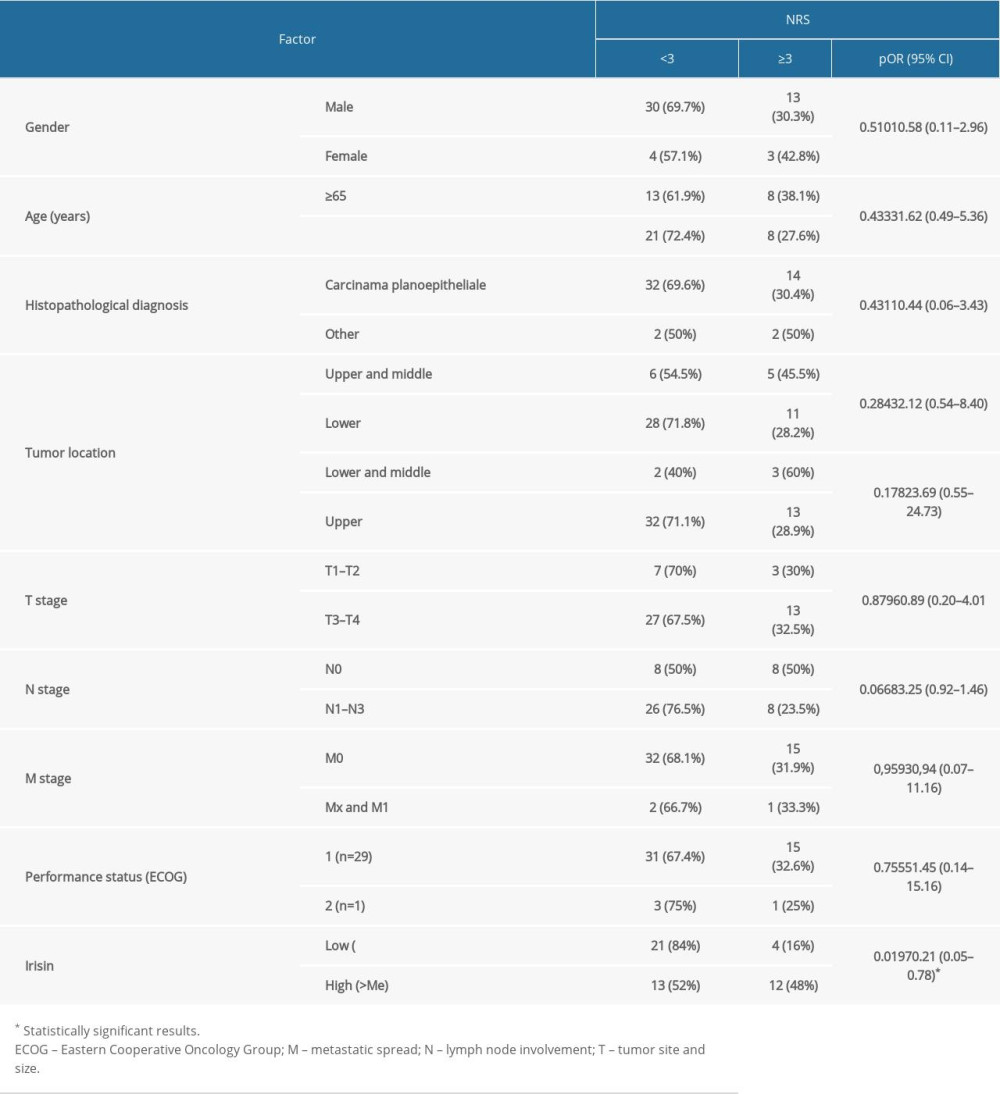
References
1. Langius JAE, Doornaert P, Spreeuwenberg MD, Radiotherapy on the neck nodes predicts severe weight loss in patients with early stage laryngeal cancer: Radiother Oncol J Eur Soc Ther Radiol Oncol, 2010; 97(1); 80-85
2. Unsal D, Mentes B, Akmansu M, Evaluation of nutritional status in cancer patients receiving radiotherapy: A prospective study: Am J Clin Oncol, 2006; 29(2); 183-88
3. Muthanandam S, Muthu J, Understanding cachexia in head and neck cancer: Asia-Pacific J Oncol Nurs [Internet], 2021; 8(5); 527-38
4. Gorenc M, Kozjek NR, Strojan P, Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy: Rep Pract Oncol Radiother, 2015; 20(4); 249-58
5. Vaitkus JA, Celi FS, The role of adipose tissue in cancer-associated cachexia: Exp Biol Med (Maywood), 2017; 242(5); 473-81
6. Małecka-Massalska T, Mlak R, Smolen A, Morshed K, Bioelectrical impedance phase angle and subjective global assessment in detecting malnutrition among newly diagnosed head and neck cancer patients: Eur Arch Otorhinolaryngol, 2016; 273(5); 1299-305
7. Hofmann T, Elbelt U, Stengel A, Irisin as a muscle-derived hormone stimulating thermogenesis – a critical update: Peptides, 2014; 54; 89-100
8. Boström P, Wu J, Jedrychowski MP, A PGC1α-dependent myokine that drives browning of white fat: Nature, 2012; 481(7382); 463-68
9. Provatopoulou X, Georgiou GP, Kalogera E, Serum irisin levels are lower in patients with breast cancer: Association with disease diagnosis and tumor characteristics: BMC Cancer, 2015; 15; 898
10. Panagiotou G, Triantafyllidou S, Tarlatzis BC, Papakonstantinou E, Serum levels of irisin and omentin-1 in breast neoplasms and their association with tumor histology: Int J Endocrinol, 2021; 2021; 6656671
11. Shahidi S, Hejazi J, Moghimi M, Circulating irisin levels and redox status markers in patients with gastric cancer: A case-control study: Asian Pac J Cancer Prev, 2020; 21(10); 2847-51
12. Petruzzelli M, Schweiger M, Schreiber R, A switch from white to brown fat increases energy expenditure in cancer-associated cachexia: Cell Metab, 2014; 20(3); 433-47
13. Zhang Z, Wan Z, Zhu Y, Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study: Nutrition, 2021; 83; 111072
14. Cao J, Xu H, Li W, Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer: Curr Probl Cancer, 2021; 45(1); 100638
15. Sobieszek G, Mlak R, Dziekanowska AS, Electrical changes in Polish patients with chronic heart failure: Preliminary observations: Medicina (Kaunas), 2019; 55(8); 484
16. Tisdale MJ, Mechanisms of cancer cachexia: Physiol Rev, 2009; 89(2); 381-410
17. Wu J, Cohen P, Spiegelman BM, Adaptive thermogenesis in adipocytes: Is beige the new brown?: Genes Dev, 2013; 27(3); 234-50
18. Ebadi M, Mazurak VC, Potential biomarkers of fat loss as a feature of cancer cachexia: Mediators Inflamm, 2015; 2015; 820934
19. Elsen M, Raschke S, Eckel J, Browning of white fat: Does irisin play a role in humans?: J Endocrinol, 2014; 222(1); R25-38
20. Perakakis N, Triantafyllou GA, Fernández-Real JM, Physiology and role of irisin in glucose homeostasis: Nat Rev Endocrinol, 2017; 13(6); 324-37
21. Aydin S, Is irisin a decisive protein in cancer cachexia and death of cancer cells?: Eur Rev Med Pharmacol Sci, 2016; 20(18); 3727-29
22. Us Altay D, Keha EE, Ozer Yaman S, Investigation of the expression of irisin and some cachectic factors in mice with experimentally induced gastric cancer: QJM, 2016; 109(12); 785-90
23. Planella-Farrugia C, Comas F, Sabater-Masdeu M, Circulating irisin and myostatin as markers of muscle strength and physical condition in elderly subjects: Front Physiol, 2019; 10; 871
24. Altay DU, Keha EE, Karagüzel E, The diagnostic value of FNDC5/irisin in renal cell cancer: Int Braz J Urol, 2018; 44(4); 734-39
Figures
 Figure 1. ROC curve showing the diagnostic usefulness of irisin in detecting malnutrition (B or C according to the SGA scale).
Figure 1. ROC curve showing the diagnostic usefulness of irisin in detecting malnutrition (B or C according to the SGA scale). Figure 2. ROC curve showing the diagnostic usefulness of irisin in detecting severe malnutrition (C according to the SGA scale).
Figure 2. ROC curve showing the diagnostic usefulness of irisin in detecting severe malnutrition (C according to the SGA scale). Figure 3. ROC curve showing the diagnostic usefulness of irisin in detecting nutritional risk (according to the NRS scale).
Figure 3. ROC curve showing the diagnostic usefulness of irisin in detecting nutritional risk (according to the NRS scale). Tables
 Table 1. Characteristic of the study group.
Table 1. Characteristic of the study group. Table 2. Influence of demographic and clinical factors on irisin level in HNC patients.
Table 2. Influence of demographic and clinical factors on irisin level in HNC patients. Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients.
Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients. Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients.
Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients. Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients.
Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients. Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients.
Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients. Table 1. Characteristic of the study group.
Table 1. Characteristic of the study group. Table 2. Influence of demographic and clinical factors on irisin level in HNC patients.
Table 2. Influence of demographic and clinical factors on irisin level in HNC patients. Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients.
Table 3. The relationship between factors reflecting malnutrition status and irisin level in HNC patients. Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients.
Table 4. The correlation between factors reflecting malnutrition status and irisin level in HNC patients. Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients.
Table 5. Influence of demographic and clinical factors on nutritional status assessed by SGA scale in HNC patients. Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients.
Table 6. Influence of demographic and clinical factors on nutritional risk assessed by NRS scale in HNC patients. In Press
05 Mar 2024 : Clinical Research
Effects of Thermal Insulation on Recovery and Comfort of Patients Undergoing Holmium Laser LithotripsyMed Sci Monit In Press; DOI: 10.12659/MSM.942836
05 Mar 2024 : Clinical Research
Role of Critical Shoulder Angle in Degenerative Type Rotator Cuff Tears: A Turkish Cohort StudyMed Sci Monit In Press; DOI: 10.12659/MSM.943703
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








