19 October 2022: Review Articles
A Review of the Role of Ultrasound Radiomics and Its Application and Limitations in the Investigation of Thyroid Disease
Wen-Wu Lu1E, Di Zhang2F, Xue-Jun Ni1E*DOI: 10.12659/MSM.937738
Med Sci Monit 2022; 28:e937738
Abstract
ABSTRACT: The incidence of thyroid disease has gradually increased in recent years. Conventional ultrasound is one of the most critical thyroid imaging methods, but it still has certain limitations. The use of B-model ultrasound (BMUS) diagnosis of thyroid disease will be affected by a doctors’ clinical experience. The ultrasound radiomics is based on ultrasound images to delineate the region of interest (ROI), and then extract features to quantify the disease information contained in the image, which helps to analyze the correlation between the image and the clinical pathology of the disease. By building a powerful model, it can be used to diagnose benign and malignant thyroid nodules, predict lymph node status in thyroid cancer, analyze molecular biological characteristics, and predict the survival of thyroid cancer patients. At present, the application of ultrasound radiomics in the thyroid is pervasive. These ultrasound radiomics studies have further promoted the progress of ultrasonic technology in the field of thyroid disease. Clinicians should be familiar with the workflow of ultrasound radiomics and understand the application of this technology to the thyroid. In this article, we first describe the workflow of ultrasound radiomics, followed by an overview of the application of ultrasound radiomics to the thyroid. Finally, some current limitations of the technology and areas for future improvement are discussed. This article aims to review the role of ultrasound radiomics and its application and limitations in the investigation of thyroid disease.
Keywords: Artificial Intelligence, Thyroid Nodule, Ultrasonography, Doppler, Humans, Ultrasonography, Thyroid Neoplasms
Background
Ultrasonography is the most common, beneficial, safe, and cost-effective method of thyroid imaging [1]. Ultrasound screening methods play an important role in the management of thyroid nodules [2]. However, doctors are affected by experience when observing ultrasound imaging to diagnose thyroid nodules, and experienced doctors have higher diagnostic accuracy than younger doctors [3]. Ultrasound imaging is a type of medical imaging that contain features of a large amount of information. In early studies, the medical image characteristics of the thyroid were analyzed to observe the abnormal areas in the organization [4,5].
Radiomics is a new field of medical image research that further expands the quantitative analysis of medical images [6]. Unlike physicians who use machines to visually observe lesions, radiomics acquires various information in images that are difficult to quantify clinical outcomes through visual observation [7]. Currently, radiomics involves extracting features from medical imaging that correlate with clinical outcomes and biological endpoints [8].
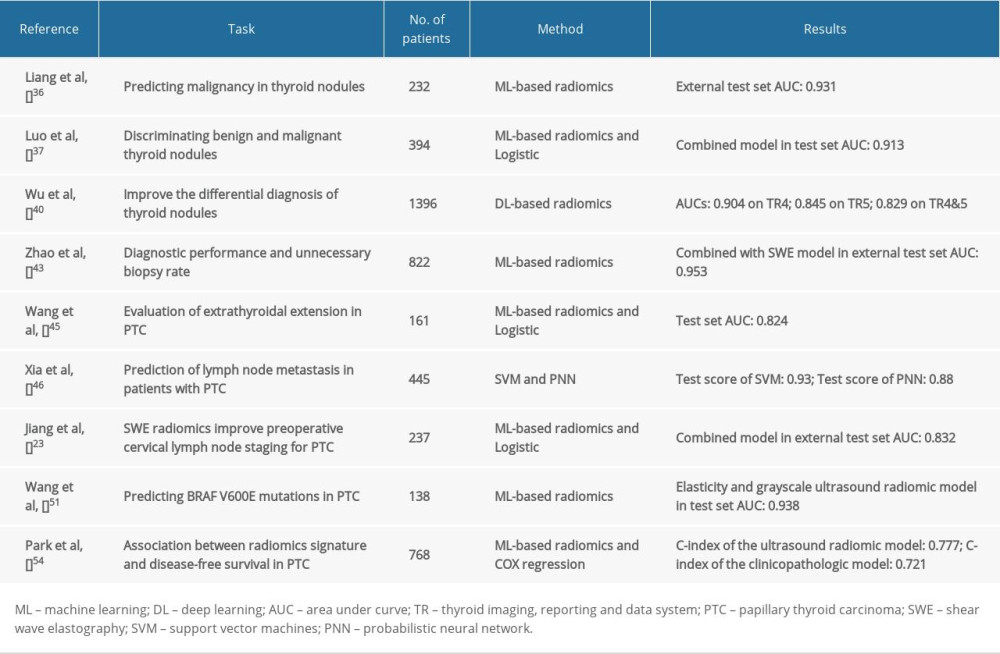
Radiomics is a powerful tool in the field of oncology and has high utility for individualized patient care [9]. Ultrasound radiomics uses image segmentation to obtain ROIs to extract features. The ROI is not necessarily a thyroid nodule or tumor, but can also be surrounding normal tissue. Imaging information in the ROI is used to develop diagnostic, predictive, or prognostic models. Currently, the application of ultrasound thyroid imaging mainly includes diagnosis of benign and malignant thyroid nodules, prediction of thyroid cancer aggressiveness to the tissue, analysis of tumor phenotype or the presence of genetic mutations, and prognostic analysis of thyroid cancer patients (Table 1). This article aims to review the workflow of ultrasound radiomics and studies on thyroid diseases.
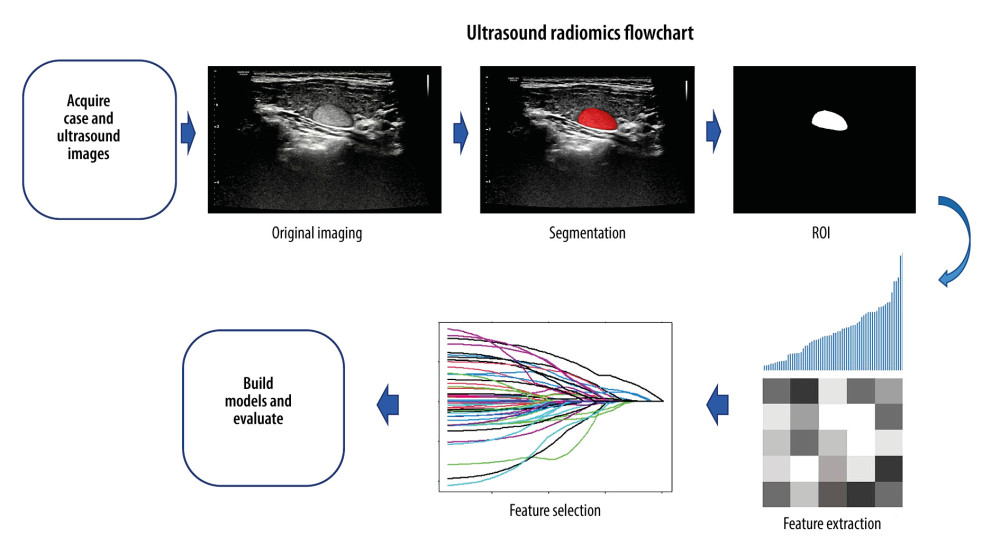
Workflow of Ultrasound Radiomics
DATA COLLECTION:
Ultrasound radiomics typically begins with image acquisition. Studying the correlation between clinical information and images requires extensive data. The medical images used for analysis generally come from different hospitals or public data centers. Physicians may perform a multi-center study or a single-center study to obtain a certain type of image, and images acquired by different operators can have large variability, which significantly limits the reliability and robustness of radiomics studies. Images from different sources will have different parameters, such as voxels or grayscale, and these differences can affect the study’s results [12]. Shafiq-UI-Hassan et al suggest that image resampling can reduce the voxel size-dependence of radiomics features [13]. In addition, studies have pointed out that using normalization in radiomics can improve the robustness of some features [14]. Ultrasound image normalization methods help to obtain reproducible measurements [15]. Researchers should pay attention to the differences in imaging and provide basic parameters of the images, such as machine name, image resolution, gray value, and gain, to achieve comparability and reproducibility among radiomics studies [16]. It is essential to eliminate unnecessary variability using a standardized imaging protocol [17].
ROI SEGMENTATION:
ROI segmentation is a fundamental step in radiomics. The target area is the region of interest (ROI), which can be a tumor lesion or normal tissue around the lesion, often determined by the research content. Commonly used segmentation methods include manual, automatic, and semi-automatic. Currently, there is no criterion standard for the target segmentation method [18]. Each segmentation method has certain challenges. Manual segmentation is very time-consuming. For CT radiomics, radiologists must observe each layer of images before segmenting the lesions, but some large lesions have dozens of tomographic images. Variability of features among operators performing segmentation makes image analysis-based machine learning (ML) nontransferable [19]. Automatic and semi-automatic segmentation methods are functions carried by some open-source software that can automatically identify and segment ROIs or partial regions. Some tumor edge contours may be poorly demarcated due to volumetric effects and may need to be refined by an experienced radiologist after using automatic or semi-automatic segmentation methods. However, some studies have found that features extracted by semi-automatic segmentation have higher repeatability and robustness than manual segmentation [20]. Heye et al compared manual and semi-automatic segmentation methods and showed that semi-automatic segmentation significantly reduced inter-observer variability [21]. Selecting an appropriate segmentation method can ensure the repeatability and reliability of features, which has a significant effect on the subsequent feature extraction [22].
Radiomics studies still use manual segmentation of ROIs. The intraclass correlation coefficient (ICC) is commonly used to evaluate radiomics features extracted by manual segmentation and to assess inter-observer and intra-observer agreement. Several ultrasound radiomics studies have used ICC-assessed radiomics features, ensuring the reproducibility of ultrasound radiomics work [23,24]. Although there are various selection methods for target segmentation, the manual segmentation method used in most studies is selected to ensure the accuracy of ROI results.
FEATURE EXTRACTION:
Radiomics was initially defined as the extraction of high-throughput features from medical images. These features are usually associated with the patient’s disease state, which is difficult for physicians to express in words, but can be converted into corresponding data information and become image features that can be quantitatively described. Most of the currently used features are obtained from segmented ROIs, which are mainly composed of voxel intensities, including morphological, first-, second-, and higher-order features [25].
Morphological features are often used to describe the surface area, volume, and diameter of ROIs. Common statistical values are available for first-order features, including maximum, minimum, entropy, and kurtosis. Second-order features are usually used to describe the texture features of images, including gray-level co-occurrence matrices and gray-level run-length matrices. The grayscale co-occurrence matrix provides pixels with the same signal intensity at adjacent frequencies as a matrix, describing the signal intensity density in a specific direction, thereby revealing differences in regional heterogeneity [26]. The grayscale run-length matrix can calculate the frequency at which the nearest neighbor pixels match in intensity, giving insight into the heterogeneity of signal intensities within the ROI [27]. Higher-order features are statistical features calculated by including multiple pixels in the matrix. Wavelet transform features are also high-order features, and many studies have used them in radiomics and ultrasound [28,29]. Higher-order feature elements are usually obtained from grayscale values by Fourier transform, which converts spatial information to frequency space and then back to the spatial domain [30].
FEATURE SELECTION:
There are usually hundreds or thousands of omics features of ROIs. Not all of these features are useful, and it is often necessary to select further features that contain critical information. At the same time, from the statistical point of view, some variables have collinearity, resulting in a significant overlap of variable information. If all are included in the model, it can eventually lead to poor model performance. To simplify the model, researchers are also required to select as few model variables as possible. This requires deleting redundant features in many radiomics features to reduce dimensionality.
Currently, feature selection methods are mainly divided into 3 categories: filtering, wrapping, and embedding methods [31]. Filtering refers to the use of univariate or multivariate methods to evaluate features and rank them. Univariate filtering methods can use the chi-square test or Mann-Whitney U test to rank the quality of features. Multivariate methods often rank features according to their relevance, consisting of a ranker and a subset selector. In many radiomics studies, the filtering method was used for preliminary feature screening [32,33]. The wrapping method evaluates the features in reverse through the model and then selects these multivariate features and subset features. The embedding method continuously evaluates features through learning in building a model and using these filtered features to build a model. In other words, the embedding method is a simultaneous feature selection and model learning process, such as least absolute shrinkage and selection operator (LASSO). LASSO is a method for feature screening and modeling used in many studies [34–37].
MODELING:
Building a model is the ultimate goal of many radiomics projects, often with finalized features, including supervised learning, unsupervised learning, and semi-supervised learning. Supervised learning refers to training a model with clinical classification labels. The most commonly used supervised learning models in ultrasound radiomics are built through logistic regression models [23,24], and other learning models such as support vector machines (SVM) and random forests [38]. Unsupervised learning does not require clinical classification labels; it continuously trains and optimizes through machine learning, and then builds the model. Commonly used model algorithms include cluster analysis [39]. Semi-supervised learning is often used to construct a semi-supervised deep learning framework to conduct related model training research, such as research on deep learning of ultrasound images in the identification of benign and malignant thyroid nodules [40].
After the model is established, the performance of the model needs to be verified or tested. It is generally believed that the independent external validation model is more accurate and reliable than the internal validation model, which is also a large-sample, multi-center study advocated by many radiomics research institutes. The performance of the model is often evaluated by the receiver operating characteristic curve (ROC), which includes indicators such as the area under the curve (AUC), sensitivity, and specificity. Calibration curves are often used to evaluate the agreement between model predictions and clinical outcomes [41]. In addition, the clinical decision curve (DCA) is also commonly used to evaluate the effectiveness of the model in clinical application.
Application of Ultrasound Radiomics in Thyroid Imaging
DIAGNOSIS OF BENIGN AND MALIGNANT THYROID NODULES BY ULTRASOUND RADIOMICS:
The American Society of Imaging (ACR) published a white paper in 2017 on the Thyroid Imaging Reporting and Data System (TI-RADS), which proposed risk grading methods for classifying thyroid nodules. The report is helpful for sonographers to standardize the description of lesions. At the same time, thyroid lesions were graded [42] by adding 5 ultrasound characteristics: composition, shape, echo, margin, and calcification scores. Ultrasonography in the diagnosis of thyroid nodules is often compared with or combined with ACR TI-RADS to evaluate the diagnostic value or utility of ultrasonography [37].
Several studies have compared the diagnostic performance of ultrasound radiomics and TI-RADS for thyroid nodules [36,40,43]. Liang et al [36] concluded that ultrasound radiomics was superior to performance of primary physicians using ACR TI-RADS for diagnosing thyroid nodules. Radiomics score was developed using the ultrasound images of the training cohort, and 19 features were screened from 1044 radiomics features using the LASSO regression model to construct an ultrasound radiomics scoring formula. Five predictive models were constructed based on the above radiomics and TI-RADS scores, respectively. The ultrasound radiomics score showed good discrimination, significantly better than the discrimination obtained by primary radiologists using the TI-RADS score in both cohorts.
Luo et al [37] extracted 286 radiomics features from ultrasound images by retrospectively collecting images of thyroid nodules. Four features were finally selected to establish the radiomics score. The Delong test and DCA showed that the method combining radiomics scoring and ACR TI-RADS had the best performance. The results indicated that the combined ultrasound radiomics score and ACR TI-RADS model was better at distinguishing benign and malignant thyroid nodules than either ACR TI-RADS or radiomics score alone. Radiomics score can improve the identification of benign and malignant thyroid nodules by ACR TI-RADS. Zhao et al [43] retrospectively collected two-dimensional ultrasound images and shear wave elastography (SWE) of patients’ thyroid nodules, and extracted features of the 2 kinds of ultrasound images. Radiomics features in images are combined with machine learning methods to establish machine learning-assisted visualization methods. The results showed that the diagnostic performance of the machine learning-assisted vision method was superior to that of ultrasound radiomics alone and ACR TI-RADS. After adding SWE image features of machine learning auxiliary visual methods, the performance of the model was further improved, and the rate of unnecessary fine-needle aspiration (FNA) was also significantly reduced. The above studies show that ultrasound radiomics can establish the correlation between ultrasound image features and malignant nodules, which will help clinicians to improve the accuracy of diagnosis based on use of ACR TI-RADS.
ULTRASOUND RADIOMICS ASSESSMENT OF THYROID CANCER AGGRESSIVENESS AND LYMPH NODE METASTASIS:
Papillary thyroid carcinoma (PTC) accounts for about 90% of thyroid cancers and generally has a good prognosis [44]. However, some PTC subtypes are often more aggressive, with extrathyroidal invasion and lymph node metastasis. The presence or absence of extrathyroidal invasion and lymph node metastasis is often an essential factor to be considered in surgery. Therefore, the non-invasive diagnosis of invasiveness of thyroid cancer to surrounding tissues and lymph nodes is the focus of many studies.
Wang et al. [45] extracted image features of lesions in the ultrasound images of PTC patients, using LASSO and multivariate logistic regression analysis to screen out the factors related to PTC invasion of surrounding tissues, and established an ultrasound radiomics diagnostic model. The results indicated that ultrasound radiomics has excellent diagnostic utility for assessing the tissue invasiveness of PTC.
Xia et al [46] retrospectively collected data on PTC patients whose cervical lymph nodes were prophylactically dissected, and predicted PTC lymph node metastasis using SVM and probabilistic neural network (PNN) models on clinicopathological factors and ultrasound characteristics. The results of the 2 models indicated that the combination of artificial intelligence algorithms and clinicopathological data can effectively predict thyroid cancer preoperative lymph node metastasis. In addition, Jiang et al [23] extracted ultrasound radiomics features from B-mode ultrasound and SWE images to construct 2 models of ultrasound radiomics scores, and then used multivariate logistic regression analysis together with clinical data to construct a nomogram. The results showed that ultrasound-reported lymph node status, multifocality, and SWE radiomics scores were independent risk factors associated with lymph node status in patients with PTC. The studies mentioned above demonstrate that ultrasound radiomics can noninvasively predict the invasiveness of thyroid cancer to surrounding tissues and lymph nodes.
ULTRASOUND RADIOMICS PREDICTS ASSOCIATIONS BETWEEN THYROID CANCER AND MOLECULAR BIOLOGICAL PROPERTIES:
Early and correct diagnosis of malignant thyroid nodules is essential for treatment. Histopathological results are generally considered to be the most accurate and reliable criterion standard for diagnosing thyroid nodules. Some early pathological examinations found that the BRAF gene of PTC patients was often accompanied by mutations [47]. Detection of these mutated genes can only be obtained using invasive testing. Later, some researchers found that some manifestations on ultrasound images may be associated with gene mutation [48]. However, the visual representation of images alone may lack objectivity, so some researchers have used ultrasound radiomics to analyze the relationship between imaging features and molecular biological features of thyroid cancer patients.
Yoon et al. [49] assessed whether ultrasound-based radiomics could predict BRAFV600E mutation in PTC patients. They used preoperative ultrasound images of 527 patients for feature extraction and generated a radiomics score by LASSO. Multivariate analysis revealed that the radiomics score was a single risk factor for BRAFV600E mutation. This suggests that radiomics features extracted from ultrasound images have value in predicting BRAFV600E mutations in PTC patients. In addition, Kwon et al. [50] extracted 86 radiomics features from ultrasound images and used 3 classifier models to evaluate whether ultrasound radiomics could predict BRAF mutation in PTC patients. The classifiers are logistic regression, SVM, and Random Forest. The results showed that in PTC, all classifier models had moderate performance in predicting BRAF gene mutations. Wang et al. [51] retrospectively collected preoperative grayscale and elastography images of 138 patients with PTC and selected ultrasound radiomics features related to BRAFV600E mutation. Finally, 8 radiomics features were extracted from grayscale ultrasound images, and 5 radiomics features were extracted from elastic ultrasound images. The results of the study showed that the radiomics model based on grayscale and elastic ultrasound has an excellent predictive value for BRAFV600E mutations in PTC patients.
ULTRASOUND RADIOMICS TO ASSESS SURVIVAL IN PATIENTS WITH PTC:
Although PTC is a histological type with a good prognosis in thyroid cancer, a small proportion of PTC cases are still aggressive. About 9.1–13.3% of patients have recurrence, and 1.4–5.2% die from thyroid cancer [52,53]. Park et al [54] developed a model based on radiomics features of ultrasound images to assess disease-free survival in PTC patients. The radiomics scoring model was constructed in COX regression, and the results showed that the ultrasound radiomics model performed better in assessing disease-free survival than the clinicopathological model (C- index: 0.777 vs 0.721).
Discussion
The characteristic of many tumors is often determined by biopsy, but some tumors are heterogeneous, and biopsy samples are not representative of the entire lesion. Ultrasound radiomics, which analyzes the overall image of the lesion, can visualize tumor heterogeneity and can serve as an intermediate step between imaging and biosy [16]. Ultrasound radiomics has yielded many achievements in the screening, diagnosis, and evaluation of thyroid tumors and can be used as an evaluation tool for clinicians to assess the pathophysiological status of various aspects of the thyroid.
As a machine learning method, the ultrasound radiomics, most studies by retrospect the clinical pathological information of patients, and use pathological diagnosis as the criterion standard, analyze the characteristics of the image, and establish a predictive model to improve the diagnosis accuracy of early thyroid nodules. ACR TI-ARDS is one of the most popular models, in which 5 risk factors related to malignant thyroid nodules can be distinguished by radiologists with human vision, which plays a significant role in clinical application. However, ultrasound radiomics often considers other meaningful risk factors based on ACR TI-RADS and has improves TI-RADS. At present, radiomics no longer only pays attention to the image features of patients, but also considers other clinical factors so that the diagnosis and treatment of patients are more individualized and comprehensive, and it is used to solve clinical problems [55].
Ultrasound radiomics also suffers from the limitations of all machine learning algorithms. First, most studies have insufficient sample sizes. Adequate sample size is key to building an excellent radiomics model. If the sample size is too small, the image features extracted may not be representative and not convincing enough. It is often manifested as overfitting or overly good results on the model. Second, most ultrasound imaging groups are influenced by clinician experience. For example, the data acquisition and target segmentation processes in the workflow are uncontrollable, and these 2 steps are the biggest challenges concerning reproducibility in radiomics research. The case data collected in most current radiomics studies are not derived from public databases, and research results are usually not transferable to applications. Therefore, most studies advocate using multi-center data to develop radiomics models, which can enhance the persuasiveness of the study and the generalizability of the model [56]. In addition, manual segmentation methods are still essential segmentation methods. This creates unavoidable errors and subjectivity. Last but not least, most ultrasound radiomics-based thyroid studies have used retrospectively collected data [23,24,36–38,43,45]. Although some prospective studies have evaluated diagnostic models for thyroid diseases and demonstrated good diagnostic performance [57,58], there have been few prospective studies on ultrasound radiomics models, and more prospective studies are needed in the future to confirm the clinical feasibility of ultrasound radiomics, not just for the thyroid.
Conclusions
In conclusion, ultrasound radiomics, as a technique for extracting image data, plays an important role in the evaluation of medical images of related diseases. However, there are some deficiencies, and more efforts are needed to standardize the discipline of ultrasound radiomics and to apply ultrasound radiomics to clinical work.
References
1. Blum M, Ultrasonography of the Thyroid: Endotext, 2000, South Dartmouth (MA)
2. Liu R, Zhang B, Role of ultrasound in the management of thyroid nodules and thyroid cancer: Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2017; 39(3); 445-50
3. Chen Y, Gao Z, He Y, An artificial intelligence model based on ACR TI-RADS characteristics for US diagnosis of thyroid nodules: Radiology, 2022; 303(3); 613-19
4. Chang Y, Paul AK, Kim N, Computer-aided diagnosis for classifying benign versus malignant thyroid nodules based on ultrasound images: A comparison with radiologist-based assessments: Med Phys, 2016; 43(1); 554
5. Jin Z, Zhu Y, Zhang S, Ultrasound Computer-Aided Diagnosis (CAD) based on the Thyroid Imaging Reporting and Data System (TI-RADS) to distinguish benign from malignant thyroid nodules and the diagnostic performance of radiologists with different diagnostic experience: Med Sci Monit, 2020; 26; e918452
6. Lambin P, Rios-Velazquez E, Leijenaar R, Radiomics: Extracting more information from medical images using advanced feature analysis: Eur J Cancer, 2012; 48(4); 441-46
7. Lohmann P, Bousabarah K, Hoevels M, Treuer H, Radiomics in radiation oncology-basics, methods, and limitations: Strahlenther Onkol, 2020; 196(10); 848-55
8. Avanzo M, Wei L, Stancanello J, Machine and deep learning methods for radiomics: Med Phys, 2020; 47(5); e185-e202
9. Yin R, Jiang M, Lv WZ, Study processes and applications of ultrasomics in precision medicine: Front Oncol, 2020; 10; 1736
10. Gillies RJ, Schabath MB, Radiomics improves cancer screening and early detection: Cancer Epidemiol Biomarkers Prev, 2020; 29(12); 2556-67
11. Kumar V, Gu Y, Basu S, Radiomics: The process and the challenges: Magn Reson Imaging, 2012; 30(9); 1234-48
12. He L, Huang Y, Ma Z, Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule: Sci Rep, 2016; 6; 34921
13. Shafiq-Ul-Hassan M, Zhang GG, Latifi K, Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels: Med Phys, 2017; 44(3); 1050-62
14. Shafiq-Ul-Hassan M, Latifi K, Voxel size and gray level normalization of CT radiomic features in lung cancer: Sci Rep, 2018; 8(1); 10545
15. Kyriacou EC, Pattichis C, Pattichis M, A review of noninvasive ultrasound image processing methods in the analysis of carotid plaque morphology for the assessment of stroke risk: IEEE Trans Inf Technol Biomed, 2010; 14(4); 1027-38
16. Lambin P, Leijenaar RTH, Deist TM, Radiomics: The bridge between medical imaging and personalized medicine: Nat Rev Clin Oncol, 2017; 14(12); 749-62
17. Sollini M, Antunovic L, Chiti A, Kirienko M, Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics: Eur J Nucl Med Mol Imaging, 2019; 46(13); 2656-72
18. Ma M, Feng Z, Peng T, Radiomics and its advances in hepatocellular carcinoma: Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2019; 44(3); 225-32
19. Muller-Franzes G, Nebelung S, Schock J, Reliability as a precondition for trust-segmentation reliability analysis of radiomic features improves survival prediction: Diagnostics (Basel), 2022; 12(2); 247
20. Parmar C, Rios Velazquez E, Leijenaar R, Robust radiomics feature quantification using semiautomatic volumetric segmentation: PLoS One, 2014; 9(7); e102107
21. Heye T, Merkle EM, Reiner CS, Reproducibility of dynamic contrast-enhanced MR imaging. Part II. Comparison of intra- and interobserver variability with manual region of interest placement versus semiautomatic lesion segmentation and histogram analysis: Radiology, 2013; 266(3); 812-21
22. van Velden FH, Kramer GM, Frings V, Repeatability of radiomic features in non-small-cell lung cancer [(18)F]FDG-PET/CT studies: Impact of reconstruction and delineation: Mol Imaging Biol, 2016; 18(5); 788-95
23. Jiang M, Li C, Tang S, Nomogram based on shear-wave elastography radiomics can improve preoperative cervical lymph node staging for papillary thyroid carcinoma: Thyroid, 2020; 30(6); 885-97
24. Zhang D, Wei Q, Wu GG, Preoperative prediction of microvascular invasion in patients with hepatocellular carcinoma based on radiomics nomogram using contrast-enhanced ultrasound: Front Oncol, 2021; 11; 709339
25. Gillies RJ, Kinahan PE, Hricak H, Radiomics: Images are more than pictures, they are data: Radiology, 2016; 278(2); 563-77
26. Azary H, Abdoos M, A semi-supervised method for tumor segmentation in mammogram images: J Med Signals Sens, 2020; 10(1); 12-18
27. Becker AS, Schneider MA, Wurnig MC, Radiomics of liver MRI predict metastases in mice: Eur Radiol Exp, 2018; 2(1); 11
28. Cheng Z, Huang Y, Huang XEffects of different wavelet filters on correlation and diagnostic performance of radiomics features: Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2019; 44(3); 244-50 [In Chinese]
29. Wang H, Li X, Yuan Y, Association of machine learning ultrasound radiomics and disease outcome in triple negative breast cancer: Am J Cancer Res, 2022; 12(1); 152-64
30. Drabycz S, Stockwell RG, Mitchell JR, Image texture characterization using the discrete orthonormal S-transform: J Digit Imaging, 2009; 22(6); 696-708
31. Bagherzadeh-Khiabani F, Ramezankhani A, Azizi F, A tutorial on variable selection for clinical prediction models: Feature selection methods in data mining could improve the results: J Clin Epidemiol, 2016; 71; 76-85
32. Liu Z, Zhang XY, Shi YJ, Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: Clin Cancer Res, 2017; 23(23); 7253-62
33. Chamming’s F, Ueno Y, Ferre R, Features from computerized texture analysis of breast cancers at pretreatment mr imaging are associated with response to neoadjuvant chemotherapy: Radiology, 2018; 286(2); 412-20
34. Wu X, Li J, Mou Y, Radiomics nomogram for identifying Sub-1 cm benign and malignant thyroid lesions: Front Oncol, 2021; 11; 580886
35. Wu S, Zheng J, Li Y, A radiomics nomogram for the preoperative prediction of lymph node metastasis in bladder cancer: Clin Cancer Res, 2017; 23(22); 6904-11
36. Liang J, Huang X, Hu H, Predicting malignancy in thyroid nodules: Radiomics score versus 2017 American College of Radiology thyroid imaging, reporting and data system: Thyroid, 2018; 28(8); 1024-33
37. Luo P, Fang Z, Zhang P, Radiomics score combined with ACR TI-RADS in discriminating benign and malignant thyroid nodules based on ultrasound images: A retrospective study: Diagnostics (Basel), 2021; 11(6); 1011
38. Qin H, Wu YQ, Lin P, Ultrasound image-based radiomics: An innovative method to identify primary tumorous sources of liver metastases: J Ultrasound Med, 2021; 40(6); 1229-44
39. Itakura H, Achrol AS, Mitchell LA, Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities: Sci Transl Med, 2015; 7(303); 303ra138
40. Wu GG, Lv WZ, Yin R, Deep learning based on ACR TI-RADS can improve the differential diagnosis of thyroid nodules: Front Oncol, 2021; 11; 575166
41. Kramer AA, Zimmerman JE, Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited: Crit Care Med, 2007; 35(9); 2052-56
42. Tessler FN, Middleton WD, Grant EG, ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS Committee: J Am Coll Radiol, 2017; 14(5); 587-95
43. Zhao CK, Ren TT, Yin YF, A comparative analysis of two machine learning-based diagnostic patterns with Thyroid Imaging Reporting and Data System for thyroid nodules: Diagnostic performance and unnecessary biopsy rate: Thyroid, 2021; 31(3); 470-81
44. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69(1); 7-34
45. Wang X, Agyekum EA, Ren Y, A radiomic nomogram for the ultrasound-based evaluation of extrathyroidal extension in papillary thyroid carcinoma: Front Oncol, 2021; 11; 625646
46. Xia E, Chi Y, Jin L, Preoperative prediction of lymph node metastasis in patients with papillary thyroid carcinoma by an artificial intelligence algorithm: Am J Transl Res, 2021; 13(7); 7695-704
47. Liu X, Yan K, Lin X, The association between BRAF (V600E) mutation and pathological features in PTC: Eur Arch Otorhinolaryngol, 2014; 271(11); 3041-52
48. Kabaker AS, Tublin ME, Nikiforov YE, Suspicious ultrasound characteristics predict BRAF V600E-positive papillary thyroid carcinoma: Thyroid, 2012; 22(6); 585-89
49. Yoon JH, Han K, Lee E, Radiomics in predicting mutation status for thyroid cancer: A preliminary study using radiomics features for predicting BRAFV600E mutations in papillary thyroid carcinoma: PLoS One, 2020; 15(2); e0228968
50. Kwon MR, Shin JH, Park H, Radiomics study of thyroid ultrasound for predicting BRAF mutation in papillary thyroid carcinoma: Preliminary results: Am J Neuroradiol, 2020; 41(4); 700-5
51. Wang YG, Xu FJ, Agyekum EA, Radiomic model for determining the value of elasticity and grayscale ultrasound diagnoses for predicting BRAF(V600E) mutations in papillary thyroid carcinoma: Front Endocrinol (Lausanne), 2022; 13; 872153
52. Cho BY, Choi HS, Park YJ, Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades: Thyroid, 2013; 23(7); 797-804
53. Londero SC, Krogdahl A, Bastholt L, Papillary thyroid carcinoma in Denmark, 1996-2008: outcome and evaluation of established prognostic scoring systems in a prospective national cohort: Thyroid, 2015; 25(1); 78-84
54. Park VY, Han K, Lee E, Association between radiomics signature and disease-free survival in conventional papillary thyroid carcinoma: Sci Rep, 2019; 9(1); 4501
55. Mayerhoefer ME, Materka A, Langs G, Introduction to radiomics: J Nucl Med, 2020; 61(4); 488-95
56. Peng S, Liu Y, Lv W, Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study: Lancet Digit Health, 2021; 3(4); e250-e59
57. Yoo YJ, Ha EJ, Cho YJ, Computer-aided diagnosis of thyroid nodules via ultrasonography: initial clinical experience: Korean J Radiol, 2018; 19(4); 665-72
58. Park VY, Han K, Seong YK, Diagnosis of thyroid nodules: Performance of a deep learning convolutional neural network model vs. radiologists: Sci Rep, 2019; 9(1); 17843
In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952